Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.8974
Peer-review started: February 21, 2022
First decision: March 24, 2022
Revised: April 4, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: September 6, 2022
Processing time: 186 Days and 7.3 Hours
Life-threatening hypoxia can occur in patients with lung cancer due to bronchial obstruction. Extracorporeal membrane oxygenation (ECMO) can be used as a bridge therapy for patients with severe hypoxia not relieved by conventional mechanical treatment. However, the usefulness of chemotherapy in patients with lung cancer receiving ECMO therapy is not well known.
A 53-year-old man visited the emergency room with worsening dyspnea for 1 mo. A series of imaging and diagnostic tests were performed, and stage IIIB (cT4N2M0) lung cancer was eventually diagnosed. On hospital day 3, he experienced dyspnea and hypoxia that was not relieved with oxygen support via a high-flow nasal cannula. ECMO was initiated because his respiratory condition did not improve even with mechanical ventilation. The patient then underwent gemcitabine/cisplatin chemotherapy without dose reduction while on ECMO. After two cycles of chemotherapy, there was a decrease in the size of the primary tumor in the right main bronchus. After the completion of concurrent chemoradiotherapy, a computed tomography scan revealed further improvement in the right main bronchus narrowing. Eight months after a lung cancer diagnosis, the patient did well without any dyspnea.
ECMO is a potential bridge therapy for respiratory failure in patients with central airway obstruction secondary to lung cancer.
Core Tip: Extracorporeal membrane oxygenation can be selected as an important salvage treatment for patients with severe cardiopulmonary dysfunction caused by lung cancer and other malignant tumors until the patients are stabilized or even cured.
- Citation: Yoo SS, Lee SY, Choi SH. Extracorporeal membrane oxygenation for lung cancer-related life-threatening hypoxia: A case report. World J Clin Cases 2022; 10(25): 8974-8979
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/8974.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.8974
Lung cancer is one of the most commonly diagnosed malignancies and is the leading cause of cancer-related deaths worldwide, with an average 5-year survival rate of 19%[1]. The clinical presentation of lung cancer varies among individuals, resulting in a range of symptoms that patients may find distressing[2]. Several common signs and symptoms associated with lung cancer can be classified as a result of the primary tumor, intrathoracic spread, distant metastases, paraneoplastic syndromes, or nonspecific symptoms[2]. Airway obstruction, superior venous cava syndrome, and massive pleural effusion secondary to lung cancer may cause dyspnea. Moreover, sudden airway obstruction can cause ventilation/perfusion mismatch or shunt, leading to life-threatening respiratory failure.
Extracorporeal membrane oxygenation (ECMO) is the only way to overcome hypoxia that persists even with conventional mechanical ventilator treatment. Patients with hematological malignancies, such as leukemia or lymphoma, may develop respiratory failure related to disease or chemotherapy, and in these cases, ECMO may be required for bridge treatment[3,4]. However, there are only a few reports on the use of chemotherapy during ECMO support as a treatment for respiratory failure in lung cancer, which has a low response rate and delayed response time compared with hematologic malignancy. Herein, we report the case of a patient treated with chemotherapy using ECMO for acute respiratory failure due to lung cancer.
In March 2021, a 53-year-old man visited the emergency room with worsening dyspnea.
The patient’s dyspnea started 1 mo ago and has progressed since then.
The patient is a 32 pack-year smoker.
The patient had a free personal and family history.
On admission, his vital signs were as follows: Blood pressure, 126/80 mmHg; body temperature, 36.5 °C; heart rate, 112 bpm; and respiratory rate, 20 breaths/min.
Atrial blood gas analysis (ABGA) showed a pH of 7.47, PCO2 of 27 mmHg, PaO2 of 67.7 mmHg, HCO3 of 19.8 mmHg, and SpO2 of 94.8% on room air.
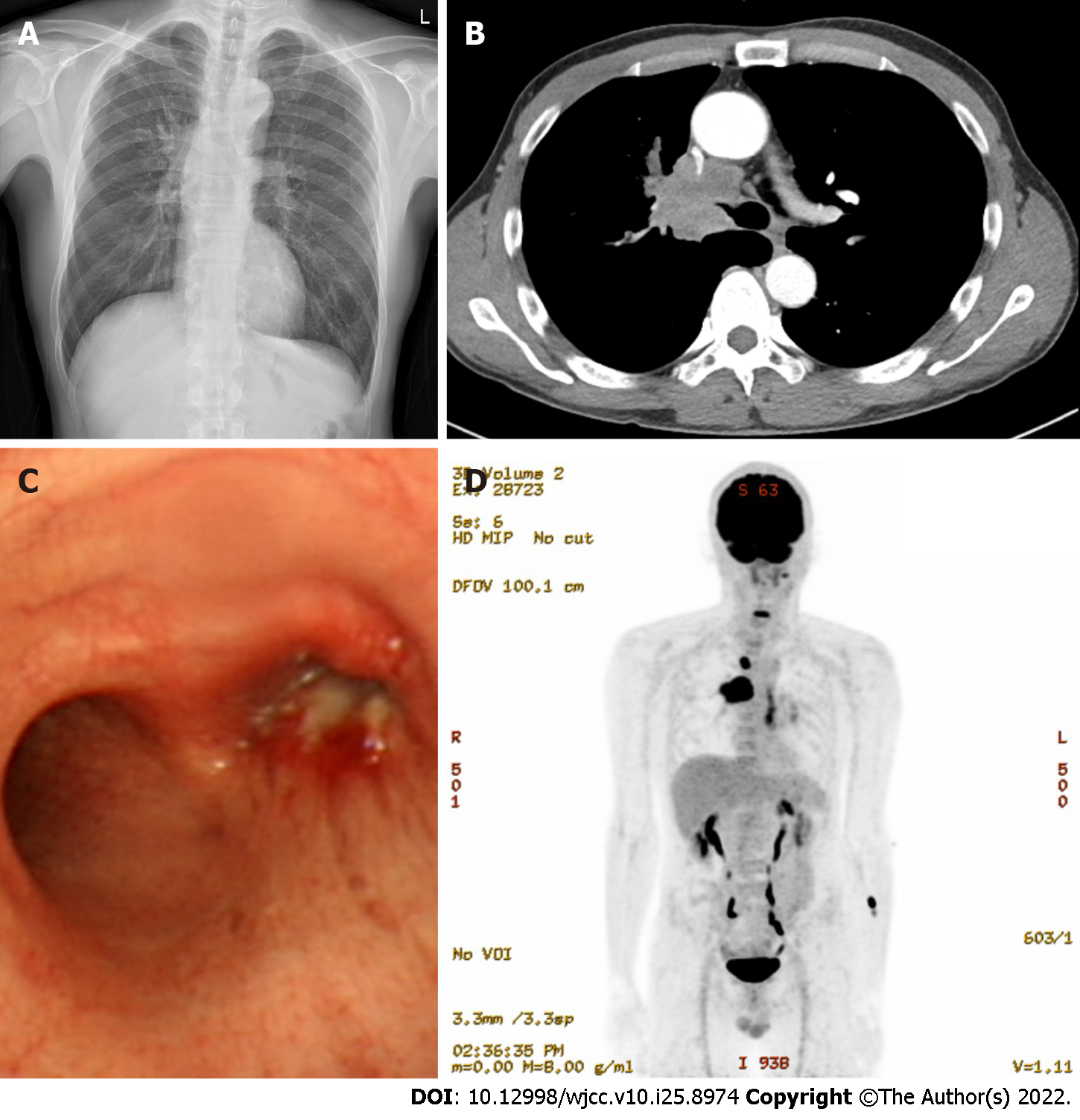
Chest radiography revealed a suprahilar mass (Figure 1A), and a chest computed tomography (CT) scan showed a 4.9-cm mass in the right upper lobe (RUL). The mass on the CT scan appeared to cause narrowing of the right main bronchus and pulmonary artery (Figure 1B). He was admitted for evaluation of the lung mass. On day 2 of hospitalization, flexible bronchoscopy revealed an obstructing mass in the right main bronchus (Figure 1C). Bronchoscopic biopsy and endobronchial ultrasound-guided transbronchial needle aspiration (EUBS-TBNA) were performed for the 4R lymph nodes. Upon suspicion of lung cancer, positron emission tomography (PET)-CT was performed (Figure 1D).
The final diagnosis of the presented case is stage IIIB (cT4N2M0) lung cancer, squamous cell carcinoma.
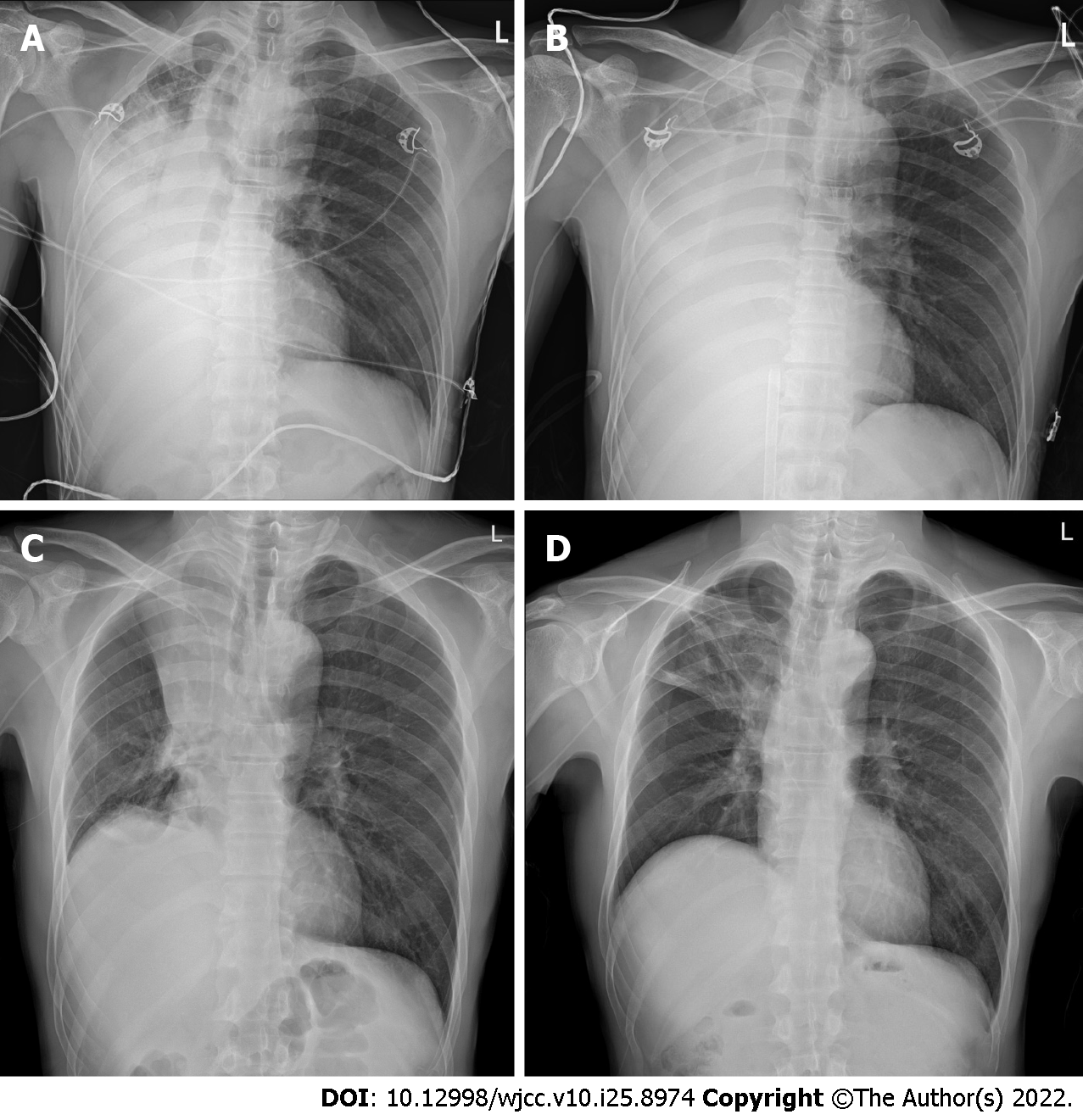
On hospital day 3, the dyspnea suddenly worsened. The O2 saturation in room air decreased from 98% to 71%. Chest radiography revealed a nearly complete collapse of the right lung (Figure 2A). A reserve mask with an oxygen flow rate of 15 L/min was used, which improved the O2 saturation to 88%-90%. A high-flow nasal cannula (HFNC) was administered, and the patient was then transferred to the intensive care unit.
On hospital day 4, ABGA showed a pH of 7.301, PCO2 of 39 mmHg, PaO2 of 44.4 mmHg, HCO3 of 18.4 mmHg, and SpO2 of 75.9% on oxygen supply via an HFNC with FiO2 of 80% and flow rate of 60 L/min. Mechanical ventilation support was started with lung-protective mechanical ventilation and pronation, according to the current protocols. However, the respiratory condition did not improve, and the patient eventually presented with refractory hypoxemia with a PaO2/FiO2 ratio of 46.7, FiO2 of 100%, and positive end-expiratory pressure (PEEP) of 12 cmH2O. Accordingly, an urgent decision was made to initiate venovenous ECMO (CardioHelp; Getinge Critical Care, Gothenburg, Sweden) by percutaneous cannulation of the right femoral vein (25-Fr draining cannula) and right jugular vein (17-Fr restitution cannula). At the start of the ECMO run, blood flow was set at 4 L/min, sweep gas flow was set at 2 L/min, and unfractionated heparin was infused to match a partial thromboplastin time ratio of 1.5- to 2-fold that of the baseline. Ultra-protective mechanical ventilation was instituted in pressure-controlled ventilation mode with a driving pressure of 8 cmH2O, PEEP of 12 cmH2O, respiratory rate of 8 breaths/min, and FiO2 of 60%. Chest radiography revealed an aggravated collapse of the right lung (Figure 2B). ABGA showed pH of 7.462, PCO2 of 25.9 mmHg, PaO2 of 373.1 mmHg, HCO3 of 20.9 mmHg, and SpO2 of 99.8% on ECMO and ventilator support.
Biopsy of the mass in the right main bronchus and 4R lymph nodes revealed squamous cell carcinoma. The patient was diagnosed with stage IIIB (cT4N2M0) lung cancer. On hospital day 6, the patient was extubated and in spontaneous breathing supported by HFNC oxygen therapy at 35 L/min with a FiO2 of 45% (awake ECMO). On hospital day 7, gemcitabine/cisplatin chemotherapy without dose reduction was initiated while the patient was on ECMO support. The doses of gemcitabine and cisplatin were 1250 mg per body surface area (BSA) and 70 mg/BSA, respectively. ECMO therapy was discontinued on day 12 of hospitalization, while HFNC was continued. On day 15 of hospitalization, the HFNC was discontinued. On hospital day 16, the total atelectasis of the right lung had improved, although RUL atelectasis remained (Figure 2C). On hospital day 20, the RUL atelectasis improved (Figure 2D). The patient was discharged on hospital day 26 without dyspnea.
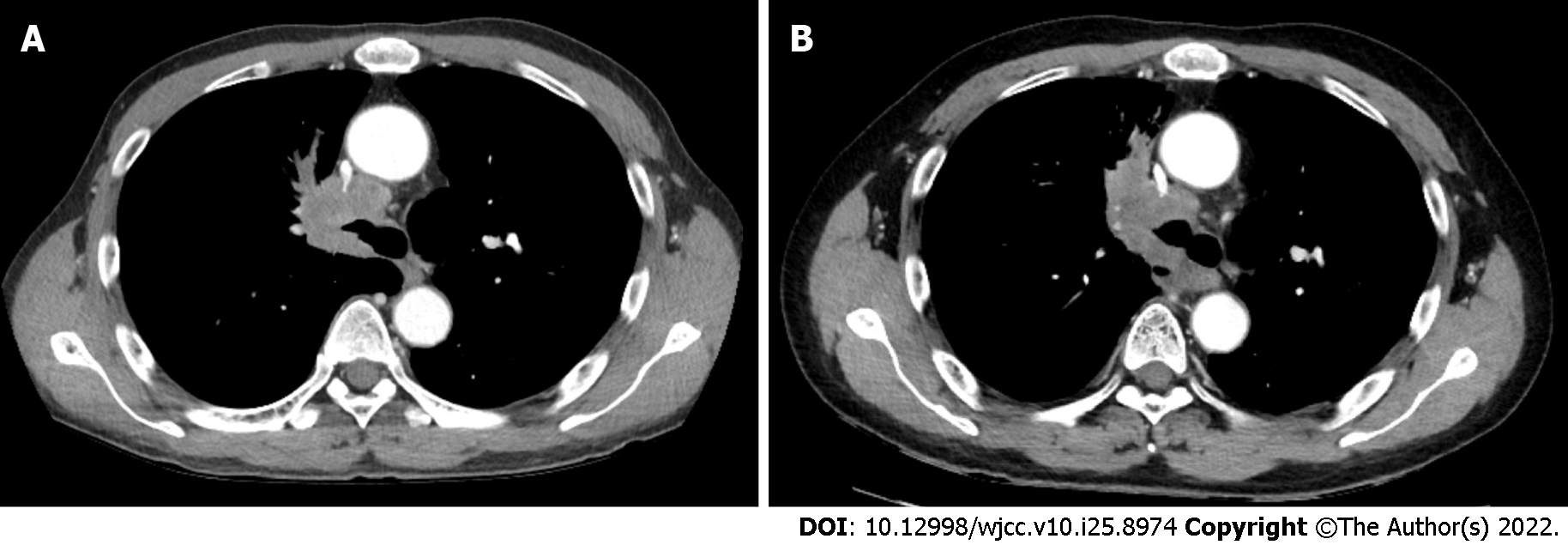
After two cycles of gemcitabine/cisplatin chemotherapy, a CT scan showed a decrease in the primary tumor size in the right main bronchus (Figure 3A). The patient received concurrent chemoradiotherapy with a weekly paclitaxel/cisplatin regimen. After the completion of concurrent chemoradiotherapy, the CT scan revealed further improvement in the right main bronchus narrowing due to the mass (Figure 3B). Eight months after lung cancer diagnosis, the patient did well without any dyspnea.
Lung cancer can cause atelectasis, which can lead to shortness of breath. In this case, sudden obstruction of the right main bronchus caused severe hypoxia, which was not relieved with oxygen supply. The mechanism of refractory hypoxia can be explained by a shunt, implying perfusion to the non-ventilated lung. Radiotherapy or endobronchial treatment with a stent is an option for intraluminal obstruction of the main bronchus. However, the patient in the current case did not have the time to receive such treatment because of life-threatening hypoxia.
ECMO can provide cardiac and pulmonary support in critically ill patients with cardiogenic shock or acute respiratory failure[5]. The use of ECMO in cancer patients who develop severe acute respiratory distress syndrome (ARDS) remains controversial[6]. However, several studies have reported the successful use of ECMO in patients who develop respiratory failure after chemotherapy for hematologic malignancies[3,4,6,7]. Chung et al[8] reported that under ECMO support, the patient underwent bronchial stent implantation and was successfully weaned off ECMO. Airway obstructions may qualify for intensive respiratory support compared with diffuse parenchymal malignancy because bronchial intervention procedures enable quick recovery from respiratory failure if airway stenosis is relieved[7,8]. In the case of solid tumors, respiratory failure may occur either during the disease itself or during the treatment course; however, only a few studies have been conducted on ECMO use in these patients. Because of the adverse effects of ECMO, specifically, infections, bleeding, and thrombocytopenia, the administration of chemotherapy is not routinely advised in patients suffering from malignancies[9]. Our experience, in this case, indicates that ARDS caused by shunting in patients with central airway lung cancer should not be considered a contraindication to ECMO treatment. With our successful treatment outcome with chemotherapy in ECMO, we suggest that chemotherapy during ECMO in solid tumors should be considered in the presence of severe hemodynamic instability or respiratory failure refractory to standard medical support.
The pharmacokinetics of chemotherapy with ECMO is not yet fully understood. Several factors may affect pharmacokinetics, including the circuit of ECMO, the changes in the volume distribution, and the alteration in drug clearance. Sherwin et al[9] reported pharmacokinetic parameters and dose recommendations for anti-infective drugs, including antibiotics, under ECMO treatment, primarily in neonates. However, information on the pharmacokinetics of anti-cancer drugs during ECMO is still lacking. The current case showed that standard doses of gemcitabine and cisplatin were effective in ECMO treatment, although further studies on chemotherapy with ECMO in solid tumors are needed. Numerous clinical studies will be necessary to determine the appropriate indications for ECMO.
In conclusion, ECMO may play an important role as a bridge therapy for respiratory failure due to ARDS in patients with central airway obstruction secondary to lung cancer. As such, this treatment approach must be considered in these patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: She K, China; Xiang T, China; Zhang YW, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15314] [Article Influence: 3062.8] [Reference Citation Analysis (4)] |
| 2. | Beckles MA, Spiro SG, Colice GL, Rudd RM. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest. 2003;123:97S-104S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Tathineni P, Pandya M, Chaar B. The Utility of Extracorporeal Membrane Oxygenation in Patients With Hematologic Malignancies: A Literature Review. Cureus. 2020;12:e9118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Lee SW, Kim YS, Hong G. Extracorporeal membrane oxygenation as a rescue therapy for acute respiratory failure during chemotherapy in a patient with acute myeloid leukemia. J Thorac Dis. 2017;9:E133-E137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015; 7: E166-E176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 175] [Reference Citation Analysis (0)] |
| 6. | Huprikar NA, Peterson MR, DellaVolpe JD, Sams VG, Lantry JH, Walter RJ, Osswald MB, Chung KK, Mason PE. Salvage extracorporeal membrane oxygenation in induction-associated acute respiratory distress syndrome in acute leukemia patients: A case series. Int J Artif Organs. 2019;42:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Wohlfarth P, Ullrich R, Staudinger T, Bojic A, Robak O, Hermann A, Lubsczyk B, Worel N, Fuhrmann V, Schoder M, Funovics M, Rabitsch W, Knoebl P, Laczika K, Locker GJ, Sperr WR, Schellongowski P; Arbeitsgruppe für hämato-onkologische Intensivmedizin der Österreichischen Gesellschaft für Internistische und Allgemeine Intensivmedizin und Notfallmedizin (ÖGIAIN). Extracorporeal membrane oxygenation in adult patients with hematologic malignancies and severe acute respiratory failure. Crit Care. 2014;18:R20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Chung JH, Yeo HJ, Cho HM, Jang JO, Ye BM, Yoon G, Shin DH, Kim D, Cho WH. Treatment of Pulmonary Tumor Embolism from Choriocarcinoma: Extracorporeal Membrane Oxygenation as a Bridge through Chemotherapy. Cancer Res Treat. 2017;49:279-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Sherwin J, Heath T, Watt K. Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature. Clin Ther. 2016;38:1976-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |