Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.663
Peer-review started: June 7, 2021
First decision: June 25, 2021
Revised: July 12, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: January 14, 2022
Processing time: 219 Days and 2 Hours
Ethambutol-induced optic neuropathy (EON) most commonly manifests as bilateral symmetrical loss of vision and often cause serious and irreversible visual impairment because of the lack of early detection and effective treatment. We followed a case of EON with rare binocular asymmetric clinical manifestations and observed the changes of visual function and retinal structure after drug withdrawal, so as to further understand the clinical characteristics of this disease.
A 54-year-old man complained of gradual visual decline in the left eye. The patient presented with best-corrected visual acuity of 20/20 in the right eye and 20/50 in the left eye. Color vision examination revealed difficulty in reading green color plates in the left eye. The visual field manifested as concentric contraction in the left eye. After nearly a month of drug withdrawal, the right eye had a similar decline in visual function. At the last visit, 19 mo after drug withdrawal, the visual function significantly recovered in both eyes. During follow-up optical coherence tomography (OCT) examination, both eyes manifested the thickness of the retinal nerve fiber layer from mild thickening to thinning and finally temporal atrophy, and the ganglion cell-inner plexiform layer showed significant thinning. The difference was that a reversible structural disorder in the outer retina of the nasal macula was detected in the left eye by macular high-definition OCT.
Nephropathy and high blood pressure, which damage the retinal microcirculation, may cause damage to the outer layer of the retina. Ethambutol may infl
Core Tip: Ethambutol-induced optic neuropathy is most commonly characterized by bilateral symmetrical loss of vision, but it may also occur successively in both eyes. Ethambutol may influence retinal photoreceptor cells and retinal ganglion cells.
- Citation: Sheng WY, Wu SQ, Su LY, Zhu LW. Ethambutol-induced optic neuropathy with rare bilateral asymmetry onset: A case report. World J Clin Cases 2022; 10(2): 663-670
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/663.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.663
Ethambutol is an antimycobacterial agent that is most commonly used in combination with other drugs for the treatment of diseases, including tuberculosis, Mycobacterium avium complex, and other species in the Mycobacterium genus. However, a common and devastating adverse effect of this drug is ethambutol-induced optic neuropathy (EON), which can lead to permanent loss of visual function[1]. It is currently reported that 1%-2% of patients receiving ethambutol may develop EON[2]. Globally, there are nearly 9.2 million new cases of tuberculosis annually, and approximately 100000 patients annually develop toxic optic neuropathy due to ethambutol treatment[3]. The clinical manifestations of EON are characterized by subacute, symmetrical, painless loss of vision, with color vision dysfunction and visual field defects. There are few reports on bilateral asymmetry onset and manifestation of EON cases. Here, we present a case of EON with bilateral asymmetric manifestations over 19 mo. A full battery of objective and subjective tests was performed. These tests show how the condition changes in detail.
A 54-year-old male patient was admitted to the Department of Ophthalmology of Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine (Hangzhou, Zhejiang Province, China), on September 1, 2019. The patient complained of gradual painless loss of vision in the left eye for 5 mo.
The patient had been diagnosed with tuberculosis of thoracic vertebrae 10 mo earlier in the Tuberculosis Department. Because this patient suffered from renal failure, the tuberculologist did not use an intensive treatment plan. He received daily combination treatment consisting of 750 mg ethambutol (11.5 mg/kg), 600 mg rifampin, and 300 mg isoniazid. After 2 wk, the patient had an allergic rash and drug-induced liver damage, so rifampin was discontinued. Therefore, the patient was finally treated with ethambutol and isoniazid for 10 mo, until loss of vision developed and he came to the ophthalmology clinic.
The patient had a history of renal dysfunction and renal hypertension (blood pressure 160/98 mmHg) for about 10 years, and he had been undergoing abdominal dialysis treatment for up to 8 years. He had no history of diabetes or any other eye diseases. The patient complained that he could correctly judge the traffic lights before the onset of the disease and denied a history of color vision dysfunction.
The patient was married and had a son. His grandparents, parents and son did not have similar episodes of loss of vision. He denied smoking and alcohol consumption.
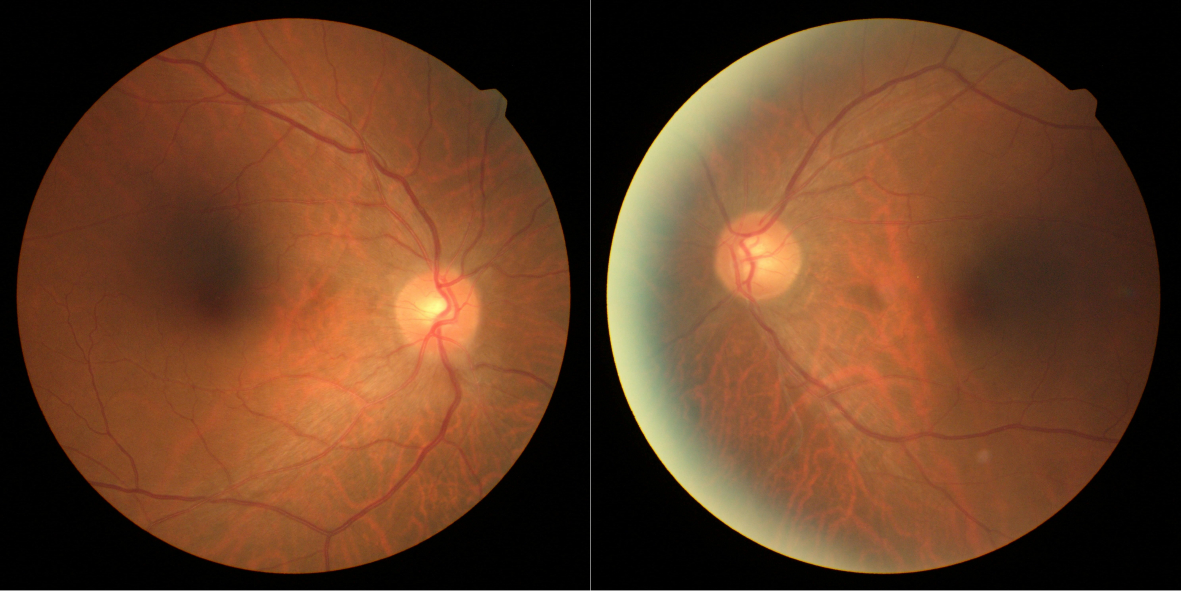
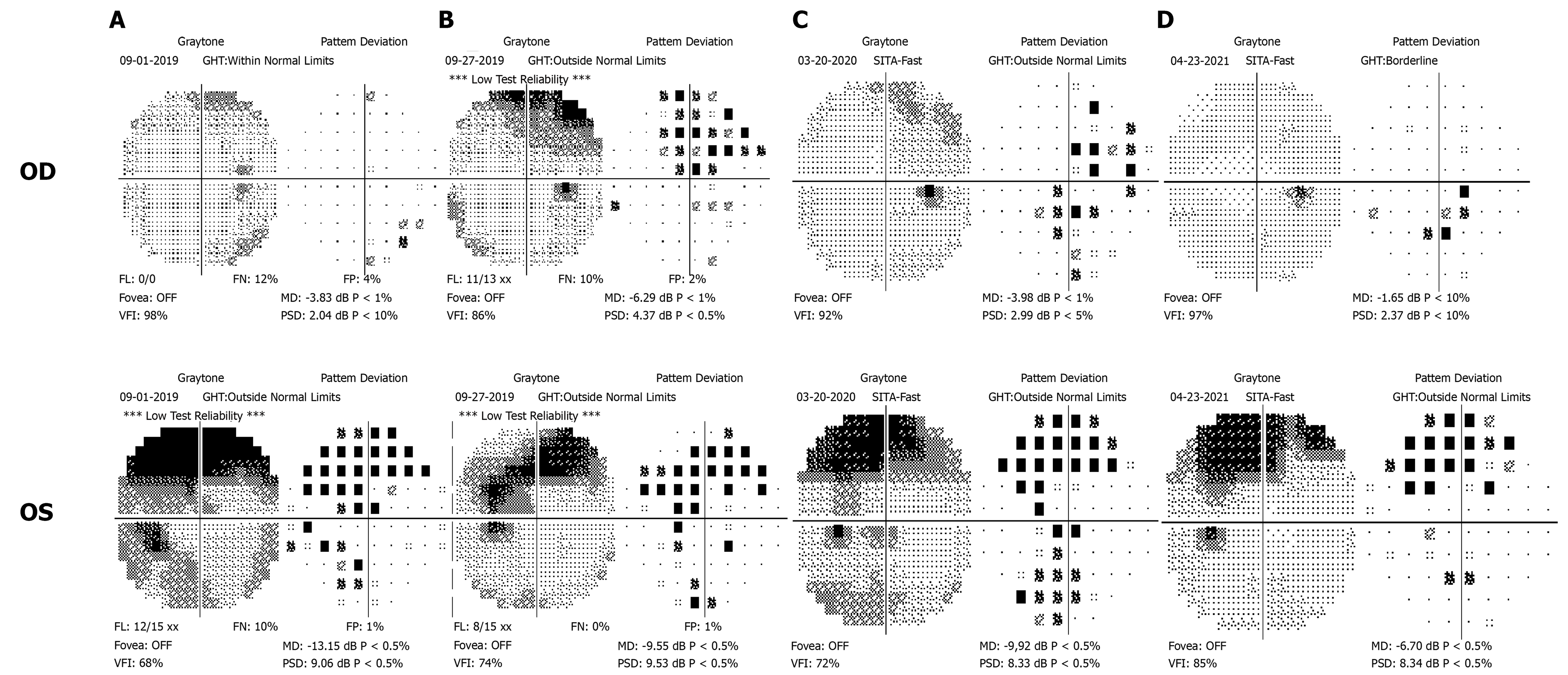
At the first visit, the best-corrected visual acuity was 20/20 in the right eye and 20/50 in the left eye. Color vision examination with Ishihara color plates revealed a difficulty in reading green color plates in the left eye. Pupillary reactions were normal with no relative afferent pupillary defect. Intraocular pressure was 15 and 14 mmHg with applanation tonometry. Slit-lamp microscopy of the bilateral anterior segments did not reveal any abnormality. Fundus examination showed normal appearance of the disk in both eyes (Figure 1). The Humphrey Field Analyzer (with SITA-FAST strategy and C30-2 program; Carl Zeiss Meditec, Dublin, CA, USA) was used for visual field examination. Concentric contraction was observed in the left eye, and there was no obvious visual field defect in the right eye (Figure 2).
The renal function was abnormal: urea nitrogen 26.51 mmol/L (normal range 3.1-8.8 mmol/L), creatinine 1123.9 μmol/L (normal range 44-133 μmol/L), uric acid 359 mmol/L (normal range 90-420 mmol/L).
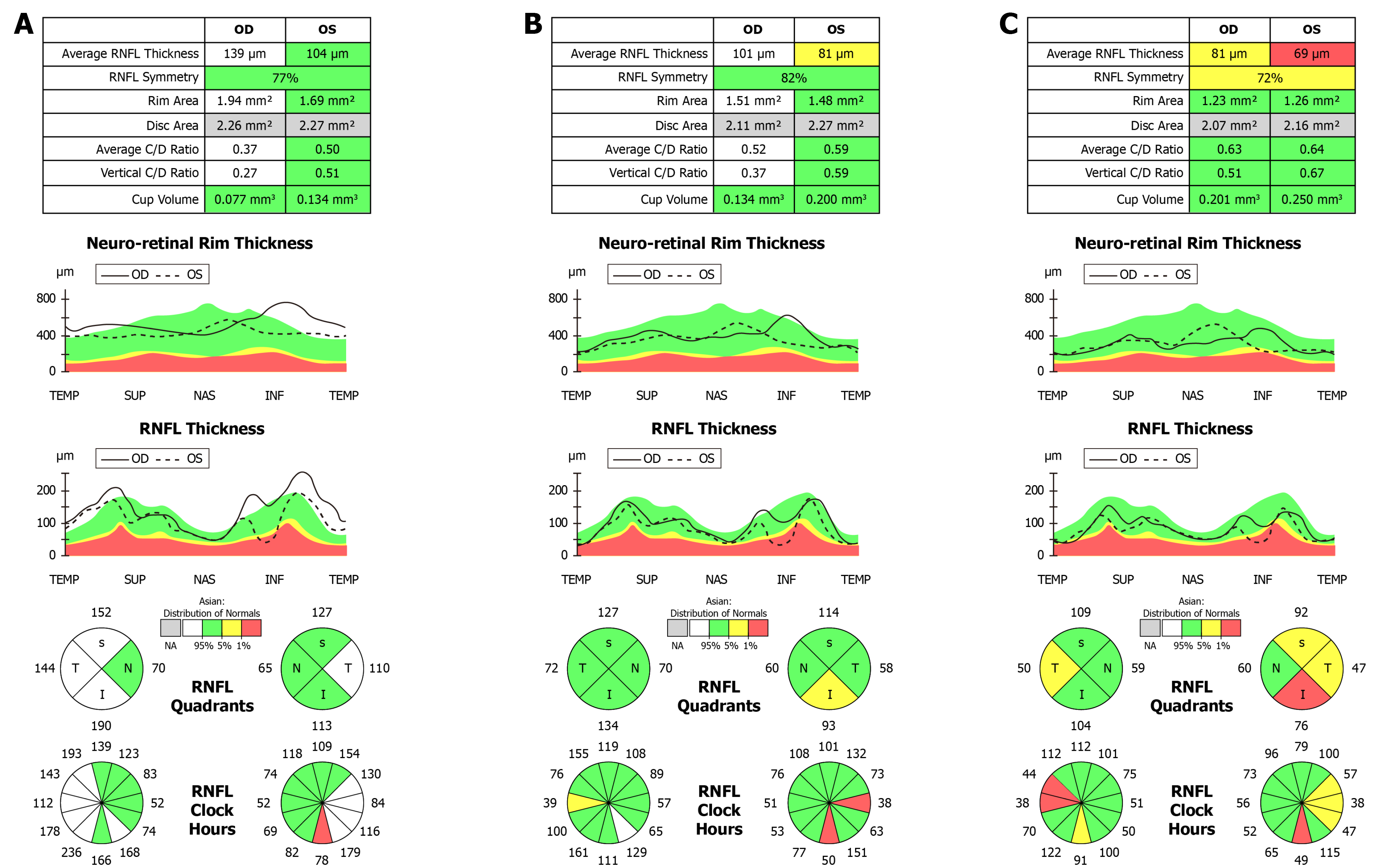
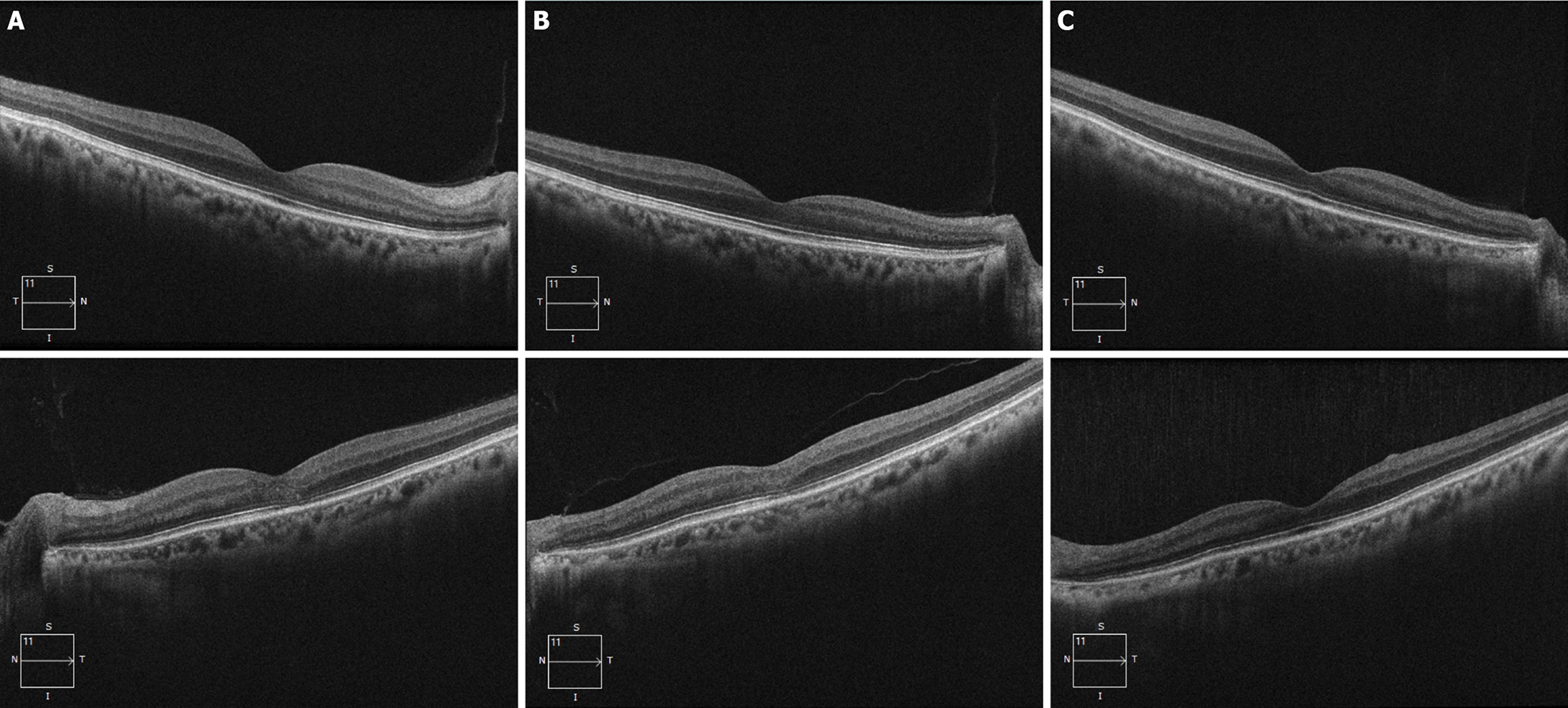
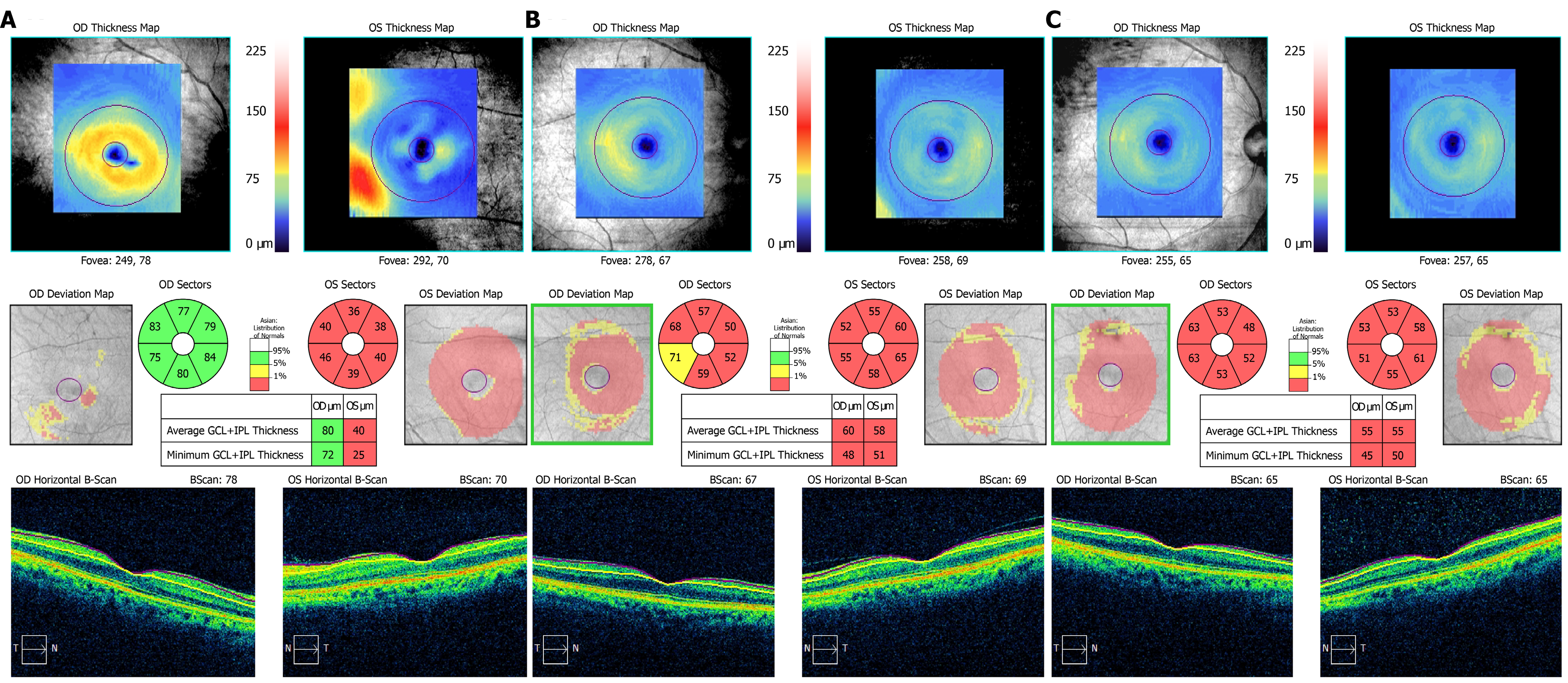
Cranial and orbit magnetic resonance examinations did not show any abnormal lesions. Cirrus high-definition optical coherence tomography (OCT) (Carl Zeiss Meditec) examination showed that, in the left eye, the peripapillary retinal nerve fiber layer (p-RNFL) slightly increased on the temporal side (Figure 3), and the thickness of the ganglion cell layer and inner plexiform layer (GCIPL) decreased (Figure 4). In the right eye, GCIPL was normal, but the thickness of p-RNFL increased on the inferior, superior and temporal sides. We found that the outer nuclear layer under the fovea and outer reflection bands representing the photoreceptor cells of the left eye were blurred (mainly the ellipsoid zone and intersection area), and the damaged inte
EON, based on ocular examination and clinical findings.
Treatment with ethambutol and isoniazid was immediately discontinued, and the patient received oral administration of vitamin B12, vitamin C, and mecobalamin for 6 mo.
At nearly 1 mo after discontinuation of ethambutol, the visual function in both eyes had deteriorated further. The best-corrected visual acuity was 20/50 in the right eye and 20/200 in the left eye. The color vision test revealed that both red and green were indistinguishable in the left and right eyes. At the third visit, 6 mo after discontinuation of ethambutol, the best-corrected visual acuity was 20/80 in the right eye and 20/200 in the left eye. Color vision examination remained red and green dyschromatopsia in both eyes. At the fourth visit, 19 mo after discontinuation of ethambutol, the best-corrected visual acuity improved to 20/20 in the right eye and 20/50 in the left eye. Color vision examination also showed recovery; only some pictures with green color were indistinguishable in both eyes.
The exact pathophysiological mechanism underlying EON is still unclear, although it may be caused by disrupted oxidative phosphorylation secondary to decreased available copper in the human mitochondria, or from inhibited lysosomal activation due to zinc chelation[4]. There have been studies stating that EON is a dose- and time-dependent adverse effect[5]. The frequency of visual impairment has been reported in 50% of patients at a dose of 60-100 mg/kg/d, 5%-6% at 25 mg/kg/d, and 1% at 15 mg/kg/d. Visual loss is typically insidious and symmetrical, occurring typically 2-8 mo after initiation of therapy[6]. However, a controlled study of 231 patients found that age > 65 years, hypertension, and kidney disease were also risk factors for the development of EON[2,7].
In > 60% of patients with EON, ocular examination reveals bilateral, painless and typically symmetric loss of visual acuity and abnormal color vision[8]. However, the onset may be unilateral, but eventually both eyes are involved[1]. Loss of color vision is typically reported for green and red, although blue–yellow color changes may also occur[9,10]. Initially, the optic disc may appear normal; however, as the disease progresses, it eventually develops into a pale optic disc[11,12]. Visual field test usually reveals central or paracentral scotoma and less commonly includes peripheral constriction, altitudinal field defects, and bilateral temporal field defects[13]. The diagnosis of EON is based on the identification of a toxic factor and exclusion of other pathologies exhibiting a similar clinical profile. Differential diagnoses include Leber’s hereditary optic neuropathy, dominantly inherited optic neuropathy, compressive or infiltrative lesion of optic chiasm, bilateral inflammatory or demyelinative optic neuropathy, maculopathies/macular dystrophy. Often, visual loss from EON can be regained after stopping the drug. The amount and time frame for visual recovery varies. If detected early and with prompt discontinuation of ethambutol, between 30% and 64% of patients show some improvement in their visual disturbances over a period of several months. However, even in patients who report improvement after therapy discontinuation, complete recovery is not always achieved[14]. Progressive worsening of vision after ethambutol discontinuation has also been documented[15].
In this report, the patient was referred to the ophthalmology department after 10 mo of antituberculosis therapy with a gradual painless loss of vision in the left eye for 5 mo. At the first visit, color visual dysfunction and visual field defects were detected only in the left eye. OCT examinations showed that the RNFL had a slight thickening and the thickness of GCIPL became thinner. Magnetic resonance imaging of the head and orbital optic nerves was normal. Although the present patient could not be checked for any mitochondrial DNA mutations, but combined with the patient’s medication history and clinical manifestations, we diagnosed EON. Although the patient was within the safe dose range, toxic optic neuropathy occurred and was attributed to renal dysfunction.
In the reported case, the eyes were asymmetric. The left eye had visual impairment 6 mo earlier than the right eye. At the second visit after nearly a month, the right eye showed the same visual dysfunction with a normal appearance of the optic disc. OCT examination of the right eye showed thinning of the GCIPL and mild thickening of the RNFL. The difference was that the macular high-definition scan of the left eye revealed structural damage to the outer nuclear layer, ellipsoid zone, and interdigitation zone mainly on the nasal side of the macula. After treatment, the macular lesions gradually disappeared, but there was no similar change in the right eye throughout follow-up.
OCT measurement of the RNFL are effective tools for the evaluation of optic neuropathies. Reports on the thickness of RNFL in EON are inconsistent[16-19]. These discrepancies may be attributed to different stages of the disease in the examined patients. Several studies have reported that the GCIPL was significantly thinner in patients with EON and suggested that, whether the p-RNFL was swollen or atrophic, loss of ganglion cells in the macular region had occurred[20-22]. As reported, retinal ganglion cells located in the papillomacular bundle have narrow caliber axons, rendering them even more susceptible to mitochondrial dysfunction, and contribute to RNFL atrophy on the temporal side and thinning of the GCIPL[20]. The RNFL and GCIPL changes in this patient were consistent with these reports.
The ellipsoid zone is composed of the inner section of the photoreceptors. The interdigitation zone is the chimera between the tip of the outer section of the photoreceptor and the microvilli on the top of the retinal pigment epithelial cells. Therefore, the macular lesion in the left eye of this patient was located in the outer layer of the macula, especially the photoreceptor layer. There is a rare disease named acute macular neuro-retinopathy (AMNR) that has similar structural manifestations on OCT examination. AMNR is a rare unilateral or bilateral macular disorder. OCT images showed focal abnormalities in the photoreceptor outer segments. The pathogenesis of the disease is still unclear. The main related factors reported so far include oral contraceptives, viral infections, adrenergic receptor agonists, trauma, and chronic kidney disease[23,24]. However, this patient does not completely rule out the presence of AMNR-like lesions in the left eye, but AMNR typically occurs in young women presenting with sudden onset of central scotomas[25]. They correspond to sharp reddish-brown areas in the macular region. These were inconsistent with the characteristics of our case.
Ethambutol poisoning causes mitochondrial dysfunction. Although the literature describes ethambutol toxicity mainly as a neuropathy, histopathological and electrophysiological evidence supports the involvement of different retinal cell layers[26]. In this regard, we consider that he had nephropathy and high blood pressure, which damaged the retinal microcirculation, resulting in insufficient blood supply to the outer layer of the retina, and damage to the outer layer of the retina. The damage to the macula may be an important factor influencing the recovery.
After withdrawal of ethambutol for as long as 19 mo, the visual function partly recovered. The thickness of the GCIPL and RNFL on the temporal side was apparently lower than normal. A previous study also reported that, even if a patient with EON can regain 1.0 visual acuity, their visual function may not recover completely[27]. Improvement in visual acuity as the nerve fiber layer progressively thinned suggests that, while some axons had irreversible damage and underwent apoptosis, the function of the remaining axons improved as the toxic effect of ethambutol waned. Presumably some axons, including those in the papillomacular bundle, did not reach a threshold for apoptosis and were able to survive and partly recover function.
There is currently no effective treatment for EON. Drug discontinuation is the only effective management that can halt the progression of visual loss and allow recovery of vision. Some authors recommend treating patients with 100-250 mg oral zinc sulfate three times per day. If vision does not improve at 10-15 wk after stopping ethambutol, parenteral administration of 40 mg/d hydroxycobalamine (vitamin B12) for 1-28 wk has been suggested[26].
EON can occur even in cases of low-dose ethambutol administration in patients with renal dysfunction. EON is most commonly characterized by bilateral symmetrical loss of vision but may also occur successively. This report highlights the need for identification of patients at risk, adjusting the dose regimen for impaired renal function, regular monitoring for early signs of ocular toxicity, and patient education.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bains L, Fernandez Escamez CS, Marques JH, Naswhan AJ, Salimi M S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Koul PA. Ocular toxicity with ethambutol therapy: Timely recaution. Lung India. 2015;32:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Chen SC, Lin MC, Sheu SJ. Incidence and prognostic factor of ethambutol-related optic neuropathy: 10-year experience in southern Taiwan. Kaohsiung J Med Sci. 2015;31:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Sadun AA, Wang MY. Ethambutol optic neuropathy: how we can prevent 100,000 new cases of blindness each year. J Neuroophthalmol. 2008;28:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 4. | Chamberlain PD, Sadaka A, Berry S, Lee AG. Ethambutol optic neuropathy. Curr Opin Ophthalmol. 2017;28:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (2)] |
| 5. | Yang HK, Park MJ, Lee JH, Lee CT, Park JS, Hwang JM. Incidence of toxic optic neuropathy with low-dose ethambutol. Int J Tuberc Lung Dis. 2016;20:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Wang MY, Sadun AA. Drug-related mitochondrial optic neuropathies. J Neuroophthalmol. 2013;33:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications: recognition and management. Drugs. 2007;67:75-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Lee EJ, Kim SJ, Choung HK, Kim JH, Yu YS. Incidence and clinical features of ethambutol-induced optic neuropathy in Korea. J Neuroophthalmol. 2008;28:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Ezer N, Benedetti A, Darvish-Zargar M, Menzies D. Incidence of ethambutol-related visual impairment during treatment of active tuberculosis. Int J Tuberc Lung Dis. 2013;17:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Woung LC, Jou JR, Liaw SL. Visual function in recovered ethambutol optic neuropathy. J Ocul Pharmacol Ther. 1995;11:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Polak BC, Leys M, van Lith GH. Blue-yellow colour vision changes as early symptoms of ethambutol oculotoxicity. Ophthalmologica. 1985;191:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Chai SJ, Foroozan R. Decreased retinal nerve fibre layer thickness detected by optical coherence tomography in patients with ethambutol-induced optic neuropathy. Br J Ophthalmol. 2007;91:895-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Kim U, Hwang JM. Early stage ethambutol optic neuropathy: retinal nerve fiber layer and optical coherence tomography. Eur J Ophthalmol. 2009;19:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Kho RC, Al-Obailan M, Arnold AC. Bitemporal visual field defects in ethambutol-induced optic neuropathy. J Neuroophthalmol. 2011;31:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Tsai RK, Lee YH. Reversibility of ethambutol optic neuropathy. J Ocul Pharmacol Ther. 1997;13:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Sivakumaran P, Harrison AC, Marschner J, Martin P. Ocular toxicity from ethambutol: a review of four cases and recommended precautions. N Z Med J. 1998;111:428-430. [PubMed] |
| 17. | Zoumalan CI, Agarwal M, Sadun AA. Optical coherence tomography can measure axonal loss in patients with ethambutol-induced optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2005;243:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Zoumalan CI, Sadun AA. Optical coherence tomography can monitor reversible nerve-fibre layer changes in a patient with ethambutol-induced optic neuropathy. Br J Ophthalmol. 2007;91:839-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Menon V, Jain D, Saxena R, Sood R. Prospective evaluation of visual function for early detection of ethambutol toxicity. Br J Ophthalmol. 2009;93:1251-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Kim YK, Hwang JM. Serial retinal nerve fiber layer changes in patients with toxic optic neuropathy associated with antituberculosis pharmacotherapy. J Ocul Pharmacol Ther. 2009;25:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Lee JY, Han J, Seo JG, Park KA, Oh SY. Diagnostic value of ganglion cell-inner plexiform layer for early detection of ethambutol-induced optic neuropathy. Br J Ophthalmol. 2019;103:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Lee JY, Choi JH, Park KA, Oh SY. Ganglion Cell Layer and Inner Plexiform Layer as Predictors of Vision Recovery in Ethambutol-Induced Optic Neuropathy: A Longitudinal OCT Analysis. Invest Ophthalmol Vis Sci. 2018;59:2104-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Peng CX, Zhang AD, Chen B, Yang BJ, Wang QH, Yang M, Wei SH. Macular thickness as a predictor of loss of visual sensitivity in ethambutol-induced optic neuropathy. Neural Regen Res. 2016;11:469-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Miller MH, Spalton DJ, Fitzke FW, Bird AC. Acute macular neuroretinopathy. Ophthalmology. 1989;96:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Li M, Zhang X, Ji Y, Ye B, Wen F. Acute Macular Neuroretinopathy in Dengue Fever: Short-term Prospectively Followed Up Case Series. JAMA Ophthalmol. 2015;133:1329-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Maschi C, Schneider-Lise B, Paoli V, Gastaud P. Acute macular neuroretinopathy: contribution of spectral-domain optical coherence tomography and multifocal ERG. Graefes Arch Clin Exp Ophthalmol. 2011;249:827-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Vistamehr S, Walsh TJ, Adelman RA. Ethambutol neuroretinopathy. Semin Ophthalmol. 2007;22:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |