Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11252
Peer-review started: May 9, 2022
First decision: August 21, 2022
Revised: August 31, 2022
Accepted: September 22, 2022
Article in press: September 22, 2022
Published online: November 6, 2022
Processing time: 170 Days and 22.4 Hours
Although lactation mastitis (LM) has been extensively researched, the incidence rate of LM remains a salient clinical problem. To reduce this incidence rate and achieve a better prognosis, early and specific quantitative indicators are particularly important. It has been found that milk electrolyte concentrations (chloride, potassium, and sodium) and electrical conductivity (EC) significantly change in the early stages of LM in an animal model. Several studies have evaluated EC for the detection of subclinical mastitis in cows. EC, chloride, and sodium content of milk were more accurate for predicting infection status than were other variables. In the early stages of LM, lactic sodium, chloride, and EC increase, but potassium decreases. However, these indicators have not been reported in the diagnosis of LM in humans. This review summarizes the pathogenesis and the mechanism of LM in terms of milk electrolyte concentration and EC, and aim to provide new ideas for the detection of sub-clinical mastitis in humans.
Core Tip: It has been found that milk electrolyte concentrations and electrical conductivity (EC) significantly change in the early stages of lactation mastitis (LM) in an animal model, allowing the early and specific diagnosis of LM. These indicators have not been reported in the diagnosis of LM in humans. We summarize the pathogenesis and the mechanism of LM in terms of milk electrolyte concentrations and EC and aim to provide new ideas for the early diagnosis of LM in humans.
- Citation: Huang Q, Zheng XM, Zhang ML, Ning P, Wu MJ. Lactation mastitis: Promising alternative indicators for early diagnosis. World J Clin Cases 2022; 10(31): 11252-11259
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11252.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11252
Lactation mastitis (LM), which is universally described as a suite of breast conditions that present with local, and often systemic, inflammatory symptoms and signs during lactation[1]. It is an enervating and common disease that affects up to 33% of lactating women[2-4]. LM has a negative impact on both the mother and the baby. Women with LM may develop pain, localized skin redness, and can have associated systemic symptoms, including fever. However, breast pain is the most common and the most distressing symptom for mothers[5]. The symptoms of LM can lead to a compromised maternal psychological state. In addition, approximately 3% of women with LM will develop a breast abscess[6], and an incidence rate of up to 11% has been reported[7], which may cause permanent damage to the shape of the breast. In order to minimize the detrimental effects of LM, researchers have explored various strategies, including the associated risk factors and etiology[8,9]. However, none of these are known to improve clinical prognosis.
To date, the clinical diagnosis of LM has mostly relied on empirical diagnoses, such as a tender, hot, swollen, wedge-shaped area of redness on the affected breast which is associated with an elevated temperature and systemic symptoms, such as a temperature of 38.5 °C or higher, chills, flu-like aching, and systemic illness[10,11]. Due to the lack of early standardized diagnostic criteria, this causes repeated outbreaks and even progresses to a breast abscess, causing great physical and mental pain in mothers with LM. In some Western countries, for example, 73% of children born in Sweden in 1996 were breastfed for 6 mo[12], but LM frequently results in the cessation of exclusive breastfeeding in the absence of appropriate treatment. As a result, it frequently reduces the protective effect of breastfeeding in mothers. For example, related data show that breastfeeding is associated with a 24% lower risk of invasive ovarian cancer[13], and aggregate results indicate that breastfeeding is inversely associated with the risk of breast cancer[14] and reduces the incidence of osteoporosis and type 2 diabetes[15]. Furthermore, LM is linked to lower levels of fat, carbohydrate, and energy in breast milk[16], which may lead to nutritional deficiencies, lowered immunity, and mental effects in infants.
Several studies have evaluated electrical conductivity (EC) for the detection of subclinical mastitis in cows, some have even identified mastitis causing pathogens using EC[17,18]. EC, chloride (Cl-), and sodium (Na+) content in milk were more accurate in predicting infection status than other variables. The electrolyte concentration and EC of milk are the physicochemical properties of milk, and EC measurements were used as an experimental screening indicator for LM in animals as early as the 1990s[19]. Paudyal et al[18] showed that absolute changes in cow’s milk electrolyte concentrations and EC can be used to screen breast milk samples for LM. A previous study provided evidence that when an inflammatory response occurs in the breast tissue of animals, breast permeability increases, and as a result, the potassium (K+) concentration reduces, but the Na+, Cl- concentrations, and EC increase[20-22]. Singh et al[23] recently conducted an observational animal study and concluded that the magnitude of changes in the milk electrolyte concentration and EC may have diagnostic and prognostic values. Due to a lack of research, more data are needed to identify the variation in human milk electrolyte concentration and EC during LM.
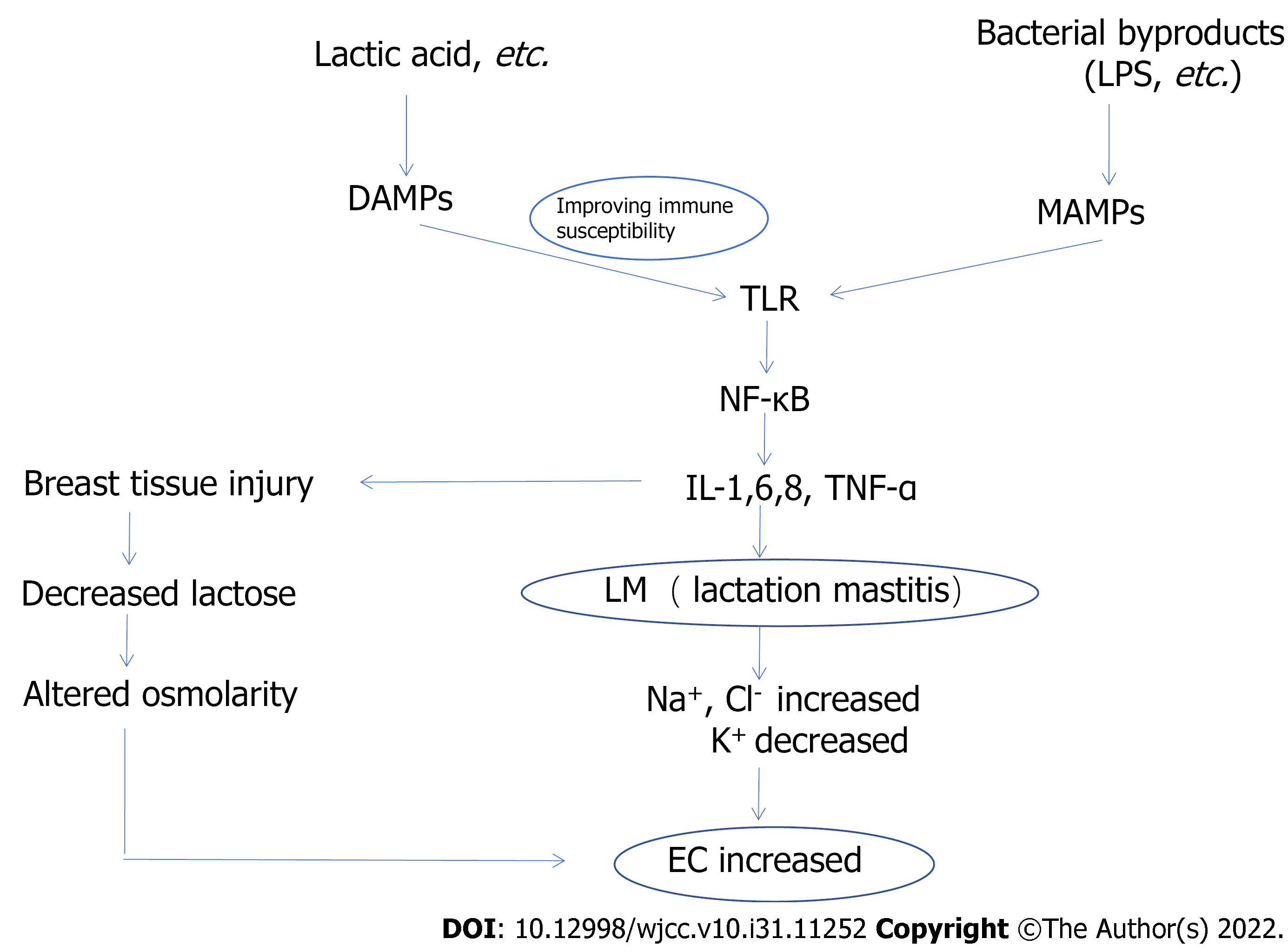
However, it is uncertain whether human milk electrolyte concentrations and EC have a positive effect on the early diagnosis of LM. To enable more patients with LM to be effectively diagnosed and treated at an early stage of the disease, the pathogenesis of LM and the relationship between LM and electrolyte concentrations and EC (Figure 1) are discussed in this review. We investigate the changes in electrolyte concentrations and EC from two perspectives: Altered cell membrane permeability and osmotic pressure level. This will provide new ideas for the detection of subclinical mastitis in humans.
A descriptive review was conducted on the mechanism of changes in milk electrolyte concentration and EC during LM. Pub-Med was searched for articles published between July 1966 and February 2022. The following Medical Subject Headings or free-text terms were used in the search: LM, milk electrolyte concentration, milk EC, early diagnosis, pathogenesis, mechanism, inflammatory injury, and altered cell membrane permeability. The search was limited to papers written in English, with no restrictions on the type of article.
The susceptibility and severity of LM are positively correlated with inflammatory factors. Some researchers consider LM to be an infectious disorder[19,24,25], but the etiology of LM has now shifted from infection to inflammation.
Due to the characteristics of LM, it was previously considered to be an infectious disease. Based on the maturation of milk culture technology, some infectious pathogenic bacteria, such as Staphylococcus aureus, Escherichia coli, and Streptococcus, have all been isolated from breast milk, confirming the importance of infective pathogenic bacteria in the pathogenesis of LM[26,27]. In recent years, Staphylococcus epidermidis, the most common species on human skin and mucosa, has also been isolated from the milk of patients with LM and may be another causative agent of LM[28-30]. Based on the fact that a variety of infectious pathogens are present in the milk of patients with LM, researchers believed that the occurrence of LM was closely related to infectious pathogens in the milk[31]. Milk stagnation, breast trauma, excessive emptying of the breast, nipple cracking, and dysbiosis of the breast flora can all contribute to the development of infectious LM.
Recently, in-depth studies on LM have revealed that the presence of infectious agents is not positively associated with the occurrence of LM[26]. In addition, there is no positive correlation between the severity of LM and the bacterial count in milk[32]. Several studies showed that the susceptibility and severity of LM are positively correlated with inflammatory factors, including C-reactive protein, interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor-α (TNF-α)[33-35]. Elevated serum cytokines IL-1, IL-6, IL-8, and TNF-α reveal the activation of transcription factor nuclear factor-kappa B (NF-κB) in the host[36]. Furthermore, activation of the NF-κB pathway in LM hosts has been demonstrated in numerous animal studies, in vitro experiments, and genetic studies[37-40].
As upstream candidate receptors of NF-κB, Toll-like receptors (TLRs) are important inflammatory mediators and their isoforms include TLR1-TLR11[41,42], which are recognized as pattern recognition receptors (PRRs) by microbe-associated molecular patterns (MAMPs) or danger-associated molecular patterns (DAMPs) and activate downstream NF-κB signaling pathways[34].
Of all the TLRs, TLR2, TLR4, and TLR5 bind to bacterial byproducts in MAMPs, such as lipopolysaccharide, phospholipid wall acids, bacterial lipopeptides, and flagellin, activate the corresponding TLRs, which activate transcription, translation, and the release of a series of inflammatory factors, chemokines, and adhesion molecules, and recruit other molecules involved in the innate immune response (neutrophils, etc.) to the site of infection[33,34]. Pathogenic byproducts bind to TLRs on the surface of PRRs, and the signal is transmitted from extracellular to intracellular, activating TLRs, followed by signal transduction, and activation of the transcription factor NF-κB. This is the pathogenesis of infectious LM via the inflammatory mechanism.
TLR activation via the DAMP pathway explains how LM can lead to disease in the absence of infectious agents. DAMPs are endogenous proteins and can activate TLRs in a sterile environment[43,44]. DAMPs can activate TLRs in two ways: first, some inflammatory mediators activated by DAMPs activate TLRs and downstream NF-κB[45]; second, DAMPs can enhance the susceptibility of the TLR immune response in a sterile environment, thus activating TLRs and downstream NF-κB leading to the development of noninfectious LM[46].
Changes in milk electrolyte concentration and EC are linked to the inflammatory response during LM, and pathological changes in breast tissues caused by inflammatory factors result in changes in milk composition. Our understanding of the changes in milk electrolyte concentration and EC during LM is linked to the research by Smith et al[47] in the 1960s.The essence of LM is the inflammatory response of the breast tissue[48], and inflammatory factors cause increased epithelial permeability and both vascular and parenchyma damage[20].
The sodium pump on the basolateral membrane, which keeps intracellular K+ high and Na+ low, and the distribution of these ions according to the gradient of electric potential across the luminal membranes are the most important characteristics in terms of Na+ and K+[49]. The Na+/K+ ratio of the intracellular fluid is maintained at approximately 1:3 and the ratio of the extracellular fluid is maintained at approximately 3:1 by active transport of the sodium–potassium ion pump. As milk is electrically positive compared to the interior of the cell, the concentrations of these ions are lower in milk. However, because milk is nearly isosmotic to plasma[50], the ratio between them is similar, with a Na+/K+ ratio of about 1:3[51,52].
When an inflammatory response occurs in the breast tissue, all of the inflammatory factors produced damage the ducts and secretary epithelial cells, disrupt the tight junctions between secretary cells, and increase capillary permeability. As a result, higher levels of Na+ and Cl- in the extracellular fluid enter the mammary gland alveolar lumen through the tight junctions that are opened, while the K+ concentration decreases in order to maintain the osmotic pressure of milk in the alveolar lumen[53]. The increase in ionic concentrations in breast milk in the presence of mastitis, such as Na+ and Cl-, is consistent with other animal studies[54-56].
Milk is rich in lactose. Lactose excretion may provide a reliable basis for fateful changes in breast permeability as lactose in food contributes minimally to the circulation[54]. Fetherston et al[57] conducted relevant studies, and the 24-h urinary excretion of lactose during LM has been extensively discussed. The correspondingly low steady-state urinary excretion of lactose demonstrates that these variations are not the result of increased paracellular pathway permeability. It indicates that the higher than normal concentrations of milk Na+ and Cl- observed in “normal breasts” could be a normal physiologic response to a lower concentration of lactose, ensuring that the osmolality of milk remains isotonic with plasma[20]. This has significant implications for the supposition that a raised Na+ concentration is an outcome of subclinical LM.
The electrolyte concentration and EC are physical properties of body fluids that are used to assess human health status and disease severity[58,59]. Previous animal studies have indicated that they can be used as an alternative method for the early diagnosis of LM[60]. In addition, Kitchen et al[61] demonstrated that the measurements of electrolyte concentration and EC in milk samples for the diagnosis of LM in animals were comparable to other diagnostic methods.
Similar to the pathological response in animals with LM[62,63], when a woman is diagnosed with LM, inflammatory factors, such as procalcitonin, IL-1, IL-6, IL-8, and TNF-α[36], cause disruption of the tight junctions between cells. Furthermore, as a result of both the decrease in available glucose, particularly during severe symptoms, and the damage or death of lactocytes due to inflammation, lactose synthesis decreases[20]. Both of these factors can lead to changes in the electrolyte concentration and EC of milk. As mentioned earlier, numerous animal studies have indicated that milk electrolyte concentration and EC can be used in the early diagnosis of LM; therefore, based on the similar pathological responses, it is feasible to use milk electrolyte concentration and EC to diagnose early LM in humans.
In a recent study, we collected milk specimens from approximately 30 healthy women and 15 patients diagnosed with LM, and measured the electrolyte concentration (including Na+, K+, and Cl-) and EC of bilateral breast milk in all patients. The final test results revealed that the concentrations of Na+, Cl- and EC in breast milk were significantly higher in women with LM than in healthy women, and in some cases several times higher. In addition, in women with LM, the concentrations of Na+, Cl- and EC in breast milk were markedly higher on the affected side than on the healthy side. Furthermore, we also found that the concentrations of Na+, Cl- and EC in milk were increased to varying degrees in patients who had symptoms but were not diagnosed with LM.
The early diagnosis and prevention of LM still face many challenges. We summarized the pathogenesis of LM, the mechanism of LM-induced changes in milk electrolyte concentration and EC, and found that changes in milk electrolyte concentration and EC in humans were primarily correlated with LM. It is clear from animal studies that there is a correlation between LM and milk electrolyte concentration and EC, and from the significant changes in these indicators, LM can be diagnosed at an early stage and thus achieve a better prognosis. However, there have been few studies carried out on this topic. As a result, more data are required to verify these findings. If the changes in milk electrolyte concentration and EC are beneficial, these will have an enormous impact on clinical practice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bairwa DBL, India; Mahmoud MZ, Saudi Arabia S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Cooper M, Lowe H, McArdle A. Development of a novel patient focussed symptom severity index for use in assessing and treating inflammatory conditions of the lactating breast: a Delphi study. Int J Evid Based Healthc. 2020;18:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Angelopoulou A, Field D, Ryan CA, Stanton C, Hill C, Ross RP. The microbiology and treatment of human mastitis. Med Microbiol Immunol. 2018;207:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Lai BY, Yu BW, Chu AJ, Liang SB, Jia LY, Liu JP, Fan YY, Pei XH. Risk factors for lactation mastitis in China: A systematic review and meta-analysis. PLoS One. 2021;16:e0251182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Barker M, Adelson P, Peters MDJ, Steen M. Probiotics and human lactational mastitis: A scoping review. Women Birth. 2020;33:e483-e491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. 2014;2:852-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (4)] |
| 6. | O'Brien C, Quinn E, Murphy M, Lehane E, O'Leary DP, Livingstone V, Paul Redmond H, Corrigan MA. Breast abscess: Not just a puerperal problem. Breast J. 2020;26:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Amir LH, Forster D, McLachlan H, Lumley J. Incidence of breast abscess in lactating women: report from an Australian cohort. BJOG. 2004;111:1378-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Kvist LJ, Rydhstroem H. Factors related to breast abscess after delivery: a population-based study. BJOG. 2005;112:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Branch-Elliman W, Golen TH, Gold HS, Yassa DS, Baldini LM, Wright SB. Risk factors for Staphylococcus aureus postpartum breast abscess. Clin Infect Dis. 2012;54:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Cooney F, Petty-Saphon N. The Burden of Severe Lactational Mastitis in Ireland from 2006 to 2015. Ir Med J. 2019;112:855. [PubMed] |
| 11. | Amir LH; Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #4: Mastitis, revised March 2014. Breastfeed Med. 2014;9:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Brown CR, Dodds L, Legge A, Bryanton J, Semenic S. Factors influencing the reasons why mothers stop breastfeeding. Can J Public Health. 2014;105:e179-e185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Babic A, Sasamoto N, Rosner BA, Tworoger SS, Jordan SJ, Risch HA, Harris HR, Rossing MA, Doherty JA, Fortner RT, Chang-Claude J, Goodman MT, Thompson PJ, Moysich KB, Ness RB, Kjaer SK, Jensen A, Schildkraut JM, Titus LJ, Cramer DW, Bandera EV, Qin B, Sieh W, McGuire V, Sutphen R, Pearce CL, Wu AH, Pike M, Webb PM, Modugno F, Terry KL. Association Between Breastfeeding and Ovarian Cancer Risk. JAMA Oncol. 2020;6:e200421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Chen J, Li Q, Huang W, Lan H, Jiang H. Association between breastfeeding and breast cancer risk: evidence from a meta-analysis. Breastfeed Med. 2015;10:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Aune D, Norat T, Romundstad P, Vatten LJ. Breastfeeding and the maternal risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Say B, Dizdar EA, Degirmencioglu H, Uras N, Sari FN, Oguz S, Canpolat FE. The effect of lactational mastitis on the macronutrient content of breast milk. Early Hum Dev. 2016;98:7-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Musser JM, Anderson KL, Caballero M, Amaya D, Maroto-Puga J. Evaluation of a hand-held electrical conductivity meter for detection of subclinical mastitis in cattle. Am J Vet Res. 1998;59:1087-1091. [PubMed] |
| 18. | Paudyal S, Melendez P, Manriquez D, Velasquez-Munoz A, Pena G, Roman-Muniz IN, Pinedo PJ. Use of milk electrical conductivity for the differentiation of mastitis causing pathogens in Holstein cows. Animal. 2020;14:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Osterman KL, Rahm VA. Lactation mastitis: bacterial cultivation of breast milk, symptoms, treatment, and outcome. J Hum Lact. 2000;16:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Fetherston CM, Lai CT, Hartmann PE. Relationships between symptoms and changes in breast physiology during lactation mastitis. Breastfeed Med. 2006;1:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Bruckmaier RM, Weiss D, Wiedemann M, Schmitz S, Wendl G. Changes of physicochemical indicators during mastitis and the effects of milk ejection on their sensitivity. J Dairy Res. 2004;71:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Pyörälä S. Indicators of inflammation in the diagnosis of mastitis. Vet Res. 2003;34:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Singh M, Yadav P, Sharma A, Garg VK, Mittal D. Estimation of Mineral and Trace Element Profile in Bubaline Milk Affected with Subclinical Mastitis. Biol Trace Elem Res. 2017;176:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Jonsson S, Pulkkinen MO. Mastitis today: incidence, prevention and treatment. Ann Chir Gynaecol Suppl. 1994;208:84-87. [PubMed] |
| 25. | Riordan JM, Nichols FH. A descriptive study of lactation mastitis in long-term breastfeeding women. J Hum Lact. 1990;6:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Kvist LJ, Larsson BW, Hall-Lord ML, Steen A, Schalén C. The role of bacteria in lactational mastitis and some considerations of the use of antibiotic treatment. Int Breastfeed J. 2008;3:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Rimoldi SG, Pileri P, Mazzocco MI, Romeri F, Bestetti G, Calvagna N, Tonielli C, Fiori L, Gigantiello A, Pagani C, Magistrelli P, Sartani A, De Silvestri A, Gismondo MR, Cetin I. The Role of Staphylococcus aureus in Mastitis : A Multidisciplinary Working Group Experience. J Hum Lact. 2020;36:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Berens P, Swaim L, Peterson B. Incidence of methicillin-resistant Staphylococcus aureus in postpartum breast abscesses. Breastfeed Med. 2010;5:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Lasagno M, Ortiz M, Vissio C, Yaciuk R, Bonetto C, Pellegrino M, Bogni C, Odierno L, Raspanti C. Pathogenesis and inflammatory response in experimental caprine mastitis due to Staphylococcus chromogenes. Microb Pathog. 2018;116:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Rodvold KA, McConeghy KW. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis. 2014;58 Suppl 1:S20-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 31. | Shuyang X, Qiang Y. Bacterial factors of mastitis in lactating women and its effect on the physical properties and chemical composition of breast milk. Cell Mol Biol (Noisy-le-grand). 2021;67:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Fetherston C. Mastitis in lactating women: physiology or pathology? Breastfeed Rev. 2001;9:5-12. [PubMed] |
| 33. | Glynn DJ, Hutchinson MR, Ingman WV. Toll-like receptor 4 regulates lipopolysaccharide-induced inflammation and lactation insufficiency in a mouse model of mastitis. Biol Reprod. 2014;90:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5843] [Cited by in RCA: 6747] [Article Influence: 449.8] [Reference Citation Analysis (0)] |
| 35. | Wambach KA. Lactation mastitis: a descriptive study of the experience. J Hum Lact. 2003;19:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Ingman WV, Glynn DJ, Hutchinson MR. Inflammatory mediators in mastitis and lactation insufficiency. J Mammary Gland Biol Neoplasia. 2014;19:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Xiao HB, Wang CR, Liu ZK, Wang JY. LPS induces pro-inflammatory response in mastitis mice and mammary epithelial cells: Possible involvement of NF-κB signaling and OPN. Pathol Biol (Paris). 2015;63:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Cao D, Luo J, Chen D, Xu H, Shi H, Jing X, Zang W. CD36 regulates lipopolysaccharide-induced signaling pathways and mediates the internalization of Escherichia coli in cooperation with TLR4 in goat mammary gland epithelial cells. Sci Rep. 2016;6:23132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Liu S, Shi X, Bauer I, Günther J, Seyfert HM. Lingual antimicrobial peptide and IL-8 expression are oppositely regulated by the antagonistic effects of NF-κB p65 and C/EBPβ in mammary epithelial cells. Mol Immunol. 2011;48:895-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Wu J, Li L, Sun Y, Huang S, Tang J, Yu P, Wang G. Altered molecular expression of the TLR4/NF-κB signaling pathway in mammary tissue of Chinese Holstein cattle with mastitis. PLoS One. 2015;10:e0118458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Sasai M, Yamamoto M. Pathogen recognition receptors: ligands and signaling pathways by Toll-like receptors. Int Rev Immunol. 2013;32:116-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Lebda MA, Elmassry IH, Taha NM, Elfeky MS. Nanocurcumin alleviates inflammation and oxidative stress in LPS-induced mastitis via activation of Nrf2 and suppressing TLR4-mediated NF-κB and HMGB1 signaling pathways in rats. Environ Sci Pollut Res Int. 2022;29:8294-8305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | McDermott MF, Tschopp J. From inflammasomes to fevers, crystals and hypertension: how basic research explains inflammatory diseases. Trends Mol Med. 2007;13:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 45. | El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111:36-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol. 2009;182:2476-2484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 47. | Smith A, Wheelock JV, Dodd FH. Effect of milking throughout pregnancy on milk yield in the succeeding lactation. J Dairy Sci. 1966;49:895-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Omranipour R, Vasigh M. Mastitis, Breast Abscess, and Granulomatous Mastitis. Adv Exp Med Biol. 2020;1252:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Peaker M. Recent advances in the study of monovalent ions movements across the mammary epithelium: relation to onset of lactation. J Dairy Sci. 1975;58:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Peaker M. Mechanism of milk secretion: milk composition in relation to potential difference across the mammary epithelium. J Physiol. 1977;270:489-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Linzell JL, Peaker M. The distribution and movements of carbon dioxide, carbonic acid and bicarbonate between blood and milk in the goat. J Physiol. 1975;244:771-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Shennan DB, McNeillie SA. High affinity (Na(+) + Cl-)-dependent taurine transport by lactating mammary tissue. J Dairy Res. 1994;61:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Prentice A, Prentice AM, Lamb WH. Mastitis in rural Gambian mothers and the protection of the breast by milk antimicrobial factors. Trans R Soc Trop Med Hyg. 1985;79:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Prosser CG, Hartmann PE. Comparison of mammary gland function during the ovulatory menstrual cycle and acute breast inflammation in women. Aust J Exp Biol Med Sci. 1983;61 (Pt 3):277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Semba RD, Kumwenda N, Taha TE, Hoover DR, Lan Y, Eisinger W, Mtimavalye L, Broadhead R, Miotti PG, Van Der Hoeven L, Chiphangwi JD. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol. 1999;6:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Brew K, Hill RL. Lactose biosynthesis. Rev Physiol Biochem Pharmacol. 1975;72:105-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Fetherston CM, Lai CT, Mitoulas LR, Hartmann PE. Excretion of lactose in urine as a measure of increased permeability of the lactating breast during inflammation. Acta Obstet Gynecol Scand. 2006;85:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Fazil Marickar YM. Electrical conductivity and total dissolved solids in urine. Urol Res. 2010;38:233-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Seifter JL. Body Fluid Compartments, Cell Membrane Ion Transport, Electrolyte Concentrations, and Acid-Base Balance. Semin Nephrol. 2019;39:368-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | de Oliveira Moura E, do Nascimento Rangel AH, de Melo MCN, Borba LHF, de Lima Júnior DM, Novaes LP, Urbano SA, de Andrade Neto JC. Evaluation of microbiological, cellular and risk factors associated with subclinical mastitis in female buffaloes. Asian-Australas J Anim Sci. 2017;30:1340-1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Kitchen BJ, Middleton G, Salmon M. Bovine milk N-acetyl-beta-D-glucosaminidase and its significance in the detection of abnormal udder secretions. J Dairy Res. 1978;45:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Kermack WO, Miller RA. Electrical conductivity and chloride content of women's milk. Part 2. The effect of factors relating to lactation. Arch Dis Child. 1951;26:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 63. | Miller RA, Jackson II. Electrical conductivity and chloride content of women's milk. Part 4. Results and their relationship to milk yield and to duration of lactation. Arch Dis Child. 1951;26:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |