Published online Jan 14, 2022. doi: 10.12998/wjcc.v10.i2.469
Peer-review started: August 26, 2021
First decision: September 29, 2021
Revised: October 8, 2021
Accepted: November 29, 2021
Article in press: November 29, 2021
Published online: January 14, 2022
Processing time: 138 Days and 16.9 Hours
A gastric stromal tumor (GST) is a mesenchymal tumor that occurs in the gastrointestinal tract; its biological characteristics are highly complex. Clinically, the severity of a GST is often evaluated by factors such as risk classification, tumor size, and mitotic figures. However, these indicators are not very accurate. Even patients classified as low risk are also at risk of metastasis and recurrence. Therefore, more accurate and objective clinical biological behavior evaluations are urgently needed.
To determine the relationship between Ki-67 and CD44 expression in GSTs and microvessel formation and prognosis.
Eighty-six GST tissue specimens from our hospital were selected for this study. The immunohistochemical staining technique was used to detect Ki-67, CD44, and microvessel density (MVD) in the collected samples to analyze the different risk grades and mitotic figures. In addition, this approach was used to determine the differences in the expression of Ki-67 and CD44 in GST tissues with varying lesion diameters.
In GSTs with positive expression of the Ki-67 protein, the proportions of patients with medium-to-high risk and more than five mitotic counts were 24.07% and 38.89%, respectively. In GSTs with positive expression of the CD44 protein, the proportions of patients with medium-to-high risk and more than five mitotic counts were 23.73% and 38.98%, respectively. In GSTs with negative expression of the Ki-67 protein, these values were relatively high (3.70% and 11.11%, respectively). The MVD in GSTs with positive and negative expression of the CD44 protein was 15.92 ± 2.94 and 13.86 ± 2.98/Hp, respectively; the difference between the two groups was significant (P < 0.05).
Ki-67 and CD44 expression in GSTs is correlated with the grade of tumor risk and mitotic figures. CD44 expression is correlated with microvessel formation in tumor tissues.
Core Tip: Microvascular plays a key role in the occurrence and development of gastric stromal tumor. Through this study, we reveal its role in tumor metastasis and invasion, and provide a basis for predicting the clinical prognosis of patients.
- Citation: Ma B, Huang XT, Zou GJ, Hou WY, Du XH. Relationship between Ki-67 and CD44 expression and microvascular formation in gastric stromal tumor tissues. World J Clin Cases 2022; 10(2): 469-476
- URL: https://www.wjgnet.com/2307-8960/full/v10/i2/469.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i2.469
A gastric stromal tumor (GST) is a type of gastrointestinal tumor. In recent years, the incidence of GSTs has been continuously increasing. Owing to the instability of their biological behavior, it is difficult to diagnose GSTs[1,2]. Immunohistochemical markers can help predict the prognosis and determine the risk of GSTs. CD44 has recently been found to be an important indicator, showing specificity in many tumors. However, its expression characteristics in GSTs remain controversial[3]. Ki-67, meanwhile, is involved in the process of cell proliferation and is highly expressed in breast cancer and neuroendocrine carcinoma[4]. The role of neovascularization in the biological process of tumorigenesis cannot be ignored. Many studies have shown that GSTs contain large amounts of pro-angiogenic factors[5], but few studies have addressed the relationship between GSTs and microvessel density (MVD). The microvasculature plays a key role in the occurrence and development of tumors. It can also induce and mediate the biological processes underlying tumorigenesis, such as participating in the processes of metastasis and tumor invasion. MVD is thus a representative quantitative indicator reflecting tumor vascular growth. It is relevant to tumor nutrition and oxygen supply[6]. This study explored the relationship between Ki-67 and CD44 expression in GST tissues and microangiogenesis.
Tissue specimens of 86 cases of GSTs that were surgically resected in our hospital from April 2016 to February 2019 were selected for this study. The inclusion criteria were as follows: (1) Updates and interpretations of the National Comprehensive Cancer Network Clinical Practice Guidelines (2019 6th version) on GSTs[7]; (2) Patients were examined prior to their operation via preoperative computer tomography and gastroscopy biopsies; and (3) Patients had no history of radiotherapy, chemotherapy, or immunological treatment before surgery. This study was approved by the Medical Ethics Committee, and all baseline data of patients were complete. The criteria for exclusion were as follows: (1) GSTs were accompanied by other types of tumor diseases; (2) Data were missing and unable to be included for statistical analysis; (3) Patients had local recurrence; or (4) Pathological examinations were lacking.
Eighty-six GST patients aged 41 to 79 years, with an average of 62.0 ± 6.8 years, were selected. There were 35 males and 51 females. The lesion sites were as follows: gastric antrum (12 cases), gastric body (19 cases), and gastric fundus (55 cases). Fifty-six cases had tumors with a diameter larger than 2.0 cm; 30 cases had a diameter equal to or less than 2.0 cm. There were 60 cases with mitotic counts equal to or less than five; 26 cases had more than five mitotic counts. The risk classifications were as follows: very low risk (32 cases), low risk (39 cases), and medium high risk (15 cases).
Paraffin sections (thickness of 4 μm) were prepared in a conventional manner. The sections were de-waxed with xylene and gradient alcohol (100%, 100%, 95%, 95%, 80%, and 70%) to water, stepwise. Distilled water was used to rinse the sections twice (3 min each time), and phosphate buffered saline (PBS) was used to rinse the sections three times (3 min each time). The samples were then rinsed with tap water and soaked in distilled water for storage. Subsequently, the sections were placed in 10 mmol of LPH6.0 citrate buffer for antigen repair. Next, the sections were rinsed in a gentle manner under running water to bring them to room temperature. Primary antibodies were added to the tissues, which were then incubated for 16 h on a shaking table at 4 ℃. After incubation, the tissues were rinsed three times with PBS (5 min each time). The primary antibodies were not added to the negative group, and only PBS was added. Then, the secondary antibodies were added and incubated for 30 min before rinsing, according to the aforementioned method. One drop of DAB was then added to each section to aid in color development, following which the sections were incubated at room temperature for 5 min. The sections were then re-stained with hematoxylin and immersed in 1% hydrochloric acid alcohol for 30 s, 1% ammonia alcohol for 45 s, and alcohol for 1 min. They were then transparentized with xylene and sealed with neutral gum.
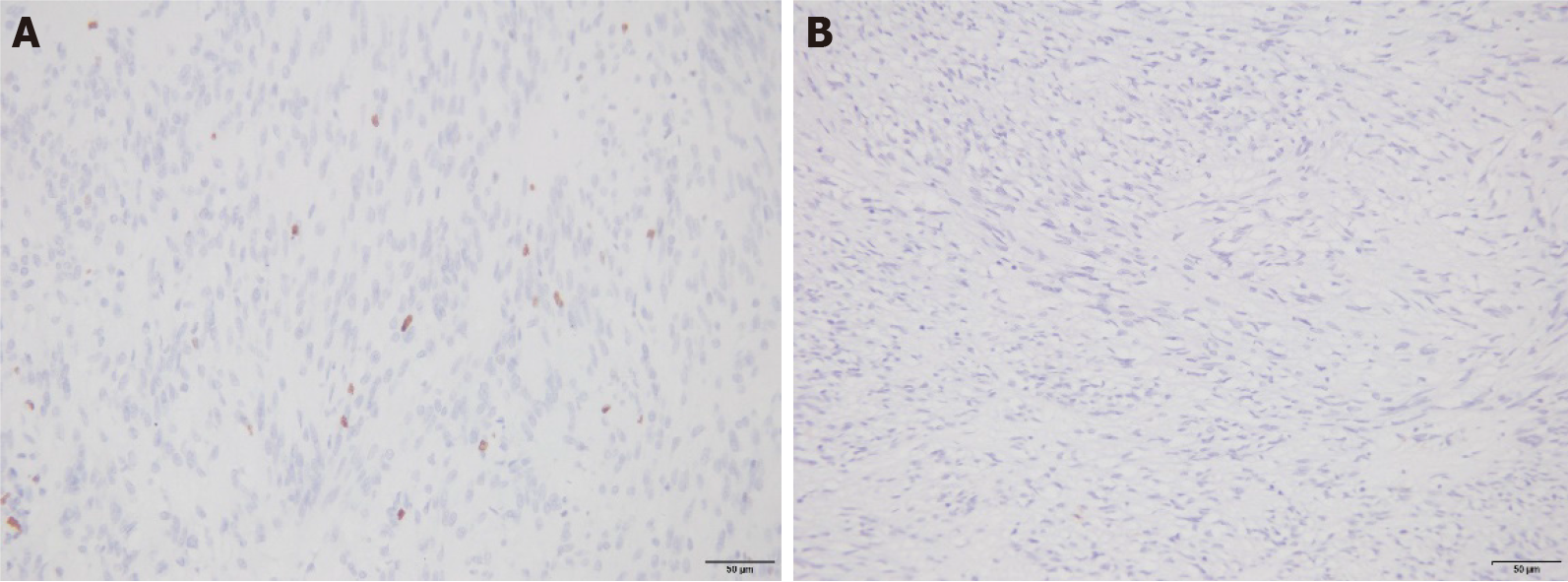
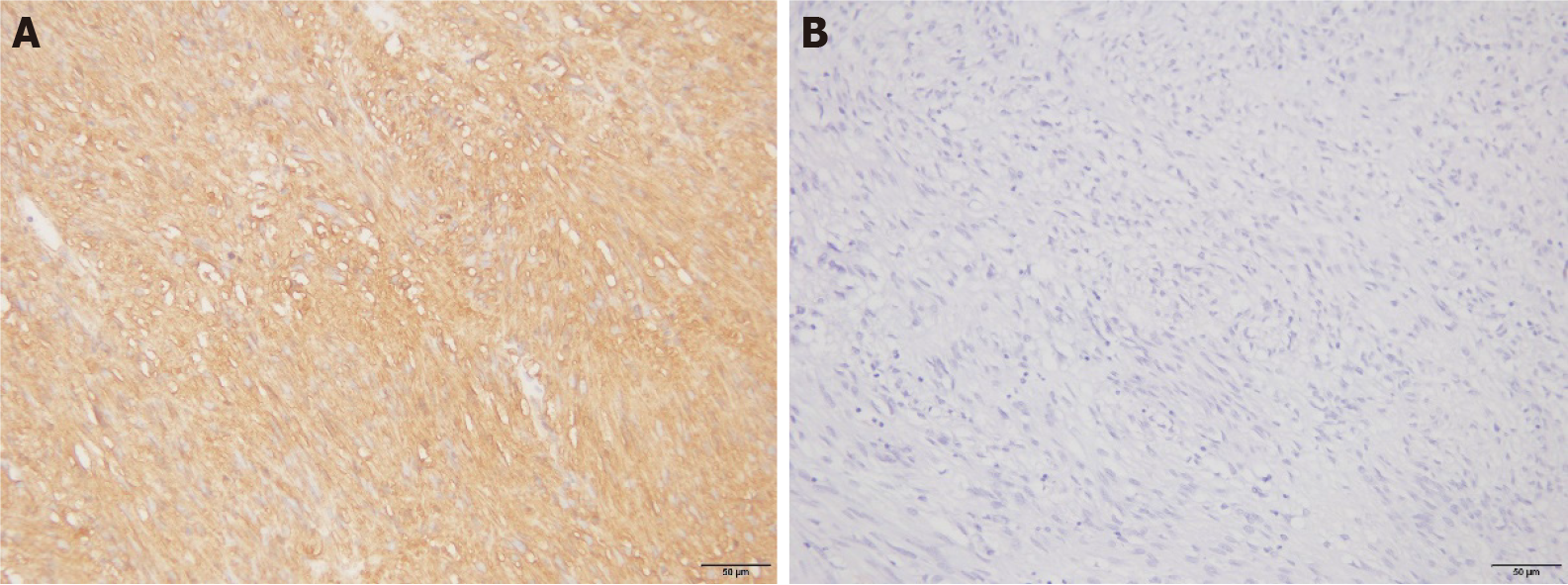
Positive staining of Ki-67 and CD44 proteins in the nucleus or cytoplasm is shown in yellow, brownish yellow, or brown: (1) According to the degree of staining, the results were categorized as follows: non-staining (0 points), only pale yellow staining (1 point), brownish yellow staining (2 points), and brown or black staining (3 points); and (2) According to the proportion of stained cells, the results were categorized as follows: equal to or less than 10% (one point), from 11% to 50% (two points), from 51% to 75% (three points), and more than 75% (four points). Products of staining degree and scores of positive cells that were less than three points were considered negative, whereas products that were equal to or greater than three points were considered positive.
The segments were reviewed by two experienced pathologists with a double-blind approach. First, high MVD regions in the tissues were identified using a low-power microscope. A high-power lens with a 200-fold microscope was then used to identify individual vascular endothelial cells with brown or tan staining. The numbers of stained microvessels were counted with a microscope at five different fold magnifications, and the average value was considered the MVD (microvessels exhibit significant differences in MVD from adjacent microvessels, tumor cells, or connective tissue components).
Statistical analysis was performed using SPSS 21.0 software. MVD in tissues with different Ki-67 and CD44 protein expression levels is presented as mean ± SD. The two groups were compared using independent sample t-tests. The positive expression rates of Ki-67 and CD44 proteins were evaluated using a χ2 test. The logistic regression model was used for multi-factor analysis. P < 0.05 was considered to represent a significant difference.
The percentages of patients with tumor risk grade (medium-to-high risk) and mitotic counts (> 5) in GSTs with positive expression of the Ki-67 protein were 24.07% and 38.89%, respectively. These values were higher than those of patients with negative expression of the Ki-67 protein (6.25% and 15.63%, respectively); the difference was significant (P < 0.05). There were no significant differences between the positive expression rates of the Ki-67 protein in GST tissues and different lesion diameters, ages, sexes, or lesion locations (P > 0.05; Table 1 and Figure 1).
| Index | Ki-67 Protein positive (n = 54) | Ki-67 Protein negative (n =32) | χ2 | P value |
| Age (yr) | 1.173 | 0.279 | ||
| ≥ 60 | 29 (53.70) | 21 (65.63) | ||
| < 60 | 25 (46.30) | 11 (34.38) | ||
| Gender | 0.844 | 0.358 | ||
| Male | 24 (44.44) | 11 (34.38) | ||
| Female | 30 (55.56) | 21 (65.63) | ||
| Lesion site | 1.250 | 0.535 | ||
| Gastric antrum | 7 (12.96) | 5 (15.63) | ||
| Body of stomach | 14 (25.93) | 5 (15.63) | ||
| Base of stomach | 33 (61.11) | 22 (68.75) | ||
| Risk classification | 7.380 | 0.025 | ||
| Very low risk | 15 (27.78) | 17 (53.13) | ||
| Low risk | 26 (48.15) | 13 (40.63) | ||
| Medium and high risk | 13 (24.07) | 2 (6.25) | ||
| Mitosis | 5.156 | 0.023 | ||
| ≤ 5 | 33 (61.11) | 27 (84.38) | ||
| > 5 | 21 (38.89) | 5 (15.63) | ||
| Lesion diameter (cm) | 1.764 | 0.184 | ||
| > 2.0 cm | 38 (70.37) | 18 (56.25) | ||
| ≤ 2.0 cm | 16 (29.63) | 14 (43.75) |
Patients classified as medium-to-high risk with CD44 protein-positive expression in GST and patients with more than five mitotic counts accounted for 23.73% and 38.98%, respectively. These values were higher than those of Ki-67-negative patients (3.70% and 11.11%, respectively); the difference was significant (P < 0.05). There were no significant differences between the positive expression rates of the CD44 protein in GST tissues and different lesion diameters, ages, sexes, or lesion locations (P > 0.05; Table 2 and Figure 2).
| Index | CD44 protein positive (n = 59) | CD44 protein negative (n = 27) | χ2 | P value |
| Age (yr) | 1.176 | 0.278 | ||
| ≥ 60 | 32 (54.24) | 18 (66.67) | ||
| < 60 | 27 (45.76) | 9 (33.33) | ||
| Gender | 0.884 | 0.347 | ||
| Male | 26 (44.07) | 9 (33.33) | ||
| Female | 33 (55.93) | 18 (66.67) | ||
| Lesion site | 1.283 | 0.256 | ||
| Gastric antrum | 7 (11.86) | 5 (18.52) | ||
| Body of stomach | 12 (20.34) | 7 (25.93) | ||
| Base of stomach | 40 (67.80) | 15 (55.56) | ||
| Risk classification | 6.534 | 0.038 | ||
| Very low risk | 18 (30.51) | 14 (51.85) | ||
| Low risk | 27 (45.76) | 12 (44.44) | ||
| Medium and high risk | 14 (23.73) | 1 (3.70) | ||
| Mitosis | 6.822 | 0.009 | ||
| ≤ 5 | 36 (61.02) | 24 (88.89) | ||
| > 5 | 23 (38.98) | 3 (11.11) | ||
| Lesion diameter (cm) | 1.584 | 0.208 | ||
| > 2.0 cm | 41 (69.49) | 15 (55.56) | ||
| ≤ 2.0 cm | 18 (30.51) | 12 (44.44) |
There was no significant difference in MVD between GST tissues with positive and negative expression of the Ki-67 protein (P > 0.05; Table 3). There was, however, a significant difference in MVD between GST tissues with positive and negative expression of the CD44 protein (P < 0.05; Table 3).
| n | MVD (/Hp) | t | P value | |
| Ki-67 expression | 0.889 | 0.377 | ||
| Positive | 54 | 15.41 ± 3.10 | ||
| Negative | 32 | 14.82 ± 2.75 | ||
| CD44 expression | 3.003 | 0.004 | ||
| Positive | 59 | 15.92 ± 2.94 | ||
| Negative | 27 | 13.86 ± 2.98 |
CD44, which is a transmembrane protein belonging to the cell adhesion molecule family, theoretically plays a certain role in tumor progression and metastasis[8,9]. A reduction in the expression level of CD44 would lead to poor adhesion between cells, making tumor cells more likely to shed and metastasize. However, studies have shown that the CD44 protein might play diverse and complex roles in the metastasis of different types of malignancies[10,11]. In this study, an immunohistochemical technique was used to detect the expression of CD44 in GSTs. It was found that the expression of CD44 was related to the risk grade and mitotic figures of GSTs, thus indicating that mitotic figures and the primary site could be independent prognostic factors[10-12]. The results of this study showed that high pathological risk grades, increased mitotic figures, and positive expression of the CD44 protein in patients with GSTs were independent risk factors for poor prognosis. CD44 could be involved in the angiogenic process in GSTs and mediate their recurrence or metastasis. Nonetheless, combining CD44 with tumor diameter and mitotic figures to more accurately evaluate and grade the risk of GSTs remains a challenge; future studies with larger sample sizes and longer follow-up times should be conducted to this effect.
Reportedly, high MVD in GSTs is related to risk classification, tumor size, and mitotic counts. MVD is an independent factor that affects the prognosis in patients[13-15]. The results of this study showed that there was a significant difference in MVD between tissues that were positive and negative for the CD44 protein. CD44 can promote tumor proliferation and further promote the generation of new blood vessels in tumor issues. However, new vascular basement membranes in tumors are not mature; their vascular walls are not closely arranged and are relatively loose. Thus, tumor cells can easily pass through these walls and enter the blood vessels, where they can diffuse. When the tumor spreads further, large numbers of blood vessels are further generated and MVD increases significantly. This, in turn, increases the opportunity for tumor cells to directly contact blood cells, thus promoting the infiltration and metastasis of the tumor cells. The increased expression level of CD44 provides sufficient blood supply and nutrition for angiogenesis and tumor cell proliferation. This study showed that the generation of microvessels in GSTs is relevant to the expression of CD44.
Some studies[9,16,17] have argued that increased Ki-67 expression level indicates that the tumor cells are growing rapidly, as Ki-67 can reflect the growth state of tumor cells. The results of the present study showed that there was a correlation between Ki-67 and the mitotic count. The mitotic count only reflects the M phase of cell proliferation, whereas Ki-67 is expressed in the G1, S, G2, and M phases of cell proliferation[18,19]. Currently, the standard of Ki-67 expression in GSTs is unclear. This is likely because Ki-67 expression is only considered a marker of tumor proliferation from quantitative to qualitative change. In addition, the present study found that Ki-67 is more reliable than tumor size in predicting tumor risk classification and different mitotic counts.
We found that there was no significant difference between the level of MVD in GST tissues and negative Ki-67 protein expression groups. There was no correlation between the formation of microvessels in GST tissues and the expression of the Ki-67 protein, but this lack of correlation might have been due to the limitation of the small sample size. Although Ki-67 expression was found to be irrelevant to MVD in GST tissues, it might be a candidate indicator for the prognostic evaluation of GSTs because of its association with tumor risk grade and mitotic counts.
CD44 expression provides a certain clinical reference value for the prognoses of GSTs. Reducing MVD and inhibiting CD44 expression could suppress angiogenesis in GSTs and provide new targets for their treatment. However, the specific mechanisms need to be studied further[20].
To date, few reports have examined the relationship between Ki-67 and CD44 protein expression and the GST risk grade, as well as the changes in mitotic counts. Therefore, it is of certain significance to elucidate the mechanisms by which Ki-67 and CD44 play a role in tumorigenesis. Although there have been various speculations regarding the mechanisms of the two genes, the synergistic effects and mechanisms thereof, as well as their expression products, in the occurrence and development of GSTs remain unclear.
In summary, the expression of Ki-67 and CD44 in GSTs has certain relationships with the tumor risk grade and mitotic changes. The expression of CD44 is related to microvessel formation in tumor tissues and the prognosis in patients with GSTs.
The incidence of gastric stromal tumors (GSTs) is increasing. The severity of a GST is often evaluated by factors such as risk classification, tumor size, and mitotic figures. However, these indicators are not very accurate.
Few studies have addressed the relationship between GSTs and microvessel density.
In this study, the authors aimed to explore the relationship between Ki-67 and CD44 expression in GST tissues and microangiogenesis.
Tissue specimens of 86 cases of GSTs were selected for this study. All cases met the inclusion and exclusion criteria.
High pathological risk grades, increased mitotic figures, and positive expression of the CD44 protein in patients with GSTs were independent risk factors for poor prognosis.
The expression of Ki-67 and CD44 in GSTs has certain relationships with the tumor risk grade and mitotic changes.
A deeper study with a larger sample size is needed to confirm this finding.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katagiri R, Sapio L S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (9)] |
| 2. | Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 3. | Montgomery E, Abraham SC, Fisher C, Deasel MR, Amr SS, Sheikh SS, House M, Lilliemoe K, Choti M, Brock M, Ephron DT, Zahuruk M, Chadburn A. CD44 loss in gastric stromal tumors as a prognostic marker. Am J Surg Pathol. 2004;28:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Miki Y, Kawamura T, Sugisawa N, Makuuchi R, Nakajima T, Tokunaga M, Tanizawa Y, Bando E, Terashima M. Risk Classification using the Ki-67 Labeling Index for Surgically-Treated Gastric Gastrointestinal Stromal Tumors. Hepatogastroenterology. 2015;62:919-923. [PubMed] |
| 5. | Sammarco G, Varricchi G, Ferraro V, Ammendola M, De Fazio M, Altomare DF, Luposella M, Maltese L, Currò G, Marone G, Ranieri G, Memeo R. Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 6. | Suzuki S, Dobashi Y, Hatakeyama Y, Tajiri R, Fujimura T, Heldin CH, Ooi A. Clinicopathological significance of platelet-derived growth factor (PDGF)-B and vascular endothelial growth factor-A expression, PDGF receptor-β phosphorylation, and microvessel density in gastric cancer. BMC Cancer. 2010;10:659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Zhao WY, Zhao G, Wang M. [Updates and interpretations of the NCCN Clinical Practice Guidelines (2019 6th version) on gastrointestinal stromal tumor]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Lourenço BN, Springer NL, Ferreira D, Oliveira C, Granja PL, Fischbach C. CD44v6 increases gastric cancer malignant phenotype by modulating adipose stromal cell-mediated ECM remodeling. Integr Biol (Camb). 2018;10:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Liang YM, Li XH, Li WM, Lu YY. Prognostic significance of PTEN, Ki-67 and CD44s expression patterns in gastrointestinal stromal tumors. World J Gastroenterol. 2012;18:1664-1671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Wu G, Song X, Liu J, Li S, Gao W, Qiu M, Yang C, Ma Y, Chen Y. Expression of CD44 and the survival in glioma: a meta-analysis. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Prochazka L, Tesarik R, Turanek J. Regulation of alternative splicing of CD44 in cancer. Cell Signal. 2014;26:2234-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Heldin P, Kolliopoulos C, Lin CY, Heldin CH. Involvement of hyaluronan and CD44 in cancer and viral infections. Cell Signal. 2020;65:109427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Chen WT, Huang CJ, Wu MT, Yang SF, Su YC, Chai CY. Hypoxia-inducible factor-1alpha is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol. 2005;35:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S. Urokinase-type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci. 2003;94:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Kawada M, Seno H, Wada M, Suzuki K, Kanda N, Kayahara T, Fukui H, Sawada M, Kajiyama T, Sakai M, Chiba T. Cyclooxygenase-2 expression and angiogenesis in gastric hyperplastic polyp--association with polyp size. Digestion. 2003;67:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Liang YM, Li XH, Chen W. [Roles of risk assessment and Ki-67 index in judging prognostic of gastrointestinal stromal tumors]. Zhonghua Yi Xue Za Zhi. 2008;88:1041-1045. [PubMed] |
| 17. | Chen XS, Shan YC, Dong SY, Wang WT, Yang YT, Liu LH, Xu ZH, Zeng MS, Rao SX. Utility of preoperative computed tomography features in predicting the Ki-67 labeling index of gastric gastrointestinal stromal tumors. Eur J Radiol. 2021;142:109840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Miller I, Min M, Yang C, Tian C, Gookin S, Carter D, Spencer SL. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018;24:1105-1112.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 418] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 19. | Varga Z, Diebold J, Dommann-Scherrer C, Frick H, Kaup D, Noske A, Obermann E, Ohlschlegel C, Padberg B, Rakozy C, Sancho Oliver S, Schobinger-Clement S, Schreiber-Facklam H, Singer G, Tapia C, Wagner U, Mastropasqua MG, Viale G, Lehr HA. How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? PLoS One. 2012;7:e37379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Nishimura S, Chung YS, Yashiro M, Inoue T, Sowa M. CD44H plays an important role in peritoneal dissemination of scirrhous gastric cancer cells. Jpn J Cancer Res. 1996;87:1235-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |