Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5884
Peer-review started: December 31, 2021
First decision: February 21, 2022
Revised: March 15, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: June 16, 2022
Processing time: 160 Days and 4.4 Hours
Small cell carcinoma (SCC) is a malignant tumour that is frequently accompanied by extensive metastasis. Primary renal SCC has typical characteristics related to SCC and is extremely rare, with no uniform treatment standard. Clinical treatment is mainly based on the literature. Here we report the diagnosis and treatment of an interesting case of primary renal SCC.
We report a tortuous course of treatment for a 68-year-old man. Four years before diagnosis, the patient developed continuous gross haematuria, during which he underwent several ureteral biopsies, ureteral stricture relief, and urine exfoliated cell examinations; however, SCC was not confirmed. One month before radical resection of the renal pelvic carcinoma, the severe haematuria recurred. Computed tomography revealed transitional cell carcinoma in the right kidney and right upper ureter. A preoperative examination exluded the possibility of a pulmonary origin of the tumour, and primary renal SCC was diagnosed. The postoperative pathology findings were suggestive of SCC. The patient was treated with combined chemotherapy but died of tumour progression at 7 mo postoperative.
Our patient's disease onset in the context of a succession of regular testing and the fact that it occurred so quickly with perirenal encroachment immediately after diagnosis reveals the cruel and unforgiving side of the disease. Furthermore, patients with poor comprehensive treatment results require new treatment regimens.
Core Tip: Our patient's onset in the context of a succession of regular testing, and the fact that it occurred so quickly, with perirenal encroachment immediately after diagnosis, reveals the cruel and cunning side of the disease. Futhermore, patients with poor comprehensive treatment results, proving the need to develop new treatment regimens.
- Citation: Xie K, Li XY, Liao BJ, Wu SC, Chen WM. Primary renal small cell carcinoma: A case report. World J Clin Cases 2022; 10(17): 5884-5892
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5884.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5884
Small cell carcinoma (SCC) usually arises from the lungs, and extrapulmonary SCC accounts for 2.5%-5% of all SCC cases[1]. SCC occurs in the urinary system is extremely rare, and most commonly occurs in the bladder[2]. Primary renal SCC is even rarer. The rarity, aggressiveness, and poor prognosis of these tumours adds to the seriousness of the disease[3]. The overall survival rate of renal SCC is worse than that of pulmonary SCC[4,5]. Given the very aggressive behaviour of renal SCC, standard treatments are required[5]. Here we report a case of primary renal SCC and discuss its rapid clinical progression, treatment, and long-term effects.
Right low back pain, abdominal distension and repeated gross hematuria for 2 wk.
The patient was a 68-year-old man admitted to The First Affiliated Hospital of Nanchang University on September 20, 2020 with a 2-wk history of right waist pain and abdominal distension with repeated gross haematuria. No special observation was noted during physical examination.
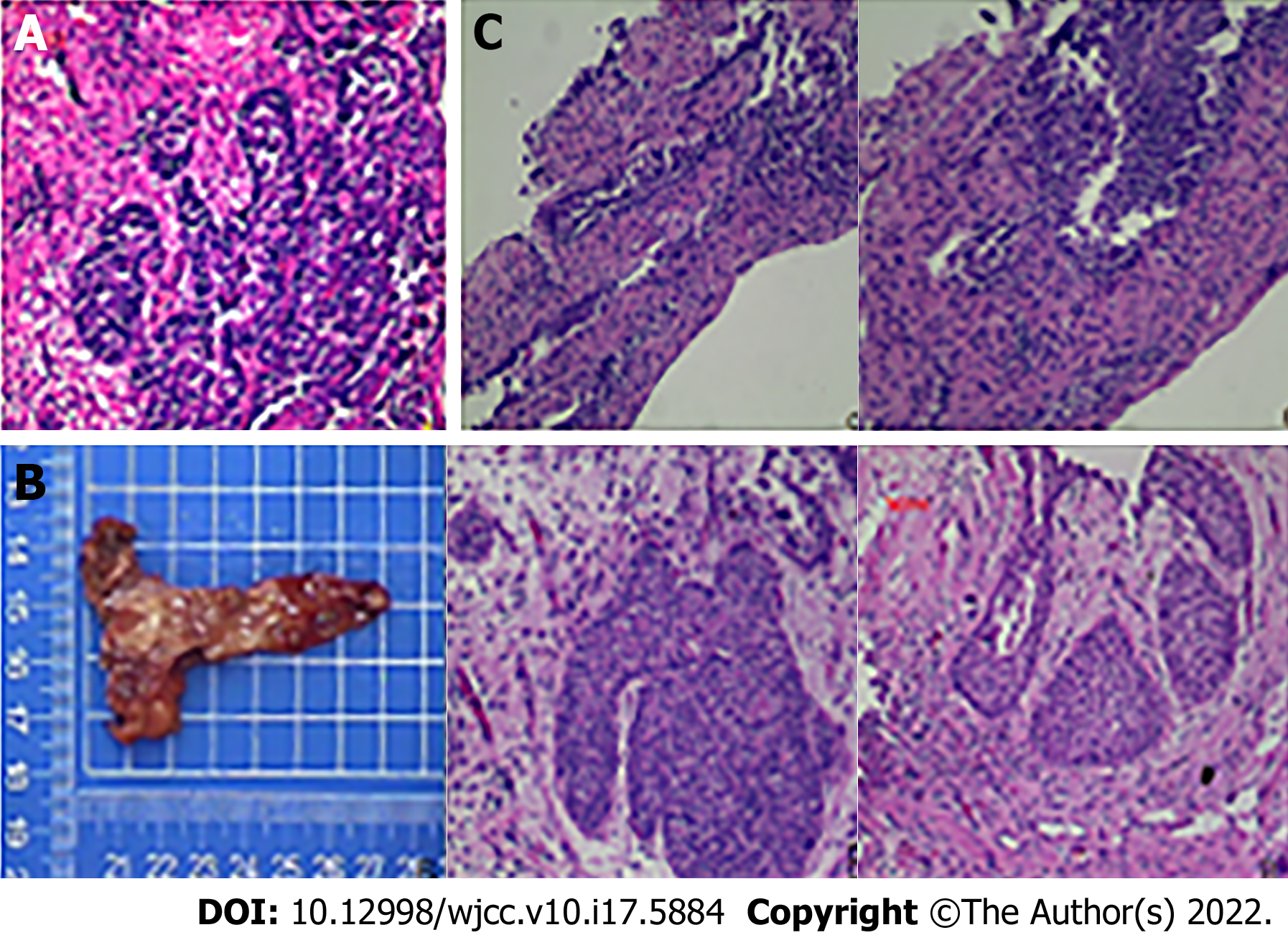
In August 2016, the patient was admitted to our hospital with repeated gross haematuria. Computed tomography (CT) showed thickening of the wall of the lower part of the right ureter suggestive of ureteral cancer, hydronephrosis of the right kidney and upper and middle ureteral segments, and right renal insufficiency. After consultation with the patient and his dependents, we decided to perform a ureteral tumour biopsy, and postoperative pathology exhibited a limited lower segment of the right ureter, suspected to be cancerous. Immunohistochemistry showed CD20 (+ mainly umbrella cells, focal whole layer +), Ki-67 (+ mainly basal cells), and p53 (-) (Figure 1A). We recommended a radical ureterectomy for this cancer; however, the patient and his family refused, and he agreed to undergo ureteral bladder replantation to treat distal ureteral strictures (right) 1 wk after the biopsy (right). Intraoperative frozen pathology and postoperative pathology of the vesicoureteral junction revealed chronic mucositis and mild atypical hyperplasia of the local urothelium (Figure 1B).
The patient’s postoperative recovery was good, but irregular gross haematuria was observed during the follow-up period. Re-examination with CT in our hospital in January 2017, March 2019, and September 2019 showed postoperative changes in the lower segment of the right ureter and slight hydronephrosis in the right kidney and upper ureteral segment. A ureteral biopsy was performed again in 2019 for gross haematuria, and the postoperative pathology findings were consistent with the morphological manifestation of a right ureteral polyp (Figure 1C). Urine exfoliative cytology was performed in August 2016 and March 2019, but the results were negative. The remainder of this paper is nothing special.
The patient had no relevant medical history.
The patient had no relevant personal or family history.
The patient’s vital signs were normal. There is percussion pain in the right renal area, normal on the left side.
The patient’s creatinine level was 114.1 μmol/L and urea nitrogen level was 4.9 mmol/L. The glomerular filtration rate (GFR) of the left kidney was 31.88 mL/min, while that of the right kidney was 14.38 mL/min. The remaining participants were not special.
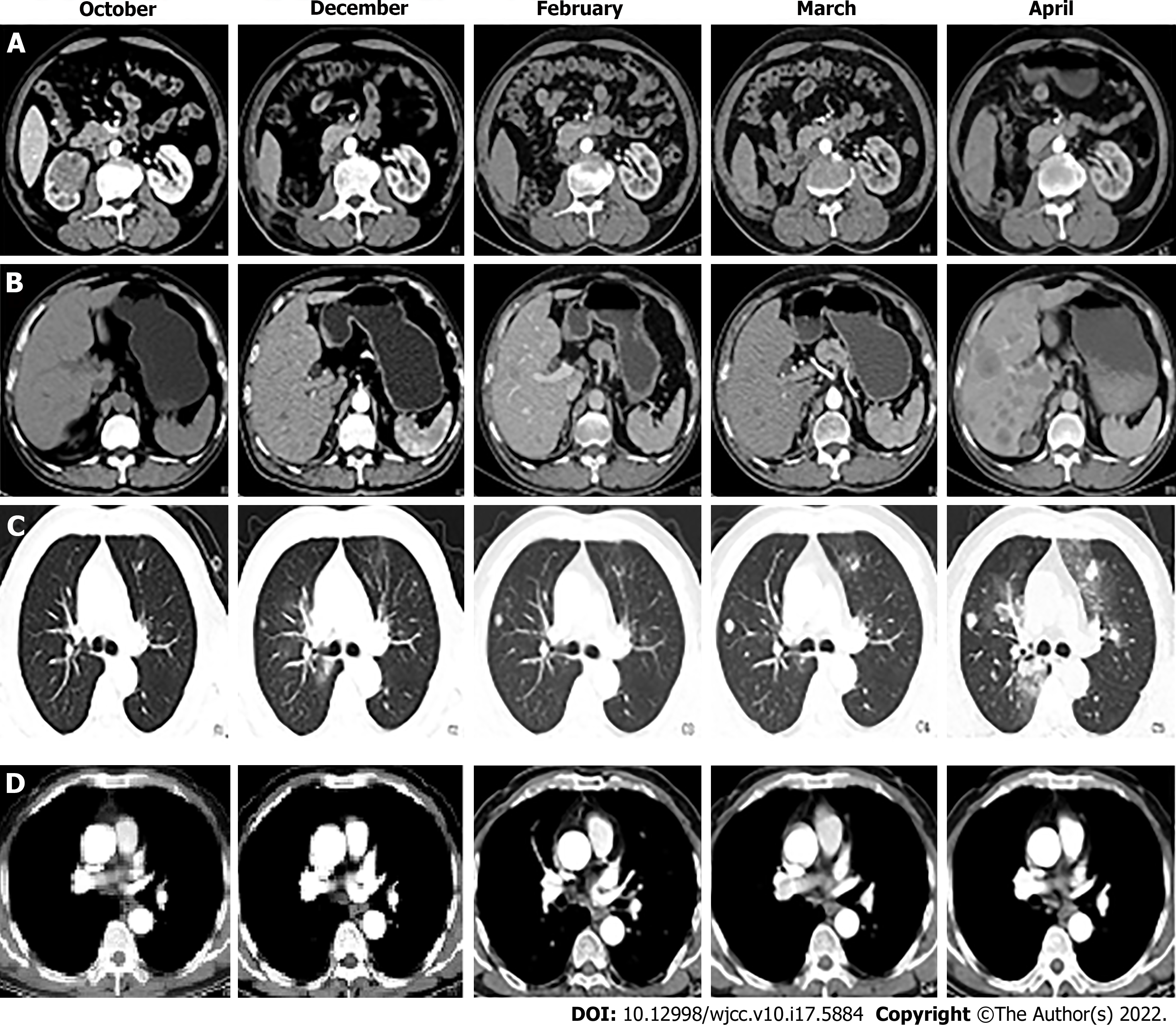
B-mode ultrasound showed the following: (1) Hydronephrosis of the right kidney suggestive of possible middle and lower ureteral obstructions; and (2) Benign prostatic hyperplasia with calcification. CT showed the following: (1) A soft-tissue tumour of the right kidney and right upper ureter segment suggestive of transitional cell carcinoma in addition to multiple enlarged lymph nodes in the right renal hilum suggestive of metastasis; (2) Blood perfusion and excretion function of the right kidney were significantly decreased; (3) Postoperative changes in the right ureter. The wall of the lower part of the right ureter near the entrance of the bladder was slightly thickened and enhanced. Therefore, an endoscopic examination was recommended; and (4) The presence of multiple nodes in both lungs suggested the possibility of metastasis (Figure 2).
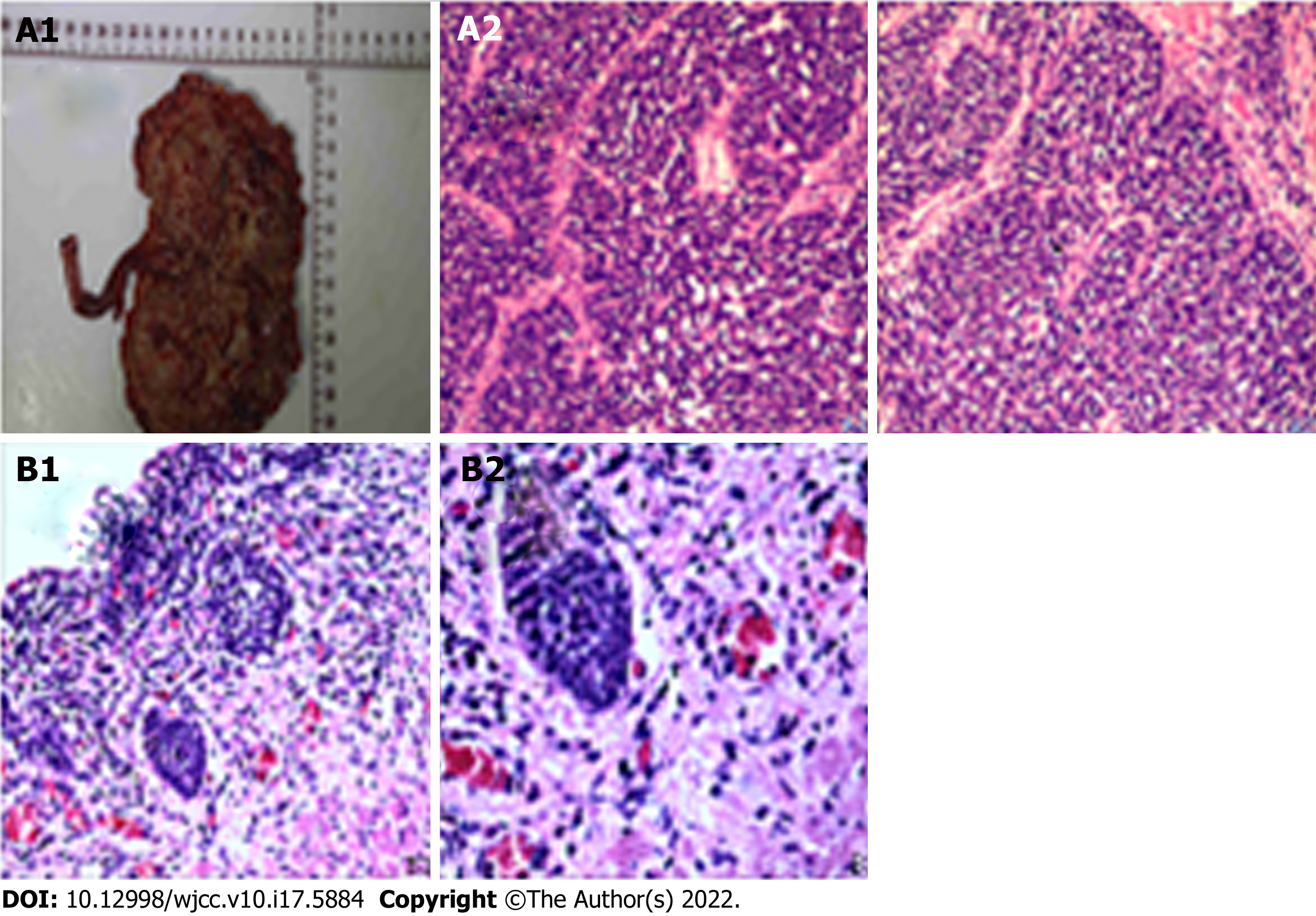
The postoperative pathology showed 60% high-grade invasive papillary urothelial carcinoma with 40% SCC in the right kidney, nervous invasion, visible tumour thrombus in vessels, and invasion of the renal parenchyma. No cancer was noted in the ureteral stump or perirenal fat. Immunohistochemistry showed GATA-3 (nest group +, flake -); CK7 (nest group +, flake -); p63 (nest group +, flake -); CGA (nest group -, flake +); syn (nest group -, flake +); CD56 (nest group -, flake +); CK20 (-); NSE (-); Ki-67 (nest group 60% +, flake 90% +); and CK (nest group strong +, flake weak +). Microscopic examination of the right lower ureter revealed lumen dilation, a partial epithelial defect, partial coverage with urothelium, and severe mechanical injury to the focal epithelial cells affecting the observation. There were two right renal pedicle lymph nodes, but no cancer metastasis was found (0 take 2). Primary renal SCC of the right kidney was also considered (Figure 3).
The preoperative diagnosis was renal pelvic carcinoma, and Laparoscopic radical resection of the tumour was performed in October 2020.
Combined with the relevant guidelines and literature recommendations, we recommended that the patient start GP chemotherapy 1 mo after surgery, such as gemcitabine 1000 mg/m2, D1 and D8 intravenous drip, and carboplatin 80 mg/m2 D1-3 intravenous drip as a 21-d cycle. The dosage would be adjusted after each cycle according to the change in the patient's body surface area. Moreover, a regular monthly review of CT scans enables observation of the changes in the disease (in January, CT examinations were not performed due to the serious epidemic situation of the novel coronavirus, but chemotherapy was still performed on schedule). CT showed tumour metastasis in the lungs and liver in December, and the mediastinum seemed to occupy space. From December to February, the space occupation of the lung seemed to improve, while the liver metastasis became increasingly serious and there was no significant change in the mediastinum. The examination in March showed that the occupation of the lung, liver, and mediastinum had increased, and there was a new mass in the right kidney area of the original operation. In April, metastatic tumours developed rapidly (Figure 2). Other than fatigue and emaciation, the patient did not experience any other discomfort and the chemotherapy drugs were not rejected. During this period, we suggested to the patient and his family that we change the chemotherapy method and add radiotherapy, immunotherapy, and other therapies according to the changes in his condition; however, the idea was rejected and he continued the original treatment plan. When the six cycles of chemotherapy were completed, we suggest that he receive further treatment, but he and his family refused. On May 5, he died of multiple organ failure.
The incidence of renal SCC is low, and studies and reports worldwide are rare[6]. We incompletely counted 92 globally published cases of renal SCC from 1984 to 2022 and summarized their clinical characteristics and treatment options (Table 1). The common clinical manifestations of renal SCC are not significantly different from those of other renal parenchymal malignant tumours and include low back pain, haematuria, abdominal mass, abdominal discomfort, weight loss, and swelling of lymph nodes on the body surface[7]. In addition, it is unrelated to paraneoplastic syndromes[8]. To date, only one case was reportedly associated with a syndrome of inappropriate antidiuretic hormone secretion[5].
| Features | Classifications | Cases (%) |
| Age (yr) | ≤ 55 | 38 (41.3) |
| > 55 | 54 (58.7) | |
| Gender | Male | 50 (54.3) |
| Female | 42 (45.7) | |
| Clinical presentation | Flank/abdominal pain | 55 (60.0) |
| Hematuria | 32 (34.7) | |
| Lump | 11 (11.9) | |
| Neurological symptoms | 5 (5.4) | |
| Other nonspecific symptoms | 7 (7.6) | |
| Size (cm) | ≤ 10 | 43 (58.9) |
| > 10 | 30 (41.1) | |
| Affected side | Right | 40 (48.1) |
| Left | 43 (51.9) | |
| pT stage | T1-T2 | 24 (27.6) |
| T3-T4 | 63 (72.4) | |
| Renal vein tumor thrombus | Yes | 16 (34.8) |
| No | 30 (65.2) | |
| Lymph node metastasis | Yes | 41 (50.0) |
| No | 39 (50.0) | |
| Distant metastasis | Yes | 23 (28.3) |
| No | 58 (71.7) | |
| Surgery | Yes | 74 (81.3) |
| No | 17 (19.8) | |
| Chemotherapy | Yes | 51 (68.0) |
| No | 24 (32.0) | |
| Cisplatin | 29 (56.9) | |
| Other | 22 (43.1) |
The diagnosis of renal SCC relies mainly on histopathological and immunohistochemical findings[9]. Renal SCC is easily misdiagnosed as other small cell tumours, such as undifferentiated carcinoma, Ewing’s sarcoma, embryonal rhabdomyosarcoma, lymphoma, and primitive neuroectodermal tumour[4]. Immunohistochemical staining and electron microscopic examinations are helpful for the identification. Besides, in the diagnosis of renal SCC, metastatic SCC, especially those originating from the lung, should be excluded[10]. The diagnosis of renal SCC must be based on the patient's clinical history and chest imaging findings. If the patient has a history of pulmonary SCC or the chest imaging examination shows lung neoplastic lesions, renal metastasis of pulmonary SCC should be considered first; however, if urothelial carcinoma is mixed with SCC, the diagnosis of primary renal SCC should be supported[11].
Histologically, renal SCC is similar to other types of SCC and is mostly mixed with other types, including urothelial carcinoma, squamous cell carcinoma, and adenocarcinoma[12]. Under a light microscope, the tumour tissue shows a solid flake or nest-like arrangement with extensive necrosis. The tumour cells are small, similar to lymphocytes, with rare cytoplasm, deep nuclear staining, unclear nucleoli, and frequent mitotic figures[4]. Immunohistochemistry can express specific neuroendocrine markers such as NSE, CD56, Syn, and CgA[13]. Among them, the Ki-67 score seems a better predictor of survival than the degree of differentiation[14]. Serum NSE levels are potentially useful in early diagnosis and treatment monitoring during chemotherapy[15], neural cell adhesion molecule (NCAM or CD56) is the most sensitive neuroendocrine marker, and chromogranin A, a protein found in neurosecretory granules, is the most specific marker[16].
Owing to the small number of primary renal SCC cases, standard treatment guidelines are lacking. Surgery and chemotherapy are currently the most widely used treatment options. Studies have found that targeted drug therapy combined with radical surgery has significant survival benefits compared to simple radical surgery, while radiotherapy is mostly used for postoperative residual lesions or distant metastases[4]. The targeted drug sunitinib is recommended for the treatment of advanced non-clear cell carcinoma[17]. The Department of Urology, Beijing Friendship Hospital Affiliated to Capital Medical University, diagnosed and treated one patient with renal cell carcinoma. The lymph nodes were fused, and the disease entered partial remission 3 mo after sunitinib treatment at 1 mo after surgery; the disease then progressed at 13 mo and the patient died of tumour metastasis after 24 mo. Patient survival was significantly prolonged after surgical resection of the affected kidney and postoperative adjuvant targeted therapy[18]. Although this patient benefited from targeted therapy, the maintenance time was short, and the late-stage treatment effect of this type of tumour requires verification in a large sample of cases.
Some scholars have proposed that simple chemotherapy has a better prognosis than surgery combined with chemotherapy, suggesting that chemotherapy should be the first choice and surgery should only be used to treat local symptoms[19]. Other scholars have proposed that, for patients whose tumours are confined to the kidney, early surgical treatment can enable long-term survival, and the prognosis of patients with clinical stage pT1-pT2 is significantly better than that of patients with pT3-pT4 disease, with a median survival time of 31 and 8 mo, respectively[20]. In a study of 14 cases of renal SCC, Si et al[21] found that one patient with SCC limited to the kidney survived tumour-free after surgery for 137 mo. However, a recent study reported no significant difference in estimated median survival across individual treatment modalities. Multimodal therapies likely merit particular investigative attention in terms of growing evidence supporting their use in treating other primary small cell malignancies of the genitourinary tract[22].
In this case, the patient's condition changed rapidly and distant metastasis occurred within 1 year. When the disease was diagnosed, the patient was already in the late stage and had missed the opportunity for early radiotherapy and chemotherapy. It is difficult to obtain suitable specimens for relevant pathological examinations without surgery, such as when the patient's urine exfoliated cells are negative. In addition, some studies reported that the early application of platinum-based chemotherapy can improve the survival rate, and patients who received platinum-based regimens had a median survival of 20 mo vs 8 mo for those who received other regimens[23]. Our patient showed an improving trend with the platinum-based chemotherapy regimen. Patrick also reported an 80-mo survival of a patient who underwent nephroureterectomy plus multiple metastasectomies followed by chemotherapy with octreotide, temozolomide, and capecitabine[13]. This is the first report of the use of a somatostatin analogue in the management of primary upper urinary tract SCC. Having no fairly large series capable of allowing a randomized study, their approach requires confirmation in broader studies. Neoadjuvant chemotherapy may also be effective at reducing the pathological stages of SCC[8,24]. However, these treatments are insufficient to achieve a cure, and other strategies are needed to improve the treatment of this deadly cancer. SCRC-1 was the first cell line derived from renal SCC[25]. However, based on this cell line and its related characteristics, further studies of the immunobiology and histogenesis of this rare malignant disease are lacking. These tumours are reportedly involved in c-kit expression and platelet-derived growth factor receptor-α (PDGFRA) mutations[26], which may be potential therapeutic targets[2]; drugs targeting c-kit and/or PDGFRA may be promising topics of future research[12]. In summary, new molecular therapies and immunotherapies for these tumours are still under active exploration and research.
The reason why our patient developed the disease so rapidly is related to the fact that it was diagnosed very late. Interestingly, the patient also underwent surgery in 2019 and did not have the disease, indicating that the tumour was highly malignant. In previous studies, renal SCC had a poor prognosis with a median overall survival, and 95% confidence interval of 9.9 mo (range, 6.9-31.6 mo), and more patients died of tumour metastasis in the short term, mostly from lung, brain, liver, and other systemic metastases. Early detection of the tumour, use of cisplatin-based chemotherapy, and careful follow-up for local recurrence or frequent metastasis within 6 mo after the primary treatment could be important for improving overall survival[27].
In conclusion, primary renal SCC is an extremely rare tumour for which neuroendocrine markers are helpful for making its pathological diagnosis. Limited available data indicate that the disease has an aggressive natural history and poor prognosis. Clinical stage, tumour composition, and sex may be important factors in determining prognosis. Close follow-up within 6 mo after the initial treatment is the key to an improved overall survival, and once metastases occur, the survival time is substantially reduced. We suggest a comprehensive treatment approach, which currently involves the combination of surgery and chemotherapy, but clinical experience is limited and more data are needed to determine its optimal treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaewput W, Thailand; Peitsidis P, Greece S-Editor: Gao CC L-Editor: A P-Editor: Cai YX
| 1. | Howard S, O'Regan K, Jagannathan J, Krajewski K, Giardino A, Ramaiya N. Extrapulmonary small cell carcinoma: a pictorial review. AJR Am J Roentgenol. 2011;197:W392-W398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Ping JH, Chen ZX, Jiong Q, Han YQ, Nong X. Small cell neuroendocrine carcinoma of the ureter: A case report and literature review. Oncol Lett. 2014;7:728-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Tombet CA, Aynaou M, Mhanna T, El Houmaidi A, Achraf M, Barki A. Low back pain revealing a primary small cell neuroendocrine carcinoma of the upper urinary tract: A case report and review of the literature. Urol Case Rep. 2020;33:101338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Kuroda N, Imamura Y, Hamashima T, Ohe C, Mikami S, Nagashima Y, Inoue K, Perez-Montiel D, Petersson F, Michal M, Hes O. Review of small cell carcinoma of the kidney with focus on clinical and pathobiological aspects. Pol J Pathol. 2014;65:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Chu C, Hu CY, Batra R, Lin AY. Small cell carcinoma of the kidney: a case report and analysis of data from the Surveillance, Epidemiology, and End Results registry. J Med Case Rep. 2019;13:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Capella C, Eusebi V, Rosai J. Primary oat cell carcinoma of the kidney. Am J Surg Pathol. 1984;8:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Jiang ZC, Zhou Qi, Ren YY. Clinicopathological features and prognostic factors of 5 cases of primary renal small cell carcinoma. Zhenduan Bingli Xue Zazhi. 2020;27:649-652. [DOI] [Full Text] |
| 8. | Rupert V, Clifton MM, Fulmer BR, Mori RL, Williams H, Park A. Primary small cell carcinoma of the upper urinary tract: A case report. Urol Case Rep. 2019;27:100995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Xu JL, Guo Y. Clinical characteristics and survival of extrapulmonary small cell carcinoma in 11 different primary tumor sites in the United States, 1975-2016. Curr Med Res Opin. 2021;37:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Uemura KI, Nakagawa G, Chikui K, Moriya F, Nakiri M, Hayashi T, Suekane S, Matsuoka K. A useful treatment for patients with advanced mixed-type small cell neuroendocrine carcinoma of the prostate: A case report. Oncol Lett. 2013;5:793-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Lee JY, Kim J. Renal Metastasis of Small Cell Lung Cancer With Urothelial Carcinoma of the Bladder Misdiagnosed as Renal Cell Carcinoma. J Med Cases. 2019;10:253-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Shimasaki N, Inoue K, Nishigawa H, Kuroda N, Shuin T. Combined small cell carcinoma and sarcomatoid squamous cell carcinoma in the renal pelvis. Int J Urol. 2005;12:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hensley PJ, Bhalodi AA, Gupta S. Primary Upper Urinary Tract Small Cell Carcinoma: A Case Series and Literature Review. J Endourol Case Rep. 2017;3:165-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Faggiano A, Mansueto G, Ferolla P, Milone F, del Basso de Caro ML, Lombardi G, Colao A, De Rosa G. Diagnostic and prognostic implications of the World Health Organization classification of neuroendocrine tumors. J Endocrinol Invest. 2008;31:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Carranza OE, Castañón E, Abella LE, Zudaire ME, Castillo A, Arévalo E, Fusco JP, Zudaire JJ, Carías R, Cambeiro M, Martínez-Monge R, Gil-Bazo I. Clinical management of small-cell carcinoma of the urinary tract: a 10-year single-center's experience. Clin Genitourin Cancer. 2013;11:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Berniker AV, Abdulrahman AA, Teytelboym OM, Galindo LM, Mackey JE. Extrapulmonary small cell carcinoma: imaging features with radiologic-pathologic correlation. Radiographics. 2015;35:152-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Wang XD, Shen HL, Yang PQ, Tian Y. A case report of sunitinib in the treatment of advanced renal small cell carcinoma. Zhonghua Miniao Waike Zazhi. 2017;38:151-152. [DOI] [Full Text] |
| 18. | Abdel-Rahman O, Fouad M. Efficacy and toxicity of sunitinib for non clear cell renal cell carcinoma (RCC): a systematic review of the literature. Crit Rev Oncol Hematol. 2015;94:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | La Rosa S, Bernasconi B, Micello D, Finzi G, Capella C. Primary small cell neuroendocrine carcinoma of the kidney: morphological, immunohistochemical, ultrastructural, and cytogenetic study of a case and review of the literature. Endocr Pathol. 2009;20:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Ouzzane A, Ghoneim TP, Udo K, Verhasselt-Crinquette M, Puech P, Betrouni N, Rouprêt M, Villers A, Leroy X, Colin P. Small cell carcinoma of the upper urinary tract (UUT-SCC): report of a rare entity and systematic review of the literature. Cancer Treat Rev. 2011;37:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Si Q, Dancer J, Stanton ML, Tamboli P, Ro JY, Czerniak BA, Shen SS, Guo CC. Small cell carcinoma of the kidney: a clinicopathologic study of 14 cases. Hum Pathol. 2011;42:1792-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Monaghan TF, Michelson KP, Suss NR, Agudelo CW, Rahman SN, Robins DJ, Flores VX, McNeil BK, Weiss JP, Winer AG. Primary Small Cell Carcinoma of the Kidney: Disease Characteristics and Treatment Outcomes. Medicines (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Majhail NS, Elson P, Bukowski RM. Therapy and outcome of small cell carcinoma of the kidney: report of two cases and a systematic review of the literature. Cancer. 2003;97:1436-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Lynch SP, Shen Y, Kamat A, Grossman HB, Shah JB, Millikan RE, Dinney CP, Siefker-Radtke A. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol. 2013;64:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Chuang CK, Shen YC, Wu JH, Tsai LH, Liao SK. Immunobiologic, cytogenetic and drug response features of a newly established cell line (SCRC-1) from renal small cell carcinoma. J Urol. 2000;163:1016-1021. [PubMed] |
| 26. | Terada T. Primary small cell carcinoma of the ureter: a case report involving immunohistochemical and molecular genetic analyses of KIT and PDGFRA genes. Pathology. 2010;42:101-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Lee SY, Hsu HH, Lin HY, Chen YC, Wong YC, Wang LJ, Ng KF, Chuang CK, Hung CC, Yang CW. Factors associated with the survival of patients with primary small cell carcinoma of the kidney. Int J Clin Oncol. 2013;18:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |