Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5146
Peer-review started: November 16, 2021
First decision: December 27, 2021
Revised: January 7, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: June 6, 2022
Processing time: 197 Days and 17.6 Hours
Advances in nanotechnology have opened new frontiers in the diagnosis and treatment of cancer. Nanoparticle-based technology improves the precision of tumor diagnosis when combined with imaging, as well as the accuracy of drug target delivery, with fewer side effects. Optimized nanosystems have demon
Core Tip: The aim of this review is to summarize nanotechnologies in gastrointestinal (GI) cancer diagnosis and therapy. Nanodevices have the advantages of enhancing the specificity of detection and reducing toxicity of drugs in diagnosis and treatment of GI cancers.
- Citation: Liang M, Li LD, Li L, Li S. Nanotechnology in diagnosis and therapy of gastrointestinal cancer. World J Clin Cases 2022; 10(16): 5146-5155
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5146.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5146
Gastrointestinal (GI) cancer is a type of cancer that affects the GI tract and other organs in the digestive system. GI cancers are malignant tumors with both high morbidity and mortality. According to the 2020 World Cancer Report from World Health Organization International Agency for Research on Cancer (IARC), in terms of worldwide cancer morbidity, colorectal, gastric, liver, and esophageal cancers occupy the third, fifth, sixth, and eight places, respectively. Global mortality of colorectal, liver, gastric, esophageal, and pancreatic cancers are 0.94, 0.83, 0.77, 0.54, and 0.47 million, respectively[1]. The clinical manifestations still lack specificity, although GI cancers have mainly symptoms such as inappetence, abdominal distension, abdominal pain, diarrhea, emaciation, etc[2]. GI cancers have the worst prognosis of all cancers and have some of the lowest overall 5-year survival rates, with only gastric cancer above 10%[3]. It is particularly important to develop high-accuracy diagnostic approaches and effective drugs for treatment.
Nanotechnology is receiving attention in many fields of chemistry, engineering, biology, and medicine. Nanoparticle (NP)-based technology has significant biocompatibility and programmability, which offers opportunities in therapeutic and diagnostic applications, especially in cancer. NPs are well used in many areas, such as drug and gene delivery, photothermal therapy, recognition, and imaging agents[4,5].
Great histocompatibility and adjustable property have given NPs a boost. Varying pH, pressure, and bacterial content in the GI tract can regulate NPs characteristics, making the GI tract an attractive target for nanotechnological applications[6]. In GI cancers, NPs have been used for identification of biomarkers, for detection of sentinel lymph nodes (SLNs), for detection of GI tumor microenvironment, and as biologically targeted contrast agents for magnetic resonance imaging (MRI)[7]. Advanced imaging approach is the gold standard for cancer diagnosis. Current imaging techniques used in cancer diagnosis include MRI, computed tomography (CT), positron emission tomography (PET), single-photon emission CT, and ultrasound. Imaging results can give accurate diagnosis and staging. Apart from diagnostic approaches, there are many types of NPs that are being used as drug delivery systems and novel therapeutic agents. These applications improve current techniques not only for early diagnosis and accurate staging of GI cancers but also for treatment approaches.
Nanodevices refer to materials with at least one of the three dimensions in the nanometer range (1-100 nm)[8]. Nanodevices for diagnosis and treatment are now being used throughout oncology due to nanotechnological developments. Nanodevices can offer many possibilities as vectors or as nanodrugs under varying conditions such as pH, transit time, pressure, and bacterial content. Most nanodevices are biocompatible and nontoxic, with high specificity and sensitivity, which have wide applications in precise diagnosis. Moreover, nanotechnology has become one of the most promising cancer treatment strategies. The nanodevices have therapeutic properties and good drug loading capacity, are bound to ligands to achieve high affinity and specificity for target cells, load multiple drugs to achieve collaborative cancer treatment. and avoid conventional drug-resistance mechanisms[9,10].
The unique properties of nanomaterials enable them to behave normally in the complex GI environment. The size, morphology, and surface functionalization of the NPs can affect the interactions of drugs and the GI tract. Size can influence cellular uptake, physical properties, and interactions with biomolecules[8]. The NP diameter should be smaller than the size of the cells so that NPs could interact with or be taken up by cells to achieve their effects in nanomedicine[11]. NP surface characteristics can be optimized for biological responses. The ζ potential accurately approximates the charge on an NP and is used to describe cell-NP interactions[12]. The charge on NPs prevents their aggregation.
The nonspecific interactions between the biomolecules and the nanodevices are other important key factors. NPs can be coated with hydrophilic polymers to remove the influence of mucosal or GI cells and reduce nonspecific interactions[13]. Most nanodevices are spherical, and the surface area-to-volume ratio can be described as 3/radius. The surface area-to-volume ratio increases as the radius decreases. NPs with high surface area-to-volume ratio have more available interaction sites, which is important for drug delivery. Nanodrugs can take advantage of the high surface area-to-volume ratio of NPs, which helps regulate the pharmacokinetics[14].
Owing to the smaller size of NPs, they can be transported easily through the GI tract and have more uniform distribution and drug release. By using nanomedicine, residence time can be increased and uptake into mucosal tissues and cells can be improved[15-17]. These advantages give nanodevices new applications for the treatment of cancer.
The GI tract is an approximately 9 m-long muscular tube including the upper and lower regions[18]. The upper GI tract consists of the mouth, pharynx, esophagus, stomach, and the first part of the small intestine, while the lower GI tract includes the other parts of the small intestine and large intestine[19]. The main functions of the GI tract are digestion of food, absorption of nutrients, and excretion of waste products[20].
GI cancers are located from the esophagus to the rectum, and the accessory digestive organs such as liver, gall bladder and pancreas. The early stages of GI cancers are asymptomatic and can be only detected by endoscopy and biopsy. GI cancers can be cured through minimally invasive endoscopic treatment or minimally invasive surgery when they are confirmed in the early stage. The prognosis is bad in the middle and late stages and the 5-year survival is < 30%, showing the importance of early diagnosis. There are major diagnostic challenges in GI cancers[21]: (1) Unable to identify accurately lesions metastases; (2) difficult to distinguish malignant and benign lesions; and (3) poor accuracy of early diagnosis. The application of NPs is of great help to the early diagnosis of GI cancers. NPs have the characteristics of high sensitivity, specificity, and permeability. Nanotechnologies are mainly used as contrast media for enhanced magnetic imaging currently. For example, superparamagnetic iron oxide NP (SPION)-based contrast agent for detecting metastases and directing surgical treatment have been completed for both esophageal and gastric cancers in clinical use[22-24]. Feridex has been successfully used to detect tumor lesions in the liver[25].
Another application of nanotechnology in GI cancers is drug or gene delivery systems. Nanodevices can load drugs at a high concentration, which are efficiently delivered to specific sites with fewer side effects. Meanwhile, cationic polymers, such as chitosan, form complexes with DNA or small interference RNA (siRNA) and may become the main type of vectors for gene therapy. Liposomes and cationic polymers are the two most common materials for in vivo siRNA delivery[26]. The polymeric NPs made with poly lactic-co-glycolic acid have used for siRNA delivery[27].
Although significant progress has been achieved, there are still issues related to nanotechnology in GI cancers: (1) Cytotoxic NPs can change the characteristics of the cell membrane and reduce cell adhesion. Newly developed NPs have reduced toxicity; however, the problem of toxicity is still the main research focus[28]; (2) NPs may react with biological macromolecules to produce biotoxicity; and (3) NPs may affect biological metabolic pathways such as the respiratory chain. For the future application of nanodrug delivery systems or gene therapy, more complete systems of pharmacology, pharmacokinetics, and toxicokinetics are needed.
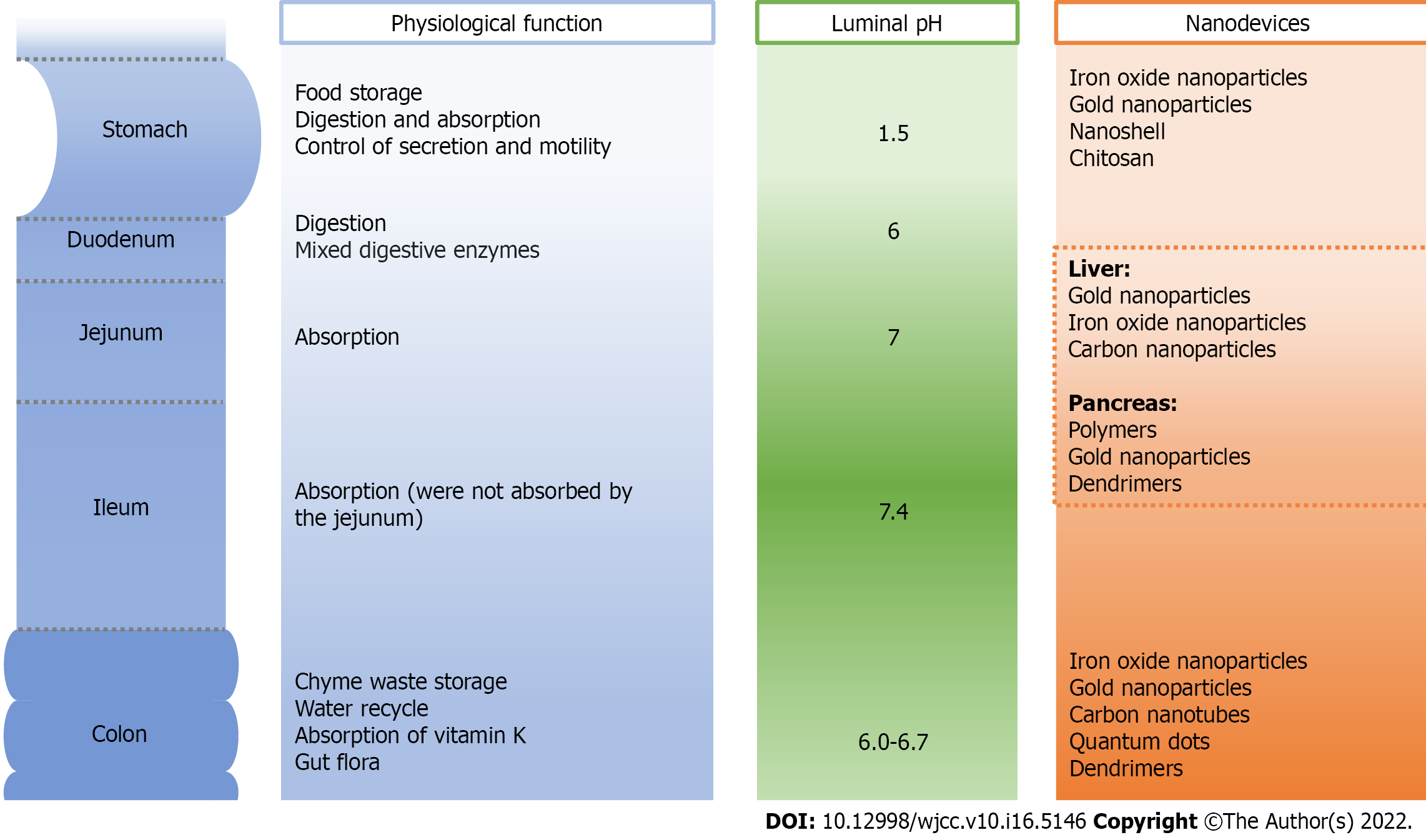
Nanodevices used in GI cancer include iron oxide NPs, quantum dots, carbon nanotubes, gold NPs, dendrimers, nanoshells, and polymers. In this review, we summarize the applications of the nanodevices in GI cancer diagnosis and therapy (Figure 1).
Nanotechnologies have the characteristics of high sensitivity, specificity, and permeability and have been applied primarily for the detection of tumors and imaging of the GI tract in MRI-based clinical applications. Nanotechnology-based drug delivery is of importance in future medical treatment, especially for cancer therapy. Owing to high biocompatibility, nanomaterials show excellent application for increasing therapeutic efficacy. Many NPs have potential for loading various drugs in order to achieve specific targeting and controlled drug release[29] (Tables 1 and 2, Figure 2).
| NP type | Properties | Advantages | Limitations | Ref. |
| Iron | Imaging: MRI contrast, lymph nodes; antigen/receptor ligand, magnetic targeting; multiple treatment opportunities | Simplicity; low cost; high reproducibility | Adverse events in clinical use: Hypotension, lumbar pain and paresthesia | [63,64] |
| QDs | Passive and active targeting; imaging through tunable autofluorescence; multiple treatment opportunities | Excellent PLQY; high photostability and biocompatibility; extreme fast synthesis | Toxicity | [65] |
| Carbon | Passive and active targeting; treatment: Therapeutic cargo delivery; imaging: Visible, infrared | Lightweight, chemically and thermally stable; high tensile strength and conductivity; high resolution and good penetration into the tissue | Adverse events in clinical use: Inflammation, fibrosis | [66] |
| Gold | Imaging: MRI contrast, fluorescence, optical properties; multiple treatment opportunities | Adjusted optical properties; high biocompatibility | Adverse events in clinical use: Nephrotoxicity | [67] |
| Polymers | Passive targeting; antigen/receptor ligand targeting; tumor microenvironment-dependent drug release | High thermal stability, biocompatibility; good biodegradability and controlled drug release ability Inhibition of bacterial growth | Toxicity | [69,70] |
| NP type | GI cancer | Application | Ref. |
| SPION | Colorectal; liver; gastric | Lymph node staging, detection of small metastatic lymph nodes.; magnetic NP-based biosensors for detection of biomarkers; companion diagnostics, evaluate accumulation and predict treatment efficacy of nanomedical cancer therapy | [35,64,71] |
| QDs | Colorectal; liver; gastric | Cancer targeting and imaging; NIR-QD for simultaneous visualization of SLNs; multicolor QD probes for diagnosis of malignant tumors | [41,72-74] |
| Carbon nanotubes | Colorectal; liver | Detection of lymph nodes and node metastasis; tumor localization | [49,52] |
| Gold NPs | Colorectal; liver; gastric; pancreatic; esophageal | Photothermal effect; hyperthermia and cellular destruction; X-ray and CT contrast agents; targeted drug delivery | [75-80] |
| Dendrimers | Pancreatic; colorectal | Dual targeting imaging; targeted drugs delivery and gene therapy; boron neutron capture therapy. | [60,81,82] |
| Nanoshell | Gastric | Contrast agents; targeted drugs delivery and gene therapy | [42,77] |
| Polymers | Colorectal; gastric; pancreatic; esophageal | Controlled drug delivery systems | [83-85] |
Iron oxide NPs belong to the ferrimagnetic class of magnetic materials, which exhibit the unique property of superparamagnetism[30]. Widder et al[31] first proposed they can be used in biomedical applications. SPION-based MRI became a revolution in the field of diagnostics[32,33]. They can be used as contrast agent in MRI to shorten the relaxation time of surrounding protons. SPIONs with a core size < 4 nm are known as ultrasmall superparamagnetic iron oxide (USPIO) NPs, which are a new type of nanomolecular contrast agent in imaging of the rectum[34], lymph nodes[35], liver[36], etc. By using USPIO and MRI, lymph nodes can be imaged in patients with rectal cancer[34]. Lymph node disease may cause poor prognosis in rectal cancer. Preoperative accurate MRI diagnosis of lymph node disease and other adverse features provides a reference for radiotherapy and chemotherapy, which can reduce the risk of relapse[37]. Ferucarbotran (Resovist®) and ferumoxide (Feridex® or Endorem®) are two types of SPIONs under clinical investigation[38,39]. Although SPION MRI contrast agent has high security, they have been shown to have some adverse events in clinical use, such as hypotension, lumbar pain, and paresthesia in 2%-10% of patients[39].
Quantum dots (QDs) are inorganic semiconductor NPs with an inorganic element core and a metal shell. The diameter of QDs ranges between 2 and 10 nm. QDs can be used as fluorescent near-infrared (NIR) probes instead of organic dyes. The fluorescence properties of QDs are affected by size and composition[40]. QDs have a variety of applications, such as drug analysis, immuno- and biosensing, and clinical diagnostics and therapeutics. QD-based in situ detection can be used to detect macrophage infiltration, tumor microvessel density, and neovascular maturity in gastric cancer. He et al[41] have presented the investigation of bioconjugating ability of NIR CdSeTe/ZnS QDs and visible CdSe QDs in immunofluorescent staining for cancer biomarkers in gastric cancer. NIR QDs show higher sensitivity and contrast for the cancer biomarkers in gastric cancer tissues. Peng et al[42] reported on a QD-based simultaneous in situ detection of infiltrating macrophages, tumor microvessel density, and neovessel maturity in gastric cancer tissues. This approach can yield combined tumor stromal features.
Carbon nanotubes (CNTs) have advantages in weight, high tensile strength, and conductivity, which make it possible for them to detect cancer cells[43,44]. CNT applications include tissue scaffolding for osteoblast proliferation, drug delivery, and thermal ablation agents[45-47]. CNTs are used to detect colorectal cancer in lymphadenectomy and for cancer prognosis. Single-walled CNTs (SWCNTs) and multiwalled CNTs are two forms[48]. SWCNTs have a smaller band gap so they are more suitable for fluorescence imaging. They are used as contrast materials in MRI for colorectal carcinoma. Gadolinium-based SWCNTs have high resolution and penetration, and the sensitivity can increase when they are radioisotope-based[49-51]. Activated carbon NP suspensions can enter lymph nodes rapidly after phagocytosis by macrophages. Carbon NPs can be used colorectal laparoscopic surgery, such as tumor localization and lymph node tracking[52].
Gold NPs work as a new type of photothermal sensor and cancer treatment drug carrier due to their tunable optical properties as well as biocompatibility[53]. Gold NPs can be used in surface enhanced Raman spectroscopy to detect changes such as the amount of nucleic acid and protein in colorectal cancer[54]. Gold nanospheres can be used to diagnose colorectal cancer as a fluorescent dye and contrast agent in CT[55]. In cancer models, small gold nanocages are more absorbed by tumors and have a higher tumor-muscle uptake ratio. The retention and accumulation of gold nanocages in tumors have been determined by PET imaging[56]. Gold nanocages with controllable biochemical properties and radioactive element labeled are used in optical coherence tomography in vivo[57]. Gold NPs have also been used as highly sensitive probes for hepatoma detection. Li et al[58] used gold NPs conjugated with redox probes on CNTs for a multi-analyte electrochemical immunoassay to detect liver cancer biomarkers.
Dendrimers are three-dimensional, highly branched monodispersed macromolecules. The diameters are usually between 1 and 10 nm. Dendritic macromolecules can perform different functions depending on shape, size, surface function, and branch length[59,60]. Dendrimer NPs acting as functional NPs are utilized for MRI or NIR fluorescence in a single probe for their unique properties such as monodispersity, modifiable surface functionality, and internal cavities. Polyamidoamines (PAMAMs) are a type of dendrimers commonly used for targeted drug delivery. PAMAM dendrimers conjugated to against CD14 or prostate-specific membrane antigen can be used as contrast agents in flow cytometry and confocal microscopy[61]. Gene therapy is a promising strategy for a plethora of diseases including cancer and inflammation. RNA interference is a post-translational gene regulation technology for gene therapy[62]. It can specifically inhibit the gene expression triggered by siRNA, genome origin miRNA, and double-stranded short hairpin RNA[63]. Many nanomaterial-based gene delivery systems have been developed for GI diseases. Polo-like kinase 1 has been formulated in stable nucleic acid lipid particles and used to evaluate patients with GI neuroendocrine tumors[64]. Dendrimers with high transfection efficiency are most commonly used in gene delivery. Heat treatment increases the dendrimer flexibility to enhance the transfection efficiency[65]. The biodegradable polymeric envelope protects and transports siRNA into the cytosol, thereby allowing siRNA to be efficiently transfected in vivo for inflammatory bowel disease[66]. The polymeric NPs made with poly lactic-co-glycolic acid are biocompatible and biodegradable polymers with low toxicity, sustained release profiles, and high stability and have emerged as suitable siRNA carriers in metastatic colorectal cancer[67].
The nanoshell is composed of a metal shell and a nonconductive core. It can adjust the plasmon resonance by changing the relative size of the metal shell and the nonconductive core[68]. Nanoshells can enhance the sensitivity and resolution of traditional contrast agents for in vivo imaging of tumors. Nanoshell-based contrast agents have become important in noninvasive imaging and are used in tumor detection and staging[69]. Nanoshells are used to carry molecular conjugates to achieve better functions. Conjugated gold NPs show stronger intensity and emission. Some conjugated gold NPs give greater sensitivity and have been applied successfully to enhance fluorescence[70] and detect gene expression level[71] and mutations[72] in colorectal cancer. The incorporation of iron or iron oxide into nanoshell structures confers some advantages for MRI. Nanoshells conjugated with diarrheagenic bacterial heat-stable peptide enterotoxin ligands have been used for the targeted delivery and ablation of colorectal cancer[73].
Polymers can adapt to the variability of conditions along the GI tract, which accelerates the design of controlled drug delivery systems for GI diseases. Polymers have the properties of adjusted size, controlled drug release, and high drug loading capacity[74]. They have wide applications in targeted drug delivery. Chitosan[75], polyethylene oxide[76], hydroxypropyl methylcellulose[77], and hyaluronic acid[78], etc are widely used for developing GI cancer drug delivery systems. In addition, liposomes[79], nanopyramids[80], and nanogels[81] are widely used in diagnosis and therapy in GI disorders. Chitosan has been considered as a vehicle for drug delivery in many GI cancers. It can effectively achieve controlled drug release, improve drug stability, reduce adverse drug reactions, and enhance drug bioavailability[82]. In a recent study, norcantharidin conjugated with carboxymethyl chitosan was successful in inducing apoptosis of gastric tumor cells and decreasing systemic toxicity[83]. Nanogels are swollen nanosized networks formed by noncovalent interactions or covalent crosslinking of polymer chains. Nanogels have been regarded as oral drug delivery systems because they are more sensitive to external stimuli than macroscopic gels are[84]. Senanayake et al[85] reported a drug conjugated with gemcitabine in cancer chemotherapy, which enables target delivered.
In this review, we summarize the current nanotechnologies in diagnosis and treatment of GI cancers. Nanodevices are biocompatible and nontoxic with high specificity and sensitivity. They have diagnostic and therapeutic properties with wide applications in precision medicine. Although there are few applications in clinical research, nanodevices have demonstrated advantages in many fields, including enhanced specificity of detection, reduced drug toxicity, enhanced effect of contrast agents, and improved diagnosis and therapy of GI cancers. Nanodevices will promote the development of personalized therapy for early diagnosis and treatment of GI cancers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Nanoscience and nanotechnology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen BH, Taiwan; Laddha UD, India S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64455] [Article Influence: 16113.8] [Reference Citation Analysis (176)] |
| 2. | Liu Z, Lin C, Mu L, Suo C, Ye W, Jin L, Franceschi S, Zhang T, Chen X. The disparities in gastrointestinal cancer incidence among Chinese populations in Shanghai compared to Chinese immigrants and indigenous non-Hispanic white populations in Los Angeles, USA. Int J Cancer. 2020;146:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11923] [Article Influence: 2980.8] [Reference Citation Analysis (4)] |
| 4. | Liu S, Lu F, Xing R, Zhu JJ. Structural effects of Fe3O4 nanocrystals on peroxidase-like activity. Chemistry. 2011;17:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Barreto JA, O'Malley W, Kubeil M, Graham B, Stephan H, Spiccia L. Nanomaterials: applications in cancer imaging and therapy. Adv Mater. 2011;23:H18-H40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 624] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 6. | Laroui H, Wilson DS, Dalmasso G, Salaita K, Murthy N, Sitaraman SV, Merlin D. Nanomedicine in GI. Am J Physiol Gastrointest Liver Physiol. 2011;300:G371-G383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Service RF. Nanotoxicology. Nanotechnology grows up. Science. 2004;304:1732-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Lamprecht A, Schäfer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001;18:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 359] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 9. | Biabanikhankahdani R, Ho KL, Alitheen NB, Tan WS. A Dual Bioconjugated Virus-Like Nanoparticle as a Drug Delivery System and Comparison with a pH-Responsive Delivery System. Nanomaterials (Basel). 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Acharya S, Dilnawaz F, Sahoo SK. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials. 2009;30:5737-5750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Lacoeuille F, Garcion E, Benoit JP, Lamprecht A. Lipid nanocapsules for intracellular drug delivery of anticancer drugs. J Nanosci Nanotechnol. 2007;7:4612-4617. [PubMed] |
| 12. | Dittgen M, Herbst B. [Zeta potential--fundamentals, measurement methods and application to pharmacy]. Pharmazie. 1987;42:641-656. [PubMed] |
| 13. | Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1019] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 14. | Hörter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev. 2001;46:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 378] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 352] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 16. | Moss DM, Curley P, Kinvig H, Hoskins C, Owen A. The biological challenges and pharmacological opportunities of orally administered nanomedicine delivery. Expert Rev Gastroenterol Hepatol. 2018;12:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Reinholz J, Landfester K, Mailänder V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018;25:1694-1705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 18. | Lin HC, Prather C, Fisher RS, Meyer JH, Summers RW, Pimentel M, McCallum RW, Akkermans LM, Loening-Baucke V; AMS Task Force Committee on Gastrointestinal Transit. Measurement of gastrointestinal transit. Dig Dis Sci. 2005;50:989-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Hua S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract - Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front Pharmacol. 2020;11:524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 20. | Bellmann S, Carlander D, Fasano A, Momcilovic D, Scimeca JA, Waldman WJ, Gombau L, Tsytsikova L, Canady R, Pereira DI, Lefebvre DE. Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:609-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Sykes PD, Neoptolemos JP, Costello E, Halloran CM. Nanotechnology advances in upper gastrointestinal, liver and pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2012;6:343-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Ishiyama K, Motoyama S, Tomura N, Sashi R, Imano H, Ogawa J, Narita K, Watarai J. Visualization of lymphatic basin from the tumor using magnetic resonance lymphography with superparamagnetic iron oxide in patients with thoracic esophageal cancer. J Comput Assist Tomogr. 2006;30:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Pultrum BB, van der Jagt EJ, van Westreenen HL, van Dullemen HM, Kappert P, Groen H, Sietsma J, Oudkerk M, Plukker JT, van Dam GM. Detection of lymph node metastases with ultrasmall superparamagnetic iron oxide (USPIO)-enhanced magnetic resonance imaging in oesophageal cancer: a feasibility study. Cancer Imaging. 2009;9:19-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Tokuhara T, Tanigawa N, Matsuki M, Nomura E, Mabuchi H, Lee SW, Tatsumi Y, Nishimura H, Yoshinaka R, Kurisu Y, Narabayashi I. Evaluation of lymph node metastases in gastric cancer using magnetic resonance imaging with ultrasmall superparamagnetic iron oxide (USPIO): diagnostic performance in post-contrast images using new diagnostic criteria. Gastric Cancer. 2008;11:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Reimer P, Tombach B. Hepatic MRI with SPIO: detection and characterization of focal liver lesions. Eur Radiol. 1998;8:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2239] [Cited by in RCA: 2383] [Article Influence: 148.9] [Reference Citation Analysis (0)] |
| 27. | Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2387] [Cited by in RCA: 2034] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 28. | Coenegrachts K, van Werven J, ter Beek L, Mzallassi Z, Bögels M, van Gulik T, Van Den Berghe I, Nederveen A, Stoker J, Rigauts H, Soenen S, De Cuyper M. Evaluation of peri-tumoral vessels surrounding colorectal liver metastases after intravenous injection of extruded magnetoliposomes in rats: correlation with 3T MRI and histopathology. JBR-BTR. 2010;93:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Nikezić AVV, Bondžić AM, Vasić VM. Drug delivery systems based on nanoparticles and related nanostructures. Eur J Pharm Sci. 2020;151:105412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Chouhan RS, Horvat M, Ahmed J, Alhokbany N, Alshehri SM, Gandhi S. Magnetic Nanoparticles-A Multifunctional Potential Agent for Diagnosis and Therapy. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Widder KJ, Senyel AE, Scarpelli GD. Magnetic microspheres: a model system of site specific drug delivery in vivo. Proc Soc Exp Biol Med. 1978;158:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 229] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Choi JY, Kim MJ, Kim JH, Kim SH, Ko HK, Lim JS, Oh YT, Chung JJ, Yoo HS, Lee JT, Kim KW. Detection of hepatic metastasis: manganese- and ferucarbotran-enhanced MR imaging. Eur J Radiol. 2006;60:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1069] [Cited by in RCA: 861] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 34. | Kumagai H, Pham W, Kataoka M, Hiwatari K, McBride J, Wilson KJ, Tachikawa H, Kimura R, Nakamura K, Liu EH, Gore JC, Sakuma S. Multifunctional nanobeacon for imaging Thomsen-Friedenreich antigen-associated colorectal cancer. Int J Cancer. 2013;132:2107-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Fortuin AS, Brüggemann R, van der Linden J, Panfilov I, Israël B, Scheenen TWJ, Barentsz JO. Ultra-small superparamagnetic iron oxides for metastatic lymph node detection: back on the block. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Thian YL, Riddell AM, Koh DM. Liver-specific agents for contrast-enhanced MRI: role in oncological imaging. Cancer Imaging. 2013;13:567-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Huertas A, Marchal F, Peiffert D, Créhange G. [Preoperative radiotherapy for rectal cancer: target volumes]. Cancer Radiother. 2013;17:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Weissleder R. Liver MR imaging with iron oxides: toward consensus and clinical practice. Radiology. 1994;193:593-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur Radiol. 2003;13:1266-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 414] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 40. | Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006;107:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 41. | He Y, Xu H, Chen C, Peng J, Tang H, Zhang Z, Li Y, Pang D. In situ spectral imaging of marker proteins in gastric cancer with near-infrared and visible quantum dots probes. Talanta. 2011;85:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Peng CW, Tian Q, Yang GF, Fang M, Zhang ZL, Peng J, Li Y, Pang DW. Quantum-dots based simultaneous detection of multiple biomarkers of tumor stromal features to predict clinical outcomes in gastric cancer. Biomaterials. 2012;33:5742-5752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Feng T, Wang Y, Qiao X. Recent Advances of Carbon Nanotubes-based Electrochemical Immunosensors for the Detection of Protein Cancer Biomarkers. Electroanalysis. 2017;29 (3):662-675. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2181] [Cited by in RCA: 1768] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 45. | Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006;6:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 347] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 46. | Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, Weisman RB, Pasquali M, Schmidt HK, Smalley RE, Curley SA. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654-2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 47. | Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1414] [Cited by in RCA: 957] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 48. | Georgakilas V, Perman JA, Tucek J, Zboril R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev. 2015;115:4744-4822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 851] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 49. | Wang LY, Li JH, Zhou X, Zheng QC, Cheng X. Clinical application of carbon nanoparticles in curative resection for colorectal carcinoma. Onco Targets Ther. 2017;10:5585-5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Eslami M, Peyghan AA. DNA nucleobase interaction with graphene like BC3 nano-sheet based on density functional theory calculations. Thin Solid Films. 2015;589:52-56. [DOI] [Full Text] |
| 51. | Eslami M, Vahabi V, Ahmadi Peyghan A. Sensing properties of BN nanotube toward carcinogenic 4-chloroaniline: A computational study. Physica E Low Dimens Syst Nanostruct. 2016;76:6-11. [DOI] [Full Text] |
| 52. | Cai HK, He HF, Tian W, Zhou MQ, Hu Y, Deng YC. Colorectal cancer lymph node staining by activated carbon nanoparticles suspension in vivo or methylene blue in vitro. World J Gastroenterol. 2012;18:6148-6154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Ahmad T, Sarwar R, Iqbal A, Bashir U, Farooq U, Halim SA, Khan A, Al-Harrasi A. Recent advances in combinatorial cancer therapy via multifunctionalized gold nanoparticles. Nanomedicine (Lond). 2020;15:1221-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Lin D, Feng S, Pan J, Chen Y, Lin J, Chen G, Xie S, Zeng H, Chen R. Colorectal cancer detection by gold nanoparticle based surface-enhanced Raman spectroscopy of blood serum and statistical analysis. Opt Express. 2011;19:13565-13577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 55. | Chen CC, Li JJ, Guo NH, Chang DY, Wang CY, Chen JT, Lin WJ, Chi KH, Lee YJ, Liu RS, Chen CL, Wang HE. Evaluation of the Biological Behavior of a Gold Nanocore-Encapsulated Human Serum Albumin Nanoparticle (Au@HSANP) in a CT-26 Tumor/Ascites Mouse Model after Intravenous/Intraperitoneal Administration. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Srivatsan A, Jenkins SV, Jeon M, Wu Z, Kim C, Chen J, Pandey RK. Gold nanocage-photosensitizer conjugates for dual-modal image-guided enhanced photodynamic therapy. Theranostics. 2014;4:163-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Ji T, Zhao Y, Wang J, Zheng X, Tian Y, Nie G. Tumor fibroblast specific activation of a hybrid ferritin nanocage-based optical probe for tumor microenvironment imaging. Small. 2013;9:2427-2431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Li Y, Zhong Z, Chai Y, Song Z, Zhuo Y, Su H, Liu S, Wang D, Yuan R. Simultaneous electrochemical immunoassay of three liver cancer biomarkers using distinguishable redox probes as signal tags and gold nanoparticles coated carbon nanotubes as signal enhancers. Chem Commun (Camb). 2012;48:537-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J Intern Med. 2014;276:579-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 60. | Talanov VS, Regino CA, Kobayashi H, Bernardo M, Choyke PL, Brechbiel MW. Dendrimer-based nanoprobe for dual modality magnetic resonance and fluorescence imaging. Nano Lett. 2006;6:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 335] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 62. | Mulligan RC. The basic science of gene therapy. Science. 1993;260:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1143] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 63. | Sioud M. RNA interference: mechanisms, technical challenges, and therapeutic opportunities. Methods Mol Biol. 2015;1218:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1323] [Cited by in RCA: 1264] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 65. | Tang MX, Redemann CT, Szoka FC Jr. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 567] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 66. | Toub N, Bertrand JR, Tamaddon A, Elhamess H, Hillaireau H, Maksimenko A, Maccario J, Malvy C, Fattal E, Couvreur P. Efficacy of siRNA nanocapsules targeted against the EWS-Fli1 oncogene in Ewing sarcoma. Pharm Res. 2006;23:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Blum JS, Saltzman WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine. J Control Release. 2008;129:66-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 68. | Chen W, Ayala-Orozco C, Biswal NC, Perez-Torres C, Bartels M, Bardhan R, Stinnet G, Liu XD, Ji B, Deorukhkar A, Brown LV, Guha S, Pautler RG, Krishnan S, Halas NJ, Joshi A. Targeting pancreatic cancer with magneto-fluorescent theranostic gold nanoshells. Nanomedicine (Lond). 2014;9:1209-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | England CG, Priest T, Zhang G, Sun X, Patel DN, McNally LR, van Berkel V, Gobin AM, Frieboes HB. Enhanced penetration into 3D cell culture using two and three layered gold nanoparticles. Int J Nanomedicine. 2013;8:3603-3617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Alagaratnam S, Yang SY, Loizidou M, Fuller B, Ramesh B. Mechano-growth Factor Expression in Colorectal Cancer Investigated With Fluorescent Gold Nanoparticles. Anticancer Res. 2019;39:1705-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Biscaglia F, Ripani G, Rajendran S, Benna C, Mocellin S, Bocchinfuso G, Meneghetti M, Palleschi A, Gobbo M. Gold Nanoparticle Aggregates Functionalized with Cyclic RGD Peptides for Targeting and Imaging of Colorectal Cancer Cells. ACS Nano. 2019;2 (10):6436-6444. [DOI] [Full Text] |
| 72. | Ravanshad R, Karimi Zadeh A, Amani AM, Mousavi SM, Hashemi SA, Savar Dashtaki A, Mirzaei E, Zare B. Application of nanoparticles in cancer detection by Raman scattering based techniques. Nano Rev Exp. 2018;9:1373551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Fortina P, Kricka LJ, Graves DJ, Park J, Hyslop T, Tam F, Halas N, Surrey S, Waldman SA. Applications of nanoparticles to diagnostics and therapeutics in colorectal cancer. Trends Biotechnol. 2007;25:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Arunraj TR, Sanoj Rejinold N, Ashwin Kumar N, Jayakumar R. Bio-responsive chitin-poly(L-lactic acid) composite nanogels for liver cancer. Colloids Surf B Biointerfaces. 2014;113:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Nataraj D, Sakkara S, Meghwal M, Reddy N. Crosslinked chitosan films with controllable properties for commercial applications. Int J Biol Macromol. 2018;120:1256-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 76. | Lee H-J, Kim J-Y, Park S-H, Rhee Y-S, Park C-W, Park E-S. Controlled-release oral dosage forms containing nimodipine solid dispersion and hydrophilic carriers. J Drug Deliv Sci Technol. 2017;37:28-37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Hu A, Chen C, Mantle MD, Wolf B, Gladden LF, Rajabi-Siahboomi A, Missaghi S, Mason L, Melia CD. The Properties of HPMC:PEO Extended Release Hydrophilic Matrices and their Response to Ionic Environments. Pharm Res. 2017;34:941-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Zhang W, Jin X, Li H, Zhang RR, Wu CW. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr Polym. 2018;186:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 79. | Martina MS, Fortin JP, Ménager C, Clément O, Barratt G, Grabielle-Madelmont C, Gazeau F, Cabuil V, Lesieur S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J Am Chem Soc. 2005;127:10676-10685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 80. | Sweeney CM, Stender CL, Nehl CL, Hasan W, Shuford KL, Odom TW. Optical properties of tipless gold nanopyramids. Small. 2011;7:2032-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Cheng G, Mi L, Cao Z, Xue H, Yu Q, Carr L, Jiang S. Functionalizable and ultrastable zwitterionic nanogels. Langmuir. 2010;26:6883-6886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Huang G, Liu Y, Chen L. Chitosan and its derivatives as vehicles for drug delivery. Drug Deliv. 2017;24:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 83. | Chi J, Jiang Z, Qiao J, Zhang W, Peng Y, Liu W, Han B. Antitumor evaluation of carboxymethyl chitosan based norcantharidin conjugates against gastric cancer as novel polymer therapeutics. Int J Biol Macromol. 2019;136:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 84. | Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl. 2009;48:5418-5429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1142] [Cited by in RCA: 932] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 85. | Senanayake TH, Warren G, Wei X, Vinogradov SV. Application of activated nucleoside analogs for the treatment of drug-resistant tumors by oral delivery of nanogel-drug conjugates. J Control Release. 2013;167:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |