Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.992
Peer-review started: February 26, 2021
First decision: October 16, 2021
Revised: October 27, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: January 21, 2022
Processing time: 323 Days and 1.8 Hours
Ankylosing spondylitis (AS) is strongly associated with the human leukocyte antigen (HLA) B27 haplotype. In regions where conventional polymerase chain reaction for HLA typing is available for antigens such as HLA B27 or HLA B51, it is common to perform the HLA B27 test for evaluation of AS. While HLA B27-associated clustered occurrences of AS have been reported in families, we report the first case series of HLA B51-related occurrences of AS in a family.
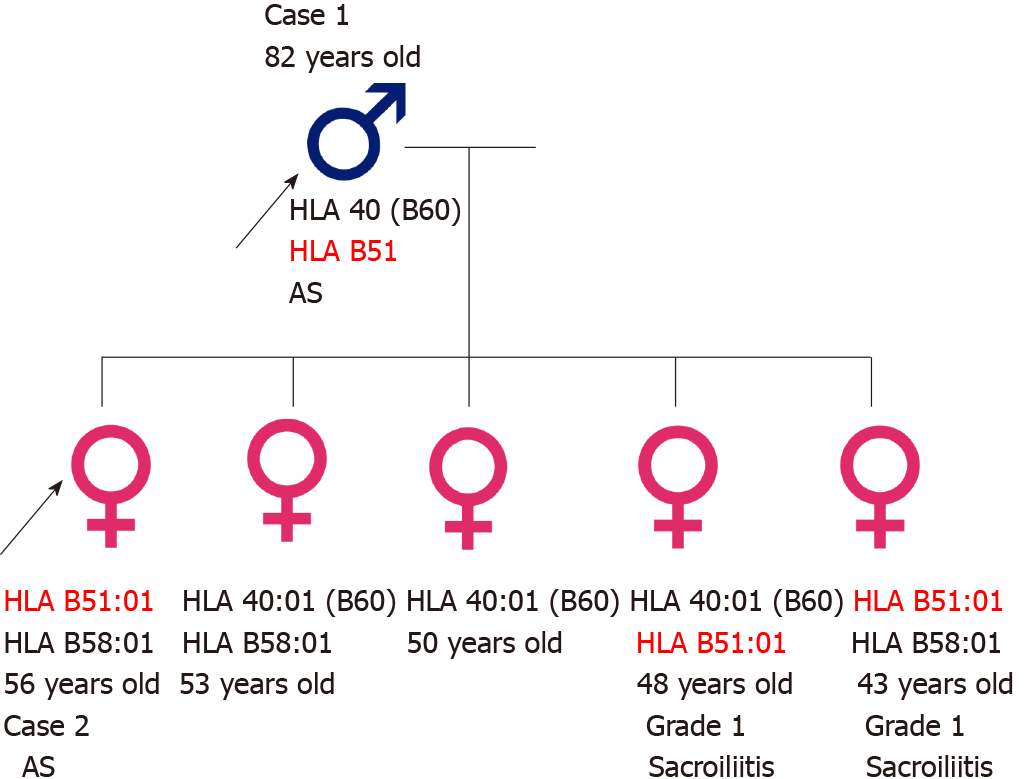
A father and his daughters were diagnosed with AS and did not have the HLA B27 haplotype. Although they were positive for HLA B51, they exhibited no signs of Behçet’s disease (BD). Of the five daughters, one had AS, and three, including the daughter with AS, were positive for HLA B51. The two daughters with the HLA B51 haplotype (excluding the daughter with AS) exhibited bilateral grade 1 sacroiliitis, whereas the daughters without the HLA B51 haplotype did not have sacroiliitis. Thus, this Korean family exhibited a strong association with the HLA B51 haplotype and clinical sacroiliitis, irrespective of the symptoms of BD.
It is advisable to check for HLA B51 positivity in patients with AS/spondyloarthropathy who test negative for HLA B27.
Core Tip: Ankylosing spondylitis (AS) is strongly associated with human leukocyte antigen (HLA) B27. In certain regions, the testing of HLA genotyping, such as HLA B27 or HLA B51, is an available medical service. Here, we report the first case series of HLA B51-related inheritance of AS in a family. No one in this family tested positive for HLA B27, and additional testing for HLA B51 revealed that family members with HLA B51 haplotype had either AS or clinical sacroiliitis without symptoms compatible with Behçet’s disease. Thus, it is advisable to perform HLA B51 testing as a genetic marker for HLA B27 negative AS/spondyloarthropathy.
- Citation: Lim MJ, Noh E, Lee RW, Jung KH, Park W. Occurrence of human leukocyte antigen B51-related ankylosing spondylitis in a family: Two case reports. World J Clin Cases 2022; 10(3): 992-999
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/992.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.992
Ankylosing spondylitis (AS) is a chronic, immune-mediated arthritis that primarily affects the spine and sacroiliac joints. Inflammation of the sacroiliac joints is a hallmark feature of the disease, and grade ≥ 2 radiological sacroiliitis on both sides or unilateral grade ≥ 3 radiological sacroiliitis are the criteria (per the modified New York criteria) for the diagnosis of AS[1] and comprise some of the criteria required for a diagnosis of spondyloarthropathy (SpA)[2]. A strong association between the human leukocyte antigen (HLA) B27 allele and AS was discovered in the early 1970s[3]. AS is also known to run strongly within families, and HLA B27 positivity was observed to be higher in familial AS patients than in their sporadic AS counterparts[4]. According to Jung et al[5], the proportion of HLA B27 positivity among Korean AS patients was 80%, and in a Korean population study, the HLA B27 positivity rate in Korean AS patients was 83.3% compared to a rate of 4.0% in healthy controls[6].
The HLA B51 antigen is a well-known genetic factor associated with Behçet’s disease (BD)[7]. In countries such as Korea and Japan where conventional polymerase chain reaction (PCR) for HLA genotyping is available for antigens such as HLA B27 or HLA B51, it is common to perform the HLA B27 test for the evaluation of AS and HLA B51 testing for BD. Interestingly, two cases of Reiter’s syndrome associated with HLA B51 have been reported[8,9] and the possibility of HLA B51-related arthropathy in individuals with HLA B27-negative reactive arthritis or seronegative SpA has also been proposed[10,11]. To date, there have been no reports of familial AS occurrence related to HLA B51. Here, we report the first cases of HLA B51-related AS in a family and the impact of HLA B51 positivity on sacroiliitis.
Case 1: In 2018, an 82-year-old man visited our clinic with the chief complaint of inflammatory low back pain.
Case 2: In 2020, the eldest daughter of the patient described in case 1 visited the clinic. She was 56 years old and complained of back pain, which had started 3 years pre
Case 1: He had previously been diagnosed with AS at another hospital. He did not complain of any additional pain in the Achilles tendon or the peripheral joints. He did not have abdominal pain or diarrhea suggestive of inflammatory bowel disease. He also did not have any symptoms of BD, such as oral or genital ulcers.
Case 2: She did not complain of any other pain in the Achilles tendon or peripheral joints. She did not have abdominal pain or diarrhea suggestive of inflammatory bowel disease. She did not have any symptoms of BD, such as oral or genital ulcers. She did not have any symptoms related to the eyes.
Case 1: He was suffering from interstitial lung disease.
Case 2: She had no previous medical history.
Case 1: There is no personal and family history.
Case 2: The patient was the first of five daughters of the patient in case 1.
Case 1: The patient’s blood pressure was 126/27 mmHg, pulse rate was 79 beats/min, and respiratory rate was 24 breaths/min at the time of presentation. The body tem
Case 2: Her blood pressure was 98/52 mmHg, pulse rate was 70 beats/minute, and respiratory rate was 20 breaths/min at the time of presentation. Her body temperature was within the normal range. No abnormal skin lesions were found, and no heart murmur was heard. Schober’s test showed a positive result of 2.5 cm. The distance between the occiput and wall and the chest wall expansion test were within normal limits.
Case 1: Laboratory tests showed a white blood cell count of 10190/μL, C-reactive protein (CRP) of 14.3 mg/L (0-5), erythrocyte sedimentation rate (ESR) of 52 mm/h (1-15) and positive antinuclear antibodies with a titer of 1:640. The tests for extractable nuclear antigen antibodies were negative. Rheumatoid factors were not observed. The patient tested negative for HLA B27 and positive for HLA B51, using conventional PCR. With help from the laboratory department, simple HLA genotyping was performed, which further confirmed the presence of HLA B51.
Case 2: Laboratory tests showed a white blood cell count of 5950/μL, CRP of 0.4 mg/L (0-5), and ESR of 14 mm/h (1-15). Neither rheumatoid factor nor anti
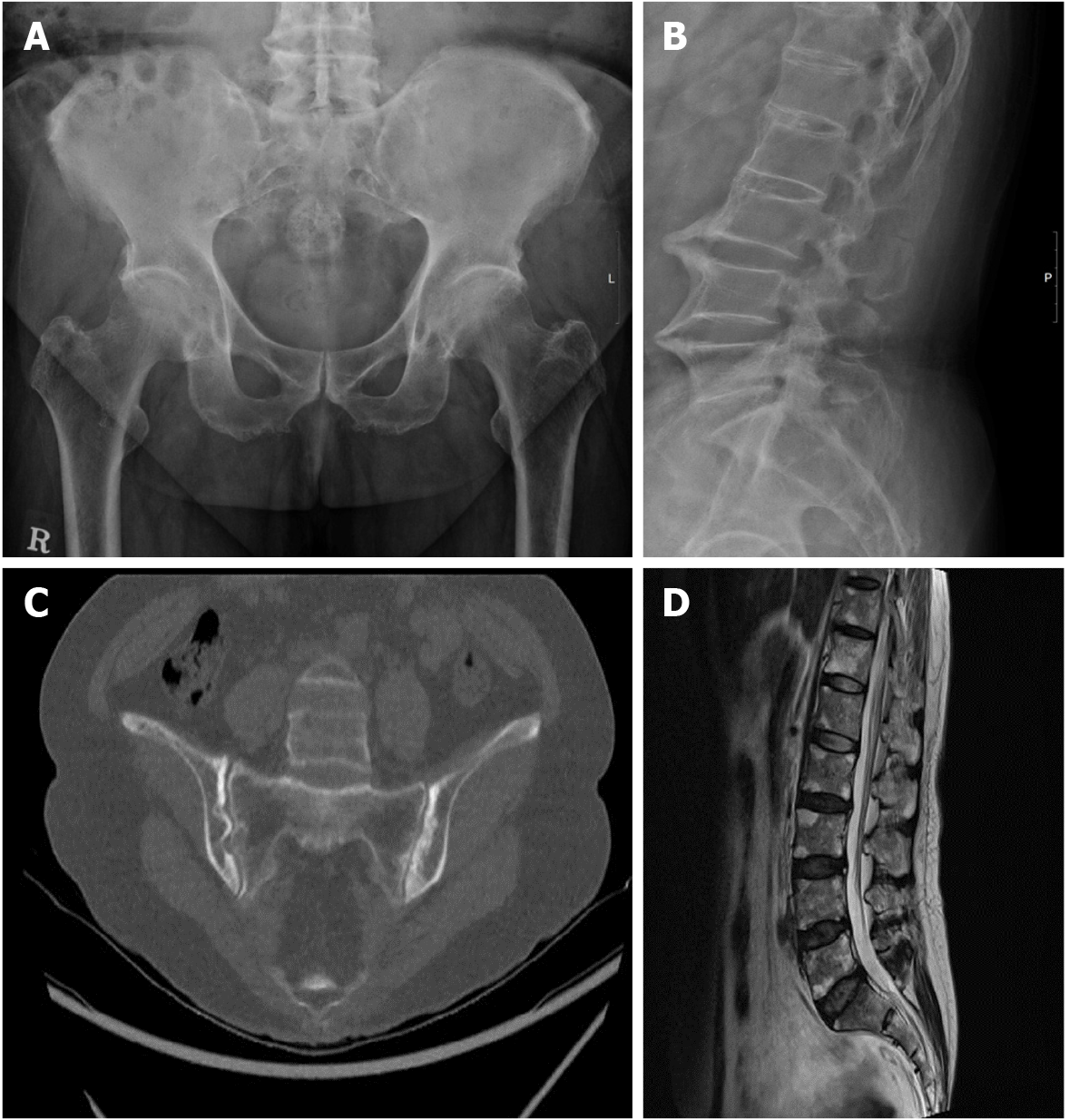
Case 1: Radiographic imaging of the sacroiliac joints revealed complete ankyloses, and his spine exhibited a “bamboo” appearance (Figures 1A and 1B). Transthoracic echocardiography revealed a sclerotic mitral and aortic valve.
Case 2: Radiographic imaging of the sacroiliac joints revealed multiple definite erosions with sclerotic changes compatible with grade III bilateral sacroiliitis (Figure 1C). Magnetic resonance imaging of her spine revealed fat deposition at the corners of the vertebral bodies, suggesting changes caused by AS (Figure 1D).
The final diagnosis in this case was AS. Laboratory findings of leukocytosis and high levels of inflammatory markers were thought to be caused by interstitial lung disease, as the patient did not complain much about back pain and no other symptoms of AS were reported.
The final diagnosis of the presented case was AS.
The patient was prescribed non-steroidal anti-inflammatory drugs (NSAIDs).
The patient was prescribed NSAIDs.
The patient reported that his back pain was under control at the 2 mo follow up. In addition, he complained of dyspnea, and 2 years after the diagnosis of AS in our hospital, the patient passed away due to worsening of interstitial lung disease.
She reported that her back pain was under control at 2 mo follow up.
The father (case 1) had five daughters, including the first daughter (case 2) who was previously diagnosed with AS. The family was concerned about the possibility of familial inheritance of AS, and all five daughters agreed to undergo full HLA-B genotyping and computed tomography (CT) of the sacroiliac joint(s) to assess the possibility of AS. HLA-B genotyping was performed using a commercially available polymerase chain reaction sequencing-based kit (AlleleSEQR HLA-B Sequencing Kit, Genome Diagnostics B. V., Utrecht, The Netherlands) for experimental purposes. This study was approved by the Institutional Review Board of Inha University Hospital (Incheon, Korea; IRB 2020-03-003), and written informed consent was obtained from all participants.
None of the daughters reported signs or symptoms of oral ulcers or genital ulcers, and only the youngest daughter complained of inflammatory back pain. No abnormal skin lesions were observed.
Three daughters, including the patient in case 2 and the youngest daughter, tested positive for HLA B51, and two other daughters were negative for HLA B51. None of the patients tested positive for HLA B27.
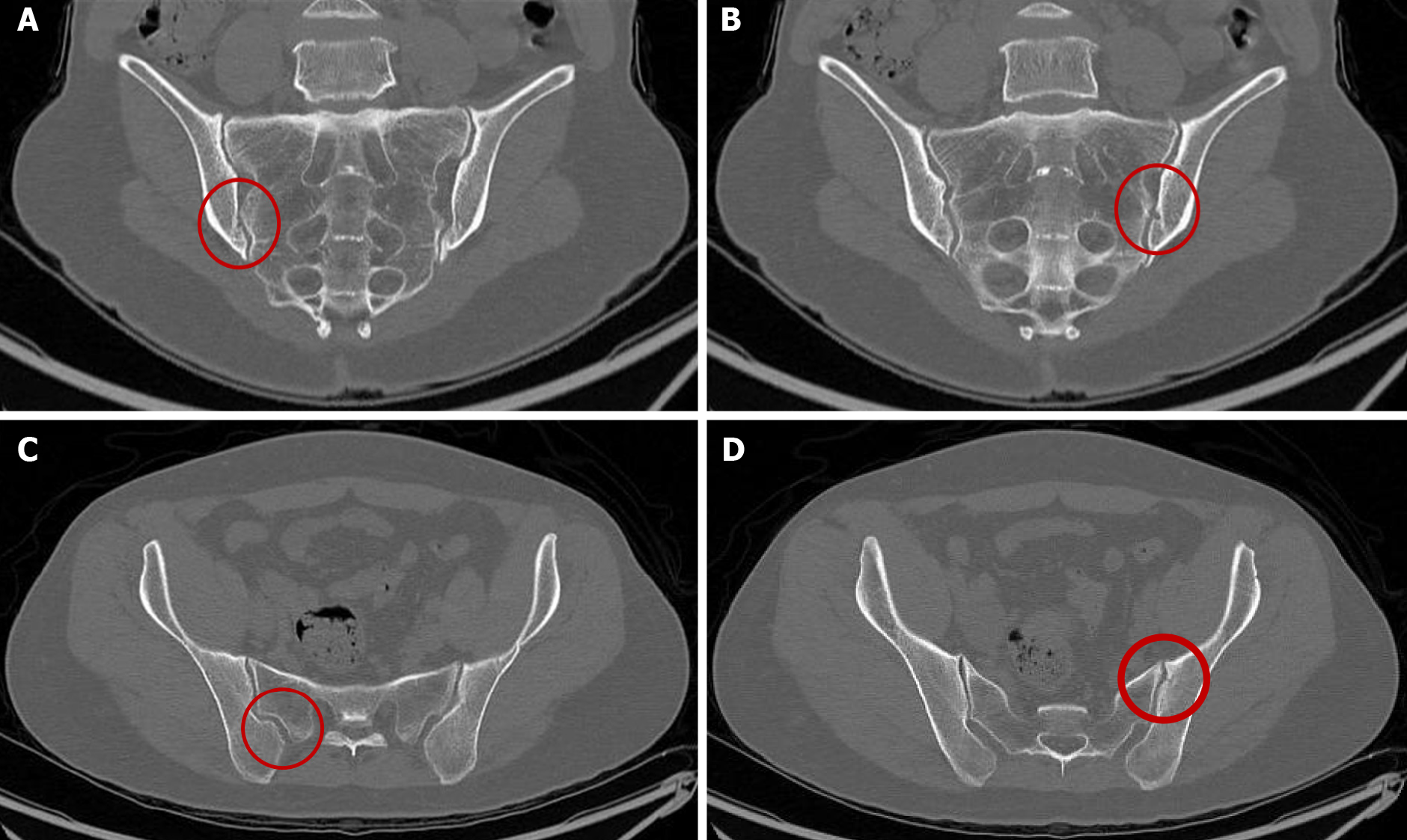
A radiologist who was blinded to patient information interpreted the images. Three daughters had the HLA B51:01 allele, among whom only the eldest daughter (case 2) was diagnosed with AS. However, the other two daughters, including the youngest daughter, were found to exhibit grade 1 sacroiliitis, upon performing pelvic bone CT [(Figures 2A and 2B for 4th daughter) and (Figures 2C and 2D for the youngest daughter)]. Two daughters without the HLA B51:01 allele did not exhibit sacroiliitis. The family pedigree is shown in Figure 3.
To the best of our knowledge, this is the first report to describe the occurrence of HLA B51-related AS in a family. Three of the five daughters had the HLA B51:01 allele and developed either AS or clinical sacroiliitis; however, the daughters without the HLA B51:01 allele did not exhibit any clinical signs or symptoms of SpA. Low-grade sacroiliitis is indicative of early AS in patients with undifferentiated SpA[12]. Thus, the high prevalence of HLA B51:01 in the daughters with sacroiliitis suggests a strong association of HLA B51 with AS/SpA in the family. It was also interesting to observe that no one in the family manifested clinical symptoms of BD, although the association between HLA B51 and BD is known to be strong.
The clinical significance of HLA B51 related familial AS familial is that this family is from Korea, where HLA B51 is highly prevalent. A previous case control study performed in Korea showed that the preva
Second, unlike in previous studies, sacroiliitis was observed in most of the daughters (three of five) in this family. There are conflicting reports regarding the prevalence of sacroiliitis in patients with BD. Chang et al[7] reported that sacroiliitis was diagnosed in 58.9% of SpA patients, 10.3% of those with BD , and 3.6% of healthy controls. Olivieri et al[16] conducted a similar study using CT scans and reported sacroiliitis in 30% of BD patients and 5% of controls. The difference in the prevalence of sacroiliitis between patients with BD and controls was clinically significant in both the aforementioned studies[7,16]. However, another study showed contrasting results with the prevalence of sacroiliitis seen in 7.4% of individuals with BD and 8% of the control group[17]. Kotevoglu et al[18] conducted a study using CT and found sacroiliitis in 5% of patients with BD and in 7% of healthy controls. In this family, sacroiliitis was found in 60% of all daughters who exhibited no clinical features of BD. Thus, the high prevalence of sacroiliitis in this family should be interpreted in the context of SpA and not a clinical feature of BD.
Lastly, all family members with sacroiliitis, tested positive for HLA B51 and negative for HLA B27. There are studies about HLA B51 and HLA B27 in SpA, and HLA B27 remains the major factor in AS. In two studies, Chang et al[7] reported that the majority of SpA patients (67.9%) were HLA B27 positive, whereas the prevalence of HLA B51 positivity was only 21.4% in those with SpA. Similarly, a study by Jung et al[5] reported that 106 of 153 patients with AS were HLA B27 positive/HLA B51 negative, whereas eight were HLA B51 positive/HLA B2-negative, and 16 patients were both HLA B27 and HLA B51 positive. In clinical practice, HLA typing is usually carried out for determining the presence of HLA B27 for the evaluation of AS, and HLA B51 for the evaluation of BD. If a patient suspected of having SpA, is negative for HLA B27, additional testing of HLA B51 could help facilitate the diagnosis of SpA even if the patient does not have the clinical features of BD.
A genome-wide association study of AS, which used “immunochip” technology, reported that the presence of the HLA B51:01 allele was associated with an increased risk of AS[3]. In addition, AS is strongly associated with the presence of specific amino acids at position 97 in HLA-B, and position 97 is associated with the cell surface expression of HLA B51[19]. Therefore, in accordance with the present case report, HLA B51 could also potentially contribute to the development of AS.
This is the first report of familial inheritance of HLA B27-negative AS and HLA B51 positivity associated with either mild or definite radiological sacroiliitis. No patient in the family exhibited any signs or symptoms of BD. Therefore, it is advisable to check for HLA B51 positivity in patients with HLA B27-negative AS or SpA, even in the absence of clinical signs of BD.
All authors are grateful to the family described in this case series for their parti
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Korean College of Rheumatology, 9-92-8.
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cure E, Kenzaka T S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3782] [Cited by in RCA: 4119] [Article Influence: 100.5] [Reference Citation Analysis (1)] |
| 2. | Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B, Olivieri I, Pasero G. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1502] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 3. | Li Z, Brown MA. Progress of genome-wide association studies of ankylosing spondylitis. Clin Transl Immunology. 2017;6:e163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Kim HW, Choe HR, Lee SB, Chang WI, Chae HJ, Moon JY, Kang J, Lee S, Song YW, Lee EY. Phenotype difference between familial and sporadic ankylosing spondylitis in Korean patients. J Korean Med Sci. 2014;29:782-787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Jung JH, Bang CH, Seok H, Choi SJ, Song GG. Clinical Findings of Ankylosing Spondylitis With and Without Human Leukocyte Antigen (HLA)-B27 and HLA-B51. Ann Acad Med Singap. 2019;48:321-329. [PubMed] |
| 6. | Cho SJ, Park MH. HLA-B27 Frequency in Korean Patients with Ankylosing Spondylitis. Korean J Lab Med. 2008;28:46-52. |
| 7. | Chang HK, Lee DH, Jung SM, Choi SJ, Kim JU, Choi YJ, Baek SK, Cheon KS, Cho EH, Won KS. The comparison between Behçet's disease and spondyloarthritides: does Behçet's disease belong to the spondyloarthropathy complex? J Korean Med Sci. 2002;17:524-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Shimamoto Y, Sugiyama H, Hirohata S. Reiter's syndrome associated with HLA-B51. Intern Med. 2000;39:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Taniguchi Y, Yorioka N, Kyuden Y, Asakimori Y. Reiter's syndrome associated with HLA-B51: a case report. J Int Med Res. 2003;31:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Matsumoto Y, Hurumura T, Nanba D, Banno S, Sugiura Y, Ueda R. HLA-B51 related arthritis belongs to seronegative spondyloarthropathy. Nihon Naika Gakkai Zasshi. 1998;87:Suppl 325. |
| 11. | Kobayashi S, Ando S. Reactive arthritis or Reiter's syndrome and B51-associated seronegative spondyloarthropathy. Intern Med. 2000;39:89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Huerta-Sil G, Casasola-Vargas JC, Londoño JD, Rivas-Ruíz R, Chávez J, Pacheco-Tena C, Cardiel MH, Vargas-Alarcón G, Burgos-Vargas R. Low grade radiographic sacroiliitis as prognostic factor in patients with undifferentiated spondyloarthritis fulfilling diagnostic criteria for ankylosing spondylitis throughout follow up. Ann Rheum Dis. 2006;65:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Chang HK, Kim JU, Cheon KS, Chung HR, Lee KW, Lee IH. HLA-B51 and its allelic types in association with Behçet's disease and recurrent aphthous stomatitis in Korea. Clin Exp Rheumatol. 2001;19:S31-S35. [PubMed] |
| 14. | Mineshita S, Tian D, Wang LM, Jian XY, Li SY, Fang GZ, Bian TY, Liao HS, Tsuchida M, Tanaka H. Histocompatibility antigens associated with Behçet's disease in northern Han Chinese. Intern Med. 1992;31:1073-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Mizuki N, Ota M, Katsuyama Y, Yabuki K, Ando H, Shiina T, Nomura E, Onari K, Ohno S, Inoko H. HLA-B*51 allele analysis by the PCR-SBT method and a strong association of HLA-B*5101 with Japanese patients with Behçet's disease. Tissue Antigens. 2001;58:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Olivieri I, Gemignani G, Camerini E, Semeria R, Pasero G. Computed tomography of the sacroiliac joints in four patients with Behçet's syndrome--confirmation of sacroiliitis. Br J Rheumatol. 1990;29:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Maghraoui AE, Tabache F, Bezza A, Abouzahir A, Ghafir D, Ohayon V, Archane MI. A controlled study of sacroiliitis in Behçet's disease. Clin Rheumatol. 2001;20:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kotevoglu N, Tasbas I, Bekiroglu N. Computed tomography does not support sacroiliitis as a feature of behçet disease: a metaanalytic review. J Clin Rheumatol. 2004;10:42-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Chen L, Shi H, Yuan J, Bowness P. Position 97 of HLA-B, a residue implicated in pathogenesis of ankylosing spondylitis, plays a key role in cell surface free heavy chain expression. Ann Rheum Dis. 2017;76:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |