Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5217
Peer-review started: September 14, 2021
First decision: October 18, 2021
Revised: October 21, 2021
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 6, 2022
Processing time: 261 Days and 1.4 Hours
The overall incidence of gastric cancer is higher in men than women worldwide. However, gastric signet-ring cell carcinoma (GSRC) is more frequently observed in younger female patients.
To analyze clinicopathological differences between sexes in GSRC, because of the limited evidence regarding association between sex-specific differences and survival.
We reviewed medical records for 1431 patients who received treatment for GSRC at the Cancer Hospital, Chinese Academy of Medical Sciences between January 2011 and December 2018 and surveyed reproductive factors. Clinicopathological characteristics were compared between female and male patients. Cox multivariable model was used to compare the mortality risks of GSRC among men, menstrual women, and menopausal women.
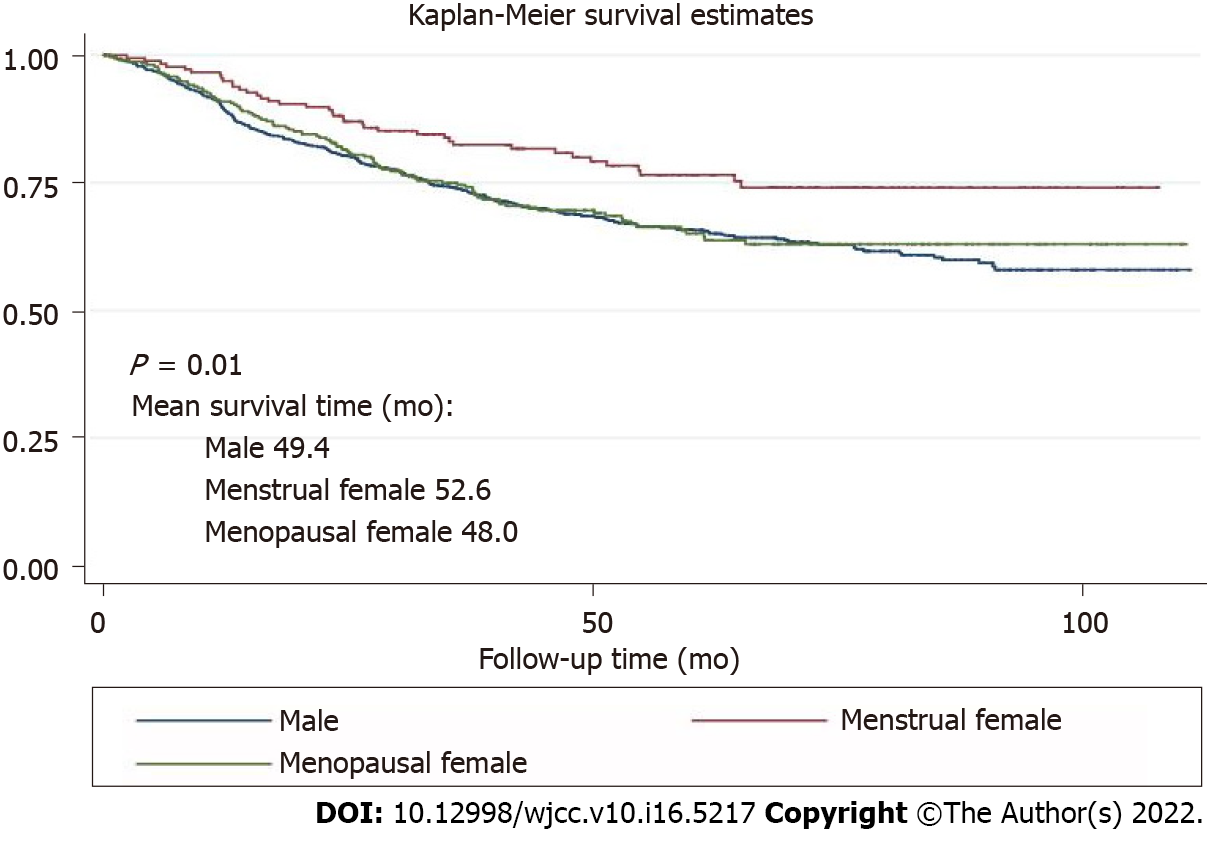
Of 1431 patients, 935 patients were male and 496 were female (181 menstrual and 315 menopausal). The 5-year overall survival in male, menstrual female and menopausal female groups was 65.6%, 76.5% and 65%, respectively (P < 0.01). Menstruation was found to be a protective factor (hazard ratio = 0.58, 95% confidence interval: 0.42–0.82).
The mortality risk of GSRC in menstrual women was lower than that in men. This study identified the protective effects of female reproductive factors in GSRC.
Core Tip: The overall incidence of gastric cancer is higher in men than in women worldwide. However, gastric signet-ring cell carcinoma (GSRC) is more frequently observed in younger female patients. Only a few studies have focused on sex-specific differences in GSRC. This study proposed clinicopathological differences between sexes to reveal sex disparities in GSRC and confirmed that female reproductive factors provided protective effects, and the mortality risk of menstrual female patients was lower. Investigation of the mechanism of action of female reproductive factors may provide new insight into the treatment of GSRC.
- Citation: Li Y, Zhong YX, Xu Q, Tian YT. Protective effects of female reproductive factors on gastric signet-ring cell carcinoma. World J Clin Cases 2022; 10(16): 5217-5229
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5217.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5217
Gastric cancer (GC) is the fifth most common adenocarcinoma and ranks third in mortality worldwide[1]. In China, an estimated 390000 people die of GC annually, which accounts for > 50% of the global deaths and imposes a severe health burden[1-3]. Commonly, the age-standardized incidence rates of GC have shown a male predominance with the male-to-female rate of more than 2:1 in most populations around the world[1,4]. The difference, however, cannot be entirely attributed to the different prevalence of established major risk factors, such as tobacco smoking[5] and Helicobacter pylori infection[6] between the sexes. A study reported that incidence of intestinal GC after menopause increased with time, and the incidence 10 years after menopause was comparable to that of men[7]. An umbrella review including 616 630 women in six observational studies showed that menopausal hormonal therapy was associated with decreased risk of GC[8]. The female reproductive factors and sex hormones may have protective effects on gastric adenocarcinoma.
Gastric signet-ring cell carcinoma (GSRC) is a distinct type of GC, and its incidence has been steadily increasing in Asia, Europe, and the United States, accounting for > 30% of new gastric adenocarcinoma cases[9]. GSRC belongs to the diffused, undifferentiated, and poorly differentiated types in the Laurén classification, Nakamura’s classification, and Japanese Gastric Cancer Association, respectively[10-13]. GSRC in early and advanced stages is more frequently observed in younger female patients than gastric adenocarcinoma[14]. A contradiction with the protective effect of female reproductive factors on gastric adenocarcinoma seems to exist. There is also evidence that female reproductive factors induce diffuse-type GC through estrogen activity[7].
Moreover, various reproductive factors have provided contradictory results in relation to the risk of GCs. The protective effects of female reproductive factors imply the potential role of sex hormones in carcinogenesis. Importantly, the explanation for the predominance of GSRC in young women might provide significant clues to the etiology of the tumors and pave the way for research on innovative prevention and treatment. The effect of female reproductive factors on GSRC tumorigenesis and tumor development remains unclear despite the advances in the understanding of its epidemiology and clinicopathology. The purpose of this study was to estimate the effects of female reproductive factors on the prognosis and combined modality treatment of GSRC.
Our study involved 1431 participants who were histologically confirmed with GC with signet-ring cells and underwent curative resection between January 2011 and December 2018 at the Cancer Hospital, Chinese Academy of Medical Sciences, China. Subtotal gastrectomy was performed for distal GCs, whereas total gastrectomy was conducted for proximal-third GCs. Patients with definitive signs of distant organ or peritoneal seeding metastases did not undergo gastrectomy and were referred for evaluation for chemotherapy instead. Based on the National Comprehensive Cancer Network Clinical Practice Guidelines, standard D2 lymphadenectomy was achieved in patients with curative intention[15]. All the surgical specimens were confirmed separately and independently by at least two experienced pathologists. Disagreements were resolved by discussion, especially on the proportion of signet-ring cells. The follow-up data were prospectively collected and regularly updated by surgeons every 6 mo after surgery. The overall survival was defined from the date of gastrectomy to the date of death or the end of follow-up period (April 30, 2020).
The demographic characteristics included age and body mass index (BMI). We considered age as an ordinal variable (< 50, 50−60, and ≥ 60 years) to approximate tertiles. We defined BMI based on the standard cutoff points established by the Work Group on Obesity in China in categories of underweight and normal: ≤ 24 kg/m2, overweight and obese: > 24.0 kg/m2[16]. Health-related lifestyle indicators, including alcohol consumption (yes: Any alcoholic beverage consumption in the last 12 mo, or no: No alcohol consumption in the last 12 mo) and smoking (yes: Any regular tobacco consumption, or no: Never smoked), were excluded from the analysis based on the variable selection outcome of best-subset selection approach and the uneven distribution. We categorized the proportion of signet-ring cells as ≤ 10%, 10%–50%, 50%–90%, and > 90%, but we used the proportion of signet-ring cells as a dichotomous variable (≤ 50% and > 50%) in the adjustments for confounding variables and to ensure that each level had a sufficient number of observations. Clinicopathological characteristics, such as BMI, proportion of signet-ring cells, T stage, N stage, adjuvant chemotherapy, neoadjuvant chemotherapy, nerve invasion, and lymphatic vessel invasion were subjected to analysis since they were closely associated with the survival of GC patients with signet-ring cell carcinoma based on a priori knowledge.
The continuous variables were expressed as mean ± SD, while categorical variables were expressed as number of observations and percentages (%). Differences in the potential covariates between the sex groups were assessed by Wilcoxon–Mann–Whitney test for continuous variables and the χ2 test for categorical variables. The proportional hazards assumption was estimated, and the Cox multivariable model was used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between the sex factors and survival of GC patients with signet-ring cell carcinoma. The model was adjusted for following potential covariates: BMI, proportion of signet-ring cells, T stage, N stage, adjuvant chemotherapy, neoadjuvant chemotherapy, nerve invasion, and lymphatic vessel invasion. Subgroup analyses were conducted by (1) BMI; (2) Signet-ring cells proportion; (3) Adjuvant chemotherapy; (4) Neoadjuvant chemotherapy; (5) Nerve invasion; and (6) Lymphatic vessel invasion to explore if the impact of the sex difference was stronger in certain groups. Interaction terms between exposures and these covariates were added into the multivariable model, and Wald tests were used to examine whether the interaction terms were statistically significant. The survival curves were estimated by the Kaplan–Meier method. In the present analysis, two-sided P < 0.05 was considered to indicate statistically significant differences. All statistical analyses (and figures created) were performed with Stata 15.0 (StataCorp LLC: College Station, TX, United States).
Table 1 displays the distributions of the demographic and potential risk factors by sex difference. Of the 1431 GC patients with signet-ring cell carcinoma, 935 (65.3%) were male, and 496 (34.7%) were female. Overall, over one-third of the participants were aged ≥ 60 (42.0%) years, with a mean age of 56.3 (SD: 11.3) years. There were no significant differences between the sex groups in terms of histological differentiation, N stage, and adjuvant or neoadjuvant chemotherapy (P > 0.05). The female subjects were more likely to be: younger, nonsmokers and nondrinkers; with diffuse Lauren type, T1 stage and metastasis; without nerve and lymphovascular invasion; and have middle and lower tumor location, lower BMI, higher signet-ring cells proportion, and more lymph nodes removed (P < 0.05).
| Characteristics | Overall (n = 1431, %) | Male (n = 935, 65.3%) | Female (n = 496, 34.7%) | P value1 |
| Age (yr) | ||||
| mean ± SD | 56.3 ± 11.3 | 57.5 ± 10.7 | 54.2 ± 12.1 | < 0.01 |
| < 50 | 386 (27.0) | 213 (55.2) | 173 (44.8) | < 0.01 |
| ≥ 50, < 60 | 444 (31.0) | 303 (68.2) | 141 (31.8) | |
| ≥ 60 | 601 (42.0) | 419 (69.7) | 182 (30.3) | |
| BMI (kg/m2) | ||||
| mean ± SD | 23.8 ± 3.4 | 24.3 ± 3.4 | 22.9 ± 3.3 | < 0.01 |
| > 24 | 659 (46.1) | 501 (76.0) | 158 (24.0) | < 0.01 |
| ≤ 24 | 772 (54.0) | 434 (56.2) | 338 (43.8) | |
| Smoke | < 0.01 | |||
| No | 822 (57.4) | 346 (42.1) | 476 (57.9) | |
| Yes | 609 (42.6) | 589 (96.7) | 20 (3.3) | |
| Drink | < 0.01 | |||
| No | 821 (57.4) | 354 (43.1) | 467 (56.9) | |
| Yes | 610 (42.6) | 581 (95.3) | 29 (4.8) | |
| Tumor site2 | < 0.01 | |||
| Upper | 334 (23.3) | 271 (81.1) | 63 (18.9) | |
| Middle | 345 (24.1) | 205 (59.4) | 140 (40.6) | |
| Lower | 662 (46.3) | 416 (62.8) | 246 (37.2) | |
| Others | 90 (6.3) | 43 (47.8) | 47 (52.2) | |
| Histology differentiation | 0.23 | |||
| Well-/moderately differentiated | 29 (2.1) | 22 (75.9) | 7 (24.1) | |
| Poorly/undifferentiated | 1326 (97.9) | 864 (65.2) | 462 (34.8) | |
| Signet-ring cell proportion (%) | 0.02 | |||
| ≤ 10 | 155 (10.8) | 117 (75.5) | 38 (24.5) | |
| > 10, ≤ 50 | 954 (66.7) | 616 (74.6) | 338 (35.4) | |
| > 50, ≤ 90 | 235 (16.4) | 143 (60.9) | 92 (39.2) | |
| > 90 | 87 (6.1) | 59 (67.8) | 28 (32.2) | |
| Lauren type | < 0.01 | |||
| Intestinal | 73 (5.1) | 61 (83.6) | 12 (16.4) | |
| Diffused | 910 (63.6) | 557 (61.2) | 353 (38.8) | |
| Mixed | 353 (24.7) | 261 (73.9) | 92 (26.1) | |
| Unknown | 95 (6.6) | 56 (59.0) | 39 (41.0) | |
| T stage | 0.02 | |||
| T1 | 411 (28.7) | 246 (59.9) | 165 (40.2) | |
| T2 | 172 (12.0) | 119 (69.2) | 53 (30.8) | |
| T3 | 322 (22.5) | 227 (70.5) | 95 (29.5) | |
| T4 | 526 (36.8) | 343 (65.3) | 183 (34.8) | |
| N stage | 0.54 | |||
| N0 | 573 (40.0) | 365 (63.7) | 208 (36.3) | |
| N1 | 192 (13.4) | 130 (67.7) | 62 (32.3) | |
| N2 | 219 (15.3) | 150 (68.5) | 69 (31.5) | |
| N3 | 447 (31.2) | 290 (64.9) | 157 (35.1) | |
| Metastasis | < 0.01 | |||
| No | 1348 (94.2) | 892 (66.2) | 456 (33.8) | |
| Yes | 83 (5.8) | 43 (51.8) | 40 (48.2) | |
| Adjuvant chemotherapy | 0.51 | |||
| No | 924 (64.6) | 598 (64.7) | 326 (35.3) | |
| Yes | 507 (35.4) | 337 (66.5) | 170 (33.5) | |
| Neoadjuvant chemotherapy | 0.72 | |||
| No | 1315 (91.9) | 861 (65.5) | 454 (34.5) | |
| Yes | 116 (8.1) | 74 (63.8) | 42 (36.2) | |
| Nerve invasion | 0.02 | |||
| No | 313 (21.9) | 184 (58.8) | 129 (41.2) | |
| Yes | 714 (49.9) | 477 (66.8) | 237 (33.2) | |
| Unknown | 404 (28.2) | 274 (67.8) | 130 (32.2) | |
| Lymphovascular invasion | 0.02 | |||
| No | 416 (29.1) | 249 (59.9) | 167 (40.1) | |
| Yes | 554 (38.7) | 373 (67.3) | 181 (32.7) | |
| Unknown | 461 (32.2) | 313 (67.9) | 148 (32.1) | |
| HER-2 | 0.28 | |||
| Negative | 1082 (85.3) | 702 (64.9) | 380 (35.1) | |
| Positive | 187 (14.7) | 129 (69.0) | 58 (31.0) | |
| Lymph nodes removed | < 0.01 | |||
| ≤ 16 | 171 (12.0) | 131 (76.6) | 40 (23.4) | |
| > 16, ≤ 30 | 585 (40.9) | 395 (67.5) | 190 (32.5) | |
| > 30 | 675 (47.2) | 409 (60.6) | 266 (39.7) |
The Cox proportional hazards regression model results are presented in Table 2. Overall, the menstrual women had a significantly lower risk of mortality (HR = 0.58, 95%CI: 0.42–0.82) than male patients in the multivariable model. We did not observe this protective effect in menopausal women (HR = 0.91, 95%CI: 0.72–1.14).
| Variable | HR (95%CI)1 | P value |
| Sex disparity | ||
| Male | Ref | |
| Menstrual female | 0.58 (0.42–0.82) | < 0.01 |
| Menopausal female | 0.91 (0.72–1.14) | 0.42 |
| BMI (kg/m2) | ||
| > 24 | Ref | |
| ≤ 24 | 1.24 (1.02–1.51) | 0.03 |
| Signet-ring cell proportion (%) | ||
| ≤ 10 | Ref | |
| > 10, ≤ 50 | 0.97 (0.72–1.32) | 0.86 |
| > 50, ≤ 90 | 0.79 (0.53–1.17) | 0.25 |
| > 90 | 1.03 (0.63–1.70) | 0.90 |
| T stage | ||
| T1 | Ref | |
| T2 | 1.58 (0.87–2.88) | 0.13 |
| T3 | 4.20 (2.57–6.84) | < 0.01 |
| T4 | 5.96 (3.67–9.69) | < 0.01 |
| N stage | ||
| N0 | Ref | |
| N1 | 1.30 (0.87–1.93) | 0.195 |
| N2 | 1.92 (1.34–2.75) | < 0.01 |
| N3 | 3.29 (2.38–4.55) | < 0.01 |
| Adjuvant chemotherapy | ||
| No | Ref | |
| Yes | 0.75 (0.62–0.91) | < 0.01 |
| Neoadjuvant chemotherapy | ||
| No | Ref | |
| Yes | 2.34 (1.79–3.04) | < 0.01 |
| Nerve invasion | ||
| No | Ref | |
| Yes | 1.50 (0.93–2.43) | 0.10 |
| Unknown | 1.47 (0.88–2.46) | 0.14 |
| Lymphovascular invasion | ||
| No | Ref | |
| Yes | 1.39 (1.02–1.90) | 0.04 |
| Unknown | 1.26 (0.89–1.80) | 0.19 |
Other variables associated with the overall survival included BMI, T stage, N stage, adjuvant or neoadjuvant chemotherapy, and lymphovascular invasion. The results of the subgroup analyses (Table 3) showed that the impact of menstruation was more significant in the female participants with lower levels of signet-ring cells (HR = 0.56, 95%CI: 0.38–0.82, P-interaction = 0.038), nerve invasion (yes vs no, HR = 0.60, 95%CI: 0.40–0.89, P-interaction = 0.029), or lymphovascular invasion (yes vs no, HR = 0.49, 95%CI: 0.30–0.80, P-interaction < 0.001), or without adjuvant chemotherapy (HR = 0.38, 95%CI: 0.22–0.65, P-interaction < 0.001) or neoadjuvant chemotherapy (HR = 0.53, 95%CI: 0.36–0.77, P-interaction < 0.001). There was a reversal effect across the strata of BMI for menopausal women (crossover interactions).
| Variable | Male | Menstrual female | Menopausal female | P-interaction3 | |||
| n1 (%) | HR (95%CI)2 | n1(%) | HR (95%CI)2 | n1 (%) | HR (95%CI)2 | ||
| BMI (kg/m2) | 0.014 | ||||||
| > 24 | 501 (53.6) | Ref | 41 (22.7) | 0.56 (0.27–1.16) | 117 (37.1) | 1.04 (0.72–1.50) | |
| ≤ 24 | 434 (46.4) | Ref | 140 (77.4) | 0.58 (0.39–0.85) | 198 (62.9) | 0.84 (0.62–1.12) | |
| Signet-ring cell proportion (%) | 0.038 | ||||||
| ≤ 50 | 733 (78.4) | Ref | 129 (71.3) | 0.56 (0.38–0.82) | 247 (78.4) | 0.94 (0.74–1.21) | |
| > 50 | 202 (21.6) | Ref | 52 (28.7) | 0.73 (0.33–1.61) | 68 (21.6) | 0.74 (0.40–1.35) | |
| Adjuvant chemotherapy | < 0.001 | ||||||
| No | 598 (64.0) | Ref | 119 (65.8) | 0.38 (0.22–0.65) | 207 (65.7) | 1.01 (0.75–1.36) | |
| Yes | 337 (36.0) | Ref | 62 (34.3) | 0.83 (0.53–1.29) | 108 (34.3) | 0.80 (0.56–1.16) | |
| Neoadjuvant chemotherapy | < 0.001 | ||||||
| No | 861 (92.1) | Ref | 165 (91.2) | 0.53 (0.36–0.77) | 289 (91.8) | 0.80 (0.35–1.82) | |
| Yes | 74 (7.9) | Ref | 16 (8.8) | 0.97 (0.76–1.25) | 26 (8.3) | 0.65 (0.33–1.26) | |
| Nerve invasion | 0.0294 | ||||||
| No | 184 (19.7) | Ref | 54 (29.8) | 0.36 (0.07–1.86) | 75 (23.8) | 0.78 (0.27–2.27) | |
| Yes | 477 (51.0) | Ref | 87 (48.1) | 0.60 (0.40–0.89) | 150 (47.6) | 0.91 (0.69–1.21) | |
| Unknown | 274 (29.3) | Ref | 40 (22.1) | 0.65 (0.31–1.39) | 90 (28.6) | 0.95 (0.60–1.49) | |
| Lymphovascular invasion | < 0.004 | ||||||
| No | 249 (26.6) | Ref | 70 (38.7) | 0.89 (0.40–1.95) | 97 (30.8) | 1.13 (0.61–2.11) | |
| Yes | 373 (39.9) | Ref | 56 (30.9) | 0.49 (0.30–0.80) | 125 (39.7.2) | 0.81 (0.59–1.10) | |
| Unknown | 313 (33.5) | Ref | 55 (30.4) | 0.68 (0.37–1.23) | 93 (29.5) | 1.23 (0.80–1.91) | |
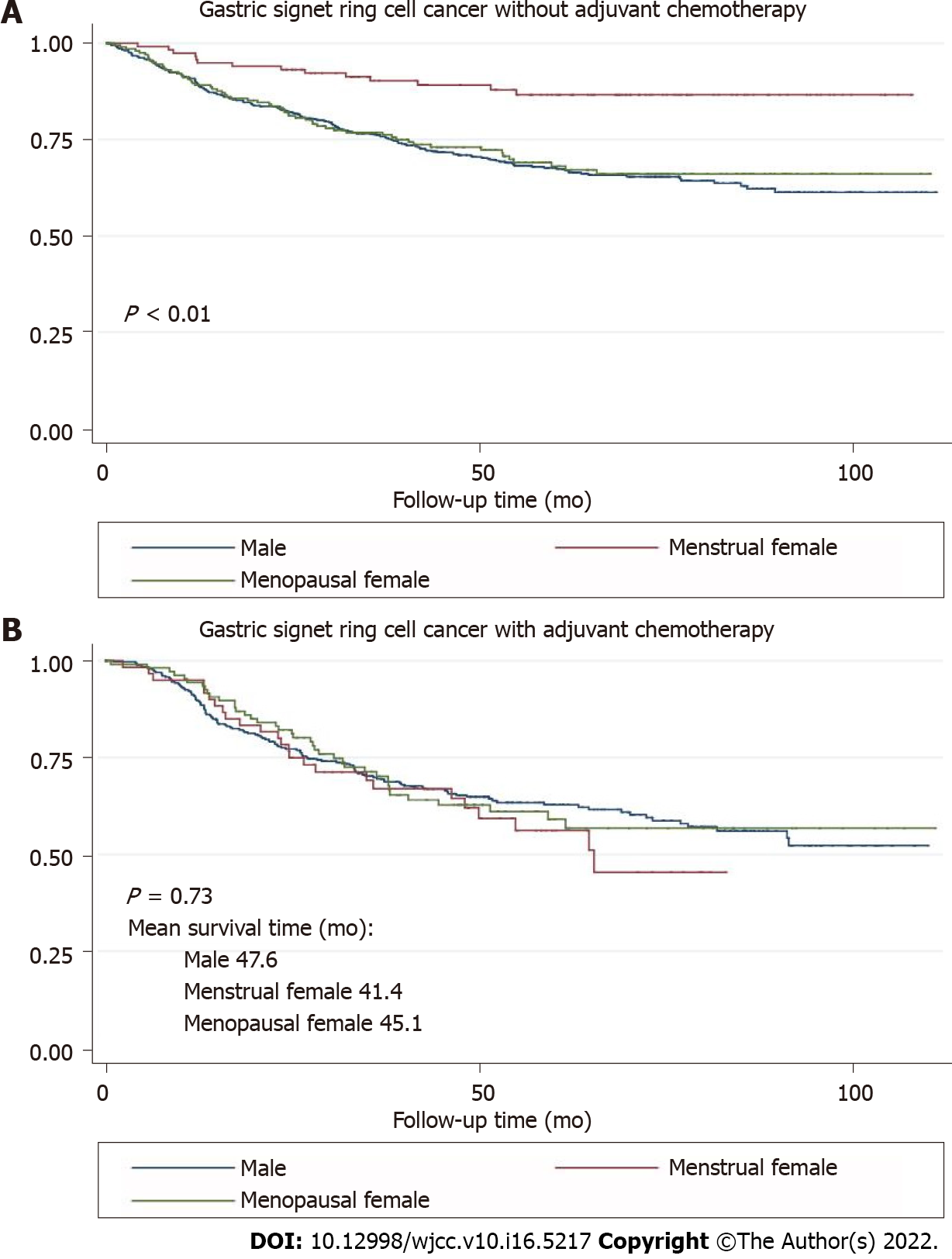
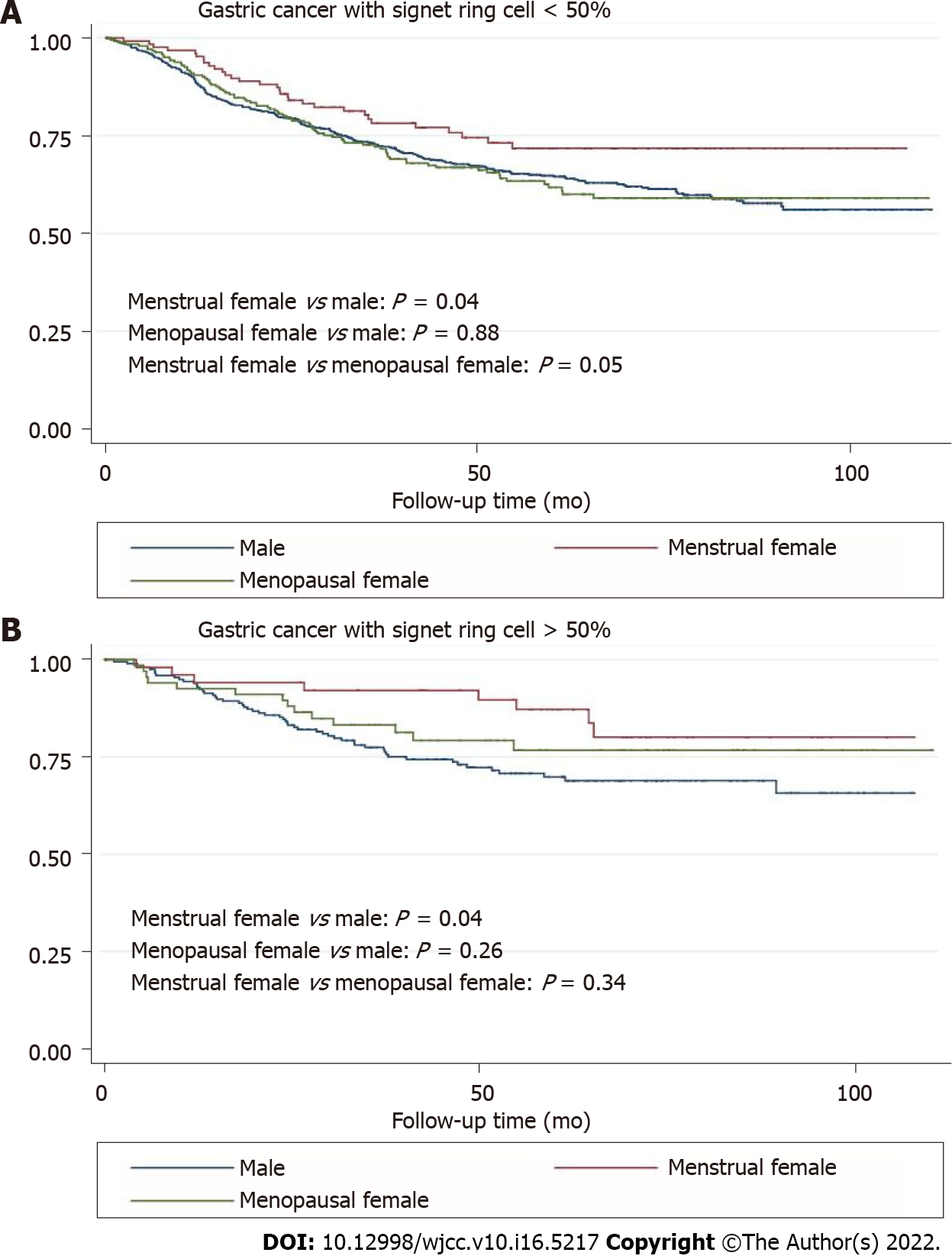
The survival curves in Figure 1 depict the survival probability based on the sex difference. Menstrual female patients had a significantly better overall survival than the male and menopausal female groups (P < 0.01). Survival analysis showed better prognosis of menstrual female patients in the non-adjuvant chemotherapy group (P < 0.01) and the inadequate survival advantages of menstrual female patients in the adjuvant chemotherapy group (P = 0.73) (Figure 2). We compared overall survival between the sexes with different levels of signet-ring cells (Figure 3). Menstrual female patients had a survival advantage compared with male and menopausal female groups; however, this advantage was not significant in the GSRC group with > 50% signet-ring cells (Supplementary Figure 1).
Although the incidence of GC has been decreasing, this disease remains the third cause of cancer mortality worldwide and in China. The proportion of GSRC in all GC cases has been increasing recently, especially in the young and female populations. There are obvious differences on morbidity of GSRC between the sexes. To find a new treatment and improve the overall survival of GSRC, this study has clinical significance to investigate the disparities of reproductive factors between male and female GSRC patients.
In this study, we focused on the influence of the sex-specific differences on the prognosis of GSRC. Our results showed that there was a stronger positive association with overall survival in menstrual female patients with GSRC, compared to that of the male or menopause female patients. The findings of the present large patient-based retrospective study may provide valuable insights into pathways for reducing GSRC mortality in the future.
The multivariate analysis results in our study showed that being a menstrual woman was a protective factor (HR = 0.58, 95%CI: 0.42–0.82) against GSRC. A similar effect was previously reported in various types of GCs[17,18]. In a study with 758 patients, Kim et al[7] proposed that female reproductive hormones might be a potentially protective factor against intestinal-type GC, and the incidence of intestinal-type GC after the menopause increased and became comparable to that in men. A Japanese study revealed that the risk of GC was lower in menstrual women (HR = 0.33, 95%CI: 0.23–0.49). Protective effects were observed in differentiated histological types (HR = 0.25, 95%CI: 0.11–0.55) and undifferentiated histological types (HR = 0.39, 95%CI: 0.23–0.63)[19]. The aforementioned consistent results demonstrate the effects of female reproductive factors in reducing the risks of different GCs. In a large Chinese prospective study with female subjects, the risks of GC increased with age of menopausal women (HR = 0.80 per 5-year increase in the menopausal age, 95%CI: 0.66–0.97)[20]. A similar finding was also obtained in a cohort study including 1 million women whose risks of GC in the menopausal age were considerably higher than those in the menstrual age (RR = 1.46, 95%CI: 1.07–2.0 and RR = 1.59, 95%CI: 1.15–2.20, respectively) from the United Kingdom[21]. Our results indicate that the effect of female reproductive factors on GSRC development is steady and robust.
Here, we hypothesize that sex hormones or their receptors, such as estrogen and estrogen receptor (ER), are the key inducers of the protective effects of female reproductive factors against GSRC. The influence of estrogen has been studied by other researchers, but the results were controversial[22]. Estrogens regulate the tissue growth, differentiation, and function, which is mediated by ER-α and ER-β. The oncological importance of estrogen and ER in carcinomas occurring in the breast and ovaries has been investigated but not in GCs. The expression of ER in the stomach is a basis on which to explore the relationship between sex hormones and survival of GC. Some researchers have demonstrated the expression of ER in gastric tissues, which provides a basis for the probable functions of estrogen in this respect[23-25]. Hess et al[26] established that ERs and female sex hormones were expressed in the male reproductive tract and may have some functions. The protective effect of estrogen was discovered also in men who received hormonal therapy for prostate cancer; notably, the results showed a decrease risk of GC[27].
ER has several types, including ERα, ERβ and ERγ. The biological actions of estrogen are mediated through two specific ERs, ERα and ERβ, which belong to the nuclear receptor superfamily[28]. Zhao et al[25] and Matsuyama et al[29] reported that both ERα, ERβ were expressed in poorly differentiated adenocarcinoma specifically in gastric signet-ring cell adenocarcinomas with characteristics of sex hormone dependency. Different effects of these two ER types were found in various studies. For example, Kameda et al[27] proposed that ERα expressed in diffuse-type GC promoted the proliferation of ERα-positive GC cells. The suggested mechanism was that the activation of the ERα pathway stimulated cancer cell proliferation by activating the hedgehog pathway in a ligand-dependent but dose-independent manner via Shh induction of diffuse-type GC. ERβ is homologous to ERα, particularly in the DNA-binding domain, but it is structurally and functionally different. ERβ manifested strong cytoplasmic staining with anti-ERβ antibody in addition to the stained nuclei[29]. This result provides evidence for the potential of GSRC treatment targets. Recently, using genomic analysis, Kang et al[29] identified ERγ as a potential tumor suppressor in GC. The molecular mechanisms of its action suggest that the activation of ERγ by the antagonizing Wnt signaling through DY131 could suppress GC cell growth and tumorigenesis. Studies on tumorigenesis regulated by estrogens or estrogen receptors are rare but will be urgently needed in the future.
Since chemoresistance is a distinct feature of GSRC, we noticed that the adjuvant chemotherapy was a protective factor in multivariate analysis. Then, we performed further subgroup analysis on adjuvant chemotherapy effects. Interestingly, protective effects exerted by female reproductive factors were observed only in the nonadjuvant chemotherapy group (HR = 0.38, 95%CI: 0.22–0.56) and the non-neoadjuvant chemotherapy group (HR = 0.53, 95%CI: 0.36–0.77). However, female reproductive factors lost their advantages and did not improve the survival after chemotherapy. It is possible that these conditions of poor survival and chemoresistance are mediated by female reproductive factors, such as ERα and ERβ, with unknown mechanism. Recently, Wang et al[30] proposed that the loss of ERβ in GSRC cells might increase the potential for malignant invasion into the deep tissues easier through the mTOR signaling pathway. Therefore, ERβ might inhibit the malignancy of GSRC and can thus become a potential target in its adjuvant treatment. The investigation of Wang did not discuss the connection between ERβ and chemotherapy. We noticed that the prognostic trends are similar in all three studied groups. However, in the subgroup analysis of adjuvant chemotherapy, the mean survival time in the menstrual female group (41.4 mo) was the shortest compared to those of the male (47.6 mo) and menopausal female (45.1 mo) groups. We considered that the probable reason was the proportion of signet-ring cells in GCs. However, no association was identified between the signet-ring cell proportion and influence of female reproductive factors on the prognosis of subjects who received chemotherapy. The results of recent studies confirm the difficulties to understand the possible mechanism between sex hormones and chemoresistance in GSRC. However, more research on this topic needs to be undertaken to clearly elucidate the association between ER and chemotherapy outcomes.
We investigated the effects of female reproductive factors on the prognosis of the GSRC with various proportions of signet-ring cells. Being a menstrual female was a protective factor (HR = 0.56, 95%CI: 0.38–0.82) and was associated with better overall survival than that of menopausal women and men with GSRC cells < 50%. However, menstrual women lost their GSRC-associated advantages over menopausal women at a proportion of the signet-ring cells > 50%. We supposed that ERβ downregulation or ectopic expression in the cytoplasm could lead to a worse prognosis of GSRC with a higher proportion of signet-ring cells. Nevertheless, the probable mechanism remains unclear. Future studies on the topic are therefore highly recommended.
This is the first large population study focused on the relationship between female reproductive factors and GSRC with an 8-year follow-up. Our results highlight the significant effect of female reproductive factors on GSRC prognosis. The clinical importance of our present findings is critical as they have outlined the prognostic role of female reproductive factors in GSRC. Therefore, further strong evidence is provided which will increase the GSRC treatment effectiveness. In this study, we have also investigated the effect of female reproductive factors on survival in a multidimensional aspect with robust statistics such as multivariable Cox proportional hazards model and different subgroups, which could considerably diminish the impact of confounders and explore the potential effect in a specific group.
Despite the aforementioned strengths of our study, several limitations have to be acknowledged. First, the data concerning the expression levels of ER and progesterone receptor (PR) were unavailable. Thus, we cannot conduct further analysis on the effects of ER and PR on GSRC prognosis and other molecular mechanisms. Further investigations are needed to clarify the biological role of estrogen or ER in the carcinogenesis and chemoresistance of GSRC. Second, future studies with a more specific measure of signet-ring cells in GC and more comprehensive long-term outcomes (e.g., the recurrence or chemoresistance, could offer more information) will be needed to verify the conclusion in this study.
Sex differences, especially female reproductive factors, might be protective against GSRC and serve as a significant prognostic factor. Further studies including more comprehensive measures will be essential to elucidate the mechanism of action of female reproductive factors on GSRC.
Gastric signet-ring cell carcinoma (GSRC) is a distinct type of gastric cancer (GC), and its incidence has been steadily increasing. GSRC in early and advanced stages is more frequently observed in younger female patients than in gastric adenocarcinomas.
The effect of female reproductive factors on GSRC tumorigenesis and tumor development remains unclear.
The purpose of this study was to estimate the effects of female reproductive factors on the prognosis of GSRC.
Our study involved 1431 participants who were histologically confirmed with GC with signet-ring cells and underwent curative resections between January 2011 and December 2018 at the Cancer Hospital, Chinese Academy of Medical Sciences, China. Cox multivariable model was used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between sex factors and survival of GC patients with signet-ring cell carcinoma. Subgroup analyses were conducted by: (1) Body mass index; (2) Signet-ring cell proportion; (3) Adjuvant chemotherapy; (4) Neoadjuvant chemotherapy; (5) Nerve invasion; and (6) Lymphatic vessel invasion to explore if the impact of the sex difference was stronger in certain groups.
The menstrual female subjects had a significantly lower risk of mortality (HR = 0.58, 95%CI: 0.42–0.82) than male participants in the multivariable model. The effect appeared to be more substantial among certain subgroup analyses.
There was a stronger positive association with overall survival in menstrual female patients with GSRC, compared to in male or menopause female patients.
Future studies with a more specific measure of signet-ring cells in GC and more comprehensive long-term outcomes (e.g., the recurrence or chemoresistance, could offer more information) will be needed to verify the conclusion in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea; Sudo T, Japan S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55774] [Article Influence: 7967.7] [Reference Citation Analysis (132)] |
| 2. | Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, Yang Z, Zou X, He J. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (1)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13205] [Article Influence: 1467.2] [Reference Citation Analysis (3)] |
| 4. | Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44:2397-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Freedman ND, Derakhshan MH, Abnet CC, Schatzkin A, Hollenbeck AR, McColl KE. Male predominance of upper gastrointestinal adenocarcinoma cannot be explained by differences in tobacco smoking in men versus women. Eur J Cancer. 2010;46:2473-2478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Ibrahim A, Morais S, Ferro A, Lunet N, Peleteiro B. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: Systematic review and meta-analysis of 244 studies. Dig Liver Dis. 2017;49:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 7. | Kim SM, Min BH, Lee J, An JY, Lee JH, Sohn TS, Bae JM, Kim JJ, Kang WK, Kim S, Choi MG. Protective Effects of Female Reproductive Factors on Lauren Intestinal-Type Gastric Adenocarcinoma. Yonsei Med J. 2018;59:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Zhang GQ, Chen JL, Luo Y, Mathur MB, Anagnostis P, Nurmatov U, Talibov M, Zhang J, Hawrylowicz CM, Lumsden MA, Critchley H, Sheikh A, Lundbäck B, Lässer C, Kankaanranta H, Lee SH, Nwaru BI. Menopausal hormone therapy and women’s health: An umbrella review. PloS Med. 2021;18:e1003731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 9. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (3)] |
| 10. | Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt at A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 11. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2422] [Article Influence: 484.4] [Reference Citation Analysis (3)] |
| 12. | Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59:251-258. [PubMed] |
| 13. | Arai T. Where does signet-ring cell carcinoma come from and where does it go? Gastric Cancer. 2019;22:651-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Kao YC, Fang WL, Wang RF, Li AF, Yang MH, Wu CW, Shyr YM, Huang KH. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer. 2019;22:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286-1312. [PubMed] |
| 16. | Chen C, Lu FC; Department of Disease Control Ministry of Health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17 Suppl:1-36. [PubMed] |
| 17. | Kaneko S, Tamakoshi A, Ohno Y, Mizoue T, Yoshimura T; JACC Study Group. Menstrual and reproductive factors and the mortality risk of gastric cancer in Japanese menopausal females. Cancer Causes Control. 2003;14:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Frise S, Kreiger N, Gallinger S, Tomlinson G, Cotterchio M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the anadian national enhanced cancer surveillance system. Ann Epidemiol. 2006;16:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Persson C, Inoue M, Sasazuki S, Kurahashi N, Iwasaki M, Ye W, Tsugane S; JPHC Study Group. Female reproductive factors and the risk of gastric cancer in a large-scale population-based cohort study in Japan (JPHC study). Eur J Cancer Prev. 2008;17:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Freedman ND, Chow WH, Gao YT, Shu XO, Ji BT, Yang G, Lubin JH, Li HL, Rothman N, Zheng W, Abnet CC. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007;56:1671-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Green J, Roddam A, Pirie K, Kirichek O, Reeves G, Beral V; Million Women Study collaborators. Reproductive factors and risk of oesophageal and gastric cancer in the Million Women Study cohort. Br J Cancer. 2012;106:210-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Brusselaers N, Maret-Ouda J, Konings P, El-Serag HB, Lagergren J. Menopausal hormone therapy and the risk of esophageal and gastric cancer. Int J Cancer. 2017;140:1693-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Wang Y, Wan Z, Liu S, Cao Y, Zeng Z. Sphingosine kinase 1 and cancer: a systematic review and meta-analysis. PloS One. 2014;9:e90362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Zhao XH, Gu SZ, Liu SX, Pan BR. Expression of estrogen receptor and estrogen receptor messenger RNA in gastric carcinoma tissues. World J Gastroenterol. 2003;9:665-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Hess RA, Cooke PS. Estrogen in the male: a historical perspective. Biol Reprod. 2018;99:27-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 27. | Kameda C, Nakamura M, Tanaka H, Yamasaki A, Kubo M, Tanaka M, Onishi H, Katano M. Oestrogen receptor-alpha contributes to the regulation of the hedgehog signalling pathway in Eralpha-positive gastric cancer. Br J Cancer. 2010;102:738-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Kang MH, Choi H, Oshima M, Cheong JH, Kim S, Lee JH, Park YS, Choi HS, Kweon MN, Pack CG, Lee JS, Mills GB, Myung SJ, Park YY. Estrogen-related receptor gamma functions as a tumor suppressor in gastric cancer. Nat Commun. 2018;9:1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Wang X, Xia X, Xu E, Yang Z, Shen X, Du S, Chen X, Lu X, Jin W, Guan W. Estrogen Receptor Beta Prevents Signet Ring Cell Gastric Carcinoma Progression in Young Patients by Inhibiting Pseudopodia Formation via the mTOR-Arpc1b/EVL Signaling Pathway. Front Cell Dev Biol. 2020;8:592919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |