Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5124
Peer-review started: November 13, 2021
First decision: January 9, 2022
Revised: January 17, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 6, 2022
Processing time: 200 Days and 12.6 Hours
Insulinomas are the most frequent type of functional pancreatic neuroendocrine tumors with a variety of neuroglycopenic and autonomic symptoms and well-defined diagnostic criteria; however, prediction of their clinical behavior and early differentiation between benign and malignant lesions remain a challenge. The comparative studies between benign and malignant cases are limited, suggesting that short clinical history, early hypoglycemia during fasting, high proinsulin, insulin, and C-peptide concentrations raise suspicion of malignancy. Indeed, malignant tumors are larger with higher mitotic count and Ki-67 proliferative activity, but there are no accurate histological criteria to distinguish benign from malignant forms. Several signaling pathways have been suggested to affect the pathophysiology and behavior of insulinomas; however, our knowledge is limited, urging a further understanding of molecular genetics. Therefore, there is a need for the identification of reliable markers of metastatic disease that could also serve as therapeutic targets in patients with malignant insulinoma. This opinion review reflects on current gaps in diagnostic and clinical aspects related to the malignant behavior of insulinoma.
Core Tip: Insulinomas are rare but potentially malignant tumors. The only sure sign of malignancy is metastases present at diagnosis. Long-term survival of patients with malignant insulinoma is poor; however, newly available agents and approaches, are reassuring. Nonetheless, it is important to distinguish between benign and malignant insulinomas early, as well as follow the non-functioning pancreatic neuroendocrine tumors and plan the appropriate treatment and follow-up. Important initial parameters include 2-3-fold higher insulin, proinsulin, and C-peptide levels, early-onset hypoglycemia during the 72-h fasting test, and high chromogranin A from the biochemical aspect, earlier recognition of neuroglycopenic symptoms from the clinical aspect, and tumor size exceeding 3 cm and higher tumor grade (G2 or G3) from the pathohistological standpoint. Molecular genetic advances are still insufficient in adding to the individualization of treatment and prognosis, but α-internexin and chromosomal instability, where available, might add to early recognition of malignancy.
- Citation: Cigrovski Berkovic M, Ulamec M, Marinovic S, Balen I, Mrzljak A. Malignant insulinoma: Can we predict the long-term outcomes? World J Clin Cases 2022; 10(16): 5124-5132
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5124.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5124
Malignant insulinoma is a rare tumor entity, with an incidence of approximately 10% of all insulinoma cases. A sure sign of malignancy, differentiating benign from malignant insulinoma, is metastases (primarily in the liver) at diagnosis. Unfortunately, some benign insulinomas alter during the disease course, and according to more current analyses, malignant forms can also arise from non-functioning pancreatic neuroendocrine tumors (PNETs), becoming clinically apparent when tumor burden and potential to secrete proinsulin increase. Although currently available therapeutic options offer symptom relief and tumor reduction even in the case of malignant insulinoma, better survival and quality of life are expected in the case of early detection of malignancy. This review summarizes the presenting characteristics that may help identify patients at risk of having malignant lesions and their therapeutic options.
The annual incidence of approximately 0.8/100000 persons makes PNETs a rare type of cancer. Functional PNETs, characterized by hypersecretion of a specific hormone, are even rarer. Of all functional PNETs, insulinomas are the most frequent, with an estimated incidence of 1-4 per million per year[1,2]. Four insulinoma characteristics are associated with the “90%” rate: 90% occur in the pancreas, 90% are benign, 90% are solitary, and 90% are less than 2 cm in diameter[3].
The clinical presentation of insulinoma includes different neuroglycopenic and autonomic symptoms through sympathetic nervous system activation due to hypoglycemia induced by periods of substrate deficiency. Insulinoma-related signs and symptoms are non-specific. On average, there is a 12-mo lag time before the insulinoma is suspected and diagnosed, giving them the fully deserved term neurological chameleon.
The gold standard for diagnosing insulinoma is hypoglycemia detected during a 72-h fasting test, accompanied by inappropriately elevated insulin, C-peptide, and proinsulin levels, with the latter being the best indicator of insulinoma. According to the Endocrine Society Clinical Practice Guidelines, endogenous hyperinsulinism is diagnosed in the absence of oral hypoglycemic agent use when hypoglycemic signs and/or symptoms are present in association with plasma glucose level < 3 mmol/L, insulin level > 18 pmol/L, C-peptide > 0.2 nmol/L, and proinsulin > 5 pmol/L[4].
While the biochemical diagnostic criteria for insulinoma, in general, are well established and prove sensitive and specific, the differences between benign and malignant insulinoma are not always clear[4].
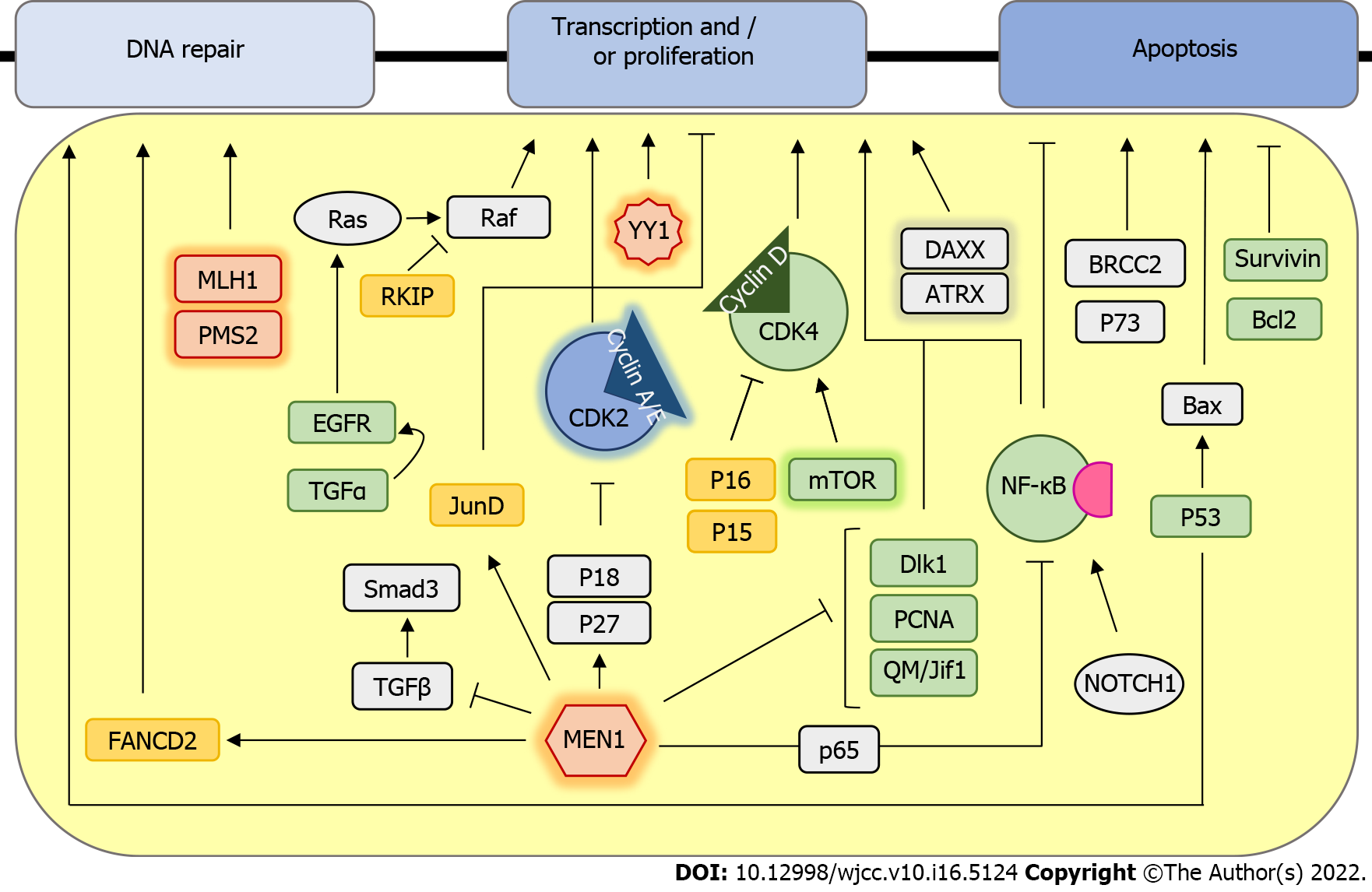
During insulinoma tumorigenesis, the cell physiology is altered through the changes in several pathways, resulting in an increased replicative potential, self-sufficiency in growth signals, evasion of apoptosis, and potential to invade other tissues[5] (Figure 1). Research on genomics and transcriptomics of human insulinomas was mainly done on hereditary forms and the MEN1 syndrome is the most common hereditary type of PNET caused by autosomal dominant germline alteration in the MEN1 (11q13) gene encoding the menin protein[6]. Because 10%-30% of individuals with MEN1 mutation develop insulinoma[7], menin was proposed as a candidate marker in the tumorigenesis of insulinoma. In pancreatic β-cells, menin has a role in maintaining the expression of cyclin-dependent kinase (CDK) inhibitors p27 and p18 to prevent enhanced pancreatic islet proliferation and subsequent tumorigenesis[8]. Moreover, menin prevents tumorigenesis by several additional mechanisms, such as the induction of transcription factor JunD to maintain normal growth regulation[9] and the induction of FANCD2 to induce the DNA repair[10]. Tumors of MEN1 syndrome patients often show an increase in the frequency of spontaneous chromosomal alterations and multiple allelic deletions[11], including the presence of mismatch-repair deficiency and a high rate of microsatellite instability in patients with malignant insulinoma[12]. Diversely, menin also acts as a repressor of SMAD3, p65-mediated transcriptional activation of NF-κB, as well as dlk1/Pref-1, Proliferating cell nuclear antigen, and QM/Jif-1, which leads to inhibition of cell growth and stimulation of apoptosis[13-14]. Apart from menin, mutations in Yin Yang1 (YY1) gene, which increases insulin secretion by β-cells, were found in approximately 20% of insulinoma patients. YY1 is a transcription factor that regulates insulin and insulin-like growth factor signaling and its mutations are only found in insulinomas, but not other malignant PNETs, suggesting that this mutation could serve for specific diagnosis of insulinoma[15].
CDK4 and its regulatory subunit cyclin D are the main regulators of cell cycle progression of Langerhans islet β-cells. Albeit there are no differences in expression of CDK4 protein[16], overexpression of cyclin D and downregulation of cyclin D inhibitors p15 and p16 have been observed in both benign and advanced lesions, suggesting their role in the tumorigenesis of insulinomas[17-18]. Apart from CDKs, that are other key regulators of cell cycle progression, and K-ras and BRAF are two key oncogenes whose somatic mutations lead to loss of cell cycle regulation.
Even though BRAF and K-ras gene mutations are rare events that are not involved in the tumorigenesis of neuroendocrine tumors[19], activation of the Ras/RAF/mitogen-activated protein kinase pathway and its inhibitor Raf-1 kinase inhibitory protein has been shown to play a role in the progression of insulinomas[20,21].
Furthermore, transforming growth factor α and its receptor epidermal growth factor receptor have been associated with the activation of the Ras signaling pathway and its oncogenic activity in malignant insulinoma[22].
mTOR is the other ubiquitous and highly conserved serine/threonine kinase that regulates several cellular functions, including cell proliferation. The expression of mTOR and P70S6K is high in insulinoma specimens, and in vitro mTOR inhibitors can prevent the proliferation of insulinoma cells[23].
Apart from cell proliferation, evasion of apoptosis is the other hallmark of most types of cancer. Even though p53 mutations in insulinoma are rarely found[24], high expression of wild-type p53 is found in almost all malignant insulinomas where it can further activate the cell death promoting factor Bax[25,26]. Furthermore, increased expression of the apoptosis inhibitor Bcl-2, found in one-third of insu
Survivin is another apoptosis inhibitor whose gene is located in the chromosome region changed in one-third of malignant insulinomas[28]. In addition, several candidate proteins, such as P73, NOTCH1, and BRCC2, play a role in the evasion of apoptosis and insulinoma progression; however, their exact roles in insulinoma tumorigenesis remain elusive[29].
Even though several signaling pathways have been suggested to affect the etiology and behavior of insulinomas, our knowledge is still limited. Detailed pathway-specific expression profiling and better model systems are required to resolve the conflicting results and further improve the understanding of pathway-specific genes involved in malignant insulinoma pathogenesis and improve the individualization of treatment.
The morbidity of insulinoma is primarily due to hypoglycemia-related symptoms, while malignant insulinomas add morbidity and mortality due to their aggressiveness and invasion of adjacent tissues and metastasis[30]. In patients who do not have an extra-pancreatic disease at the initial presentation, the prediction of clinical behaviour is difficult as comparative studies between benign and malignant cases are limited[31,32].
Although there is a new 2019 NETs classification[33,34], in most insulinoma studies the tumor classification is based on the macroscopic (tumor size and invasion of surrounding tissue/vascular invasion), microscopic (mitotic count per 10 high power fields), and immunohistochemical features (Ki-67 proliferation index and neuroendocrine markers).
Tumors are graded as: G1 or well-differentiated (low grade) tumors: Size up to 2 cm, mitotic count < 2 mitoses/10 HPFs, Ki-67 index < 2%, and no local invasion or regional or distant metastases; G2 (intermediate grade): Tumor size > 2 cm, limited to the pancreas or with minimal local extension, mitotic count of 2-20 mitoses/10 HPFs, and Ki-67 index of 3-20%, with or without metastases; G3 (high grade): Local invasion, mitotic count > 20/10 HPFs, and/or Ki-67 index > 20%, with or without metastases[34,35].
Histologically, there are no accurate criteria to distinguish benign from malignant insulinomas. Malignant tumors are usually larger than 2, even 3 cm, showing higher Ki-67 proliferative activity, mostly G2 or even G3 NETs[35,36].
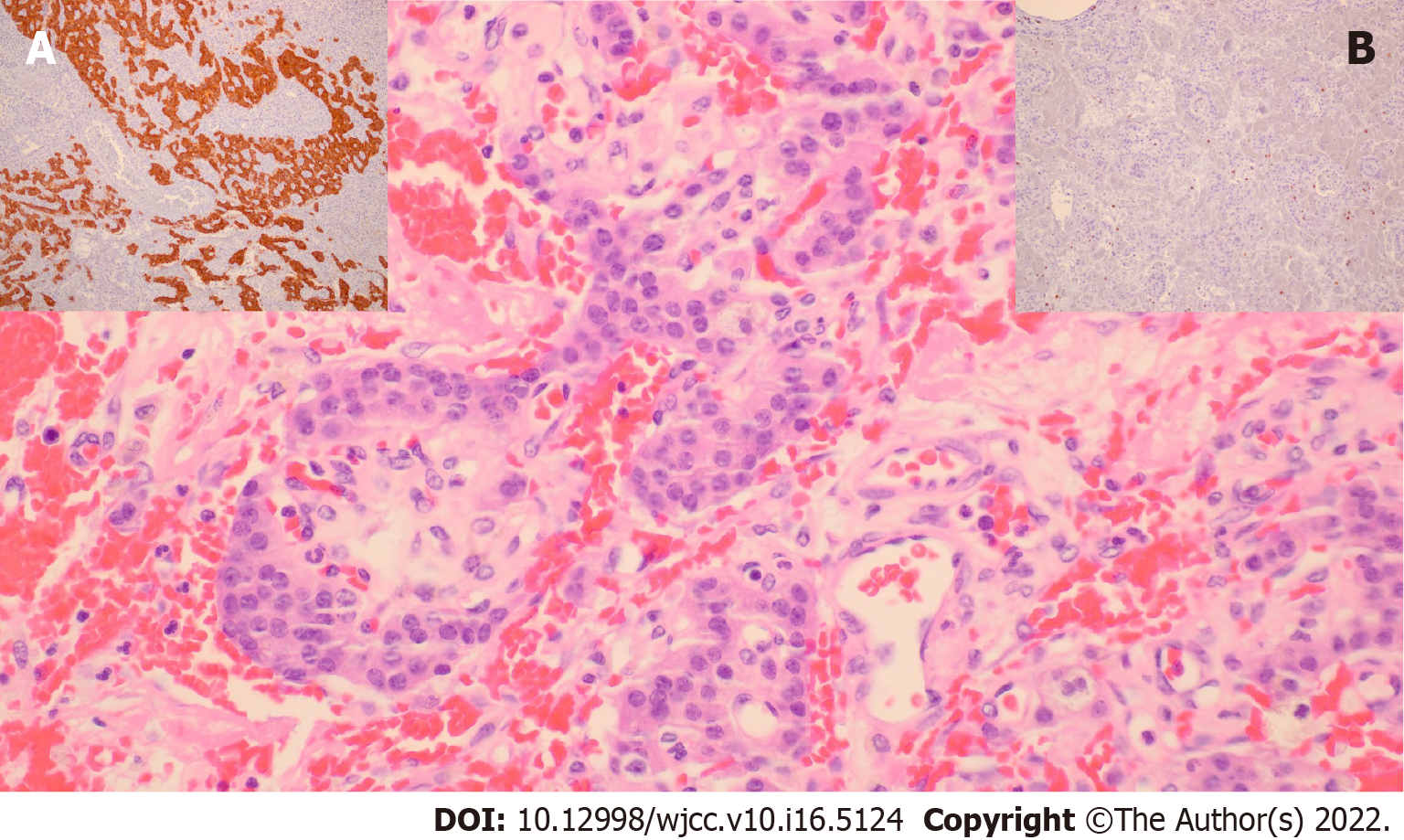
Microscopically, tumors show a trabecular, nested, and solid growth pattern with uniform cells and small round nuclei showing pepper-like chromatin. Their cytoplasm is eosinophilic and abundant. Immunohistochemically, cells are positive for neuroendocrine markers like synaptophysin and chromogranin; insulin staining is not mandatory for the diagnosis (Figure 2). Insulinoma tumor cells contain less insulin and secretory granules than normal β-cells but higher levels of proinsulin.
Malignant forms can be expected in 4-14% of cases and occur more often in younger age (< 50 years) and in males (77%)[37]. Insulinemia and 2 to 3-fold higher C-peptide levels with hypoglycemia occurring within the first 8 h of the 72-h fast might suggest malignancy[38,39]. However, metastases are a sure sign of malignancy.
A retrospective series of 311 patients treated for insulinoma in Mayo Clinic showed biochemical and pathological differences between benign and malignant insulinoma[35]. Malignant cases had higher than expected insulin and proinsulin levels and tumors of a larger size (4.2 cm compared to 1.8 cm seen in patients with benign insulinoma). Both malignant and benign insulinoma occurred sporadically and in patients with MEN1 syndrome[35].
In the past decades, much effort has been made to form the risk stratification of pNETs, and staging and grading according to the Ki67 index have been proposed as a tool to differentiate between benign and malignant tumor forms.
In addition, chromosomal instability (6q loss, and gains in 12q, 14q, and 17q) was found to be a strong predictor of the metastatic potential of insulinoma, and when used combined with histopathologic parameters, tumor size, and Ki67 index, it might improve the early diagnosis of malignant insulinoma[40].
Patients presenting earlier with clinical symptoms of neuroglycopenia during the fast have distinctly worse histopathological tumor characteristics. Insulin concentrations at the beginning and at the end of the fast correlate with the Ki-67 index and tumor size, linking grading and size with hormonal activity in insulinoma. When applying the Ki67 5% cut-off between the patients in the GI and GII/III group, higher staged patients suffer from significantly lower glucose concentrations, and have increased insulin and C-peptide levels, and a higher insulin secretion index compared to the GI group. Additionally, the tumor recurrence rate is about threefold higher in GII/GIII stage when the same Ki67 cut-off is applied compared to GI stage tumors[41].
A series of 103 insulinoma patients from Brazil showed a greater preponderance of malignant forms in males, with higher chromogranin A levels, shorter duration of symptoms, lower blood glucose (BG) levels, higher insulin and C-peptide levels, larger tumor size, and a tumor primarily located in the pancreatic head. Malignant tumors were quite aggressive, with a mortality rate nearing 85% and five-year average survival of 24%. Interestingly, among the malignant insulinomas, time to death varied from few months to up to 25 years after the diagnosis. Thus, some malignant insulinomas seemed to have more aggressive and others more indolent behavior. In this case series, most patients (77%) had malignant insulinoma at the time of diagnosis, and 23% had this diagnosis during follow-up[36].
Similar survival rates were recorded in a widely cited 50-year-long follow-up of 237 insulinoma patients (among whom 13 with metastatic forms) at Mayo Clinic where the 10-year postoperative survival was 88% of patients with benign compared to 29% of patients with metastatic disease[42]. In a more recent study, based on the data of 81 patients with metastatic insulinoma from 30 European NET registries, a 5-year survival improved to 55.6%[43].
More contemporary analysis of malignant insulinoma cases suggests their origin from non-functioning PNETs, developing phenotypic shift characterized by metachronous insulin hypersecretion after several years. Non-functioning PNET cells originally probably harbor rare cells expressing insulin and/or proinsulin, but the quantity of their concentration in circulation is initially too small to cause clinically recognizable symptoms. Only after the tumor burden grows large enough, usually with liver metastasis, the tumor cells can produce the amounts of insulin and proinsulin large enough to cause hypoglycemia[44]. Therefore, nonfunctioning PNETs, especially when accompanied by elevated chromogranin A levels (an ubiquitous NET marker which is usually low in case of benign tumors) as well as pancreastatin, need to be surveilled more closely, while they potentially give rise to malignant tumors. Moreover, at the time of diagnosis, in case benign insulinoma is initially suspected, additional molecular and genetic markers, such as α-internexin, might add awareness of malignant potential[45].
Recurrence of the tumor after initial surgery has always been accompanied by hypoglycemia, therefore continuing regular monitoring of BG levels is needed during patients visits[36].
Additionally, there was an attempt to analyze postoperative hyperglycemia as a prognostic sign after resection. The data coming from a small patient series could not differentiate immediate or perioperative glucose levels (as a threshold BG > 10 mmol/L) by tumor size, insulin production, and/or malignancy, making it an unreliable marker for surgical cure/residual tumor[46]. On the other hand, high proinsulin levels and large proinsulin/insulin molar ratio (although at present there is no unique cut-off value) are indicative of malignant insulinoma, therefore a new term “proinsulinoma” might be more appropriate for malignant tumor forms[45].
A comprehensive approach is required to treat malignant insulinoma, and includes medication, surgery, and interventional therapy.
Regardless of benign or malignant insulinoma, behavior dietary modifications, treatment with diazoxide, and octreotide or pasireotide represent plausible options for hypoglycemia avoidance[37,47-49] and patients with the malignant type more often demonstrate pronounced neuroglycopenic symptoms[50].
In case of malignant insulinoma, extensive surgical procedures, including complete oncological resection along with regional lymph nodes dissections, are warranted[35]. Since more than 80% of the patients have liver metastasis[50], hepatic burden can be treated by hepatic resection or hepatic artery embolization combined with doxorubicin or streptozotocin[51,52]. Liver transplantation represents a treatment option for macroscopic and concealed micrometastases in the liver[53] after curative resection of primary insulinoma, for selected patients with less than 50% of liver involvement and Ki-67 index of less than 5 to 10%[54].
Antiproliferative effect of everolimus and sunitinib prolongs progression-free survival in patients with unresectable or metastatic insulinoma[55,56]. In case of failure, chemotherapy protocol with streptozotocin, doxorubicin, and/or 5-fluorouracil for G1 and G2, or cisplatin with etoposide for G3 malignant insulinoma should be considered[57].
In patients with somatostatin receptor positive tumors, peptide receptor radionuclide therapy with radiolabelled peptides yttrium (90Y) or lutetium (177Lu) linked to a somatostatin analogue significantly extends progression-free survival[58,59].
In context of insulinoma management, surgical procedures can be potentially curable, in addition treatments with newer agents prolong progression-free survival of patients with malignant insulinoma. Cytoreductive surgery is favorable to control hormone secretion. Moreover, patients’ quality of life is improved by new hypoglycemia detection technological tools (continuous glucose monitoring systems-CGM) that help patients in real time glucose monitoring and through audio and vibration-based alerts prevent severe hypoglycemic episodes[60].
Biochemical and imaging follow-up in patients with insulinoma should be done frequently: Every 3 to 6 mo for G1 and G2, and every 2 to 3 mo for G3, along with yearly somatostatin receptor imaging or FDG PET (if positive at diagnosis)[61].
The diagnostic and therapeutic pathway of an insulinoma patient mostly relies on the awareness of clinical symptoms prerogative to a single specialist such as gastroenterologists, endocrinologists, or oncologists. However, an integrated approach in a form of a multidisciplinary team combined with current NET related guidelines should aspire to improve clinical management and outcomes[62].
Malignant insulinomas, besides hypoglycemia-related symptoms, cause morbidity and mortality due to their local aggressiveness. To our current knowledge, the presence of metastasis differentiates benign from malignant insulinoma since histological criteria are insufficient. Detailed pathway-specific expression profiling would improve the understanding of malignant insulinoma pathogenesis, and identification of specific genes could predict the long-term outcomes of insulinoma management. The small number of affected patients precludes quality research of therapeutic options. Further research is mandatory to improve the individualization of treatment for patients with insulinoma, particularly malignant variants.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Sahin TT, Turkey; Yang F, China S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Vaidakis D, Karoubalis J, Pappa T, Piaditis G, Zografos GN. Pancreatic insulinoma: current issues and trends. Hepatobiliary Pancreat Dis Int. 2010;9:234-241. [PubMed] |
| 2. | Mehrabi A, Fischer L, Hafezi M, Dirlewanger A, Grenacher L, Diener MK, Fonouni H, Golriz M, Garoussi C, Fard N, Rahbari NN, Werner J, Büchler MW. A systematic review of localization, surgical treatment options, and outcome of insulinoma. Pancreas. 2014;43:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 3. | Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World J Gastroenterol. 2013;19:829-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (4)] |
| 4. | Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 746] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 5. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19515] [Article Influence: 780.6] [Reference Citation Analysis (0)] |
| 6. | Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ, Hou BH. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020;26:2305-2322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (5)] |
| 7. | Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA Jr, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1115] [Cited by in RCA: 907] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 8. | Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A. 2005;102:14659-14664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci U S A. 2003;100:10770-10775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Jin S, Mao H, Schnepp RW, Sykes SM, Silva AC, D'Andrea AD, Hua X. Menin associates with FANCD2, a protein involved in repair of DNA damage. Cancer Res. 2003;63:4204-4210. [PubMed] |
| 11. | Hessman O, Skogseid B, Westin G, Akerström G. Multiple allelic deletions and intratumoral genetic heterogeneity in men1 pancreatic tumors. J Clin Endocrinol Metab. 2001;86:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Starr J, Puebla G, McMillan J, Lewis JT, Kasi PM. Microsatellite Instability-High, Malignant Insulinoma With Brain Metastasis. Cureus. 2021;13:e16969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | La P, Schnepp RW, D Petersen C, C Silva A, Hua X. Tumor suppressor menin regulates expression of insulin-like growth factor binding protein 2. Endocrinology. 2004;145:3443-3450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Liu L, Broaddus RR, Yao JC, Xie S, White JA, Wu TT, Hamilton SR, Rashid A. Epigenetic alterations in neuroendocrine tumors: methylation of RAS-association domain family 1, isoform A and p16 genes are associated with metastasis. Mod Pathol. 2005;18:1632-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Cao Y, Gao Z, Li L, Jiang X, Shan A, Cai J, Peng Y, Li Y, Huang X, Wang J, Wei Q, Qin G, Zhao J, Jin X, Liu L, Wang W, Ning G. Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1. Nat Commun. 2013;4:2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | Vax VV, Bibi R, Diaz-Cano S, Gueorguiev M, Kola B, Borboli N, Bressac-de Paillerets B, Walker GJ, Dedov II, Grossman AB, Korbonits M. Activating point mutations in cyclin-dependent kinase 4 are not seen in sporadic pituitary adenomas, insulinomas or Leydig cell tumours. J Endocrinol. 2003;178:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Tomita T. Cyclin-dependent kinase (cdk6) and p16 in pancreatic endocrine neoplasms. Pathology. 2004;36:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Lubomierski N, Kersting M, Bert T, Muench K, Wulbrand U, Schuermann M, Bartsch D, Simon B. Tumor suppressor genes in the 9p21 gene cluster are selective targets of inactivation in neuroendocrine gastroenteropancreatic tumors. Cancer Res. 2001;61:5905-5910. [PubMed] |
| 19. | Allen A, Qin ACR, Raj N, Wang J, Uddin S, Yao Z, Tang L, Meyers PA, Taylor BS, Berger MF, Yaeger R, Reidy-Lagunes D, Pratilas CA. Rare BRAF mutations in pancreatic neuroendocrine tumors may predict response to RAF and MEK inhibition. PLoS One. 2019;14:e0217399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Tannapfel A, Vomschloss S, Karhoff D, Markwarth A, Hengge UR, Wittekind C, Arnold R, Hörsch D. BRAF gene mutations are rare events in gastroenteropancreatic neuroendocrine tumors. Am J Clin Pathol. 2005;123:256-260. [PubMed] |
| 21. | Lai TH, Ahmed M, Hwang JS, Zada S, Pham TM, Elashkar O, Kim DR. Transcriptional Repression of Raf Kinase Inhibitory Protein Gene by Metadherin during Cancer Progression. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Hsieh ET, Shepherd FA, Tsao MS. Co-expression of epidermal growth factor receptor and transforming growth factor-alpha is independent of ras mutations in lung adenocarcinoma. Lung Cancer. 2000;29:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Zhan HX, Cong L, Zhao YP, Zhang TP, Chen G, Zhou L, Guo JC. Activated mTOR/P70S6K signaling pathway is involved in insulinoma tumorigenesis. J Surg Oncol. 2012;106:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Jonkers YM, Claessen SM, Veltman JA, Geurts van Kessel A, Dinjens WN, Skogseid B, Ramaekers FC, Speel EJ. Molecular parameters associated with insulinoma progression: chromosomal instability vs p53 and CK19 status. Cytogenet Genome Res. 2006;115:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Pavelic K, Hrascan R, Kapitanovic S, Vranes Z, Cabrijan T, Spaventi S, Korsic M, Krizanac S, Li YQ, Stambrook P, Gluckman JL, Pavelic ZP. Molecular genetics of malignant insulinoma. Anticancer Res. 1996;16:1707-1717. [PubMed] |
| 26. | Batta K, Kundu TK. Activation of p53 function by human transcriptional coactivator PC4: role of protein-protein interaction, DNA bending, and posttranslational modifications. Mol Cell Biol. 2007;27:7603-7614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Wang DG, Johnston CF, Buchanan KD. Oncogene expression in gastroenteropancreatic neuroendocrine tumors: implications for pathogenesis. Cancer. 1997;80:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Ekeblad S, Lejonklou MH, Stålberg P, Skogseid B. Prognostic relevance of survivin in pancreatic endocrine tumors. World J Surg. 2012;36:1411-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Jonkers YM, Claessen SM, Feuth T, van Kessel AG, Ramaekers FC, Veltman JA, Speel EJ. Novel candidate tumour suppressor gene loci on chromosomes 11q23-24 and 22q13 involved in human insulinoma tumourigenesis. J Pathol. 2006;210:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Queiroz Almeida M, Machado MC, Correa-Giannella ML, Giannella-Neto D, Albergaria Pereira MA. Endogenous hyperinsulinemic hypoglycemia: diagnostic strategies, predictive features of malignancy and long-term survival. J Endocrinol Invest. 2006;29:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Bégu-Le Corroller A, Valéro R, Moutardier V, Henry JF, Le Treut YP, Gueydan M, De Micco C, Sierra M, Conte-Devolx B, Oliver C, Raccah D, Favre R, Digue L, Heim M, Seitz JF, Delpero JR, Vialettes B. Aggressive multimodal therapy of sporadic malignant insulinoma can improve survival: a retrospective 35-year study of 12 patients. Diabetes Metab. 2008;34:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Baudin E, Caron P, Lombard-Bohas C, Tabarin A, Mitry E, Reznick Y, Taieb D, Pattou F, Goudet P, Vezzosi D, Scoazec JY, Cadiot G, Borson-Chazot F, Do Cao C; Société française d’endocrinologie; Groupe d’étude des tumeurs endocrines. Malignant insulinoma: recommendations for characterisation and treatment. Ann Endocrinol (Paris). 2013;74:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Kloeppel G. Pancreatic neuroendocrine neoplasias. In: The WHO Classification of Endocrine Tumors. Lyon, France: IARC Press; 2017: 209-240. |
| 34. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 35. | Sada A, Yamashita TS, Glasgow AE, Habermann EB, Thompson GB, Lyden ML, Dy BM, Halfdanarson TR, Vella A, McKenzie TJ. Comparison of benign and malignant insulinoma. Am J Surg. 2021;221:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 36. | Câmara-de-Souza AB, Toyoshima MTK, Giannella ML, Freire DS, Camacho CP, Lourenço DM Jr, Rocha MS, Bacchella T, Jureidini R, Machado MCC, Almeida MQ, Pereira MAA. Insulinoma: A retrospective study analyzing the differences between benign and malignant tumors. Pancreatology. 2018;18:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Brown E, Watkin D, Evans J, Yip V, Cuthbertson DJ. Multidisciplinary management of refractory insulinomas. Clin Endocrinol (Oxf). 2018;88:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798-7803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 39. | Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L, Tomassetti P, Delle Fave G, Falconi M. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011;29:2372-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 40. | Jonkers YM, Claessen SM, Perren A, Schmid S, Komminoth P, Verhofstad AA, Hofland LJ, de Krijger RR, Slootweg PJ, Ramaekers FC, Speel EJ. Chromosomal instability predicts metastatic disease in patients with insulinomas. Endocr Relat Cancer. 2005;12:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Wolf P, Winhofer Y, Smajis S, Anderwald CH, Scheuba C, Niederle B, Gessl A, Luger A, Krebs M, Koperek O. Clinical presentation in insulinoma predicts histopathological tumour characteristics. Clin Endocrinol (Oxf). 2015;83:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991;66:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 542] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 43. | Lepage C, Ciccolallo L, De Angelis R, Bouvier AM, Faivre J, Gatta G; EUROCARE working group. European disparities in malignant digestive endocrine tumours survival. Int J Cancer. 2010;126:2928-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | de Mestier L, Hentic O, Cros J, Walter T, Roquin G, Brixi H, Lombard-Bohas C, Hammel P, Diebold MD, Couvelard A, Ruszniewski P, Cadiot G. Metachronous hormonal syndromes in patients with pancreatic neuroendocrine tumors: a case-series study. Ann Intern Med. 2015;162:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Yu R, Nissen NN, Hendifar A, Tang L, Song YL, Chen YJ, Fan X. A Clinicopathological Study of Malignant Insulinoma in a Contemporary Series. Pancreas. 2017;46:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Nockel P, Tirosh A, El Lakis M, Gaitanidis A, Merkel R, Patel D, Nilubol N, Sadowski SM, Cochran C, Gorden P, Kebebew E. Incidence and management of postoperative hyperglycemia in patients undergoing insulinoma resection. Endocrine. 2018;61:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Gill GV, Rauf O, MacFarlane IA. Diazoxide treatment for insulinoma: a national UK survey. Postgrad Med J. 1997;73:640-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC; North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 444] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 49. | Hendren NS, Panach K, Brown TJ, Peng L, Beg MS, Weissler J, Mirfakhraee S. Pasireotide for the treatment of refractory hypoglycaemia from malignant insulinoma. Clin Endocrinol (Oxf). 2018;88:341-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Yu J, Ping F, Zhang H, Li W, Yuan T, Fu Y, Feng K, Xia W, Xu L, Li Y. Clinical Management of Malignant Insulinoma: a single Institution's experience over three decades. BMC Endocr Disord. 2018;18:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Steinmüller T, Kianmanesh R, Falconi M, Scarpa A, Taal B, Kwekkeboom DJ, Lopes JM, Perren A, Nikou G, Yao J, Delle Fave GF, O'Toole D; Frascati Consensus Conference participants. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 52. | Kress O, Wagner HJ, Wied M, Klose KJ, Arnold R, Alfke H. Transarterial chemoembolization of advanced liver metastases of neuroendocrine tumors--a retrospective single-center analysis. Digestion. 2003;68:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, Beal EW, Felekouras E, Vernadakis S, Fung JJ, Pawlik TM. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery. 2017;162:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 54. | Lim C, Lahat E, Osseis M, Sotirov D, Salloum C, Azoulay D. Liver Transplantation for Neuroendocrine Tumors: What Have We Learned? Semin Liver Dis. 2018;38:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2117] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 56. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1829] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 57. | Eriksson B, Annibale B, Bajetta E, Mitry E, Pavel M, Platania M, Salazar R, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: chemotherapy in patients with neuroendocrine tumors. Neuroendocrinology. 2009;90:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O'Dorisio MS, O'Dorisio TM, Howe JR, Cremonesi M, Kwekkeboom DJ, Zaknun JJ. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 532] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 59. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2239] [Article Influence: 279.9] [Reference Citation Analysis (0)] |
| 60. | Rodbard D. Continuous Glucose Monitoring: A Review of Recent Studies Demonstrating Improved Glycemic Outcomes. Diabetes Technol Ther. 2017;19:S25-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 289] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 61. | Knigge U, Capdevila J, Bartsch DK, Baudin E, Falkerby J, Kianmanesh R, Kos-Kudla B, Niederle B, Nieveen van Dijkum E, O'Toole D, Pascher A, Reed N, Sundin A, Vullierme MP; Antibes Consensus Conference Participants; Antibes Consensus Conference participants. ENETS Consensus Recommendations for the Standards of Care in Neuroendocrine Neoplasms: Follow-Up and Documentation. Neuroendocrinology. 2017;105:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 62. | Spada F, Rossi RE, Kara E, Laffi A, Massironi S, Rubino M, Grimaldi F, Bhoori S, Fazio N. Carcinoid Syndrome and Hyperinsulinemic Hypoglycemia Associated with Neuroendocrine Neoplasms: A Critical Review on Clinical and Pharmacological Management. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |