Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12208
Peer-review started: September 14, 2022
First decision: September 26, 2022
Revised: October 6, 2022
Accepted: October 31, 2022
Article in press: October 31, 2022
Published online: November 26, 2022
Processing time: 69 Days and 22.9 Hours
Crohn’s disease (CD) is a chronic inflammatory bowel disorder that progresses to bowel damage (BD) over time. An image-based index, the Lémann index (LI), has been developed to measure cumulative BD.
To characterize the long-term progression of BD in CD based on changes in the LI and to determine risk factors for long-term progression.
This was a single-center longitudinal cohort study. Patients who had participated in prospective studies on the accuracy of magnetic resonance imaging using endoscopy as a gold standard and who had a follow-up of at least 5 years were re-evaluated after 5-12 years.
Seventy-two patients were included. LI increased in 38 patients (52.8%), remained unchanged in 9 patients (12.5%), and decreased in 25 patients (34.7%). The small bowel score and surgery subscale significantly increased (P = 0.002 and P = 0.001, respectively), whereas the fistulizing subscale significantly decreased (P = 0.001). Baseline parameters associated with BD progression were ileal location (P = 0.026), CD phenotype [stricturing, fistulizing, or both (P = 0.007, P = 0.006, and P = 0.035, respectively)], disease duration > 10 years (P = 0.019), and baseline LI stricturing score (P = 0.049). No correlation was observed between BD progression and baseline clinical activity, biological markers, or severity of endoscopic lesions.
BD, as assessed by the LI, progressed in half of the patients with CD over a period of 5-12 years. The main determinants of BD progression were ileal location, stricturing/fistulizing phenotype, and disease duration.
Core Tip: The aim of the study was to characterize the long-term progression of bowel damage (BD) in patients with Crohn’s disease based on changes in the Lémann index. Predictors of BD progression were a baseline stricturing and fistulizing Crohn’s disease phenotype, ileal location, disease duration of more than 10 years, and a higher Lémann index stricturing score. Strict monitoring of BD-associated lesions during treatment, especially in those patients with a higher baseline Lémann index score, may help clinicians to improve treatment strategies in order to halt BD progression, adapting treatment based on risk factors identified in this study.
- Citation: Fernández-Clotet A, Panés J, Ricart E, Castro-Poceiro J, Masamunt MC, Rodríguez S, Caballol B, Ordás I, Rimola J. Predictors of bowel damage in the long-term progression of Crohn’s disease. World J Clin Cases 2022; 10(33): 12208-12220
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12208.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12208
The notion that Crohn’s disease (CD) is a progressive disease is well established. The proportion of patients that require surgery, either due to refractory inflammatory disease or stricturing/fistulizing complications increases over time[1]. Changing this long-term progressive course is one of the recognized unmet therapeutic needs in patients with CD. In order to develop new therapeutic strategies that are effective in changing the course of the disease, a reliable tool to measure bowel damage (BD) progression is crucial. To that end, the Lémann index (LI) has been developed and validated[2,3]. The LI consists of a scoring system based on a comprehensive assessment of structural BD, which includes the identification of stricturing and penetrating lesions based on cross-sectional imaging and endoscopy, and previous surgery. The LI is a measure of intestinal damage ranging from a minimum value corresponding to absence of damage to a maximum theoretical value corresponding to complete resection of the entire gastrointestinal tract.
The second aspect that is required for efficient design of studies on disease modification is characterization of the kinetics and risk factors for BD progression. Given that the development of the LI is relatively recent, studies determining damage severity have mostly consisted of transversal studies, and the few longitudinal studies evaluating changes in BD measured by the LI involve a relatively short period of observation, whereas damage accumulates over long periods of time.
The objectives of the current study were to characterize the long-term progression of BD in patients with CD based on changes in the LI, to identify which components of the index are the main determinants of progression, and to identify risk factors for long-term progression. To that end, we took advantage of our patient cohorts that had participated in past studies on the accuracy of magnetic resonance imaging (MRI) for characterizing CD inflammatory activity using endoscopy as the gold standard. We invited patients that had undergone these examinations within the past 5 years to 12 years to be re-evaluated in the context of the current study.
We performed a longitudinal cohort study in the tertiary referral center Hospital Clinic of Barcelona from April 2018 to December 2019. The study was approved by the local ethics committee (Reg. HCB/2018/0160) and was conducted according to the European Medicines Agency’s good clinical practice guidelines (CMPM/ICH/135/95, July 2002). All patients provided written informed consent before inclusion.
Patients were included if they met the following criteria: ≥ 18-years-old, with an established diagnosis of CD according to the European Crohn’s and Colitis Organisation guidelines[4], had undergone an MRI or computed tomography (CT) scan and an ileocolonoscopy between 2006 and 2013, had a follow-up of at least 5 years, and signed a written informed consent to be re-evaluated. Patients with formal contraindications for a new MRI or colonoscopy were excluded from the study. We used our local database to identify candidates and invited them to participate when they attended the outpatient clinic during the recruitment period.
In those patients who had more than one MRI examination during 2006-2013, the first assessment within this period was considered as the baseline examination.
Demographic and clinical characteristics were captured at two timepoints: Baseline and the second assessment. For baseline, the following variables were recorded: Sex, age at diagnosis, date of CD diagnosis, disease duration at the time of first assessment, CD location and phenotype according to Montreal Classification, current or past history of perianal disease, smoking status, previous intestinal surgeries, previous treatments (exposure to immunosuppressants or biologic therapies), current treatment, and C-reactive protein (CRP) level. Clinical disease activity was assessed according to the CD Activity Index, and active disease was classified as a CD activity index ≥ 150 points. Endoscopic disease activity was recorded using the CD Endoscopic Index of Severity. The cutoffs for remission, mild disease, and severe disease were < 3.5, 3.5-7.0, and > 7.0 points, respectively.
The following clinical variables were recorded at the second assessment: changes in treatment (exposure to immunosuppressants and/or biologic therapy) and surgery requirements (number, type, and indication) during the period between the two assessments.
The MRI examinations were performed using the clinical 1.5 or 3T systems (TrioTim /Aera, Siemens Medical Solutions, Germany). T2 sequences with and without fat saturation in the axial plane and without fat saturation in the coronal plane were acquired. Next, three-dimensional non-enhanced and contrast-enhanced T1 sequences with fat signal saturation were acquired in the coronal and axial planes. CT examinations were acquired using a multidetector CT scan (Siemens Somaton 64, Germany) with thin (2 mm) axial and coronal plane image reconstructions during the enterographic phase following iodinated contrast injection.
BD and its progression over time were assessed for each patient using the LI[2]. Imaging examinations (either MRI or CT scan) were performed in all patients at baseline and at the second assessment. Intestinal segments were assessed as normal or abnormal. The length of each abnormal intestinal segment, wall thickness, presence of ulcers, stricturing lesions (including caliber of luminal narrowing and pre-stenotic diameter), and fistulas or abscesses were collected. Stricturing and penetrating lesions were defined by the imaging results, graded, and recorded according to severity on an ordinal scale (from 0: absent to 3: maximum). A documented expert radiologist in gastrointestinal imaging with 15 years of experience in bowel imaging (JR) graded the severity of the lesions according to the LI rules. Imaging procedures were not read by a gastroenterologist since this type of assessment requires expertise in the field, and gastroenterologists in Spain are not formally trained in cross-sectional enterographic image interpretation. Additional investigations were recorded based on disease location: physical examination and a pelvic MRI in case of perianal disease and endoscopic studies in cases of upper gastrointestinal and/or colonic involvement.
At baseline, all of the procedures to calculate the LI were performed within 120 d. For the second assessment, almost all procedures were performed within this period. However, as previously described in other studies using the LI methodology, endoscopies performed less than 1 year prior to the imaging procedures were used in those cases in which it had been performed[5].
The extent of the damage performed in the surgery was documented and graded based on the medical reports. The length of resection was obtained from the pathologist’s report.
Radiological, endoscopic, and surgical information were generated for each intestinal segment and recorded in the excel file published by the LI development study[2]. The LI was calculated globally (at a patient level), for each organ, and for each subscale (stricturing, fistulizing, and surgery). BD progression was defined as any increase in the LI between the two evaluations.
The aims of the study were to characterize the long-term (> 5 years) progression of BD in CD based on changes in the LI, to establish which components of the LI are the main determinants of progression, and to identify risk factors for long-term progression.
The study aimed to enroll a cohort of approximately 70-80 patients; the sample size was not prespecified or based on statistical considerations. To analyze factors associated with BD progression, we classified patients according to any increase or no increase/decrease of the global LI between the two assessments. Descriptive statistics were used to summarize patient baseline characteristics. Continuous variables were expressed as mean ± standard deviation, while discrete variables were expressed as frequencies and percentages and/or absolute values. For comparisons of continuous variables, the Student’s t-test was used as appropriate, and for comparisons of categorical variables the χ2 test was applied. Logistic regression modeling was performed to analyze predictors of BD progression. Covariates tested included sex, age at diagnosis, age at inclusion, disease duration, smoking status, CD location, CD phenotype, family history of irritable bowel disease, previous surgery, prior treatment with biological drugs, baseline treatment, baseline CRP, baseline disease activity measured by the CD Activity Index, and endoscopic activity measured by CDEIS. Univariate modeling was performed, and covariates with a univariate significance of P ≤ 0.10 were included in the multivariate model. Results were evaluated by means of odds ratios and their 95% confidence intervals. A receiver operating characteristic curve was used to define the discriminative ability of the logistical model to predict BD progression.
A P value < 0.05 was considered statistically significant. All analyses were performed with the SPSS statistical package V.23.
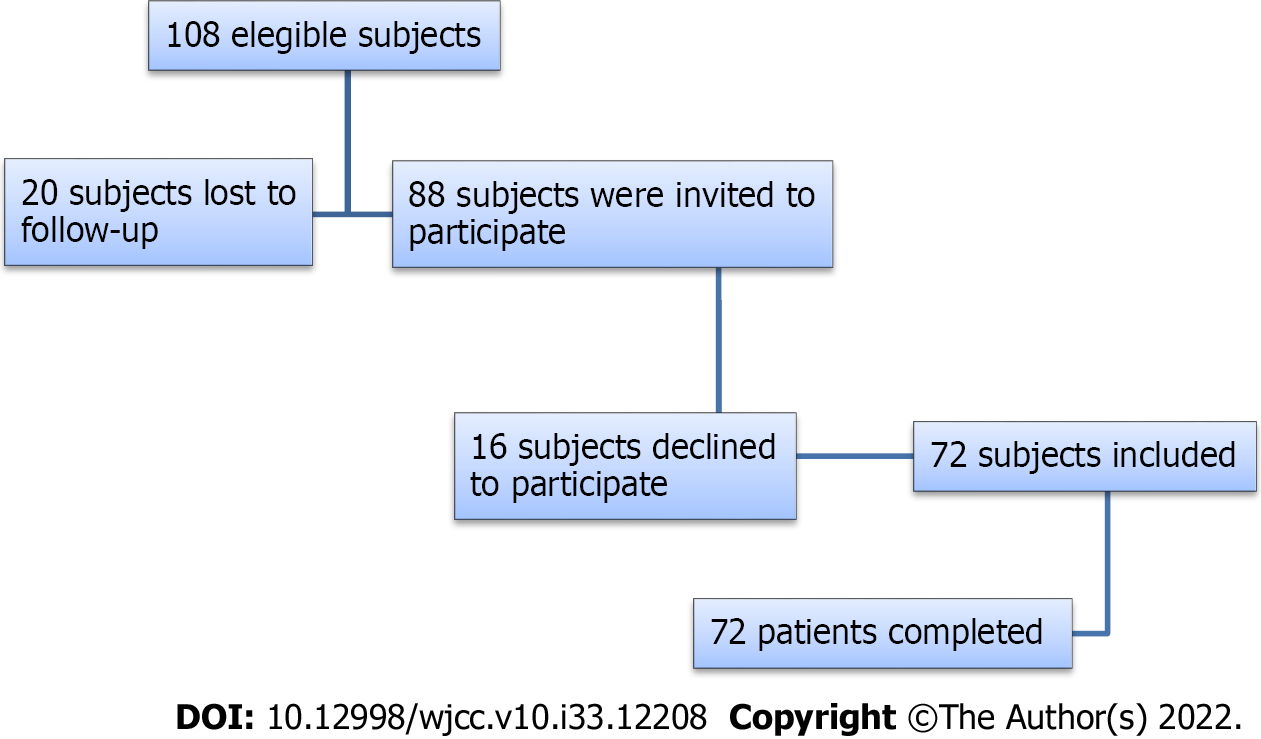
A total of 108 patients were eligible for the study. Twenty patients could not participate because they were lost to follow-up during the previous 12 years. Eighty-eight subjects were invited to participate; 16 declined and 72 accepted and were included in the study (Figure 1). Table 1 summarizes the baseline demographic and clinical characteristics of the patients included in this study.
| Variable | n = 72 |
| Female sex | 42 (58.3) |
| Age at inclusion in yr, mean (SD) | 34.41 (± 11.1) |
| Age at diagnosis in yr | |
| < 16 | 8 (11.1) |
| 17-40 | 57 (79.2) |
| > 40 | 7 (9.7) |
| Disease duration in yr, mean (SD) | 8.09 (± 7.3) |
| Disease duration in yr | |
| < 2 | 21 (29.2) |
| 2-10 | 31 (43.1) |
| >10 | 20 (27.8) |
| Smoking status | |
| Never smoker | 28 (38.9) |
| Current smoker | 31 (43.1) |
| Past smoker | 13 (18.1) |
| CD location | |
| Terminal ileum | 27 (37.5) |
| Colon | 8 (11.1) |
| Ileocolic | 37 (51.4) |
| CD upper tract involvement | 6 (8.3) |
| CD phenotype | |
| Inflammatory | 30 (41.7) |
| Stricturing | 15 (20.8) |
| Penetrating | 14 (19.4) |
| Stricturing and penetrating | 13 (18.1) |
| Current or past history of perianal disease | 14 (19.4) |
| Family history | 5 (6.9) |
| Previous resective surgery | |
| No | 51 (70.8) |
| 1 surgery | 13 (18.1) |
| > 1 surgery | 8 (11.1) |
| Biological naïve at baseline | |
| Yes | 46 (63.9) |
| No | 26 (36.1) |
| Treatment at baseline | |
| None | 18 (25.0) |
| Corticosteroids | 4 (5.6) |
| Immunosuppressants | 32 (44.4) |
| TNF-α inhibitors | 8 (11.1) |
| Immunosuppressants plus TNF-α inhibitors | 10 (13.9) |
| CRP in mg/L, mean (SD) | 2.89 (± 4.2) |
| CRP | |
| Normal: < 1 mg/L | 30 (41.7) |
| Elevated: ≥ 1 mg/L | 42 (58.3) |
| CDAI, mean (SD) | 194.73 (± 95.8) |
| Clinical activity according to CDAI score | |
| Active disease | 51 (69.9) |
| Clinical remission | 21 (28.8) |
| CDEIS, mean (SD) | 7.8 (± 6.7) |
| CDEIS | |
| < 3.5 | 20 (29.9) |
| 3.5-7.0 | 18 (26.8) |
| > 7.0 | 29 (43.3) |
| NA | 5 |
Changes in the LI: Calculation of the LI at baseline for each individual was based on cross-sectional enterographies, either MRI (n = 71) or CT (n = 1), 67 colonoscopies, 1 upper endoscopy, 3 capsule endoscopy studies, and 6 pelvic MRIs (in those patients with active perianal disease). Calculation of the LI at the second assessment for each individual was based on cross-sectional enterographies (68 MRIs and 4 CT scans), 46 colonoscopies, 1 upper endoscopy, and 6 pelvic MRIs (in those patients with active perianal disease). One patient developed perianal disease in the interval between the two assessments.
The mean LI at baseline was 5.75 (± 7.54) and ranged from 0 to 58. The second LI assessment was performed between 5 years and 12 years after baseline [mean of 8.81 (± 2.17) years]. The mean LI at this point was 7.26 (± 9.04) and ranged from 0 to 52. The mean organ damage evaluations and the mean LI subscales at baseline and follow-up assessments are summarized in Table 2.
| Parameter | LI at baseline | LI at second assessment | Changes in LI |
| Global Lémann index | 5.75 ± 7.57 | 7.26 ± 9.04 | 1.51 ± 6.51 (P = 0.054) |
| Organ location | |||
| Upper digestive tract | 0.03 ± 0.18 | 0.04 ± 0.35 | 0.01 ± 0.33 (P = 0.721) |
| Small bowel damage score | 1.79 ± 1.62 | 2.46 ± 2.51 | 0.67 ± 1.75 (P = 0.002) |
| Colon/rectum damage score | 3.19 ± 3.94 | 3.39 ± 3.81 | 0.2 ± 3.38 (P = 0.622) |
| Anus damage score | 0.80 ± 4.32 | 1.38 ± 5.95 | 0.58 ± 4.28 (P = 0.253) |
| LI subscales | |||
| Stricturing subscale | 1.12 ± 1.38 | 0.88 ± 1.44 | -0.24 ± 1.77 (P = 0.262) |
| Fistulizing subscale | 1.95 ± 2.39 | 1.01 ± 1.99 | -0.94 ± 2.19 (P = 0.001) |
| Surgery subscale | 2.72 ± 6.78 | 5.32 ± 8.52 | 2.60 ± 5.82 (P = 0.001) |
Overall, the mean LI change between the baseline and follow-up assessments was an increase of 1.51 (± 6.51) points (P = 0.054). The LI increased in 38 patients (52.8%), remained unchanged in 9 patients (12.5%), and decreased in 25 patients (34.7%). BD progression was defined as any progression in the LI between the two evaluations (of note, in all cases the progression was greater than 0.3 points, as previously set as the BD progression cutoff)[6]. The small bowel score was the only organ evaluation that significantly increased (P = 0.002). The fistulizing subscale significantly decreased (P = 0.001), whereas the surgery subscale significantly increased (P = 0.001) between the two assessments.
Surgery between baseline and the final assessments was the main determinant of LI progression. Twenty-four patients (33.3%) required surgery in the period between the two assessments, with a total of 29 surgeries. Indications for surgery included: Stricturing lesions (18 cases), penetrating lesions (6 cases), stricturing and penetrating lesions (1 case), refractoriness to medical treatment (3 cases), and reconstruction of the intestinal tract (colostomy closure with segmental resection of the colon, 1 case). Furthermore, a stricturing baseline LI score was correlated with a future risk of surgery (P = 0.002) in contrast to the fistulizing baseline LI score, which was not significantly associated with a risk of surgery (P = 0.051).
Factors associated with BD progression over time: The associations between demographic and CD characteristics and BD progression are summarized in Table 3. BD progression was significantly associated with CD phenotype at baseline (P = 0.001), with a progression noted in 71.4% of patients with a penetrating phenotype, in 80.0% of patients with a stricturing phenotype, and in 69.2% of patients with both a stricturing and penetrating phenotype compared to 23.2% of those patients with an inflammatory phenotype. Disease duration at baseline was also associated with BD progression (P = 0.001), with 80.0% of patients with a duration of > 10 years showing progression compared to 38.1% of those with a newly diagnosed disease (< 2 years).
| LI progression, n = 38 | No LI progression, n = 34 | P value | |
| Sex | 0.352 | ||
| Male | 18 (60.0) | 12 (40.0) | |
| Female | 20 (47.6) | 22 (52.4) | |
| Age at diagnosis in yr | 0.961 | ||
| < 16 | 4 (50.0) | 4 (50.0) | |
| 17-40 | 30 (52.6) | 27 (47.4) | |
| > 40 | 4 (57.1) | 3 (42.9) | |
| Age at baseline, mean (SD) | 25.17 (± 10.4) | 27.61 (± 10.3) | 0.323 |
| CD location at baseline | 0.091 | ||
| Terminal ileum | 17 (66.6) | 9 (33.3) | |
| Colon | 2 (25.0) | 6 (75.0) | |
| Ileocolic | 18 (48.7) | 19 (51.3) | |
| CD upper tract involvement | 0.677 | ||
| Yes | 4 (66.7) | 2 (33.3) | |
| No | 34 (51.5) | 32 (48.5) | |
| CD phenotype at baseline | 0.001 | ||
| Inflammatory | 7 (23.3) | 23 (76.7) | |
| Stricturing | 12 (80.0) | 3 (20.0) | |
| Penetrating | 10 (71.4) | 4 (28.6) | |
| Stricturing and penetrating | 9 (69.2) | 4 (30.8) | |
| Current or past history of perianal disease at baseline | 0.151 | ||
| Yes | 10 (71.4) | 4 (28.6) | |
| No | 28 (48.3) | 30 (51.7) | |
| Disease duration at inclusion, mean (SD) | 10.36 (± 8.4) | 5.54 (± 4.8) | 0.001 |
| Disease duration at inclusion in yr | 0.001 | ||
| < 2 | 8 (38.1) | 13 (61.9) | |
| 2-10 | 14 (45.2) | 17 (54.8) | |
| >10 | 16 (80.0) | 4 (20.0) | |
| Smoking status | 0.342 | ||
| Never smoker | 15 (53.6) | 13 (46.4) | |
| Current smoker | 14 (45.2) | 17 (54.8) | |
| Past smoker | 9 (69.2) | 4 (30.8) | |
| Family history | 0.541 | ||
| Yes | 2 (40.0) | 3 (60.0) | |
| No | 35 (53.0) | 31 (47.0) | |
| Immunosuppressant treatment between intervals | 0.741 | ||
| Yes | 35 (52.2) | 32 (47.8) | |
| No | 3 (40.0) | 2 (60.0) | |
| Biological naïve at baseline | 0.143 | ||
| Yes | 21 (45.7) | 25 (54.3) | |
| No | 17 (65.4) | 9 (34.6) | |
| Biological treatment between intervals | 0.591 | ||
| Yes | 34 (54.0) | 29 (46.0) | |
| No | 4 (44.4) | 5 (55.6) | |
| Biological treatment between intervals | 21 (47.7) | 23 (52.3) | 0.443 |
| TNF-α inhibitors | 8 (61.5) | 5 (38.5) | |
| TNF-α inhibitors and ustekinumab | 2 (66.7) | 1 (33.3) | |
| TNF-α inhibitors and vedolizumab | 2 (100.0) | 0 (0) | |
| TNF-α inhibitors, vedolizumab and ustekinumab | 1 (100.0) | 0 (0) | |
| Previous surgery at inclusion | 0.192 | ||
| Yes | 14 (66.7) | 7 (33.3) | |
| No | 24 (47.1) | 27 (52.9) | |
| Autologous stem-cell transplantation between intervals | 0.602 | ||
| Yes | 2 (66.7) | 1 (33.3) | |
| No | 36 (52.2) | 33 (47.8) | |
| Surgery between intervals | 0.001 | ||
| Yes | 23 (95.8) | 1 (4.2) | |
| No | 15 (31.3) | 33 (68.8) | |
| Baseline LI score evaluation, mean (SD) | |||
| Total LI score | 5.60 (± 4.40) | 5.90 (± 10.10) | 0.860 |
| Upper tract score | 0.05 (± 0.30) | 0 | 0.211 |
| Small bowel score | 2.16 (± 1.60) | 1.37 (± 1.60) | 0.040 |
| Colon/rectum score | 2.98 (± 3.00) | 3.42 (± 4.80) | 0.652 |
| Anus score | 0.51 (± 1.90) | 1.13 (± 6.00) | 0.542 |
| Stricturing score | 1.43 (± 1.60) | 0.78 (± 1.00) | 0.045 |
| Fistulizing score | 1.69 (± 2.50) | 2.23 (± 2.30) | 0.341 |
| Surgical score | 2.54 (± 3.40) | 2.92 (± 9.30) | 0.823 |
| CRP at baseline, mean (SD) | 2.93 (± 4.70) | 2.84 (± 3.60) | 0.921 |
| CRP at baseline | 0.692 | ||
| Normal: < 1 mg/L | 15 (50.0) | 15 (50.0) | |
| Elevated: ≥ 1 mg/L | 23 (54.8) | 19 (45.2) | |
| CDAI, mean (SD) | 199.69 (± 94.9) | 189.19 (± 97.8) | 0.673 |
| Clinical activity at baseline according to CDAI score | 0.610 | ||
| Active disease | 28 (54.9) | 23 (45.1) | |
| Clinical remission | 10 (47.6) | 11 (52.4) | |
| CDEIS activity at baseline, mean (SD) | 6.2 (5.4) | 9.44 (1.3) | 0.052 |
| CDEIS activity at baseline | 0.190 | ||
| < 3.5 | 12 (60.0) | 8 (20.0) | |
| 3.5-7.0 | 11 (61.1) | 7 (38.9) | |
| > 7.0 | 11(37.9) | 18 (62.1) |
When analyzing which LI components at baseline were associated with BD progression, we observed that patients with BD progression had a significantly higher baseline small bowel LI score (P = 0.040) and a significantly higher baseline stricturing LI score (P = 0.045).
Neither inflammatory markers (CRP) nor clinical or endoscopic severity were associated with LI progression. Although comparisons of endoscopic severity between patients with and without BD progression were of borderline significance, unexpectedly, CDEIS was numerically higher in the groups without CD progression.
Regarding medical therapies used between the two assessments, 67 patients (93.1%) received immunosuppressant therapy (thiopurines or methotrexate), and 63 patients (87.5%) received biological treatment (51 combination therapies). The biological therapy class is detailed in Table 3. Additionally, 3 patients with refractory disease underwent autologous stem cell transplantation. The fact that around 90% of patients included in the study were treated during the long follow-up period with biological or immunosuppressive drugs precludes any analysis of the influence of these treatments on damage progression.
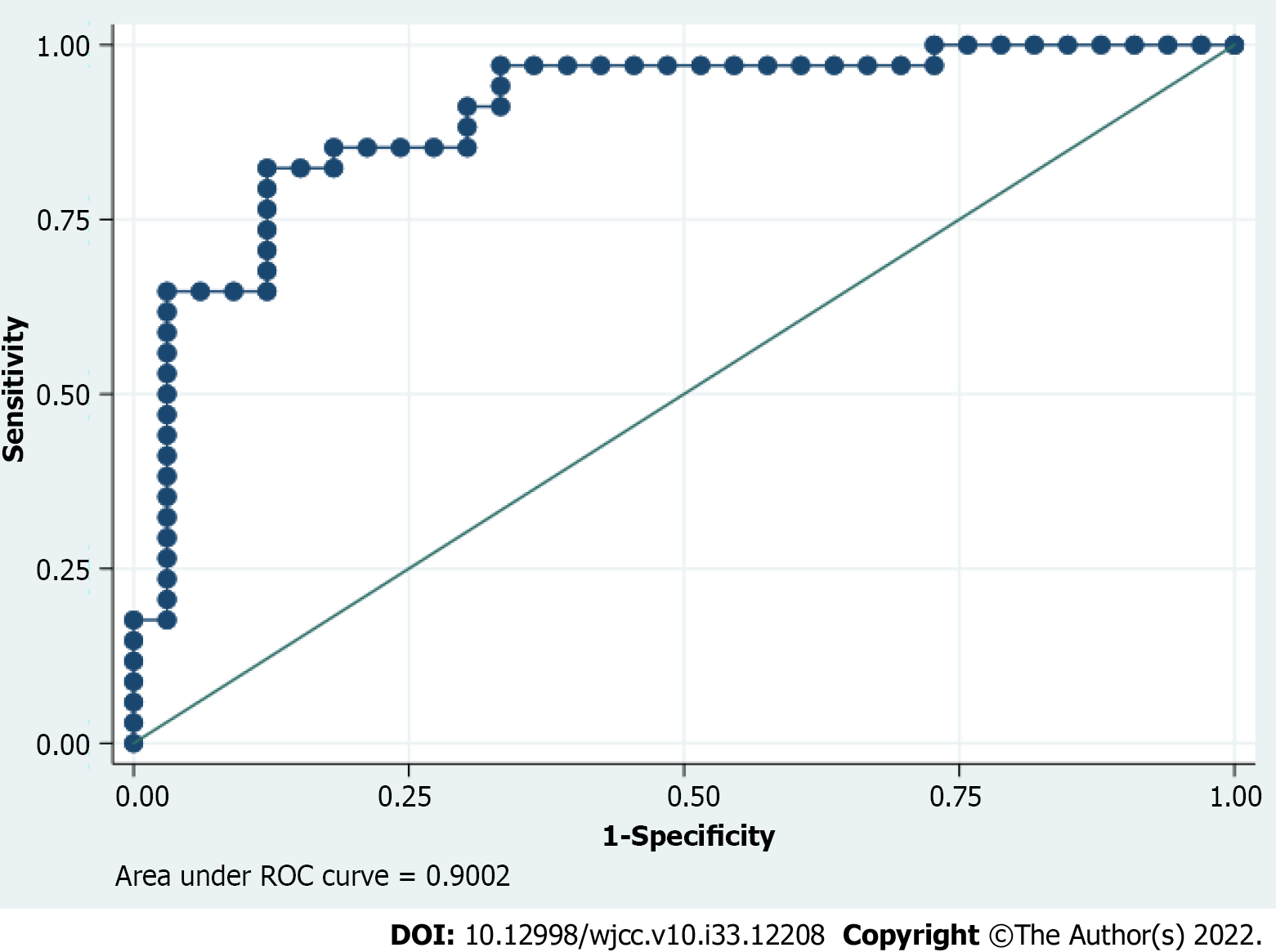
Regarding multivariate logistic regression analysis, only ileal location, a CD stricturing or fistulizing phenotype, disease duration of more than 10 years, and a baseline LI stricturing score were associated with BD progression (Table 4). The area under the receiver operator characteristics curve of the logistic model for predicting BD progression was 0.900 (95% confidence interval: 0.824-0.976, P < 0.01) (Figure 2).
| Risk factor | OR | 95%CI | P value |
| CD location | |||
| Terminal ileum | 8.307 | 1.296-53.251 | 0.026 |
| Colon | 0.580 | 0.014-24.658 | 0.776 |
| CD phenotype | |||
| Stricturing | 18.447 | 2.219-153.321 | 0.007 |
| Fistulizing | 17.085 | 2.217-131.673 | 0.006 |
| Stricturing and fistulizing | 12.296 | 1.190-127.022 | 0.035 |
| Disease duration at inclusion in yr | |||
| 2-10 | 2.174 | 0.374-12.652 | 0.387 |
| > 10 | 15.196 | 1.557-148.332 | 0.019 |
| Baseline LI stricturing score | 1.929 | 1.004-3.709 | 0.049 |
| Baseline LI small bowel score | 0.690 | 0.383-1.243 | 0.216 |
| CDEIS activity at baseline | 0.948 | 0.837-1.074 | 0.400 |
The results of the current study show that in an unselected population of patients with CD, BD progression occurs in about half of them after a long period of follow-up (5 years to 12 years). The main contributors to BD progression, as assessed by the LI, were a stricturing LI score and surgical components, whereas the fistulizing component significantly decreased during follow-up. Baseline factors that predicted BD progression were a disease duration of more than 10 years, ileal location, and the presence of a stricturing or fistulizing phenotype.
Few studies have assessed BD progression over time based on the LI, and the observation period is, in general, limited. In addition, there is the possibility that changes observed in the components of the LI stem from variations in the inflammatory component rather than true BD progression[6,7]. In this regard, some recent studies have evaluated whether the LI is sensitive to changes, but only a few of them have a prospective design[6,7] with a short period of time between evaluations. In fact, in some cases, there is only one morphologic evaluation[5]. In this study, we provide longer term information on BD progression in patients with CD that may be crucial for selecting populations at risk of progression in disease modification trials and to establish the follow-up time required to detect a sufficient number of events.
The mean global LI at baseline and second assessment were 5.75 (± 7.57) and 7.26 (± 9.04) respectively, without significant differences in the LI scores between the two assessment timepoints in the overall population (P = 0.054). The magnitude of LI increase over time in the current study is lower than other results reported in similar previous studies[8]. This may be, at least in part, due to the retrospective nature of other studies, which can result in the selection of patients who had a complicated disease and were thus subjected to additional MRI studies. Such a design differs from our study in which the assessment of BD progression was based on examinations performed specifically to that end.
Surgery was the major contributor to LI progression, in agreement with prior studies[6], and this would be expected considering the impact that the LI has on intestinal resection. We found that a higher stricturing score at first evaluation predicted LI progression, in most cases due to the need for surgery. Interestingly, the fistulizing score was not associated with LI progression over time as many of these lesions can heal after medical treatment. Although more data is needed to confirm our observation, a re-evaluation of fistulizing lesions when assessing BD may be required.
We found that ileal location and a higher stricturing LI score at baseline were related to BD progression. Lunder et al[9] previously reported a high risk of progression in ileocolonic disease vis-à-vis the presence of stricturing lesions. Our group has also reported that severe inflammatory lesions evaluated with MRI were more likely to heal in the colon as compared to the terminal ileum[10]. Additionally, we found that the persistence of the ileal inflammation over time, resulting in fibroblast activation and further development of stricturing lesions, might explain the observed higher LI scores in small bowel locations.
In the current study we did not observe any relationship between the severities of inflammatory lesions as measured by clinical indices, biomarkers (CRP), or endoscopy and future BD progression. Previous studies reported conflicting results. Lunder et al[9] reported a positive association between CRP levels and LI score, whereas Straksyte et al[11] could not confirm such a correlation. A prior retrospective population-based cohort study evaluating 156 patients over a period of 20 years similarly did not find any relationship between severe clinical activity (defined as a Harvey Bradshaw index > 8 points) and the LI[9]. As for the predictive value of inflammatory lesions at endoscopy, the current results are similar to a prior observation showing that MRI strictures and fistulas, but not deep ulceration detected at endoscopy, were associated with the future risk of surgery[12].
It has been proposed that components of BD can be reversible with the use of TNF-α inhibitors. Therefore, this therapy may prevent BD progression[13]. The study conducted by Fiorino et al[6] prospectively evaluated 30 patients with active disease who had begun taking TNF-α inhibitors during a median period of 32 mo and found that biological treatment may induce BD regression. Another study conducted by Ribaldone et al[14] retrospectively evaluated 91 patients (31 treated with adalimumab and 60 with azathioprine) for 12 mo and found that adalimumab therapy halted BD progression while azathioprine treatment did not. Bodini et al[15] retrospectively evaluated 104 CD patients divided according to the treatment received (biological therapy, azathioprine, and mesalazine) for a median time of 29.5 mo and concluded that the LI did not progress in the group receiving biological therapy but increased in the other groups. This suggests that the resolution of inflammation may be associated with halting BD progression[15]. These three studies had a short follow-up period and focused on the early changes in the LI related to the treatment received. In the current study we did not observe any correlation between biological treatment during follow-up and BD progression or regression. However, it must be noted that 87.5% of the population included in the study were exposed to biologics at some point during follow-up, and no firm conclusion can be drawn regarding this finding.
The main strength of our study is the long follow-up time (5-12 years), the longitudinal design, and the prospective design used to assess BD progression. Our design avoided any selection bias with severely ill populations undergoing repeated evaluations as clinically indicated. In addition, LI was calculated according to the methodology published in the development study, and MRIs were evaluated by an experienced radiologist in the field. However, there are certain limitations that must be acknowledged. The size of the cohort was limited and based on a single center cohort, and a majority of patients received biological therapy at some point during follow-up. The latter precludes any study of the association between biologic therapies and BD progression.
BD is progressive and accumulative as confirmed with the continued progression of LI over a long period of time in patients with CD. The main indication for surgery was stricturing disease and not the presence of ulcers at endoscopy. Predictors of BD progression were a baseline stricturing and fistulizing CD phenotype, ileal location, disease duration of more than 10 years, and a higher LI stricturing score. Strict monitoring of BD-associated lesions during treatment, especially in those patients with a higher baseline LI score, may help clinicians to improve treatment strategies in order to halt BD progression. Finally, such monitoring should likely be adapted according to the presence of those risk factors identified in the current study.
Crohn’s disease (CD) progresses to bowel damage (BD) over time. An image-based index, the Lémann index (LI), has been developed and validated to measure cumulative BD. The LI consists of a scoring system based on a comprehensive assessment of structural BD, which includes the identification of stricturing and penetrating lesions based on cross-sectional imaging and endoscopy, and previous surgery.
Risk factors for BD progression are not well identified. Studies that evaluate damage severity have a short period of observation, whereas damage accumulates over long periods of time.
To characterize the long-term progression of BD in patients with CD based on changes in the LI, to identify which components of the index are the main determinants of progression, and to identify risk factors for long-term progression.
We performed a longitudinal cohort study in the tertiary referral center Hospital Clinic of Barcelona from April 2018 to December 2019. We took advantage of our patient cohorts that had participated in past studies on the accuracy of magnetic resonance imaging for characterizing CD inflammatory activity using endoscopy as the gold standard. We invited patients that had undergone these examinations within the past 5 years to 12 years to be re-evaluated in the context of the current study. BD and its progression over time were assessed for each patient using the LI and calculated at baseline and at the second assessment.
Seventy-two patients were included. LI increased in 38 patients (52.8%), remained unchanged in 9 patients (12.5%), and decreased in 25 patients (34.7%). The small bowel score and surgery subscale significantly increased (P = 0.002 and P = 0.001, respectively), whereas the fistulizing subscale significantly decreased (P = 0.001). Baseline parameters associated with BD progression were ileal location (P = 0.026), CD phenotype (stricturing, fistulizing, or both with P = 0.007, P = 0.006, and P = 0.035, respectively), disease duration > 10 years (P = 0.019), and baseline LI stricturing score (P = 0.049).
BD, as assessed by the LI, progressed in half of the patients with CD over a period of 5-12 years. The main determinants of BD progression are ileal location, stricturing/fistulizing phenotype, and disease duration.
The timepoint to evaluate BD progression is still not yet established. Some treatment can prevent BD progression, but we still do not have robust data to confirm these findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fabbri N, Italy; Liu G, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus E V. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 571] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 2. | Pariente B, Mary JY, Danese S, Chowers Y, De Cruz P, D’Haens G. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology. 2015;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 3. | Pariente B, Torres J, Burisch J, Arebi N, Barberio B, Duricova D. Validation and Update of the Lémann Index to Measure Cumulative Structural Bowel Damage in Crohn’s Disease. Gastroenterology. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohn’s Colitis. 2010;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 792] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 5. | Lauriot dit Prevost C, Azahaf M, Nachury M, Branche J, Gerard R, Wils P. Bowel damage and disability in Crohn’s disease: a prospective study in a tertiary referral centre of the Lémann Index and Inflammatory Bowel Disease Disability Index. Aliment Pharmacol Ther. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Fiorino G, Bonifacio C, Allocca M, Repici A, Balzarini L, Malesci A, Peyrin-Biroulet L, Danese S. Bowel Damage as Assessed by the Lémann Index is Reversible on Anti-TNF Therapy for Crohn's Disease. J Crohns Colitis. 2015;9:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Amitai MM, Zarchin M, Lahat A, Yablecovitch D, Neuman S, Levhar N. Structural bowel damage in quiescent Crohn’s disease. Dig Liver Dis. 2017;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Gilletta C, Lewin M, Bourrier A, Nion-Larmurier I, Rajca S, Beaugerie L. Changes in the Lémann Index Values During the First Years of Crohn’s Disease. Clin Gastroenterol Hepatol. 2015;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Lunder AK, Jahnsen J, Bakstad LT, Borthne A, Hov JR, Vatn M. Bowel Damage in Patients With Long-term Crohn’s Disease, Assessed by Magnetic Resonance Enterography and the Lémann Index. Clin Gastroenterol Hepatol. 2018;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Rimola J, Fernàndez-Clotet A, Capozzi N, Rojas-Farreras S, Alfaro I, Rodríguez S, Masamunt MC, Ricart E, Ordás I, Panés J. Pre-treatment magnetic resonance enterography findings predict the response to TNF-alpha inhibitors in Crohn's disease. Aliment Pharmacol Ther. 2020;52:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Straksyte V, Kiudelis G, Gineikiene I, Janciauskas D, Basevicius A, Lukosevicius S. Lemann Index for Assessment of Crohn’s Disease: Correlation with the Quality of Life, Endoscopic Disease activity, Magnetic Resonance Index of Activity and C- Reactive Protein. Open Med. 2019;14(1):785-91. [PubMed] |
| 12. | Jauregui-Amezaga A, Rimola J, Ordás I, Rodríguez S, Ramírez-Morros A, Gallego M. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut. 2015;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Fiorino G, Bonifacio C, Allocca M, Danese S. Impact of therapies on bowel damage in Crohn’s disease. United European Gastroenterology Journal. 2020;8:410-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Ribaldone DG, Caviglia GP, Pellicano R, Vernero M, Italia A, Morino M. Adalimumab vs azathioprine to halt the progression of bowel damage in Crohn’s disease: application of Lémann Index. Scand J Gastroenterol. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Bodini G, Giannini EG, De Maria C, Dulbecco P, Furnari M, Marabotto E. Anti-TNF therapy is able to stabilize bowel damage progression in patients with Crohn’s disease. A study performed using the Lémann Index. Dig Liver Dis. 2017;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |