Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8443
Peer-review started: February 9, 2022
First decision: April 28, 2022
Revised: May 9, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: August 26, 2022
Processing time: 187 Days and 14.1 Hours
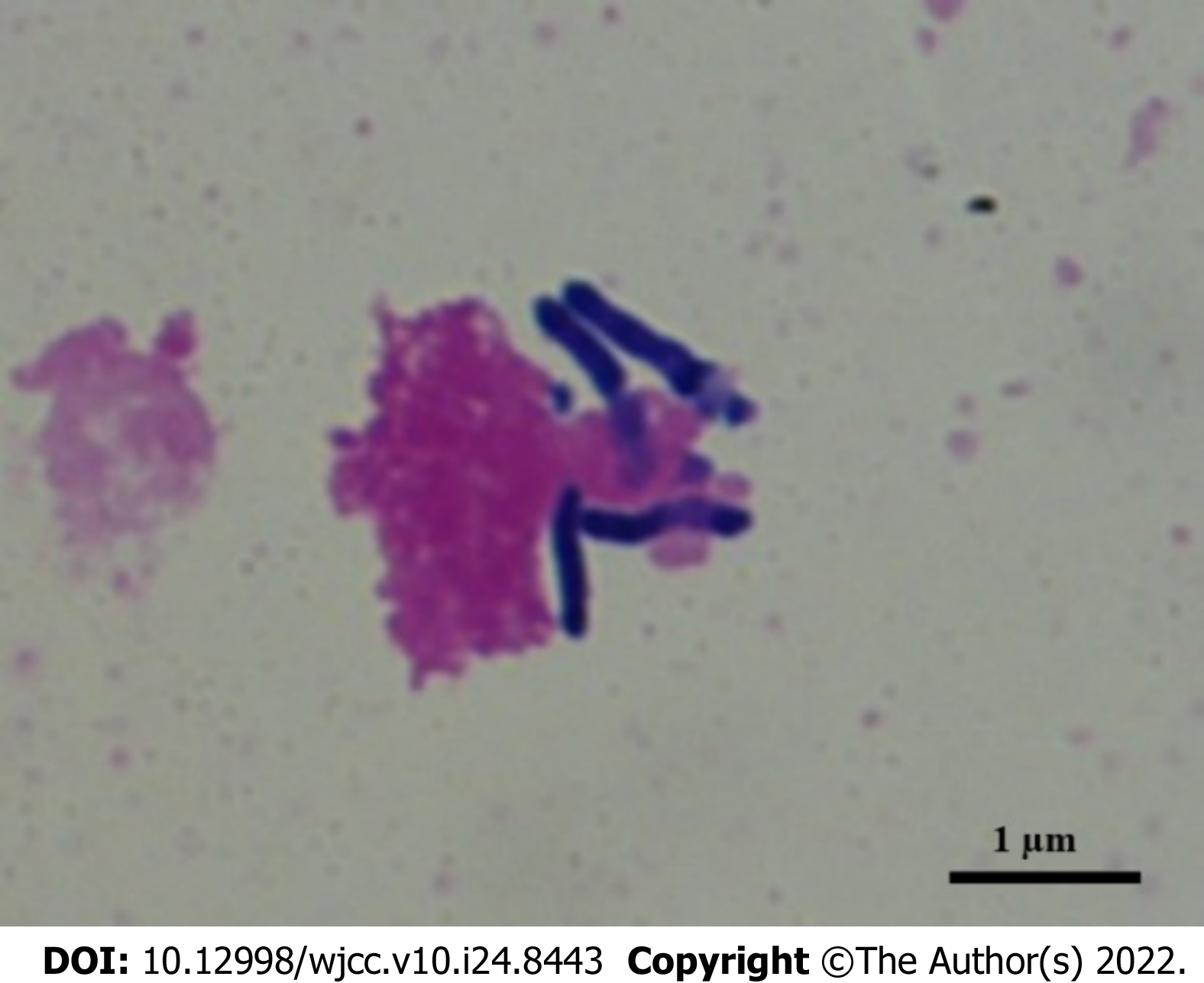
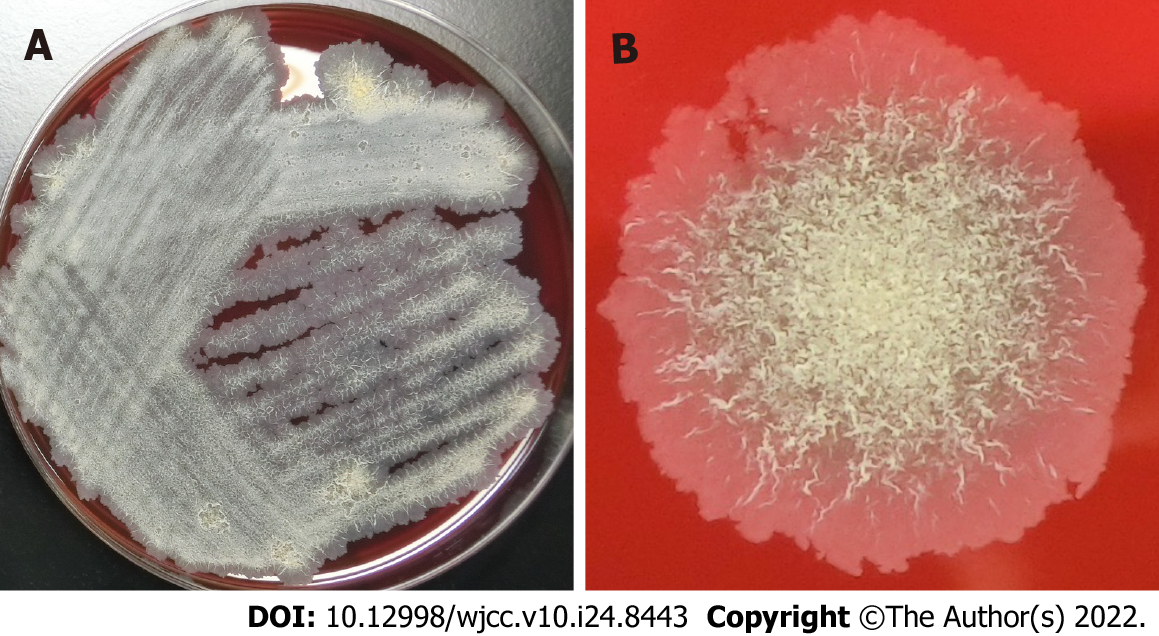
Tsukamurella species are obligate aerobic, gram-positive, weak acid-fast, nonmotile bacilli. They are found in various environments, such as soil, water, sludge, and petroleum reservoir wastewater, and belong to the order Actinomycetales. In 2016, there was a reclassification of species within the genus Tsukamurella, merging the species Tsukamurella tyrosinosolvens (T. tyrosinosolvens) and Tsukamurella carboxydivorans. Tsukamurella species are clinically considered to be a rare opportunistic pathogen, because most reported cases have been related to bacteremia and intravascular prosthetic devices and immunosuppression. To date, it has been isolated only from human specimens, and has always been associated with clinical disease; human infections are very rare. Reported infections have included pneumonia, brain abscesses, catheter-related bloodstream infections, ocular infections, bacteremia, and sepsis presenting with septic pulmonary emboli in patients who are immunocompromised. To date, there is no commercially available test for identification. On the other hand, sequence-based identification, including matrix-assisted laser desorption ionization time-of-flight mass spectrometry, is an alternative method for identifying clinical isolates that are either slow growers or difficult to identify through biochemical profiling. The golden standards for diagnosis and optimal management still remain to be determined. However, newer molecular biological techniques can provide accurate identification, and contribute to the appropriate selection of definitive therapy for infections caused by this organism. Combinations of several antimicrobial agents have been proposed for treatment, though the length of treatment for infections has yet to be determined, and should be individualized according to clinical response. Immunocompromised patients often experience severe cases due to infection, and life-threatening T. tyrosinosolvens events associated with dissemination and/or failure of source control have occurred. Favorable prognoses can be achieved through earlier identification of the cause of infection, as well as successful management, including appropriate antibiotic therapy together with source control. Further analyses of similar cases are required to establish the most adequate diagnostic methods and treatment regimens for infections.
Core Tip: Tsukamurella species are obligate aerobic, gram-positive, weak acid-fast, nonmotile bacilli that are found in various environments, including soil, water, and sludge. In 2016, there was a reclassification of species within the genus Tsukamurella, merging the species Tsukamurella tyrosinosolvens (T. tyrosinosolvens) and Tsukamurella carboxydivorans. To date, human infections are very rare, and reported infections include pneumonia, brain abscesses, catheter-related bloodstream infections, ocular infections, and bacteremia in patients who are immunocompromised. The golden standards for diagnosis and optimal management still remain to be determined. Immunocompromised patients often experience severe cases due to infection, and life-threatening T. tyrosinosolvens events associated with dissemination and/or failure of source control have occurred. Favorable prognoses can be achieved through earlier identification of the cause of infection, as well as successful management, including appropriate antibiotic therapy together with source control. Further analyses of similar cases are required to establish the most adequate diagnostic methods and treatment regimens for infections.
- Citation: Usuda D, Tanaka R, Suzuki M, Shimozawa S, Takano H, Hotchi Y, Tokunaga S, Osugi I, Katou R, Ito S, Mishima K, Kondo A, Mizuno K, Takami H, Komatsu T, Oba J, Nomura T, Sugita M. Obligate aerobic, gram-positive, weak acid-fast, nonmotile bacilli, Tsukamurella tyrosinosolvens: Minireview of a rare opportunistic pathogen. World J Clin Cases 2022; 10(24): 8443-8449
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8443.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8443
Tsukamurella species, which are members of the order Actinomycetales, can be found in a variety of different environments, including soil, water, sludge, and wastewater from petroleum reservoirs; these nonmotile bacilli are obligate aerobic, gram-positive, and weak acid-fast[1-4]. They were initially isolated from bedbug mycetoma and ovaries, in 1941[5]. The name Tsukamurella comes from Tsukamura and Mizuno[6], the microbiologists who described the first Gordona aurantiaca strain in 1971, which had been isolated from the sputum of a patient suffering from a chronic lung pathology[5-7]. The genus Tsukamurella currently contains 12 species with validly published names that include Tsukamurella tyrosinosolvens (T. tyrosinosolvens), and was first described by Collins et al[8] in 1988. Following a 2016 reclassification of Tsukamurella species, the species T. tyrosinosolvens and Tsukamurella carboxydivorans have been merged[4]. The genus is phylogenetically related to other mycolic acid-containing genera of the order Actinomycetales, including Nocardia, Gordonia, Streptomyces, Rhodococcus, Corynebacterium, and Mycobacterium, because of their similar phenotypic properties[1,7,9,10].
Tsukamurella species infections are rare in humans, as the species is a type of saprophyte bacterium[5]. This genus has the potential to causes various infections in humans, and more and more infections have been reported in Asia, America, Europe, and Africa, suggesting a global emergence of diseases caused by this group of bacteria[11]. Tsukamurella spp. are clinically considered a rare opportunistic pathogen, as most reported cases have been related to bacteremia and intravascular prosthetic devices, such as catheters or cardiac pacemaker implants, and immunosuppression (hematological malignancy, post chemotherapy, chronic renal failure, graft-versus-host disease after bone marrow transplant, and acquired immunodeficiency syndrome)[1,12-14]. Catheter-related infection range varies, from infections of the local insertion site to metastatic deep-seated infections[9]. In addition, a case has been reported of a septic pulmonary embolism, secondary to a central venous catheter-related bloodstream infection (CRBSI)[15]. Other examples include cutaneous infection, meningitis, brain abscess, lung infection, peritonitis, knee prosthesis infection, and ocular infection[2,11,16]. Among these, a striking similarity has been noted between the clinical features of lung disease with Tsukamurella and those of mycobacterial infections[16]. There is a great likelihood that Tsukamurella lung disease incidence is significantly underestimated in areas with high tuberculosis incidence rates; it is conceivable that Tsukamurella has greater community prevalence than currently recognized[16].
To date, T. tyrosinosolvens has been isolated only from human specimens, and has been associated with clinical disease in all cases; human infections are very uncommon[7]. The infections reported have included pneumonia, brain abscesses, CRBSI, ocular infections, bacteremia, and sepsis presenting with septic pulmonary emboli[1,7,15,17].
On the other hand, there has been a reported case of coinfection with T. tyrosinosolvens and another microorganism (e.g., Mycobacterium bovis pneumonia) in an immunocompromised patient[17].
To date, no test to identify Tsukamurella spp. is commercially available[7]. The genus bears a phy
One alternative method for identifying clinical isolates that are either slow growers or difficult to identify through biochemical profiling is sequence-based identification[7]. Because clinical laboratories most frequently use the 16S rRNA gene as a target for molecular identification, and for the identification of unusual pathogens, sequencing serves as a comparatively rapid and reliable new molecular technique[1,7,13,18]. Previous studies have shown that the majority of Tsukamurella species have highly similar 16S rRNA gene sequences in common (> 99% nucleotide identities); as a result, these species cannot be confidently identified using this gene target[18]. Despite this, 16S rRNA gene sequences have been found to be insufficiently discriminative for the purpose of identifying certain species, including Tsukamurella species, due to the minor differences that exist within various Tsukamurella species’ 16S rRNA gene sequences[1,7,9]. However, in previously reported criteria used to interpret partial 16S rRNA gene sequences, the sequence provided 100% identification for T. tyrosinosolvens (GenBank Accession numbers AY238514, Y12246, Y12245, and Y12247)[9].
An isolate digested by HinfI (440 bp) and MspI (313/135 bp) leaves unique fragments compatible with Tsukamurella species identification[1]. Other bacterial genera have been successfully identified through other gene targets, including ssrA (stable small RNA), secA (the secretion ATPase), rpoB (beta-subunit of RNA polymerase), and groEL (heat shock protein 60)[5,18,19]. On the other hand, the groEL gene is reportedly useful for speciation of T. tyrosinosolvens[1,7,11]. However, two gene sequences for Tsukamurella species were found to be available within the GenBank database: T. tyrosinosolvens (GenBank accession No. U90204) and T. paurometabola (GenBank accession No. AF352578)[1]. This indicates that further studies of groEL gene sequencing of multiple strains of each Tsukamurella species would be merited, as verification of suitability for speciation of Tsukamurella[1,11]. Furthermore, regarding the biodegradation of hydrocarbons, two alkane catabolic pathways have been discovered within the T. tyrosinosolvens PS2 genome, unlike the above-mentioned representatives of the genus Tsukamurella[4]. One of these pathways contains an alkane 1-monooxygenase gene (alkB; GenBank accession number KZL97795), two rubredoxin genes (rubA; GenBank accession numbers KZL97794 and KZL97793), one rubredoxin-reductase gene (rubB), and one regulatory protein TetR gene (alkU; GenBank accession number KZL97792)[4]. This pathway resembles that of the alkane-degrading actinomycete T. tyrosinosolvens strain MH1, in accordance with the complete genome[4]. In addition, a gene known to perform a key alkane degradation role was found within the T. tyrosinosolvens PS2 genome (GenBank accession number KZL95198), homologous to cytochrome P450 alkane 1-monooxygenase, from the Gordonia spp. strain TF6 (GenBank accession number BAF95905)[4]. Consequently, these two systems may benefit this particular strain in highly polluted environments, and may additionally offer insights into the ecological role played by this bacterium[4].
PCR sequencing is not yet used in clinical laboratories as a routine method of identification, due to its expensive, time-consuming nature and its technical demands[11]. To date, none of the 60 isolates have been correctly identified at the species level using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) with the original Bruker database V.6.0.0.0[11]. By using the Bruker database, expanded with 15 type and reference strains that cover all 11 Tsukamurella species recognized to date, 59 of the 60 isolates were accurately identified at the species level, with scores of ≥ 2.0[11]. Therefore, MALDI-TOF MS should prove beneficial for the routine identification of Tsukamurella species in clinical microbiology laboratories, once the database has been optimized[11]. However, because of its inability to correctly identify species of Tsukamurella, it is necessary to continually expand the MALDI-TOF MS databases to add more gram-positive rods[11]. Tsukamurella isolates have also been identified at the species level through PCR-restriction fragment length polymorphism analysis, as well as through 16S rRNA gene sequencing[1,7,20,21]. One identification scheme based on PCR-restriction fragment length polymorphism, using an amplified 440-bp segment of hsp65, has been described as being performed with the primers TB11 (5’-ACCAACGATGGTGTGTCCAT-3’) and TB12 (5’-CTTGTCGAACCGCATACCCT-3’)[1].
Regarding other genetic analysis, it has been confirmed through DNA-DNA hybridization that these are distinct from the other known species within the genus Tsukamurella (26.2% ± 2.4% to 36.8% ± 1.2% DNA-DNA relatedness), in line with results of in silico genome-to-genome comparison (32.2%-40.9% Genome-to-Genome Distance Calculator and 86.3%-88.9% average nucleotide identity values)[19].
For diagnoses, physicians should ask for detailed information about patients’ working and living environments, as well as their histories of animal contact, and perform routine bacterial smear tests[16]. The first step towards a reliable diagnosis of Tsukamurella infections is a high level of suspicion, and a low threshold for microbiological sampling[16]. Ideally, molecular diagnostic assays should serve as the routine laboratory method[16]. Given the rising number of Tsukamurella infections, new Tsukamurella species implementations in matrix-assisted laser desorption ionization time-of-flight databases need to be more discriminant[22].
The optimal management of Tsukamurella bacterial infections has yet to be determined[1]. Based on treatment principles for nocardiosis and atypical mycobacterial infections, a number of combinations of antimicrobial agents have been proposed as potential treatments for Tsukamurella infections[1,5]. To date, there remains no recommended standard susceptibility method for Tsukamurella species; however, in most case reports, a susceptibility to amikacin, clarithromycin, imipenem, ciprofloxacin, and trimethoprim-sulfamethoxazole has been noted in Tsukamurella isolates, as well as a resistance to penicillin, cefoxitin, and expanded-spectrum cephalosporins, determined through a standard in vitro antibiotic disk diffusion susceptibility assay[1,9]. Immunocompromised patients often experience severe cases due to infection, and life-threatening T. tyrosinosolvens events associated with dissemination and/or failure of source control have occurred[14,15]. Favorable prognoses can be achieved through earlier identification of T. tyrosinosolvens as the cause of infection, as well as successful management, including appropriate antibiotic therapy with source control[9,14]. In particular, good clinical outcomes are possible for CRBSI caused by Tsukamurella species, through a combination of appropriate antibiotics and catheter removal; this is likely to be the most effective management strategy for patients[1,2,9].
The length of treatment for Tsukamurella bacterial infections has yet to be determined, and should be individualized based on clinical response[1]. On the other hand, expect frequent relapses, especially in hosts who are immunocompromised; it is recommended to administer prolonged antibiotic treatment and oral suppressive treatment[1,17].
Multiple lapses in infection control have been identified; the most likely cause of the outbreak was the clinic improperly preparing the saline flush syringes[23]. It has been demonstrated, through this outbreak, that oncology patient bloodstream infections can be the result of improper infection control practices; this serves to highlight the critical necessity of increased attention to, and supervision of, infection control measures in outpatient oncology settings[23].
This mini-review aims to highlight the difficulty of identifying the genus Tsukamurella. The golden standards for diagnosis and optimal management still remain to be determined. However, accurate identification can be achieved through newer molecular biological techniques, thus contributing to appropriate selection of definitive therapy for infections due to this organism. Further analysis of similar cases is required in order to establish the most adequate diagnostic methods and treatment regimens for T. tyrosinosolvens infections. In addition, future studies should aim to establish better guidelines for the effective management of Tsukamurella infections.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: García-Mena J, Mexico; Zhang HF, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Sheng WH, Huang YT, Chang SC, Hsueh PR. Brain abscess caused by Tsukamurella tyrosinosolvens in an immunocompetent patient. J Clin Microbiol. 2009;47:1602-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Cho AR, Kim HR, Lee MK, Choi SH, Yun SW. A Case Report of Tsukamurella pulmonis Infection Misidentified as Atypical Mycobacteria. Korean J Clin Microbiol. 2010;13:93-97. [DOI] [Full Text] |
| 3. | Zhang H, Han L, Jiang B, Long C. Identification of a phosphorus-solubilizing Tsukamurella tyrosinosolvens strain and its effect on the bacterial diversity of the rhizosphere soil of peanuts growth-promoting. World J Microbiol Biotechnol. 2021;37:109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Romanova VA, Boulygina EA, Siniagina MN, Markelova MI, Grigoryeva TV, Laikov AV. Draft Genome Sequence of a Medium- and Long-Chain n-Alkane-Degrading Bacterium, Tsukamurella tyrosinosolvens Strain PS2, with Two Genetic Systems for Alkane Degradation. Microbiol Resour Announc. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Safaei S, Fatahi-Bafghi M, Pouresmaeil O. Role of Tsukamurella species in human infections: first literature review. New Microbes New Infect. 2018;22:6-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Tsukamura M, Mizuno S. A new species Gordona aurantiaca occurring in sputa of patients with pulmonary disease. Kekkaku. 1971;46:93-98. [PubMed] |
| 7. | Ménard A, Degrange S, Peuchant O, Nguyen TD, Dromer C, Maugein J. Tsukamurella tyrosinosolvens--an unusual case report of bacteremic pneumonia after lung transplantation. Ann Clin Microbiol Antimicrob. 2009;8:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Collins M, Smida J, Dorsch M, Stackebrandt E. Tsukamurella gen. nov. harboring Corynebacterium paurometabolum and Rhodococcus aurantiacus. Int J Syst Evol Microbiol. 1988;38:385-391. |
| 9. | Elshibly S, Doherty J, Xu J, McClurg RB, Rooney PJ, Millar BC, Shah H, Morris TC, Alexander HD, Moore JE. Central line-related bacteraemia due to Tsukamurella tyrosinosolvens in a haematology patient. Ulster Med J. 2005;74:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Yassin AF, Müller J. Development of real-time polymerase chain reaction assay for specific detection of Tsukamurella by targeting the 16S rRNA gene. Diagn Microbiol Infect Dis. 2012;72:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Teng JLL, Tang Y, Wong SSY, Fong JYH, Zhao Z, Wong CP, Chen JHK, Ngan AHY, Wu AKL, Fung KSC, Que TL, Lau SKP, Woo PCY. MALDI-TOF MS for identification of Tsukamurella species: Tsukamurella tyrosinosolvens as the predominant species associated with ocular infections. Emerg Microbes Infect. 2018;7:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Schwartz MA, Tabet SR, Collier AC, Wallis CK, Carlson LC, Nguyen TT, Kattar MM, Coyle MB. Central venous catheter-related bacteremia due to Tsukamurella species in the immunocompromised host: a case series and review of the literature. Clin Infect Dis. 2002;35:e72-e77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Karunakaran R, Halim HA, Ng KP, Hanifah YA, Chin E, Jaafar FL, Abubakar S. Tsukamurella tyrosinosolvens intravascular catheter-related bacteremia in a haematology patient: a case report. Eur Rev Med Pharmacol Sci. 2011;15:1343-1346. [PubMed] |
| 14. | Sheridan EA, Warwick S, Chan A, Dall'Antonia M, Koliou M, Sefton A. Tsukamurella tyrosinosolvens intravascular catheter infection identified using 16S ribosomal DNA sequencing. Clin Infect Dis. 2003;36:e69-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Romano L, Spanu T, Calista F, Zappacosta B, Mignogna S, Sali M, Fiori B, Fadda G. Tsukamurella tyrosinosolvens and Rhizobium radiobacter sepsis presenting with septic pulmonary emboli. Clin Microbiol Infect. 2011;17:1049-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Yang L, Cao Y, Dan Z, Wang Z, Wang X. Community-acquired Tsukamurella pneumonia in a young immunocompetent adult: a case misdiagnosed as pulmonary tuberculosis and literature review. Postgrad Med. 2017;129:563-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Chen CH, Lee CT, Chang TC. Tsukamurella tyrosinosolvens bacteremia with coinfection of Mycobacterium bovis pneumonia: case report and literature review. Springerplus. 2016;5:2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Teng JL, Tang Y, Chiu TH, Cheung CL, Ngan AH, Ngai C, Wong SS, Que TL, Lau SK, Woo PC. The groEL Gene Is a Promising Target for Species-Level Identification of Tsukamurella. J Clin Microbiol. 2017;55:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Teng JLL, Fong JYH, Fok KMN, Lee HH, Chiu TH, Tang Y, Ngan AHY, Wong SSY, Que TL, Lau SKP, Woo PCY. Tsukamurella asaccharolytica sp. nov., Tsukamurella conjunctivitidis sp. nov. and Tsukamurella sputi sp. nov., isolated from patients with bacteraemia, conjunctivitis and respiratory infection in Hong Kong. Int J Syst Evol Microbiol. 2020;70:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Liu CY, Lai CC, Lee MR, Lee YC, Huang YT, Liao CH, Hsueh PR. Clinical characteristics of infections caused by Tsukamurella spp. and antimicrobial susceptibilities of the isolates. Int J Antimicrob Agents. 2011;38:534-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Zhao K, Zhao C, Liao P, Zhang Q, Li Y, Liu M, Ao X, Gu Y, Liao D, Xu K, Yu X, Xiang Q, Huang C, Chen Q, Zhang L, Zhang X, Penttinen P. Isolation and antimicrobial activities of actinobacteria closely associated with liquorice plants Glycyrrhiza glabra L. and Glycyrrhiza inflate BAT. in Xinjiang, China. Microbiology (Reading). 2016;162:1135-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Leroy AG, Persyn E, Guillouzouic A, Ruffier d'Epenoux L, Launay E, Takoudju EM, Juvin ME, Chantreau F, El Khobzi J, Bémer P, Corvec S. Catheter-related bloodstream infection due to Tsukamurella pulmonis identified by MALDI-TOF spectrometry, 16S rRNA gene sequencing, and secA1 gene sequencing in an immunocompromised child: a case report and literature review. Diagn Microbiol Infect Dis. 2020;97:115052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | See I, Nguyen DB, Chatterjee S, Shwe T, Scott M, Ibrahim S, Moulton-Meissner H, McNulty S, Noble-Wang J, Price C, Schramm K, Bixler D, Guh AY. Outbreak of Tsukamurella species bloodstream infection among patients at an oncology clinic, West Virginia, 2011-2012. Infect Control Hosp Epidemiol. 2014;35:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |