Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9404
Peer-review started: May 8, 2022
First decision: May 30, 2022
Revised: June 12, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 16, 2022
Processing time: 111 Days and 18.5 Hours
In trauma patients, bleeding is an immediate major concern. At the same time, there are few cases of acute vascular occlusion after blunt trauma, and it is unclear what assessment and diagnosis should be considered for these cases. Herein, we describe a patient diagnosed with antiphospholipid syndrome after a hypercoagulable workup for acute renal and splenic vascular occlusion due to blunt trauma.
A 20-year-old man was admitted to the emergency department with abdominal pain after hitting a tree while riding a sled 10 h ago. He had no medical history. Radiological investigations revealed occlusion of the left renal artery with global infarction of the left kidney and occlusion of branches of the splenic artery with infarction of the central portion of the spleen. Attempted revascularization of the left renal artery occlusion through percutaneous transluminal angioplasty failed due to difficulty in passing the wire through the total occlusion. Considering the presence of acute multivascular occlusions in a young man with low cardio
A hypercoagulable workup can be considered in trauma patients with acute multivascular occlusion, especially in young patients with low cardiovascular risk.
Core Tip: Acute vascular occlusion following blunt trauma has been reported in a few trauma patients. We describe a case diagnosed with antiphospholipid syndrome after a hypercoagulable workup for acute multivascular occlusion due to blunt trauma. In trauma patients with acute multivascular occlusion or a hypercoagulable state, especially in young patients with low cardiovascular risk, a hypercoagulable workup may help evaluate other risk factors.
- Citation: Lee NA, Jeong ES, Jang HS, Park YC, Kang JH, Kim JC, Jo YG. Antiphospholipid syndrome with renal and splenic infarction after blunt trauma: A case report. World J Clin Cases 2022; 10(26): 9404-9410
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9404.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9404
Except for head injuries, bleeding is the immediate major concern for most patients experiencing trauma[1,2]. In contrast, there are few cases of acute vascular occlusion and consequent infarction after trauma. In blunt trauma, blood vessels can be compressed or stretched. Intimal injury from vascular stretching can lead to vessel dissection or intramural thrombosis, resulting in vessel stenosis or total occlusion[3]. This can progress to organ infarction.
Usually, splenic and renal infarctions are related to cardiac thromboembolism[4], other systemic pathologies[5], sickle cell trait carriers[6], and infection[7]. The incidence of renal or spleen infarction in patients with blunt trauma is unknown. However, there are reports that 16.7%-30.8% of patients with renal infarction have renal artery injury[8,9], 20% of patients with spleen infarction have splenic artery injury, and 20% of patients with spleen and renal infarctions have trauma[9].
This case report describes a patient with acute infarction of the left kidney and the central portion of the spleen due to vascular occlusion after blunt trauma. The patient was diagnosed with antiphospholipid syndrome (APS) after a hypercoagulable workup. Through this case report, we suggest that additional evaluation of hypercoagulability is needed in patients with acute multivascular occlusion after blunt trauma.
A 20-year-old man was admitted to the emergency department with abdominal pain after hitting a tree while riding a sled 10 h ago.
The patient’s symptoms started after the trauma, and the abdominal pain worsened over time.
The patient had no previous medical history.
The patient did not smoke and had no relevant family history.
At the time of admission, the patient’s Glasgow Coma scale was 15/15, blood pressure was 120/70 mmHg, body temperature was 36.3 °C, heart rate was 94 bpm, respiratory rate was 20 breaths per minute, and oxygen saturation in room air was 98%. A physical examination revealed tenderness without rebound pain over the left upper quadrant.
The results of the laboratory investigations were as follows: white blood cell count, 21.8 × 103/μL (normal range, 4.8-10.8); neutrophils, 86.8% (50-75); hemoglobin, 13.1 g/dL (12-18); platelet count, 172 × 103/μL (130-450); fibrin degradation products, 16.4 μg/mL (0-5); D-dimer, 6.19 mg/L (0-0.55); creatinine, 1.37 mg/dL (0.5-1.3); modification of diet in renal disease estimated glomerular filtration rate (eGFR), 66.938 mL/min/1.73 m2 (71.11-214.2); and chronic kidney disease epidemiology collaboration eGFR, 74.24 mL/min/1.73 m2 (79.1-157.0). Lactate, fibrinogen assay, activated partial thromboplastin time, and prothrombin time (PT) values were within normal limits.
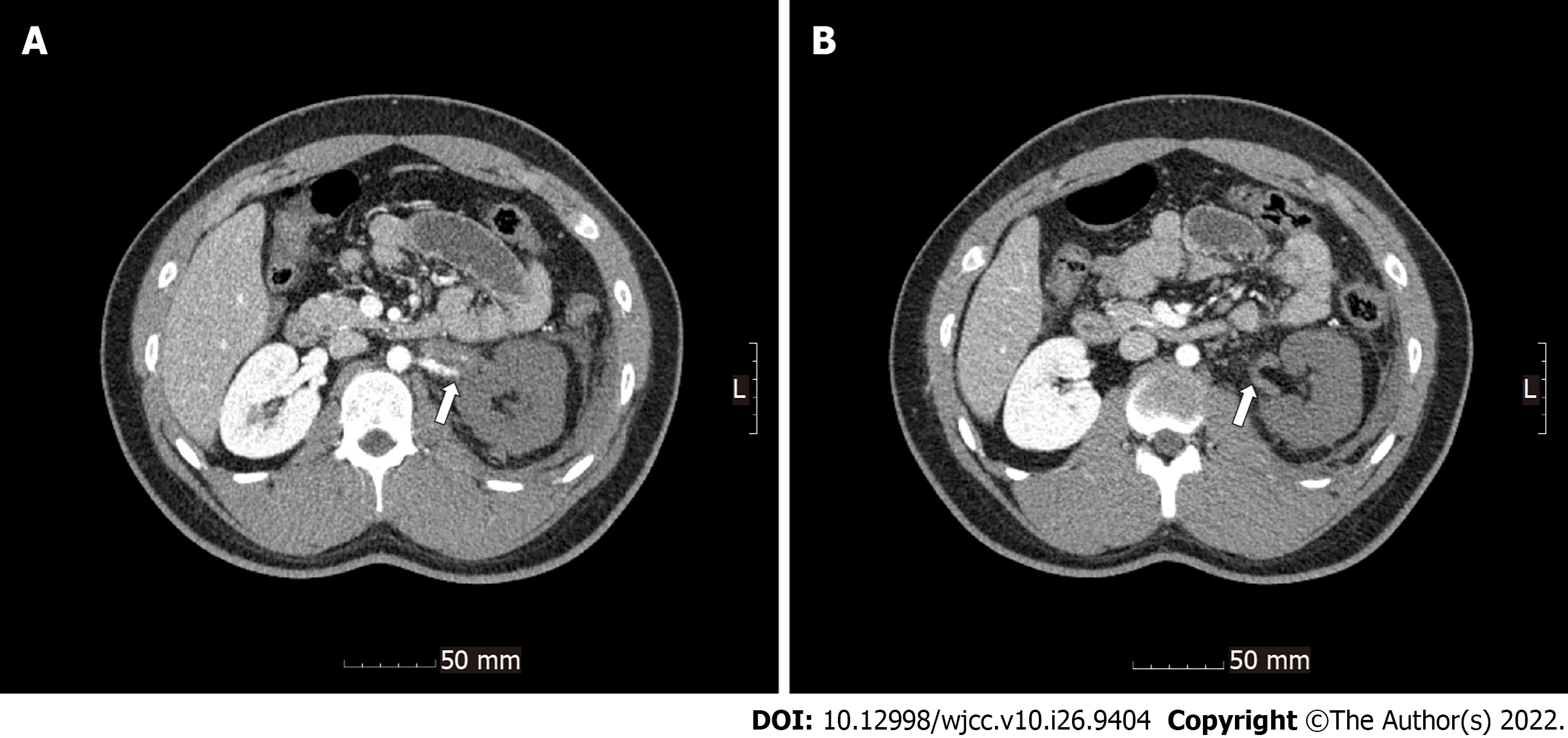
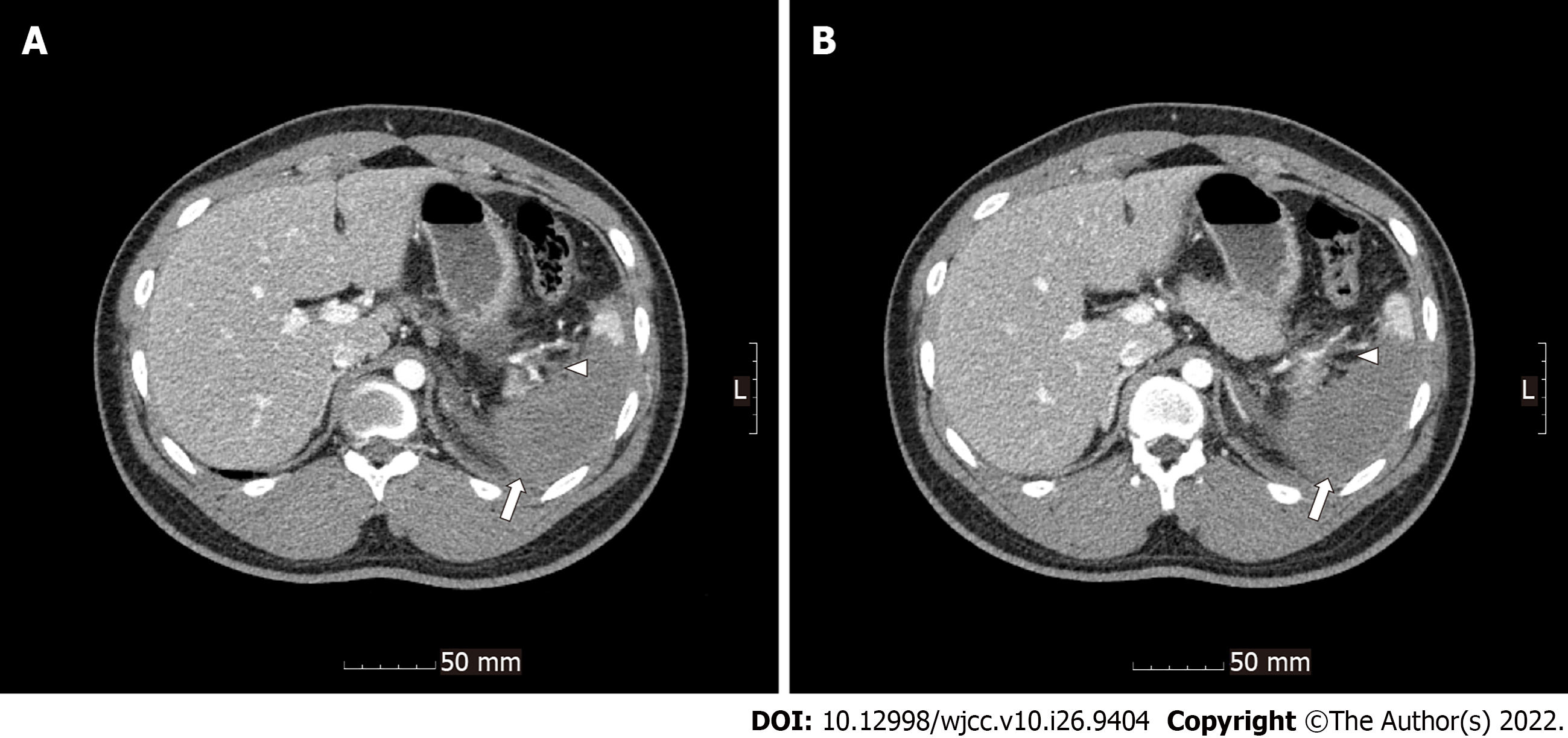
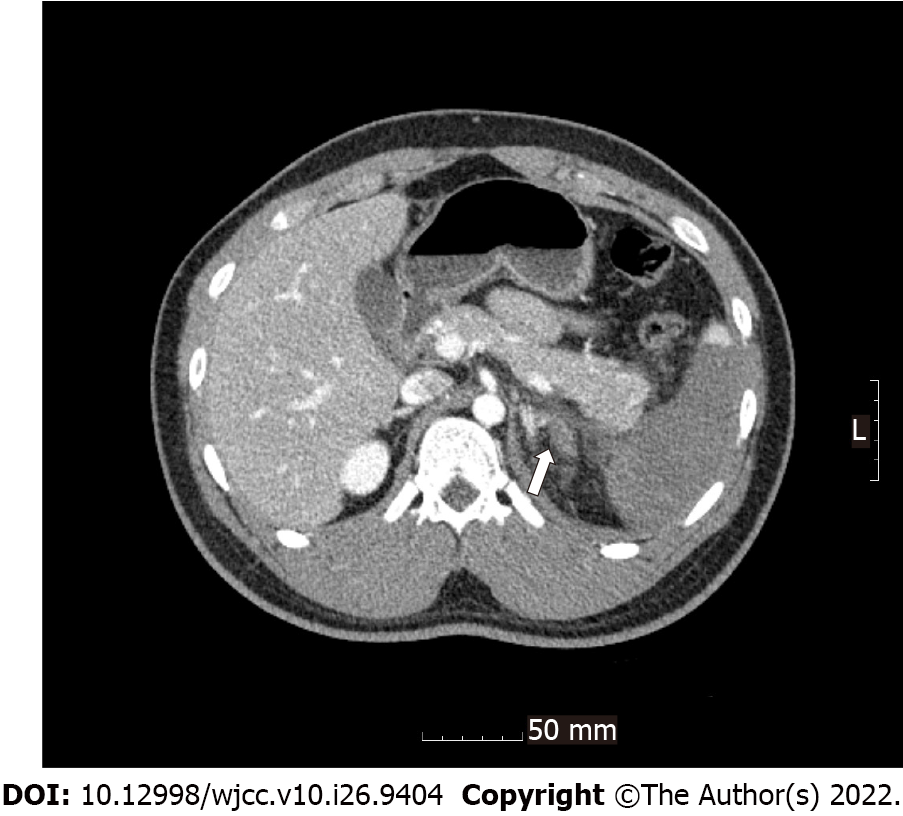
Radiological investigations revealed occlusion of the left renal artery with global infarction of the left kidney (Figure 1), occlusion of the branches of the splenic artery with infarction of the central portion of the spleen (Figure 2), a small amount of hemoperitoneum, and left adrenal hematoma (Figure 3).
The percutaneous transluminal angioplasty attempted to revascularize the left renal artery occlusion failed due to difficulty in passing the wire through the total occlusion. In addition, considering the acute splenic and renal infarctions with multivascular occlusion in this young male patient without underlying disease, a laboratory evaluation of hypercoagulability was performed to exclude the possibility of other diseases. Lupus anticoagulant (LA), antinuclear antibody (ANA) titer, antineutrophil cytoplasmic antibody (ANCA), anti-cardiolipin (aCL) immunoglobulin M (IgM) and immunoglobulin G (IgG), and anti-β2-glycoprotein I (GPI) IgM and IgG tests were performed.
Laboratory tests were reported on day 7 after the injury and were as follows: LA, weak positive (1.41) (0-1.30, negative; 1.31-1.50, weak positive; 1.51-2.0, moderate positive; > 2.0, strong positive; LA1 screening reagent, LA2 confirmation reagent; Siemens Healthcare, Marburg, Germany); ANA titer, negative (< 1:80, negative; ANA HEp-2 standard kit; Aesku Diagnostics, Wendelsheim, Germany); ANCA, negative (< 1:20, negative; ANCA Ethanol; Aesku Diagnostics, Wendelsheim, Germany); aCL IgM, negative (< 7 U/mL, negative); aCL IgG, negative (< 10 U/mL, negative; aCL IgM, IgG; Orgentec Diagnostika, Mainz, Germany); anti-β2-GPI IgM, negative (< 7 U/mL, negative); and anti-β2-GPI IgG, negative (< 7 U/mL, negative; Phadia EliA; Thermofisher, Freiburg, Germany).
APS is diagnosed when the patient meets at least one clinical criterion and one laboratory criterion. Clinical criteria include clinical episodes of arterial, venous, or small vessel thrombosis or pregnancy morbidity. Vascular thrombosis of clinical criteria can be diagnosed with pathologic confirmation or appropriate imaging studies. Laboratory criteria include LA, aCL IgG and/or IgM, or anti-β2-GPI IgG and/or IgM detected twice or more within an interval of at least 12 wk[10]. This patient had acute splenic and renal infarctions with complete vessel occlusion confirmed by arteriography. This event meets the clinical criteria. For the laboratory criteria, the LA test showed weak positivity (1.41). Although the presence of LA must be reconfirmed after 12 wk, LA is an antibody with high specificity[11]. Therefore, it was judged that the possibility of APS was high, and inpatient treatment for APS was performed by a rheumatologist.
Treatment with a subcutaneous injection of enoxaparin [low-molecular-weight heparin (LMWH)] was started. In addition, the department of infectious diseases considered the patient to have functional asplenia due to the near-total splenic infarction; thus, asplenia vaccination was performed.
After 2 wk, the LMWH was changed to warfarin. Thereafter, if the follow-up LA test is positive, lifelong warfarin therapy should be considered. The patient was discharged 3 wk after the injury.
The result of the LA test performed at the rheumatology outpatient clinic was 1.51, moderate positive (0-1.30, negative; 1.31-1.50, weak positive; 1.51-2.0, moderate; > 2.0, strong). The patient was diagnosed with APS. He continues to visit the rheumatology outpatient clinic and is taking warfarin. Follow-up ultrasonography revealed atrophic changes in the left kidney, resolved splenic infarction in the superior pole, and resolution of the fluid collection in the perisplenic space without abscess. In addition, creatinine and eGFR were within the normal range.
Antiphospholipid syndrome is an autoimmune syndrome characterized by vascular or pregnancy morbidity in the presence of persistent laboratory evidence of antiphospholipid antibodies (aPL)[12]. In the absence of a thromboembolic event or pregnancy morbidity, the presence of aPL alone is not sufficient for a diagnosis of APS. Furthermore, aPL can be a temporary finding due to other acute diseases, such as systemic lupus erythematosus, bacterial infection (syphilis, Lyme disease), viral infection (human immunodeficiency virus, hepatitis), or the use of medications such as chlorpromazine, phenytoin, quinidine, hydralazine, or amoxicillin[13-17]. Therefore, the diagnosis of APS is based on the presence of aPL on two or more occasions at least 12 wk apart in addition to vascular thrombosis or pregnancy morbidity[10,18].
In this case, characteristically, the patient was young, had no medical and family history, had low cardiovascular risk, had thromboembolic events rather than hemorrhage, and progressed to renal and splenic infarction. As a result, hypercoagulability was evaluated and APS was diagnosed. Given that this patient had not experienced a previous thromboembolic event, the trauma may have triggered the formation of aPL and thromboembolism[19]. Acute multivascular occlusion is triggered by trauma-induced intimal injury of blood vessels and consequent thrombosis. This suggests that it is necessary to consider the possibility of a hypercoagulable state and evaluate its causes, not only in cases of nontraumatic vascular thrombosis but also in cases of acute vascular thrombosis related to trauma.
For the initial management of patients with suspected APS, the use of LMWH and conversion to warfarin is preferred rather than the use of direct oral anticoagulants (DOACs). This is because DOACs are less effective than warfarin for recurrent thrombosis prevention in arterial (rather than venous) thrombosis, particularly among younger patients[20,21]. Most patients with APS have a high probability of experiencing recurrent thromboembolic events. Therefore, lifelong anticoagulation therapy is recommended. Standard-intensity warfarin (target international normalized ratio range of PT, 2-3) anticoagulation is recommended for patients with venous thrombosis, while standard-intensity warfarin plus aspirin is recommended for patients with arterial thrombosis. However, standard-intensity warfarin alone can be considered for patients with arterial thrombosis that have low cardiovascular risk factors[22]. In this case, the patient had arterial thrombosis with APS, but he was a young patient with low cardiovascular risk. Therefore, he was prescribed only warfarin for his anticoagulant therapy.
This case is significant in that it suggests the importance of a hypercoagulable workup for patients with acute thromboembolism after trauma.
A hypercoagulable workup can be considered for trauma patients with acute multivascular occlusion or a hypercoagulable state, particularly for young patients with low cardiovascular risk.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Emergency medicine
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sandoval C, Chile; Yi XL, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73:S431-S437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1120] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 3. | Lee TS, Ducic Y, Gordin E, Stroman D. Management of carotid artery trauma. Craniomaxillofac Trauma Reconstr. 2014;7:175-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Argiris A. Splenic and renal infarctions complicating atrial fibrillation. Mt Sinai J Med. 1997;64:342-349. [PubMed] |
| 5. | Arnold MH, Schrieber L. Splenic and renal infarction in systemic lupus erythematosus: association with anti-cardiolipin antibodies. Clin Rheumatol. 1988;7:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Novak RW, Brown RE. Multiple renal and splenic infarctions in a neonate following transfusion with sickle trait blood. Clin Pediatr (Phila). 1982;21:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Salord F, Allaouchiche B, Gaussorgues P, Boibieux A, Sirodot M, Gerard-Boncompain M, Biron F, Peyramond D, Robert D. Severe falciparum malaria (21 cases). Intensive Care Med. 1991;17:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Bourgault M, Grimbert P, Verret C, Pourrat J, Herody M, Halimi JM, Karras A, Amoura Z, Jourde-Chiche N, Izzedine H, François H, Boffa JJ, Hummel A, Bernadet-Monrozies P, Fouque D, Canouï-Poitrine F, Lang P, Daugas E, Audard V. Acute renal infarction: a case series. Clin J Am Soc Nephrol. 2013;8:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Romano S, Scaglione M, Gatta G, Lombardo P, Stavolo C, Romano L, Grassi R. Association of splenic and renal infarctions in acute abdominal emergencies. Eur J Radiol. 2004;50:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4924] [Cited by in RCA: 4723] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 11. | Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med. 2018;378:2010-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 489] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 12. | Lim W. Antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program. 2013;2013:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Taraborelli M, Leuenberger L, Lazzaroni MG, Martinazzi N, Zhang W, Franceschini F, Salmon J, Tincani A, Erkan D. The contribution of antiphospholipid antibodies to organ damage in systemic lupus erythematosus. Lupus. 2016;25:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Prieto J, Yuste JR, Beloqui O, Civeira MP, Riezu JI, Aguirre B, Sangro B. Anticardiolipin antibodies in chronic hepatitis C: implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology. 1996;23:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Cervera R, Asherson RA. Clinical and epidemiological aspects in the antiphospholipid syndrome. Immunobiology. 2003;207:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Triplett DA. Many faces of lupus anticoagulants. Lupus. 1998;7 Suppl 2:S18-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Dlott JS, Roubey RA. Drug-induced lupus anticoagulants and antiphospholipid antibodies. Curr Rheumatol Rep. 2012;14:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, De Groot PG; Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 855] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 19. | Barak N, Orion Y, Cordoba M, Mekori YA. Catastrophic antiphospholipid syndrome triggered by trauma. J Rheumatol. 1999;26:1835-1836. [PubMed] |
| 20. | Pengo V, Denas G, Zoppellaro G, Jose SP, Hoxha A, Ruffatti A, Andreoli L, Tincani A, Cenci C, Prisco D, Fierro T, Gresele P, Cafolla A, De Micheli V, Ghirarduzzi A, Tosetto A, Falanga A, Martinelli I, Testa S, Barcellona D, Gerosa M, Banzato A. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 528] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 21. | Dufrost V, Risse J, Reshetnyak T, Satybaldyeva M, Du Y, Yan XX, Salta S, Gerotziafas G, Jing ZC, Elalamy I, Wahl D, Zuily S. Increased risk of thrombosis in antiphospholipid syndrome patients treated with direct oral anticoagulants. Results from an international patient-level data meta-analysis. Autoimmun Rev. 2018;17:1011-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 22. | Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |