Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12247
Peer-review started: April 13, 2022
First decision: June 16, 2022
Revised: June 21, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: November 26, 2022
Processing time: 223 Days and 11 Hours
Loeys-Dietz syndrome (LDS) is a rare autosomal dominant syndrome characterized by heterozygous mutations causing multisystemic alterations. It was recently described in 2005, and today at least six different subtypes have been identified. Classically presenting with aortic root enlargement or aneurysms and craniofacial and skeletal abnormalities, with specific arterial tortuosity at any site. The differential diagnosis of LDS includes atypical Marfan syndrome, vascular Ehlers-Danlos syndrome, Shprintzen-Goldberg craniosynostosis, and familial aortic aneurysm and dissection syndrome.
We present a case study of a 35-year-old female who came to the emergency department due to lower gastrointestinal bleeding and severe abdominal pain. Computed tomography revealed vascular tortuosity in almost every abdominal vein.
This case report will help us analyze the infrequent presentation of LDS type 4 and the numerous complications that it implies, underlying the importance of publishing more cases in order to expand our knowledge and offer better treatment for these patients. Differential diagnosis, clinical presentation and treatment options for this syndrome are discussed in this article.
Core Tip: Diagnosing and treating Loeys-Dietz syndrome within its many comorbidities is very challenging for most clinicians. Suspected Loeys-Dietz syndrome in a patient without aortic aneurysm should not be discarded. We describe a case to enhance the clinical suspicion of this genetic disease for proper management within a variety of medical specialties. The management and follow-up depend upon each patient and their manifestations.
- Citation: Azrad-Daniel S, Cupa-Galvan C, Farca-Soffer S, Perez-Zincer F, Lopez-Acosta ME. Unusual presentation of Loeys-Dietz syndrome: A case report of clinical findings and treatment challenges. World J Clin Cases 2022; 10(33): 12247-12256
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12247.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12247
Loeys-Dietz syndrome (LDS) is an autosomal dominant syndrome characterized by heterozygous mutations in the genes transforming growth factor β receptor (TGFBR1 or TGFBR2) and other related genes such as SMAD2, SMAD3 (Small Mothers Against Decapentaplegic), TGFB2, and TGFB3, which modify the physiological development and function of the extracellular matrix, resulting in cardiovascular and multisystem abnormalities[1,2]. The clinical classic manifestations consist of bifid uvula and/or cleft palate, hypertelorism, and tortuous aortic and arterial aneurysms[3]. Although these were the most typical characteristics when it was first described in 2005, a wide range in the involvement of many different organ systems was also observed due to its variable clinical expression. In the first descriptions of this syndrome, LDS patients were classified into two categories, according to craniofacial or cutaneous traits. Nonetheless, these findings are now believed to be part of a spectrum within the LDS syndrome[4].
The greater number of individuals with LDS 1 and 2 show vascular features compelling critical aortic dissection and large aortic aneurysms, thus early death has been reported at a median of 37 years[5]. LDS type 4 syndrome has been rarely reported, therefore its clinical presentation is not yet completely known. It is puzzling that deletions and loss-of-function mutations of TGFB2 induce increased activity of TGF-β[6]. Some dermatologic features such as easy bruising, velvety and translucent skin and atrophic scarring, are additionally reported to various degrees (Table 1).
| LDS type | Gene symbol | Location | Gene/locus MIM number | Clinical signs | Other syndromes related |
| LDS 1 | TGFBR1 | 9q22.33 | 190181 | -Micrognathia | -TAAD (previously LDS1a, 1b, 2a, 2b) |
| -Hypertelorism | |||||
| -Exotropia | |||||
| -Blue sclerae | |||||
| -Arterial tortuosity, generalized | |||||
| -Patent ductus arteriosus | |||||
| -Ascending aortic aneurysm | |||||
| -Ascending aortic dissection | |||||
| -Pulmonary artery aneurysm | |||||
| -Bifid uvula | |||||
| -Malar hypoplasia | |||||
| -Joint laxity | |||||
| -Translucent skin | |||||
| LDS 2 | TGFBR2 | 3p24.1 | 190182 | -Micrognathia | -TAAD |
| -Hypertelorism | -MFS2 (previously LDS 1a, 1b, 2a, 2b) | ||||
| -Exotropia | |||||
| -Blue sclerae | |||||
| -Proptosis | |||||
| -Hypertelorism | |||||
| -Bifid uvula | |||||
| -Arterial tortuosity | |||||
| -Pectus deformity | |||||
| -Malar hypoplasia | |||||
| -Brachydactyly | |||||
| -Patent ductus arteriosus | |||||
| -Ascending aortic aneurysm and dissection | |||||
| -Pulmonary artery aneurysm | |||||
| LDS 3 | SMAD3 | 15q22.33 | 603109 | -Aneurysms-osteoarthritis syndrome | --- |
| -High-arched palate | |||||
| -Mitral valve prolapse and regurgitation | |||||
| -Aortic insufficiency | |||||
| -Left ventricular hypertrophy | |||||
| -Atrial fibrillation | |||||
| -Intervertebral disc degeneration | |||||
| -Dural ectasia | |||||
| -Spondylysis | |||||
| -Camptodactyly | |||||
| -Uncovertebral (C3-C7) joint osteoarthritis | |||||
| LDS 4 | TGFB2 | 1q41 | 190220 | -Retrognathia | --- |
| -High-arched palate | |||||
| -Hypertelorism | |||||
| -Mitral valve prolapse | |||||
| -Aortic root aneurysm | |||||
| -Aortic dissection | |||||
| -Arterial tortuosity | |||||
| -Cerebrovascular aneurysm | |||||
| -Fusiform dilation and tortuosity of cerebrovascular arteries | |||||
| -Pectus deformity | |||||
| -Inguinal hernia | |||||
| -Flat feet | |||||
| LDS 5 | TGFB3 | 14q24.3 | 190230 | -Tall stature | ---- |
| -Retrognathia, mild | |||||
| -Long face | |||||
| -Blue sclerae | |||||
| -Downslanting palpebral fissures | |||||
| -Bifid uvula | |||||
| -Cleft palate | |||||
| -Mitral insufficiency | |||||
| -Aortic root dilation | |||||
| -Aneurysm of thoracic aorta | |||||
| -Aneurysm of abdominal aorta | |||||
| -Aneurysmal dissection or rupture | |||||
| -Elastic fiber fragmentation in aneurysmal aortic wall | |||||
| -Pectus excavatum | |||||
| -Pectus carinatum | |||||
| -Hiatal hernia | |||||
| -Kyphoscoliosis | |||||
| LDS 6 | SMAD2 | 18q21.1 | 601366 | -Tall stature | --- |
| -Dolichocephaly | |||||
| -Midface hypoplasia | |||||
| -Retrognathia | |||||
| -Hypertelorism | |||||
| -Downslanting palpebral fissures | |||||
| -High-arched palate | |||||
| -Broad uvula | |||||
| -Any cardiac valve prolapse or insufficiency | |||||
| -Thoracic aortic aneurysm | |||||
| -Tortuosity of aorta | |||||
| -Arterial tortuosity and aneurysms | |||||
| -Pectus deformity | |||||
| -Diaphragmatic hernia | |||||
| -Diverticulosis | |||||
| -Dolichocephaly | |||||
| -Positive wrist and thumb sign | |||||
| -Genu valgum | |||||
| -Easy bruising | |||||
| -Varicose veins | |||||
| -Migraine |
The diagnosis of LDS is established in a person without a known family history of LDS who has a heterozygous pathogenic variant in SMAD2, SMAD3, TGFB2, TGFB3, TGFBR1, or TGFBR2 and EITHER of the following[7]: (1) Aortic root enlargement (defined as an aortic root z-score ≥ 2.0) or type A dissection; and (2) Compatible systemic features including characteristic craniofacial, skeletal, cutaneous, and/or vascular manifestations found in combination. Additional emphasis is given to arterial tortuosity, prominently including the head and neck vessels, and to aneurysms or dissections involving medium-to-large muscular arteries throughout the arterial tree.
Some of the alterations described include skeletal, craniofacial, cutaneous and ocular alterations. Whilst LDS shows clinical overlap with Marfan syndrome (MFS), it can be clinically distinguished from the latter. Some clinical manifestations that can be found in both LDS and Marfan syndrome involve pectus deformities, aortic root aneurysm, arachnodactyly and scoliosis. Discriminating features in LDS are micrognathia, cranial alterations, hypertelorism, cleft palate or bifid uvula, intervertebral disc degeneration, club feet, and primarily dilated and tortuous arteries and early aortic rupture (Table 1). In LDS there is no typical marfanoid habitus or lens displacement.
Histologic analysis of the original series reported diminished elastin content and chaotic elastic fibers in the aortic media of patients with classic MFS or mutations in TGFBR2 compared with samples from age-matched controls[2]. Morphologic analysis revealed loss of intimate spatial association between elastin deposits and vascular smooth muscle cells. These features were noted in young children and in the absence of inflammation, expressing a dire imperfection in elastogenesis alternatively to secondary elastic fiber elimination. Furthermore, a noticeable overabundance of aortic wall collagen in individuals with MFS compared with age-matched controls was highlighted in individuals with TGFBR2 mutations. As multiple collagens normally expressed in the aorta are derived from early-induced TGF-β target genes (including COL1A1 and COL3A1), this consists of increased (rather than decreased) TGF-β signaling.
The differential diagnosis of LDS includes atypical Marfan syndrome, Shprintzen-Goldberg craniosynostosis, vascular Ehlers-Danlos syndrome, and familial aortic aneurysm and dissection syndrome. Arterial tortuosity syndrome is a closely related syndrome that is also characterized by severe tortuosities, stenosis, and aneurysms of large and mid-sized arteries.
We present a 35-year-old Hispanic woman who came to the emergency department of a private hospital in Mexico City due to lower gastrointestinal (GI) bleeding and abdominal pain. Her severe abdominal pain was localized in the hypogastrium and was accompanied by diarrhea with clots and hematochezia for the last 48 h that was not relieved by over-the-counter medications.
Recent prescription for hormonal contraceptives.
Her past medical history revealed prior lower GI bleedings during childhood that resolved spontaneously, hypothyroidism and a recent diagnosis of polycystic ovary syndrome that required her to take hormonal contraceptives for five months prior to her visit.
The patient’s mother has diabetes mellitus type 2.
On physical examination she was tachycardic (112 bpm) with normal blood pressure (112/75 mmHg) and 90% oxygen saturation. She did not have a fever or cough. She had sinus tachycardia with a clear systolic heart murmur and diffuse severe abdominal pain. She also had upper back pain consistent with muscular contracture. The rest of the physical examination was normal.
The only abnormal findings in her laboratory tests were low albumin (3.1 mg/dL) and an elevated D dimer at 10000 μg/mL (normal value: less than 0.5 μg/mL). We ordered laboratory tests to search for hematologic disturbances and thrombophilias such as factor V Leiden, homocysteine, lupus anticoagulant, antineutrophil cytoplasmic antibodies (ANCAs), extractable nuclear antigen (ENA) panel (ENAs), rheumatoid factor, and anticardiolipin antibody, all which were normal.
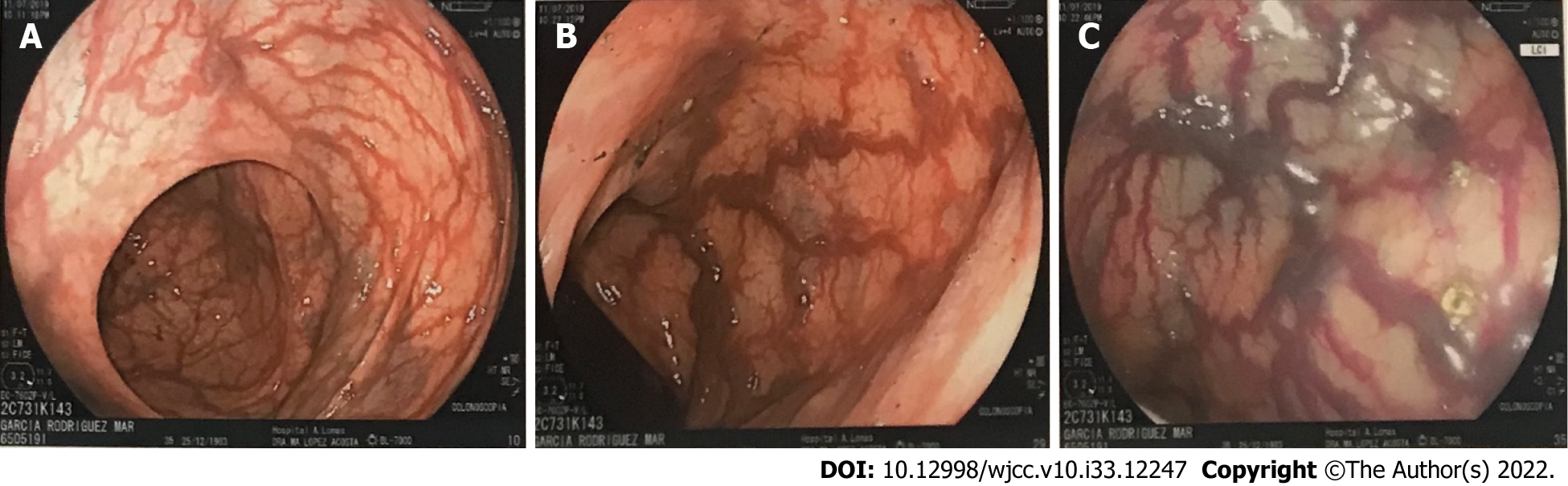
Our team of gastroenterologists performed an upper endoscopy and colonoscopy to identify the etiology of her lower GI bleeding. Colonoscopy revealed tortuous internal hemorrhoid veins and increased caliber in colon vessels as well as in the terminal ileum (Figure 1), which increased suspicion for an ongoing venous thrombosis vs mesenteric ischemia. Biopsies were obtained.
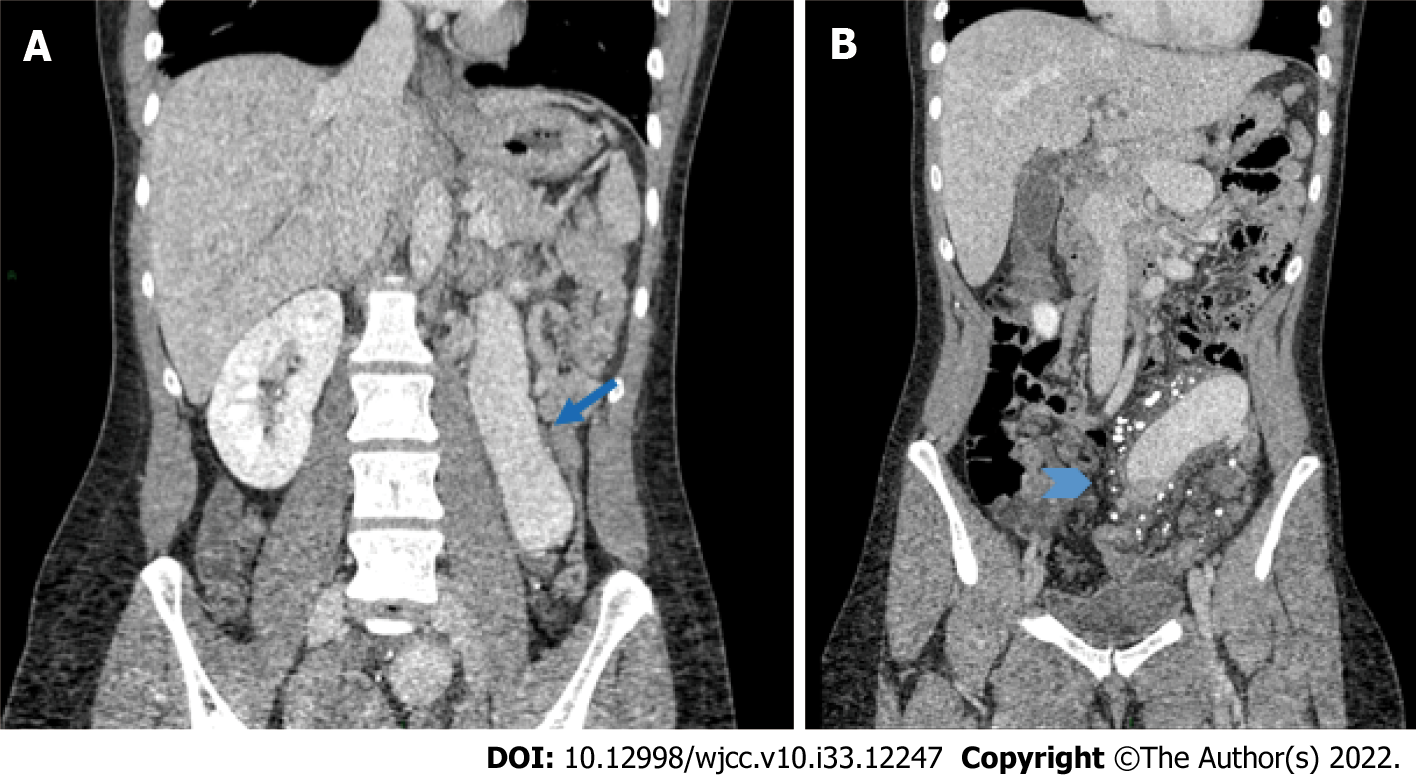
A thoracoabdominal computed tomography (CT) scan with IV contrast revealed segmental pulmonary thromboembolism with bilateral pulmonary infarctions as well as abnormal vessel distribution around the abdomen consistent with tortuous and bizarre vessels (Figure 2). There was portal vein atresia with no hepatic arteries easily found. We found a venous shunt from the left mesenteric vein to the right colonic vein and we observed significant varicose dilatation of the inferior mesenteric vein and confluent branches of the splenic vein, congestive conflicting veins of the superior mesenteric system, as well as engorgement of the inferior vena cava, renal veins, common iliac veins and internal right iliac vein. She was admitted to the intensive care unit for treatment.
An echocardiogram showed preserved ejection fraction with increased pulmonary venous pressure estimated to be 62 mmHg. She was started on enoxaparin (1 mg/kg) to treat her pulmonary embolism at therapeutic doses. During the first 48 h of admission, she developed grade three anemia (hemoglobin 8.7 mg/dL) and continued to bleed even more through her lower GI tract, worsening her tachycardia. She required blood transfusions and we were forced to hold her anticoagulation for around 72 h to prevent massive bleeding. Oral iron was started at this time.
We immediately programed her for an angiography procedure to stop her anomalous vessels from bleeding into her colon. Embolization of her left mesenteric vein and her right colon vein immediately stopped the bleeding.
Due to the severe abdominal pain that persisted despite multiple pain killers including opioids, she was unable to feed herself, so we decided to give her total parenteral nutrition.
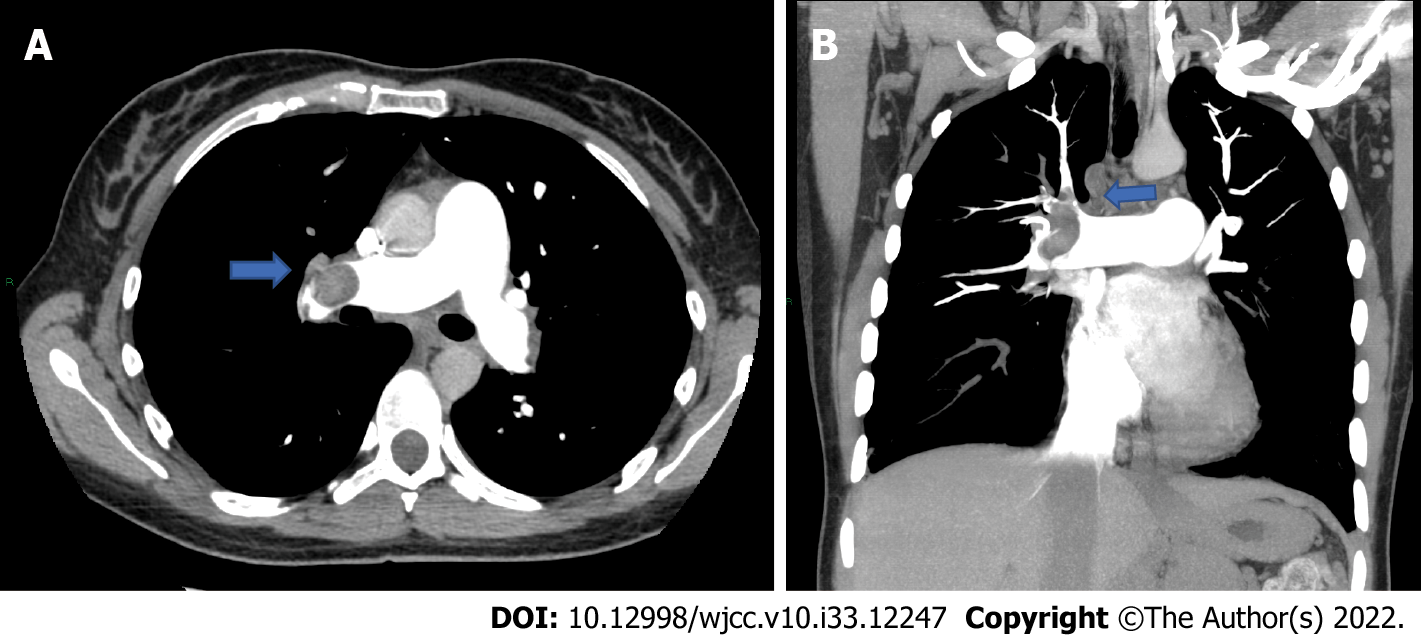
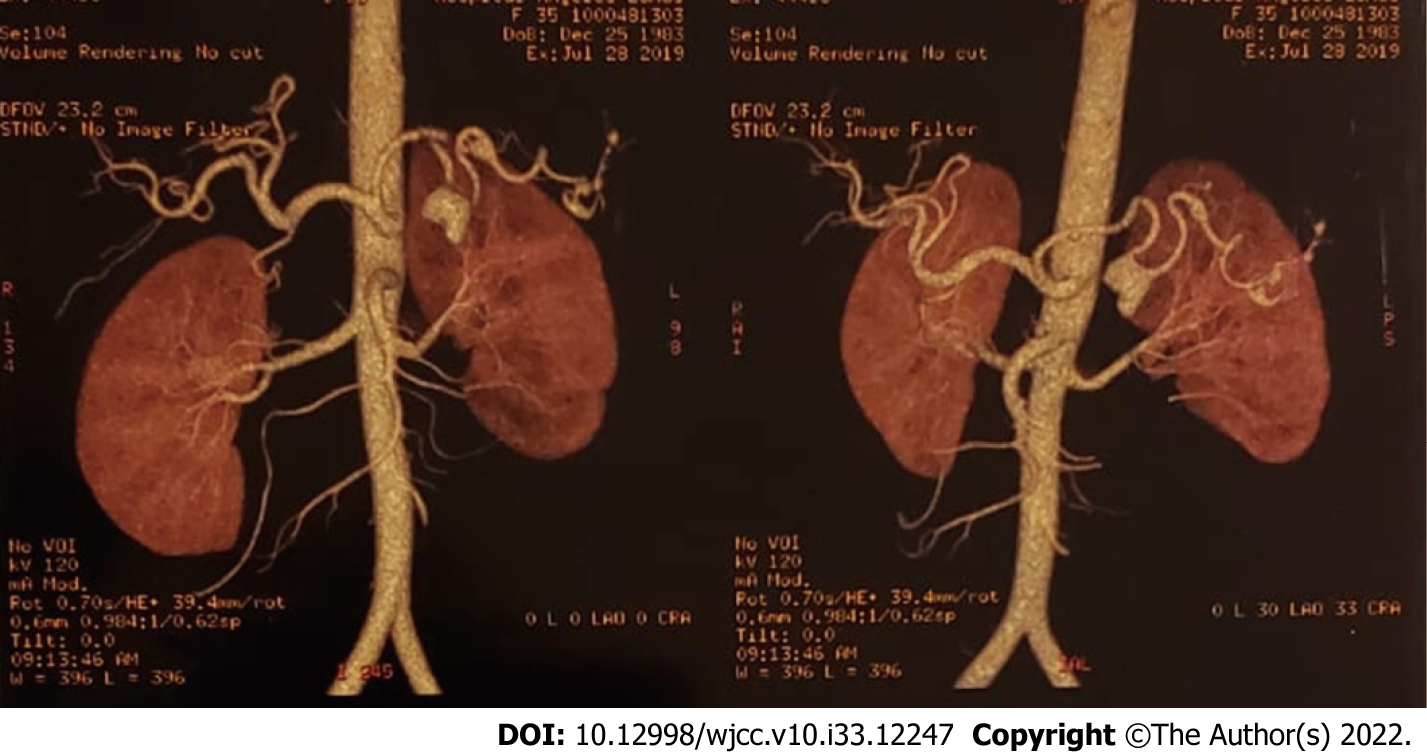
At 72 h after suspending anticoagulation she developed sudden dyspnea, her oxygen saturation dropped to 76% and her heartbeat increased up to 145 bpm. Her blood pressure dropped to 80/60 mmHg. Due to the high suspicion of a new pulmonary embolism, a thoracic CT scan was conducted which showed complete obstruction of the right pulmonary vein (Figure 3) that required thrombolysis and thrombectomy which was immediately performed. During this procedure she needed several blood transfusions and an inferior vena cava filter was inserted. At this point, a heparin infusion was started in order to control her anticoagulation. She subsequently continued to bleed transvaginally, not corresponding to menstruation, signaling that there was more pressure on other venous shunts that we had not seen in the previous angiography. A second angiography found at least three more aneurysmatic lesions in the splenic artery (Figure 4) which required more coiling, achieving successful control of the bleeding. More blood transfusions were needed at this time.
We also ordered genetic tests to confirm our suspicion of a collagen related genetic disorder. A whole exome sequence and copy number variation were requested. The results showed heterozygous missense mutation in the TGFB2 gene, variant c.439C>T p. (Prol47Ser) in Exon 3, categorized as uncertain significance mutation was consistent with Loeyz-Dietz syndrome type 4. After eleven days of hospitalization, the patient was discharged and her management was continued at home.
After resolving her anatomic abnormalities, the main treatment was anticoagulation.
After eleven days of hospitalization, the patient was discharged and her management was continued at home. She has been taking her anticoagulation to prevent the development of new embolic events and her follow-up visits are every six months with a complete blood count to identify signs of bleeding. Colonoscopy and transthoracic echocardiogram are performed annually.
The abnormal presentation of this genetic syndrome during adulthood is very bizarre. Most previous reports of LDS type 4 have described the clinical presentation during childhood, which almost always included aortic aneurysm and mental retardation, which were not observed in our patient. Few reports have described the late presentation of LDS with aortic aneurysm[8]; nevertheless, our patient was 35 years old, when the actual life expectancy has been estimated at 37 years.
LDS is a bizarre autosomal dominant disorder with extensive widespread implications. The recently proposed nosology states that a mutation in any of the previously described four genes in addition to a documented arterial/venous aneurysm or dissection should be considered as diagnostic for LDS. Consequently, in our case we describe sporadic signs of LDS4 that need to be considered within the LDS spectrum even in the absence of aortic aneurysm.
TGFB2 has a universal role in chondrocyte maturation and endochondral ossification as it is expressed by chondrocytes during the late stage of hypertrophic differentiation[10]. It participates in bone remodeling, modulating both bone deposition and turnover[11], contributes to the survival of osteoblasts[12], interacts with collagen[13], and enhances bone formation and cortical development[14]. Reports have described a marked association between the low expression of TGFB2 and hip dysplasia[15].
The data indicate that the TGF-β latency profile in chondrocytes undergoing maturation and endochondral ossification is convoluted, a reflection of the potency of this factor and the pleiotropic effects evoked by it. Therefore, there is a need for multiple regulatory levels to control TGF-β activity[10].
Reports from previous case series with LDS show that approximately 55% to 60% of patients have aneurysmal disease beyond the ascending aorta, and both report late mortality of 9% and 14%[16]. Our patient´s anatomical abnormalities including portal atresia directed us at first to search for other genetic causes including Abernathy syndrome, characterized by diversion of portal blood away from the liver. There is either an absence of portal vein, type I congenital extrahepatic portosystemic shunt or presence of thin portal vein radicles (congenital extrahepatic portosystemic shunt, although it never really described all her anatomic alterations. Our patient had mild typical facies and no joint laxity.
The main clinical challenge with this patient was to control her massive GI and vaginal bleeding caused by abnormal tortuous veins while treating her pulmonary embolism and preventing a new one with anticoagulant therapy. Also, with the many blood transfusions required, the tissue sample for the genetic test had to be taken from the saliva and sent to Germany for further analysis. When the diagnosis of LDS is made, it is critical to search for further typical or atypical features such as cerebral aneurysms or venous tortuosity that could compromise the person’s life. Furthermore, it is important to consider optimal treatment with calcium and vitamins given the skeletal fragility and low bone mass seen in LDS as in other connective tissue diseases[17]. Many different aspects including a variety of specialists have to be considered in order to make valuable recommendations for these patients including cardiologic, gastrointestinal, hematologic, oncologic and orthopedic guidance. The follow-up visits of our patient included anticoagulant monitoring, annual colonoscopy and echocardiogram.
Fundamentally, prudence must be taken with regard to recommendations for patients with LDS syndrome. Moreover, it is plausible that the former LDS reports have recorded the most severe features of the disorder, giving the unreal conclusion of a life-threatening natural history[16], although this may not be the case in every patient.
As diagnostic methods have improved and the LDS spectrum is more recognized, we have been identifying phenotypically less severe forms and treating them much earlier in their clinical spectrum. These reports provide additional understanding of the behavior of this disorder among the medical community, and as we continue to treat and study patients with LDS, this knowledge will undoubtedly continue to evolve resulting in further improved outcomes and lengthen life expectancy. The more case reports on this syndrome that are published, our medical knowledge on the wide clinical features will expand and we will become more experienced providing these patients with better counseling to improve life quality and reduce mortality.
We want to thank our dear patient for letting us publish her case. We would also like to thank everyone involved in the treatment and diagnosis of this disease, including the radiologist team, the cardiologist, and the geneticist.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Physicians, No. 01682939.
Specialty type: Medicine, general and internal
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: DeSousa K, India; Ding X, China; Fontana P, Italy; Schoenhagen P, United States S-Editor: Wu YXJ L-Editor: Webster JR P-Editor: Wu YXJ
| 1. | Camerota L, Ritelli M, Wischmeijer A, Majore S, Cinquina V, Fortugno P, Monetta R, Gigante L; Marfan Syndrome Study Group Tor Vergata University Hospital; Sangiuolo FC, Novelli G, Colombi M, Brancati F. Genotypic Categorization of Loeys-Dietz Syndrome Based on 24 Novel Families and Literature Data. Genes (Basel). 2019;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1239] [Cited by in RCA: 1243] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 3. | Van Laer L, Dietz H, Loeys B. Loeys-Dietz syndrome. Adv Exp Med Biol. 2014;802:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Ritelli M, Chiarelli N, Dordoni C, Quinzani S, Venturini M, Maroldi R, Calzavara-Pinton P, Colombi M. Further delineation of Loeys-Dietz syndrome type 4 in a family with mild vascular involvement and a TGFB2 splicing mutation. BMC Med Genet. 2014;15:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1212] [Cited by in RCA: 1141] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 6. | Fontana P, Genesio R, Casertano A, Cappuccio G, Mormile A, Nitsch L, Iolascon A, Andria G, Melis D. Loeys-Dietz syndrome type 4, caused by chromothripsis, involving the TGFB2 gene. Gene. 2014;538:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Harky A, Garner M, Roberts N. A late presentation of Loeys-Dietz syndrome associated with an aortic root aneurysm. Ann R Coll Surg Engl. 2017;e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Gu W, Zhang F, Lupski JR. Mechanisms for human genomic rearrangements. Pathogenetics. 2008;1:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 451] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 10. | Dangelo M, Sarment DP, Billings PC, Pacifici M. Activation of transforming growth factor beta in chondrocytes undergoing endochondral ossification. J Bone Miner Res. 2001;16:2339-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Erlebacher A, Filvaroff EH, Ye JQ, Derynck R. Osteoblastic responses to TGF-beta during bone remodeling. Mol Biol Cell. 1998;9:1903-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Dufour C, Holy X, Marie PJ. Transforming growth factor-beta prevents osteoblast apoptosis induced by skeletal unloading via PI3K/Akt, Bcl-2, and phospho-Bad signaling. Am J Physiol Endocrinol Metab. 2008;294:E794-E801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Yang M, Wang X, Zhang L, Yu C, Zhang B, Cole W, Cavey G, Davidson P, Gibson G. Demonstration of the interaction of transforming growth factor beta 2 and type X collagen using a modified tandem affinity purification tag. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:493-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Ripamonti U, Roden LC. Induction of bone formation by transforming growth factor-beta2 in the non-human primate Papio ursinus and its modulation by skeletal muscle responding stem cells. Cell Prolif. 2010;43:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Shi LW, Zhao Q, Zhang LJ, Li LY, Gao H. [Distribution and expression of TGF-ß2 in the capsule of children with developmental dysplasia of the hip]. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:641-644. [PubMed] |
| 16. | Williams JA, Hanna JM, Shah AA, Andersen ND, McDonald MT, Jiang YH, Wechsler SB, Zomorodi A, McCann RL, Hughes GC. Adult surgical experience with Loeys-Dietz syndrome. Ann Thorac Surg. 2015;99:1275-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | MacCarrick G, Black JH 3rd, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, Sponseller PD, Loeys B, Dietz HC 3rd. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 363] [Article Influence: 33.0] [Reference Citation Analysis (0)] |