Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5586
Peer-review started: January 6, 2022
First decision: February 14, 2022
Revised: March 1, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Processing time: 153 Days and 21.3 Hours
Early thrombolytic therapy is crucial to treat acute cerebral infarction, especially since the onset of thrombolytic therapy takes 1-6 h. Therefore, early diagnosis and evaluation of cerebral infarction is important.
To investigate the diagnostic value of magnetic resonance multi-delay three-dimensional arterial spin labeling (3DASL) and diffusion kurtosis imaging (DKI) in evaluating the perfusion and infarct area size in patients with acute cerebral ischemia.
Eighty-four patients who experienced acute cerebral ischemia from March 2019 to February 2021 were included. All patients in the acute stage underwent magnetic resonance-based examination, and the data were processed by the system’s own software. The apparent diffusion coefficient (ADC), average diffusion coefficient (MD), axial diffusion (AD), radial diffusion (RD), average kurtosis (MK), radial kurtosis (fairly RK), axial kurtosis (AK), and perfusion parameters post-labeling delays (PLD) in the focal area and its corresponding area were compared. The correlation between the lesion area of cerebral infarction under MK and MD and T2-weighted imaging (T2WI) was analyzed.
The DKI parameters of focal and control areas in the study subjects were compared. The ADC, MD, AD, and RD values in the lesion area were significantly lower than those in the control area. The MK, RK, and AK values in the lesion area were significantly higher than those in the control area. The MK/MD value in the infarct lesions was used to determine the matching situation. MK/MD < 5 mm was considered matching and MK/MD ≥ 5 mm was considered mismatching. PLD1.5s and PLD2.5s perfusion parameters in the central, peripheral, and control areas of the infarct lesions in MK/MD-matched and -unmatched patients were not significantly different. PLD1.5s and PLD2.5s perfusion parameter values in the central area of the infarct lesions in MK/MD-matched and -unmatched patients were significantly lower than those in peripheral and control areas. The MK and MD maps showed a lesion area of 20.08 ± 5.74 cm2 and 22.09 ± 5.58 cm2, respectively. T2WI showed a lesion area of 19.76 ± 5.02 cm2. There were no significant differences in the cerebral infarction lesion areas measured using the three methods. MK, MD, and T2WI showed a good correlation.
DKI parameters showed significant difference between the focal and control areas in patients with acute ischemic cerebral infarction. 3DASL can effectively determine the changes in perfusion levels in the lesion area. There was a high correlation between the area of the infarct lesions diagnosed by DKI and T2WI.
Core Tip: Through the analysis and research of patients in the hospital, we have concluded that diffusion kurtosis imaging (DKI) parameters show that there is a significant difference between the lesions of patients with acute ischemic cerebral infarction and the control area. Three-dimensional arterial spin labeling can effectively determine the changes in the perfusion level of the diseased area. The infarct size diagnosed by DKI is highly correlated with T2-weighted imaging.
- Citation: Jiang YY, Zhong ZL, Zuo M. Three-dimensional arterial spin labeling and diffusion kurtosis imaging in evaluating perfusion and infarct area size in acute cerebral ischemia. World J Clin Cases 2022; 10(17): 5586-5594
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5586.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5586
Cerebral infarction mainly occurs in geriatric patients, and it is a common clinical cerebrovascular disease with high disability and fatality rates. Therefore, it can severely affect patients’ quality of life and health. Clinical practice has demonstrated that early and effective thrombolytic therapy can significantly improve the prognosis of patients with acute cerebral infarction, and that treatment options vary according to the severity of the infarction[1]. Therefore, it is important to explore effective methods for the accurate diagnosis of cerebral infarction to improve the treatment modalities and prognosis.
Magnetic resonance imaging (MRI) has important advantages in the diagnosis of central nervous system diseases due to its high soft tissue resolution. Multi-delay three-dimensional arterial spin labeling (3DASL) can accurately evaluate cerebral blood flow (CBF). Diffusion kurtosis imaging (DKI) is a clinical imaging method used to describe the non-Gaussian diffusion of water molecules in tissues; it can accurately reflect the complexity and heterogeneity of neural tissue microstructure by quantifying the diffusion characteristics of water molecules[2,3]. Our study aimed to investigate the diagnostic value of magnetic resonance multi-delay 3DASL and DKI in evaluating the perfusion and infarct area size in patients with acute cerebral ischemia.
A total of 84 patients who experienced acute cerebral ischemia from March 2019 to February 2021 in our hospital were selected. The inclusion criteria were as follows: Patients (1) Aged 57–82 years; (2) diagnosed with unilateral acute ischemic cerebral infarction (diagnostic criteria of the Fourth National Academic Conference on Cerebrovascular Diseases in 1996)[4]; (3) with dizziness, vomiting, limb numbness, and headache as the main clinical manifestations; (4) who were hospitalized within 24 h of the onset of the disease and who underwent MRI; and (5) who provided informed consent before the relevant examination. The exclusion criteria were as follows: Patients with (1) Cerebrovascular hemorrhagic diseases (hypertensive cerebral hemorrhage, aneurysm, arterial malformation); (2) intracranial tumors; (3) a history of craniotomy; (4) a history of acute myocardial infarction and had an implanted pacemaker placed less than 3 mo previously; (5) cochlear implants and other such complications; (6) mental illness and hyperthyroidism; and (7) a history of drug allergy. The study tender and related materials was implemented after the decision of the medical ethics committee.
A 3DASL scan was performed using our MRI scanner (1.5 T, 8-channel cranial coil) from the cranial top to the lower margin of the foramen magnum. Conventional transverse T1-weighted imaging, T2-weighted imaging (T2WI), and coronal fat-suppressed (FS) + fluid-attenuated inversion recovery imaging were performed. The 3DASL sequence adopted 3D spiral fast spin echo technology. The scanning parameters were as follows: echo time (TE), 10.5 ms; repetition time (TR), 4548 ms; labeling delay time, 1.5; layer thickness, 4 mm; and post-labeling delay (PLD), 1525. The CBF values of PLD1.5s and PLD2.5s were obtained.
The DKI sequence scanning parameters were as follows: TR, 6000 ms; layer thickness, 5 mm; TE, minimum; layer spacing, 1.5 mm; field of vision, 240 mm × 240 mm; matrix, 96 × 130; diffusion direction, 15; and B = 0, 1000, and 2000 s/mm2. The images obtained were analyzed by the supporting software for the following DKI parameters: apparent diffusion coefficient (ADC), axial tensor (AD), mean diffusion coefficient (MD), radial tensor (RD), mean kurtosis (MK), radial kurtosis (RK), and axial kurtosis (AK).
The original data were processed using a GEMR processing workstation. Two imaging physicians with a senior professional title in our hospital agreed to select the infarction area, abnormal ASL perfusion area, mismatching area, and corresponding contralateral normal brain tissue as regions of interest (ROIs). The CBF value of each ROI area and the ADC value were measured and calculated.
In this study, the DKI parameters, CBF values under PLD1.5s and PLD2.5s, and other measurement indices were consistent with the approximate normal distribution or the normal distribution by a normal distribution test, and they are expressed as mean ± SD. The t-test was used for group comparisons. The Statistical Package for the Social Sciences (version 21.0; IBM, Armonk, NY) was used for data analysis. The inspection level was α = 0.05.
The age, body mass index, and time from onset to admission of the study subjects were 57-82 (average, 68.8 ± 5.8) years, 23.8 ± 2.1 kg/m2, and 9-24 (average, 14.2 ± 4.0) h, respectively. The study subjects included 48 males and 36 females. A total of 27 and 30 patients smoked and consumed alcohol, respectively. Moreover, 29, 15, 16, and 34 patients had hypertension, diabetes, coronary heart disease, and hyperlipidemia, respectively. The National Institutes of Health Stroke Scale (NIHSS) score within 24 h after admission ranged from 11–18 points, and the average NIHSS score was 14.8 ± 2.2 points.
The DKI parameters of the focal and control areas in the study subjects were compared. The ADC, MD, AD, and RD values in the lesion area were significantly lower than those in the control areas (P < 0.05). The MK, RK, and AK values in the lesion area were significantly higher than those in the control area (P < 0.05) (Table 1).
| Indexes | The lesion area (n = 84) | The control area (n = 84) | t value | P value |
| ADC | 576.3 ± 94.2 | 756.0 ± 102.1 | -11.856 | 0.000 |
| MD | 0.651 ± 0.150 | 0.847 ± 0.167 | -8.003 | 0.000 |
| AD | 0.830 ± 0.167 | 1.305 ± 0.204 | -16.513 | 0.000 |
| RD | 0.531 ± 0.093 | 0.644 ± 0.122 | -6.751 | 0.000 |
| AK | 1.281 ± 0.224 | 0.760 ± 0.115 | 18.964 | 0.000 |
| MK | 1.256 ± 0.241 | 0.922 ± 0.207 | 9.636 | 0.000 |
| RK | 1.328 ± 0.304 | 0.987 ± 0.185 | 8.782 | 0.000 |
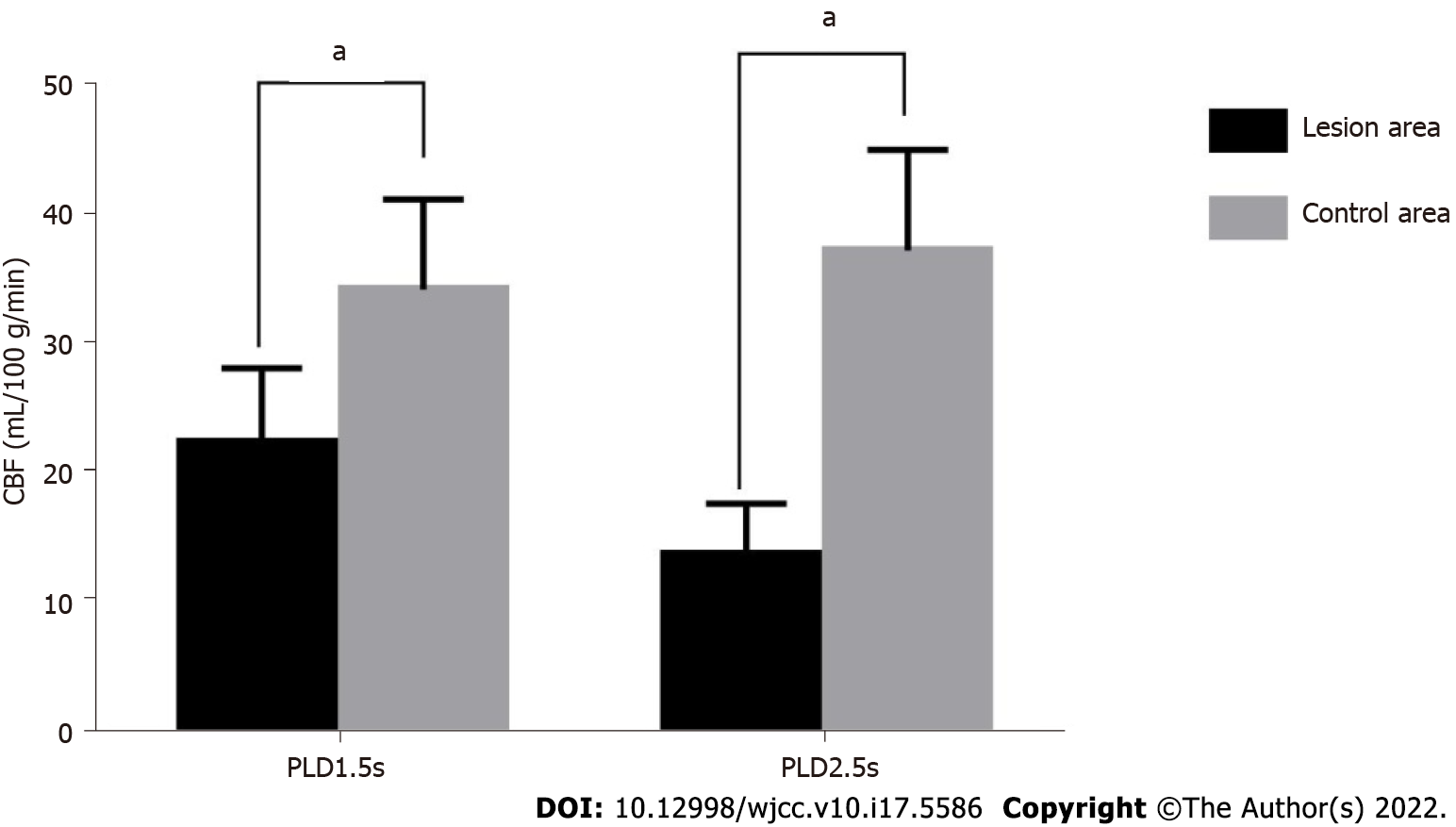
The CBF values of the focal area of the study subjects at PLD1.5s and PLD2.5s were significantly lower than that of the control area (P < 0.05). The results are presented in Table 2 and Figure 1.
| Groups | n | PLD1.5s | PLD2.5s |
| Focal area | 84 | 22.64 ± 5.81 | 14.03 ± 3.91 |
| Control area | 84 | 34.51 ± 7.03 | 37.58 ± 7.76 |
| t value | -11.929 | -24.839 | |
| P value | 0.000 | 0.000 |
The MK/MD value in the infarct lesions was used to determine the matching situation. MK/MD < 5 mm was considered matching, whereas MK/MD ≥ 5 mm was considered mismatching. The PLD1.5s perfusion parameters in the central, peripheral, and control areas of the infarct lesions in MK/MD-matched and -unmatched patients were not significantly different (P > 0.05). The PLD1.5s perfusion parameter values in the central area of infarct lesions in MK/MD-matched and -unmatched lesions were significantly lower than those in peripheral and control areas (P < 0.05) (Table 3).
The PLD2.5s perfusion parameters in the central, peripheral, and control areas of the infarct lesions in MK/MD-matched and -unmatched patients were not significantly different (P > 0.05). The values of the PLD2.5s perfusion parameters in the central area of infarct lesions in MK/MD-matched and -unmatched patients were significantly lower than those in peripheral and control areas (P < 0.05) (Table 4).
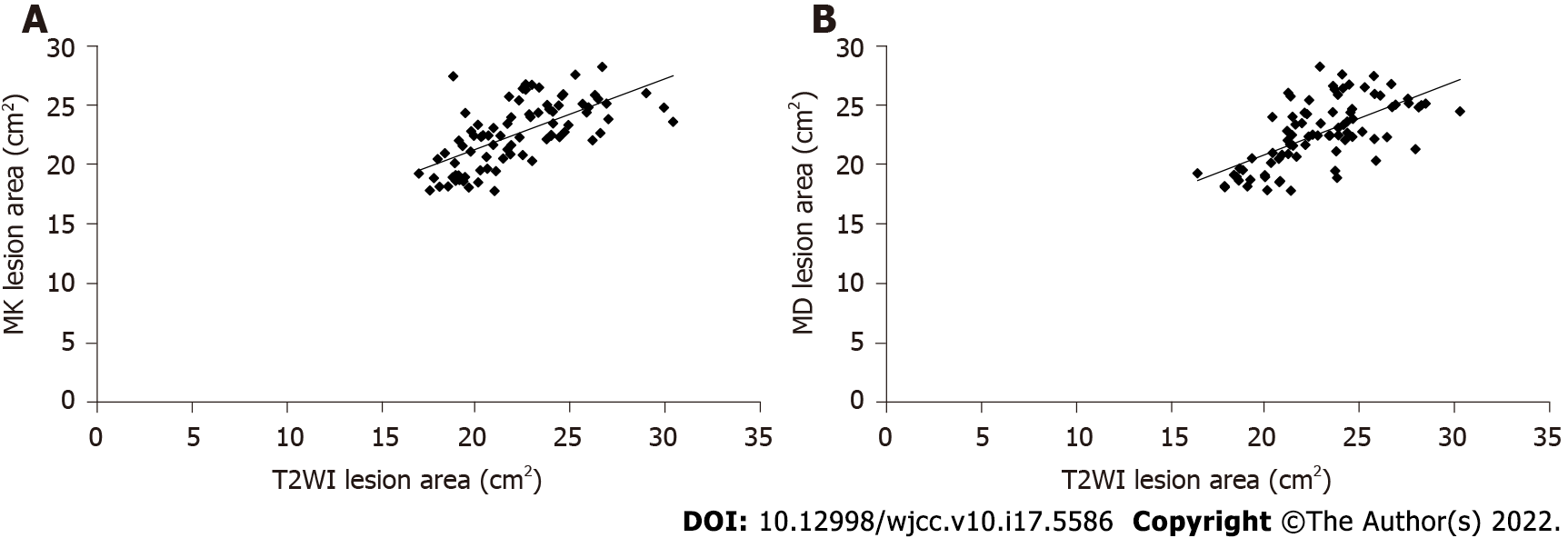
The MK map showed an infarct area of 20.08 ± 5.74 cm2, and the MD map showed an area of 22.09 ± 5.58 cm2 in the study subjects. T2WI showed a lesion area of 19.76 ± 5.02 cm2. There were no significant differences in the cerebral infarction areas measured using these three methods (F = 2.094, P = 0.227). MK, MD, and T2WI showed a good correlation (r = 0.617, r = 0.620, P < 0.05) (Figure 2A and B).
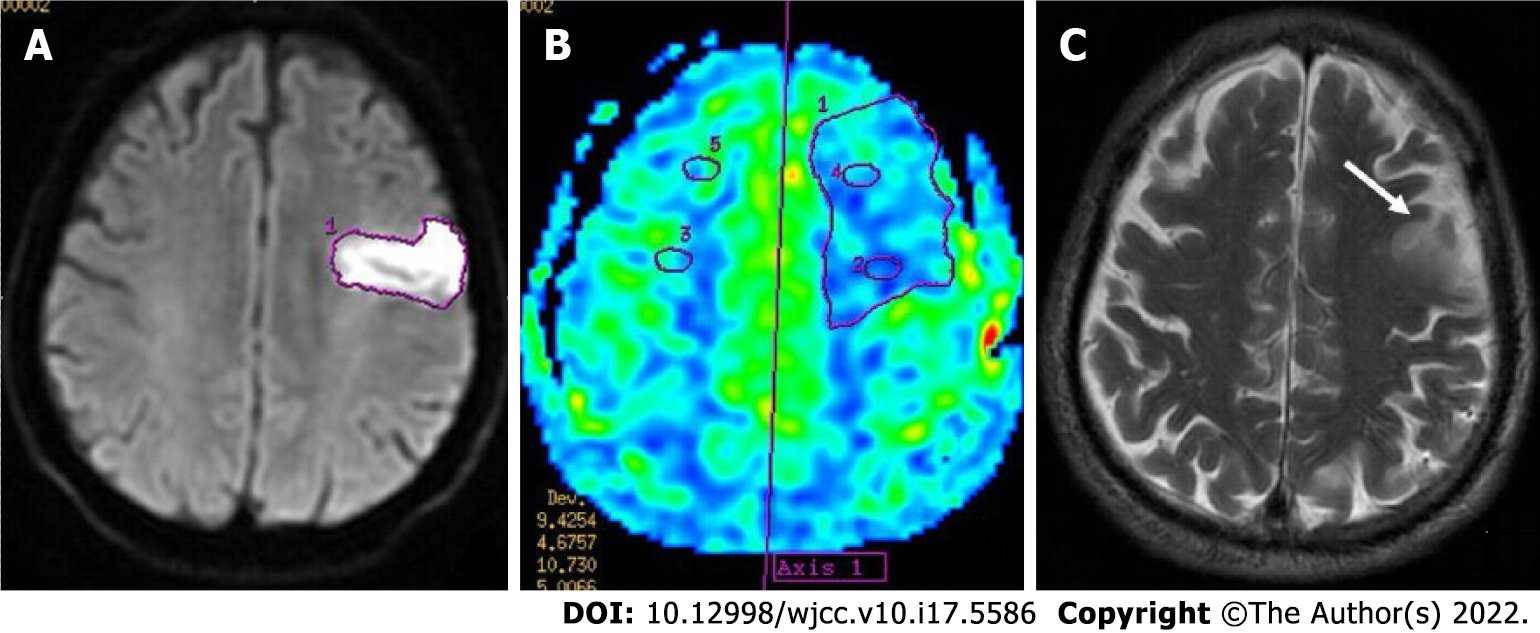
A 67-year-old male patient was admitted to the hospital due to dizziness, vomiting, and limb dysfunction for 12 h (Figure 3).
The traditional method used for clinical diagnosis of cerebral infarction relies on computed tomography (CT), which evaluates the absorption of different types of radiation by different tissues. However, some studies[5-7] have suggested that CT diagnosis and evaluation of cerebral infarction often cannot be performed within 6 h of onset, thus affecting the treatment.
DKI technology analyzes the motion of water molecules in tissues in a non-Gaussian distribution, which is closer to the real motion characteristics of water molecules, and is an extension of magnetic resonance diffusion tensor imaging and magnetic resonance diffusion-weighted imaging[8,9]. In our study, the ADC, MD, AD, and RD values in the lesion area were significantly lower than those in the control group, whereas the MK, RK, and AK values were significantly higher than those in the control group. DKI was more prone to uneven signals due to the large variation in DKI parameters when taken soon after the cerebral infarction occurred, suggesting that DKI has a higher sensitivity in differentiating ischemic brain injuries and can be used as an indicator of complexity and heterogeneity of the changes in the microenvironment inside the cerebral infarction tissue. The movement of water molecules in brain tissue is restricted by the cell membrane, organelles, axons, and myelin sheath; therefore, water molecules in brain tissue cannot show the ideal normal distribution diffusion movement because of restricted movement. Therefore, DKI is more sensitive to pathological changes in brain tissue after ischemia than DWI and DTI, and it is conducive for a more comprehensive analysis of the micro
3DASL technology is a type of perfusion imaging with total brain volume coverage and can be used to determine the appropriate treatment method for stroke. In this study, the CBF values of the focal area in the study subjects at PLD1.5s and PLD2.5s were significantly lower than that of the control area. 3DASL is a non-invasive and quantitative diagnostic method, which can accurately evaluate blood flow in the entire brain, determine the blood flow status of the local infarction area, quantitatively analyze blood flow velocity, and evaluate the establishment of collateral circulation, which is of crucial significance for the formulation of a treatment plan and the evaluation of its curative effect[13-15].
The comparison of the PLD1.5s and PLD2.5s perfusion parameters between the two groups showed that the values of the PLD1.5s perfusion parameters in the central area of infarct lesions in MK/MD-matched and -unmatched patients were significantly lower than those in the peripheral and control areas. 3DASL, as a whole-brain non-invasive volume perfusion evaluation technology, can provide quantitative and accurate whole-brain blood perfusion information and help in the precise and early diagnosis and treatment of stroke. Moreover, the selection of the PLD is important for the analysis of ASL results. PLD1.5s showed the perfusion behavior and the compensatory ability of rapid collateral circulation and PLD2.5s showed the perfusion result. The results of this study also suggested that 3DASL can effectively evaluate and determine the infarct area[16].
The results of the low perfusion area measurement indicated that MK, MD, and T2WI showed no significant differences in the measurement of the area of cerebral infarction lesions in the study subjects, suggesting that these three modalities showed good correlation. The MK and MD values are dependent on the complexity of the tissue microstructure in the ROI and are the most representative parameters of DKI, which can show the degree of limited diffusion of water molecules and the complexity of tissue microstructure. The more complex the structure, the more evident the limited diffusion of water molecules and the larger the MK and MD values[17-20]; whereas there was a significant increase in CBF value on T2WI of the cerebral infarction area, which was closely correlated with MK, MD, and T2WI.
In conclusion, the difference in DKI parameters between the focal and control areas in patients with acute ischemic cerebral infarction is significant, which is important for the diagnosis of infarction. 3DASL can effectively determine the changes in perfusion levels in the lesion area. There was a high correlation between the area of the infarct lesions diagnosed by DKI and T2WI.
Early thrombolytic therapy is crucial to treat acute cerebral infarction, especially since the onset of thrombolytic therapy takes 1–6 h. Therefore, early diagnosis and evaluation of cerebral infarction is important.
This study explored the methods for assessing perfusion and infarct size in patients with acute cerebral ischemia.
The study aimed to investigate the diagnostic value of magnetic resonance multi-delay three-dimensional arterial spin labeling (3DASL) and diffusion kurtosis imaging (DKI) in evaluating the perfusion and infarct area size in acute cerebral ischemia patients.
Eighty-four patients who experienced acute cerebral ischemia from March 2019 to February 2021 were included.
The apparent diffusion coefficient, average diffusion coefficient (MD), axial diffusion, and radial diffusion values in the lesion area were significantly lower than those in the control area. The average kurtosis (MK), radial kurtosis, and axial kurtosis values in the lesion area were significantly higher than those in the control area. parameters post-labeling delays (PLD) 1.5s and PLD2.5s perfusion parameters in the central, peripheral, and control areas of the infarct lesions in MK/MD-matched and -unmatched patients were not significantly different. PLD1.5s and PLD2.5s perfusion parameter values in the central area of the infarct lesions in MK/MD-matched and -unmatched patients were significantly lower than those in peripheral and control areas. There were no significant differences in the cerebral infarction lesion areas measured using the three methods.
DKI parameters showed significant difference between the focal and control areas in patients with acute ischemic cerebral infarction. 3DASL can effectively determine the changes in perfusion levels in the lesion area. There was a high correlation between the area of the infarct lesions diagnosed by DKI and T2-weighted imaging.
3DASL and DKI have broader application value in assessing perfusion and infarct size in patients with acute cerebral ischemia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cvijic M, Slovenia; Narain R, Canada S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Zhu LH, Zhang ZP, Wang FN, Cheng QH, Guo G. Diffusion kurtosis imaging of microstructural changes in brain tissue affected by acute ischemic stroke in different locations. Neural Regen Res. 2019;14:272-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Yang Z, Rong Y, Cao Z, Wu Y, Zhao X, Xie Q, Luo M, Liu Y. Microstructural and Cerebral Blood Flow Abnormalities in Subjective Cognitive Decline Plus: Diffusional Kurtosis Imaging and Three-Dimensional Arterial Spin Labeling Study. Front Aging Neurosci. 2021;13:625843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Mao C, Fu Y, Ye X, Wu A, Yan Z. [Study of 3D-pcASL in differentiation of acute cerebral infarction and acute encephalitis]. Zhonghua Yi Xue Za Zhi. 2015;95:1846-1848. [PubMed] |
| 4. | Frank L, Burigk L, Lehmbecker A, Wohlsein P, Schütter A, Meyerhoff N, Tipold A, Nessler J. Meningioma and associated cerebral infarction in three dogs. BMC Vet Res. 2020;16:177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kong LM, Zeng JY, Zheng WB, Shen ZW, Wu RH. Effects of Acute Alcohol Consumption on the Human Brain: Diffusional Kurtosis Imaging and Arterial Spin-Labeling Study. AJNR Am J Neuroradiol. 2019;40:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Zhou IY, Guo Y, Igarashi T, Wang Y, Mandeville E, Chan ST, Wen L, Vangel M, Lo EH, Ji X, Sun PZ. Fast diffusion kurtosis imaging (DKI) with Inherent COrrelation-based Normalization (ICON) enhances automatic segmentation of heterogeneous diffusion MRI lesion in acute stroke. NMR Biomed. 2016;29:1670-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Sun PZ, Wang Y, Mandeville E, Chan ST, Lo EH, Ji X. Validation of fast diffusion kurtosis MRI for imaging acute ischemia in a rodent model of stroke. NMR Biomed. 2014;27:1413-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Lu D, Jiang Y, Ji Y, Zhou IY, Mandeville E, Lo EH, Wang X, Sun PZ. JOURNAL CLUB: Evaluation of Diffusion Kurtosis Imaging of Stroke Lesion With Hemodynamic and Metabolic MRI in a Rodent Model of Acute Stroke. AJR Am J Roentgenol. 2018;210:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Arab A, Ruda-Kucerova J, Minsterova A, Drazanova E, Szabó N, Starcuk Z Jr, Rektorova I, Khairnar A. Diffusion Kurtosis Imaging Detects Microstructural Changes in a Methamphetamine-Induced Mouse Model of Parkinson's Disease. Neurotox Res. 2019;36:724-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Tan ZR, Zhang C, Tian FF. Spectrum of clinical features and neuroimaging findings in acute cerebral infarction patients with unusual ipsilateral motor impairment- a series of 22 cases. BMC Neurol. 2019;19:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Suman G, Rusin JA, Lebel RM, Hu HH. Multidelay Arterial Spin Labeling MRI in the Assessment of Cerebral Blood Flow: Preliminary Clinical Experience in Pediatrics. Pediatr Neurol. 2020;103:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Qu Y, Zhou L, Jiang J, Quan G, Wei X. Combination of three-dimensional arterial spin labeling and stretched-exponential model in grading of gliomas. Medicine (Baltimore). 2019;98:e16012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Tortora D, Severino M, Rossi A. Arterial spin labeling perfusion in neonates. Semin Fetal Neonatal Med. 2020;25:101130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Uetani H, Kitajima M, Sugahara T, Muto Y, Hirai K, Kuroki Y, Nakaura T, Tateishi M, Yamashita Y. Perfusion abnormality on three-dimensional arterial spin labeling in patients with acute encephalopathy with biphasic seizures and late reduced diffusion. J Neurol Sci. 2020;408:116558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Aoike S, Sugimori H, Fujima N, Suzuki Y, Shimizu Y, Suwa A, Ishizaka K, Kudo K. Three-dimensional Pseudo-continuous Arterial Spin-labeling Using Turbo-spin Echo with Pseudo-steady State Readout: A Comparison with Other Major Readout Methods. Magn Reson Med Sci. 2019;18:170-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Shinohara Y, Kato A, Kuya K, Okuda K, Sakamoto M, Kowa H, Ogawa T. Perfusion MR Imaging Using a 3D Pulsed Continuous Arterial Spin-Labeling Method for Acute Cerebral Infarction Classified as Branch Atheromatous Disease Involving the Lenticulostriate Artery Territory. AJNR Am J Neuroradiol. 2017;38:1550-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Wei Y, Wu L, Wang Y, Liu J, Miao P, Wang K, Wang C, Cheng J. Disrupted Regional Cerebral Blood Flow and Functional Connectivity in Pontine Infarction: A Longitudinal MRI Study. Front Aging Neurosci. 2020;12:577899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Wang C, Miao P, Liu J, Wei S, Guo Y, Li Z, Zheng D, Cheng J. Cerebral blood flow features in chronic subcortical stroke: Lesion location-dependent study. Brain Res. 2019;1706:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Lin L, Chen X, Jiang R, Zhong T, Du X, Xu G, Duan Q, Xue Y. Differentiation between vestibular schwannomas and meningiomas with atypical appearance using diffusion kurtosis imaging and three-dimensional arterial spin labeling imaging. Eur J Radiol. 2018;109:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Hoehn M, Küstermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Föcking M, Arnold H, Hescheler J, Fleischmann BK, Schwindt W, Bührle C. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A. 2002;99:16267-16272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 526] [Article Influence: 22.9] [Reference Citation Analysis (0)] |