Published online Aug 16, 2022. doi: 10.12998/wjcc.v10.i23.8124
Peer-review started: December 17, 2021
First decision: February 21, 2022
Revised: March 2, 2022
Accepted: June 30, 2022
Article in press: June, 30, 2022
Published online: August 16, 2022
Processing time: 227 Days and 2.5 Hours
Spontaneous pneumoperitoneum (SP) without gastrointestinal perforation rarely occurs in neonates, with most SP cases being idiopathic. Although SP usually follows a benign clinical course with favorable prognosis, it can become life-threatening in certain situations. In these cases, urgent surgical intervention may be required. Therefore, it may be difficult to decide when or how to perform prompt interventions.
To demonstrate the distinct clinical features of SP to guide appropriate management by comparing characteristics between SP and typical pneumoperitoneum secondary to gastrointestinal perforation.
We retrospectively reviewed electronic medical records and identified 37 neonates with radiological evidence of pneumoperitoneum who were treated at our institution. Clinical variables were compared between neonates with SP without gastrointestinal perforation (Group A) and those with pneumoperitoneum secondary to gastrointestinal perforation (Group B). Clinical variables between groups were compared using Student’s t-test and the chi-square test. The risk factors related to mortality were examined using multi-logistic regression analysis.
Group A comprised 35.1% (13/37) of the patients. The frequency of persistent pulmonary hypertension (53.8%) and pneumothorax (46.2%) before the development of pneumoperitoneum was significantly higher in group A than in group B (P = 0.004). Platelet count and partial pressure of arterial oxygen (PaO2) were significantly lower in group A (P = 0.015 and 0.025, respectively). Overall mortality was significantly higher in group A than in group B (76.9% vs 16.7%, P = 0.001). Only preterm infants were significantly associated with high mortality (P = 0.041; odds ratio = 18.0). Accompaniment with persistent pulmonary hypertension and pneumothorax were also significantly high (P = 0.004) in group A, but these were not strongly associated with high mortality.
This study identified a higher mortality rate in patients with SP than that described in previous reports. Neonates with SP were more likely to have thrombocytopenia, pneumothorax, and persistent pulmonary hypertension. Prematurity was the most significant factor affecting mortality.
Core Tip: This study shows a higher mortality rate in a spontaneous pneumoperitoneum (SP) group than pneumoperitoneum secondary to gastrointestinal perforation, contrary to previous studies. Additionally, neonates with SP were more likely to have thrombocytopenia and accompany pneumothorax and persistent pulmonary hypertension. Preterm infants were the most significant factor affecting its mortality. These distinctive clinical features should be considered in the management of SP.
- Citation: Kim SH, Cho YH, Kim HY. Distinctive clinical features of spontaneous pneumoperitoneum in neonates: A retrospective analysis. World J Clin Cases 2022; 10(23): 8124-8132
- URL: https://www.wjgnet.com/2307-8960/full/v10/i23/8124.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i23.8124
Pneumoperitoneum refers to the abnormal presence of intraperitoneal free air and is usually identified by radiography in extremely low-birth weight neonates with gastrointestinal perforation as most common cause[1-4]. Necrotizing enterocolitis and congenital anomalies causing intestinal perforation are common clinical findings[5,6]. Pneumoperitoneum generally requires emergent surgical intervention and may show variable clinical courses according to its etiology. However, spontaneous pneumoperitoneum (SP) not associated with gastrointestinal perforation rarely occurs in neonates[7,8]. Most cases of SP are idiopathic; however, SP may occur as a consequence of inadequate mechanical ventilation or massive cardiopulmonary resuscitation in critical neonates[9-11]. Although SP usually follows a benign clinical course with favorable prognosis, it can become life-threatening in certain situations. In these cases, urgent surgical intervention may be required. Therefore, it may be difficult to decide when or how to perform prompt interventions.
This study aimed to demonstrate the distinct clinical features of SP to guide appropriate management by comparing characteristics between SP and typical pneumoperitoneum secondary to gastrointestinal perforation.
We enrolled 37 neonates managed for pneumoperitoneum at our institution between January 2009 and December 2020. Pneumoperitoneum was diagnosed primarily based on radiological findings. Patients were divided into two groups: SP without gastrointestinal perforation (Group A) and pneumoperitoneum secondary to gastrointestinal perforation (Group B). This study was approved by the Institutional Review Board (IRB No. 05-2020-044) and was conducted in accordance with the recommendations of the IRB committee.
Patients’ electronic medical records were retrospectively reviewed to collect data regarding demographics, gestational data, clinical history before diagnosis (use of mechanical ventilation or high-frequency oscillation ventilation; history of persistent pulmonary hypertension, respiratory distress syndrome with bronchopulmonary dysplasia, pneumothorax, or cardiopulmonary resuscitation; and history of vasopressor infusion), laboratory parameters indicating inflammatory process (blood sugar level around diagnosis, C-reactive protein level, white blood cell count with segmented neutrophil count, platelet count), respiratory condition [pH, partial pressure of arterial oxygen (PaO2)], and treatment outcomes.
Clinical variables between groups were compared using Student’s t-test and the chi-square test. The risk factors related to mortality in group A were examined using multi-logistic regression analysis (IBM SPSS Statistics v26, IBM Corp., Armonk, NY, United States). A P value of < 0.05 was considered statistically significant.
Of the 37 patients, 13 (35.1%) had SP without gastrointestinal perforation (Group A), and 24 (64.9%) had pneumoperitoneum secondary to gastrointestinal perforation (Group B). Their demographic characteristics are presented in Table 1. There was a high proportion of male, preterm, and cesarean deliveries in both groups, but there were no significant differences between groups. Gestational age, birth weight, and 1- and 5-min Apgar scores were high in group B, while postnatal age at diagnosis was high in Group A. However, there were no significant differences (Table 1).
| Variables | Group A, n = 13 | Group B, n = 24 | P value |
| Sex (male/female) | 12/1 | 17/7 | 0.224 |
| GA, wk | 29.5 ± 5.3 (23-39) | 31.7 ± 5.2 (23-39) | 0.216 |
| Preterm/full term | 10/3 (76.9%) | 16/8 (66.7%) | 0.515 |
| Birth weight, g | 1479.2 ± 1071.2 (450-3670) | 1888.2 ± 986.5 (670-3450) | 0.189 |
| Delivery (NSVD/CS) | 1/12 | 10/14 | 0.057 |
| Postnatal age at diagnosis, d | 18.8 ± 29.9 (0-111) | 12.1 ± 18.5 (1~ 79) | 0.499 |
| Apgar at 1 min | 4.0 ± 2.5 (0-8) | 4.3 ± 1.5 (0-8) | 0.936 |
| Apgar at 5 min | 5.8 ± 2.0 (1-9) | 6.5 ± 1.5 (0-9) | 0.825 |
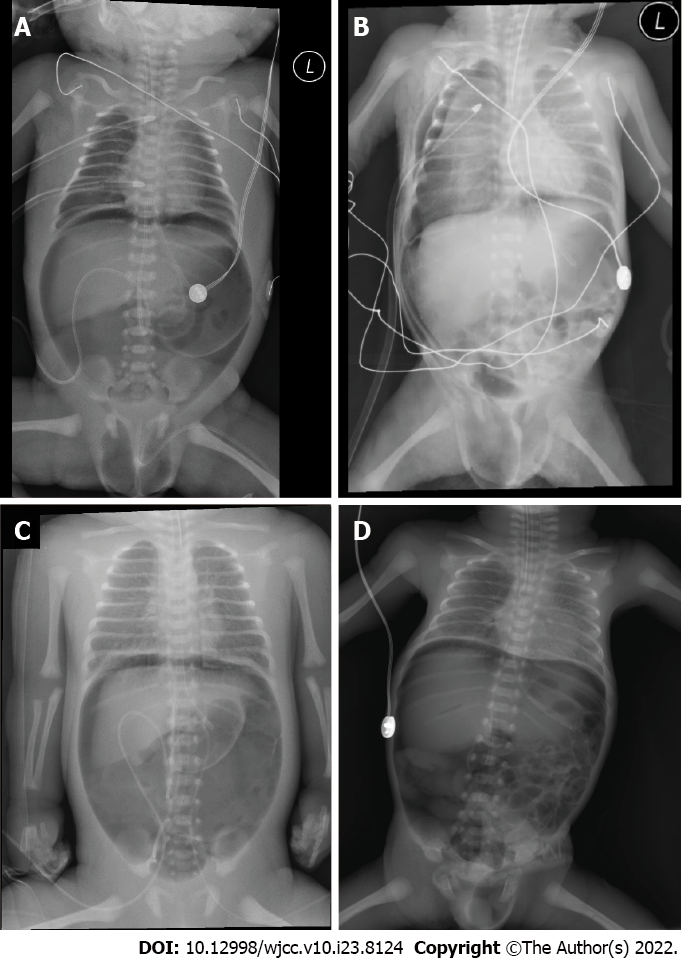
Persistent pulmonary hypertension (53.8%) and pneumothorax (46.2%) were significantly more common in group A (Figure 1A and B) than in group B (Figure 1C and D) (P = 0.004). There were no other significant differences in clinical characteristics between the groups, although patients in group A were more likely to have received mechanical ventilation at birth and of high-frequency oscillation ventilation and have a history of respiratory distress syndrome, cardiopulmonary resuscitation before diagnosis, and vasopressor infusion. Patients in group A also had a longer duration of mechanical ventilation compared with group B (Table 2).
| Variables | Group A, n = 13 | Group B, n = 24 | P value | Odds ratio, 95%CI |
| MV at birth (yes/no) | 12/1 | 17/7 | 0.216 | 4.333 |
| HFOV (yes/no) | 5/13 | 3/24 | 0.100 | 4.375 |
| PPHN (yes/no) | 7/6 | 2/22 | 0.004 | 12.833 |
| RDS (yes/no) | 11/2 | 15/9 | 0.262 | 3.3 |
| Pneumothorax (yes/no) | 6/7 | 1/23 | 0.004 | 19.7 |
| CPR (yes/no) | 3/10 | 2/22 | 0.321 | 3.3 |
| Vasopressor infusion (yes/no) | 11/2 | 12/12 | 0.074 | 5.5 |
| MV duration, d | 17.1 ± 30.2 (1-111) | 12.4 ± 21.7 (2-79) | 0.067 | - |
Blood sugar levels were higher in group A than in group B without significancy. By contrast, C-reactive protein level, white blood cell count, and neutrophil count were higher in group B, but the difference was not significant. Platelet count, pH, and PaO2 were lower in group A than in group B, and the differences in platelet count and PaO2 were significant lower in group A (P = 0.015 and 0.025, respectively). Overall mortality was significantly higher in group A than in group B (76.9% vs 16.6%, P = 0.001) (Table 3).
| Variables | Group A, n = 13 | Group B, n = 24 | P value |
| Blood sugar, mg% | 152.1 ± 58.2 (69-235) | 148.3 ± 51.4 (85-294) | 0.790 |
| CRP, IU/L | 1.28 ± 1.60 (0.01-4.88) | 2.24 ± 4.03 (0.03-17.94) | 0.649 |
| WBC, 103/μL | 14641.5 ± 7008.6 (3810-25470) | 14568.8 ± 9349.4 (4410-37360) | 0.561 |
| Segmented neutrophil, % | 57.5 ± 17.4 (20.3-87.0) | 62.1 ± 12.8 (34.1-81.4) | 0.479 |
| PLT, 103/μL | 156.1 ± 115.9 (6.0-371.0) | 272.9 ± 143.8 (83.0-565.0) | 0.015 |
| Thrombocytopenia | 8 / 13 | 5 / 24 | 0.013 |
| pH | 7.26 ± 0.15 (6.95-7.43) | 7.27 ± 0.17 (6.92-7.48) | 0.696 |
| PaO2, mmHg | 55.4 ± 19.2 (18.0-86.8) | 100.9 ± 58.3 (46.3-227.2) | 0.025 |
| Overall mortality, % | 76.9 (10/13) | 16.6 (4/24) | 0.001 |
Five patients with SP were managed by non-operative treatment, and 8 patients underwent surgery due to the presence of abdominal rigidity and clinical deterioration during non-surgical management. Surgical exploration did not reveal any obvious underlying conditions. The overall mortality rate among patients with SP was 76.9%. Mortality rate was higher in preterm infants, those treated by non-operative management, and in cases accompanied by pneumothorax, persistent pulmonary hyper
| Variables | No of cases (%) | P value | Odd ratio (95%CI) |
| Management | |||
| Non-operative | 5 (38.5) | ||
| Operation | 8 (61.5) | ||
| Mortality, overall | 10/13 (76.9) | ||
| Mortality in | |||
| Non-operative | 5/5 (100) | ||
| Operative | 5/8 (62.5) | 0.231 | 0.625 (0.365-1.069) |
| Pneumothorax (+) | 6/6 (100) | ||
| Pneumothorax (-) | 4/7 (57.1) | 0.067 | 1.750 (0.921-3.324) |
| PPHN (+) | 6/7 (85.7) | ||
| PPHN (-) | 4/6 (66.7) | 0.416 | 3.0 (0.199-45.244) |
| RDS (+) | 9/11 (81.8) | ||
| RDS (-) | 1/2 (50.0) | 0.326 | 4.5 (0.190-106.8) |
| HFOV (+) | 4/5 (80.0) | ||
| HFOV (-) | 6/8 (75.0) | 0.912 | 1.333 (0.088-20.108) |
| Vasopressor (+) | 9/11 (81.8) | ||
| Vasopressor (-) | 1/2 (50.0) | 0.326 | 4.5 (0.190-106.823) |
| CPR (+) | 3/3 (100) | ||
| CPR (-) | 7/10 (70.0) | 0.279 | 1.429 (0.952-2.143) |
| Thrombocytopenia (+) | 7/8 (87.5) | ||
| Thrombocytopenia (-) | 3/5 (60.0) | 0.51 | 4.667 (0.197-73.384) |
| Full-term | 1/3 (33.3) | ||
| Preterm | 9/10 (90.0) | 0.041 | 18.0 (0.758-427.291) |
Among neonates with pneumoperitoneum with gastrointestinal perforation, there were 7 and 7 cases of gastric perforation and necrotizing enterocolitis, respectively, 2 cases of single intestinal perforation, 4 cases of Hirschsprung’s disease, 2 cases of esophageal atresia with distal tracheoesophageal fistula, and 1 case of intestinal atresia. The mortality rate was 16.6% (Table 5).
| Variable | Cases, n (%) |
| Associated gastrointestinal conditions | |
| Gastric perforation | 7 (29.2) |
| NEC | 7 (29.2) |
| SIP | 2 (8.3) |
| HD | 4 (16.6) |
| Intestinal atresia | 1 (4.2) |
| EA /c TEF | 2 (8.3) |
| Malrotation | 1 (4.2) |
| Mortality, overall | 4 (16.6) |
Gastrointestinal perforation is the most common cause of pneumoperitoneum and requires prompt surgical exploration[1-4]. In neonates, these cases present a clinical challenge for neonatologists and pediatric surgeons. Meanwhile, SP not associated with gastrointestinal perforation during the neonatal period has been described sporadically[12-15]. The clinical course of SP is variable; some patients are asymptomatic and do not require surgical intervention, while others can become critically unwell. Further, there are some controversial issues in diagnosis and management due to the lack of a clear etiology. A few previous reports described SP as a benign pneumoperitoneum because it generally presents with asymptomatic intraperitoneal air without signs or symptoms of peritonitis and suggested careful management to avoid unnecessary surgery if possible[7,8,15,16]. There are not many reports about its prevalence and consensus regarding the optimal treatment protocol, but it is estimated to contribute to 5.4%-7.8% of all cases of neonatal pneumoperitoneum and the laparotomy rate for this condition is known to be as high as 28%[6,17]. This study revealed an unexpectedly high prevalence of SP, which contributed to approximately one-third of all pneumoperitoneum cases. Generally, pneumoperitoneum is more likely to occur in extremely low birth weight neonates due to necrotizing enterocolitis. However, we found no significant differences in gestational age and birth weight between neonates with SP and those with pneumoperitoneum with gastrointestinal perforation, although those with SP showed low gestational age and lower birth weight. Therefore, it is important to differentiate SP from cases of pneumoperitoneum associated with gastrointestinal perforation to ensure appropriate management.
Several possible origins of free air in cases of SP were presumed: Intrathoracic, gynecologic, intra-abdominal, iatrogenic, and miscellaneous[2,18,19]. Among those, there were some suggested clinical factors with including inadequate mechanical ventilation, a massive cardiopulmonary resuscitation, air leak syndrome, and other pulmonary conditions associated with prematurity[9,10,20]. Of these, pneumothorax is a frequent preceding sign and has been demonstrated to show a favorable clinical course in most of the reported cases[2,7,8]. There was also a relatively high proportion of prematurity regardless of gastrointestinal perforation in this study, but the mortality rate was significantly higher in SP cases than in cases associated with gastrointestinal perforation (76.9% vs 16.7%, P = 0.001). Even though surgical exploration was performed in some cases with abdominal rigidity or clinical deterioration despite conservative management, no obvious cause for SP was identified in any of our patients. In contrast, we found several well-known causes of gastrointestinal perforation in this study.
There were no significant differences in demographic findings and laboratory results at the time of diagnosis, including white blood cell count, segmented neutrophil proportion, and C-reactive protein level, implying an inflammatory process regardless of gastrointestinal perforation. Therefore, it is difficult to predict the characteristics of pneumoperitoneum based on only typical clinical presentations, such as abdominal distention and radiologic finding. Regarding clinical conditions before the occurrence of pneumoperitoneum, there were important differences between groups. Of note, some respiratory problems (persistent pulmonary hypertension, pneumothorax, and respiratory distress syndrome) and consequent supportive management (application of mechanical ventilation and its duration, high-frequency oscillation ventilation, vasopressor infusion, and cardiopulmonary resuscitation) were relatively common in the SP group. These findings may also be considered as possible causes described in previous reports[10,11,15,16,21], but more studies are needed to reach a suitable conclusion. This study showed a significantly high proportion of pneumothorax and persistent pulmonary hypertension in the SP group (P = 0.004), but these were not significantly associated with the risk of pneumoperitoneum. Instead, patients with SP had a significantly decreased platelet count (thrombocytopenia) and PaO2 compared with those in the pneumoperitoneum secondary to gastrointestinal perforation group. Additionally, a significantly high mortality rate (76.9%) in the SP group was an interesting finding. It is likely that the above laboratory findings could affect morbidity and mortality. These critical factors should be considered, as they may influence the progress and management of SP cases.
The management of patients with SP depends on their condition, but deciding between non-operative or operative management presents a treatment dilemma for clinicians. Typical clinical and laboratory findings are not sufficient to support such a decision. In our study, there were no significant differences in types of management (surgical or non-surgical). Surgical management was performed in 53.8% of SP cases when clinical examination revealed abdominal rigidity and clinical deterioration suggestive of perforation during conservative care. The rate of surgical management of SP in our study was higher than that cited by previous reports[6,17]. During conservative management, surgical procedure was performed due to unusual and not improved clinical situations. However, a previous case reported good outcomes in a patient with idiopathic SP after a period of observation, suggesting that surgical intervention should only be performed in necessary cases to avoid unnecessary procedures[4,7,8,15,16,21]. On the contrary, this study revealed a relatively high mortality rate in patients who did not undergo surgery (100% vs 62.5%). In this study, we could not reveal clear factors related to its high mortality. Nevertheless, it is presumed to be caused by combination of some clinical situations. Of note, more than two-thirds of cases with SP were preterm infants with a mean gestational age of 29.5 wk. Therefore, the high mortality rate may be related to underlying pulmonary conditions, especially persistent pulmonary hypertension and pneumothorax, and other critical situations requiring vasopressor infusion and cardiopulmonary resuscitation. However, this study did not find a significant correlation between these variables with high mortality. Instead, only preterm infants were significantly associated with high mortality (P = 0.041; odds ratio = 18.0, 95% confidence interval 0.758-427.29).
Compared to a pneumoperitoneum secondary to gastrointestinal perforation, SP showed some specific clinical features; (1) A high association with proceeding clinical condition, persisted pulmonary hypertension and pneumothorax; (2) Frequently accompanied with a thrombocytopenia and lower partial pressure of arterial oxygen; and (3) A high mortality, especially in preterm neonates.
This study had a few limitations. First, this was a retrospective study conducted at a single institution and all diagnoses were based solely on radiologic findings; therefore, we cannot exclude the possibility of selection bias. Second, the sample size was small, which may limit the interpretation of the results. Considering the rarity of SP, further studies with larger sample sizes are warranted to improve our understanding of this condition. Finally, the results are limited by the lack of a comparative study for this clinical situation with inclusion of a control group without pneumoperitoneum. Nevertheless, this study is valuable as it furthers our understanding of the distinctive features of SP.
SP is associated with a higher mortality rate than pneumoperitoneum secondary to gastrointestinal perforation, and this rate was higher than that reported by previous studies. Additionally, neonates with SP were more likely to have thrombocytopenia and accompany pneumothorax and persistent pulmonary hypertension. Preterm infants were the most significant factor affecting its mortality. These distinctive clinical features should be considered in the management of SP. Further studies with larger sample sizes are warranted to validate these results.
Spontaneous pneumoperitoneum (SP) without gastrointestinal perforation rarely occurs in neonates, with most cases being idiopathic. Although it usually follows a benign clinical course with favorable prognosis, it can become life-threatening in certain situations.
SP is associated with a higher mortality rate than pneumoperitoneum secondary to gastrointestinal perforation, and this rate was higher than that reported by previous studies.
To demonstrate the distinct clinical features of SP to guide appropriate management by comparing characteristics between SP and typical pneumoperitoneum secondary to gastrointestinal perforation.
Retrospectively reviewed electronic medical records and identified 37 neonates with radiological evidence of pneumoperitoneum who were treated at our institution. Clinical variables were compared between neonates with SP without gastrointestinal perforation (Group A) and those with pneumoperitoneum secondary to gastrointestinal perforation (Group B).
Compared to a pneumoperitoneum secondary to gastrointestinal perforation, SP showed some specific clinical features: (1) A high association with proceeding clinical condition, persisted pulmonary hypertension and pneumothorax; (2) Frequently accompanied with a thrombocytopenia and lower partial pressure of arterial oxygen; and (3) A high mortality, especially in preterm neonates.
This study identified a higher mortality rate in patients with SP than that described in previous reports. Neonates with SP were more likely to have thrombocytopenia, pneumothorax, and persistent pulmonary hypertension. Prematurity was the most significant factor affecting mortality.
There were a few limitations: First, this was a retrospective study conducted at a single institution and all diagnoses were based solely on radiologic findings; second, the sample size was small which may limit the interpretation of the results; third, the results are limited by the lack of a comparative study for this clinical situation with inclusion of a control group without pneumoperitoneum. Nevertheless, this study is valuable as it furthers our understanding of the distinctive features of SP.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hefny AF, United Arab Emirates; Zhang ZQ, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Ye N, Yuan Y, Xu L, Pfister RE, Yang C. Successful conservative treatment of intestinal perforation in VLBW and ELBW neonates: a single centre case series and review of the literature. BMC Pediatr. 2019;19:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Mularski RA, Sippel JM, Osborne ML. Pneumoperitoneum: a review of nonsurgical causes. Crit Care Med. 2000;28:2638-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Gutkin Z, Iellin A, Meged S, Sorkine P, Geller E. Spontaneous pneumoperitoneum without peritonitis. Int Surg. 1992;77:219-223. [PubMed] |
| 4. | Morsi AH, Omar HR, Osama A, Khodary AR. Clinical spectrum of neonates presenting with pneumoperitoneum: A retrospective study. Afr J Paediatr Surg. 2016;13:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Calisti A, Perrelli L, Nanni L, Vallasciani S, D'Urzo C, Molle P, Briganti V, Assumma M, De Carolis MP, Maragliano G. Surgical approach to neonatal intestinal perforation. An analysis on 85 cases (1991-2001). Minerva Pediatr. 2004;56:335-339. [PubMed] |
| 6. | Khan TR, Rawat JD, Ahmed I, Rashid KA, Maletha M, Wakhlu A, Kureel SN. Neonatal pneumoperitoneum: a critical appraisal of its causes and subsequent management from a developing country. Pediatr Surg Int. 2009;25:1093-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Duan SX, Sun ZB, Wang GH, Zhong J, Ou WH, Fu MX, Wang FS, Ma SH, Li JH. Diagnosis and treatment of pediatric benign pneumoperitoneum: A case report series of 9 patients. Medicine (Baltimore). 2017;96:e5814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Gupta R, Bihari Sharma S, Golash P, Yadav R, Gandhi D. Pneumoperitoneum in the newborn: is surgical intervention always indicated? J Neonatal Surg. 2014;3:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Briassoulis GC, Venkataraman ST, Vasilopoulos AG, Sianidou LC, Papadatos JH. Air leaks from the respiratory tract in mechanically ventilated children with severe respiratory disease. Pediatr Pulmonol. 2000;29:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Park J, Jung E. Spontaneous pneumoperitoneum in two extremely preterm infants during nasal intermittent positive pressure ventilation. Pediatr Int. 2019;61:424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | De Rose DU, Romano V, Priolo F, Zecca C, Maggio L, Vento G, & Gallini F. An Unusual Pneumoperitoneum in an Extremely Low Birth Weight Preterm Newborn. Acta Biomed. 2021;92:e2021213. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Shah RS, Patel MP, Pikale HS, Kulkarni BK, Borwankar SS. Benign neonatal pneumoperitoneum--an enigma. J Postgrad Med. 1992;38:84-85. [PubMed] |
| 13. | Vohra K, Jenkins K, Klotz DH, French JH. Neonatal pneumoperitoneum of uncertain etiology. J Natl Med Assoc. 1992;84:633-635. [PubMed] |
| 14. | PORTER A. Spontaneous pneumoperitoneum in the newborn; report of a case. N Engl J Med. 1956;254:694-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | He TZ, Xu C, Ji Y, Sun XY, Liu M. Idiopathic neonatal pneumoperitoneum with favorable outcome: A case report and review. World J Gastroenterol. 2015;21:6417-6421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Karaman A, Demirbilek S, Akin M, Gürünlüoğlu K, Irşi C. Does pneumoperitoneum always require laparotomy? Pediatr Surg Int. 2005;21:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. |
R Gupta.
Spontaneous pneumoperitoneum: Discerning from radiological imaging |
| 18. | van Gelder HM, Allen KB, Renz B, Sherman R. Spontaneous pneumoperitoneum. A surgical dilemma. Am Surg. 1991;57:151-156. [PubMed] |
| 19. | Tallant C, Tallant A, Nirgiotis J, Meller J. Spontaneous pneumoperitoneum in pediatric patients: A case series. Int J Surg Case Rep. 2016;22:55-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Gummalla P, Mundakel G, Agaronov M, Lee H. Pneumoperitoneum without Intestinal Perforation in a Neonate: Case Report and Literature Review. Case Rep Pediatr. 2017;2017:6907329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Broekaert I, Keller T, Schulten D, Hünseler C, Kribs A, Dübbers M. Peritoneal drainage in pneumoperitoneum in extremely low birth weight infants. Eur J Pediatr. 2018;177:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |