Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4301
Peer-review started: December 29, 2021
First decision: January 25, 2022
Revised: February 4, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: May 6, 2022
Processing time: 121 Days and 21.7 Hours
Primary intracranial extraskeletal myxoid chondrosarcoma (EMC) is an extremely rare low- to intermediate-grade malignant soft tissue sarcoma, and only 15 cases have been reported in the literature. Due to its rarity, clinical data and research on this tumor type are extremely limited, the pathogenesis and histological origin are still unclear, and the diagnostic and standard clinical treatment strategies for intracranial EMC remain controversial and undefined.
We reported a case of a 52-year-old male who was admitted to the hospital with headache and dizziness for 1 mo, and his health status deteriorated during the last week. CT of the head showed a well-defined low-density lesion situated in the left cavernous sinus. Brain magnetic resonance imaging (MRI) showed a 3.4 cm × 3.0 cm sized, well-defined, round-shaped and heterogeneously enhanced lesion located in the left cavernous sinus. The entire lesion was removed via supratentorial craniotomy and microsurgery. Postoperative pathological diagnosis indicated primary intracranial EMC. Subsequently, the patient underwent 45 Gy/15 F stereotactic radiotherapy after discharge. At present, it is 12 mo after surgery, with regular postoperative follow-up and regular MRI examinations, that there are no clinical symptoms and radiographic evidence indicating the recurrence of the tumor, and the patient has returned to normal life.
Currently, the most beneficial treatment for primary intracranial EMC is gross total resection combined with postoperative radiotherapy. Long-term follow-up is also necessary for patients.
Core Tip: Primary intracranial extraskeletal myxoid chondrosarcoma (EMC) is an extremely rare intracranial neoplasm, and only 15 cases have been reported in the literature. We report herein an extremely rare case, which is also the first case of primary EMC occurring in the cavernous sinus. Primary intracranial EMC is indolent in growth yet has a high recurrence rate after total resection. In these cases, we observed the importance of postoperative radiotherapy that can improve the outcome of patients with primary intracranial EMC. Surgical total resection combined with postoperative radiotherapy can prolong progression-free survival and decrease the recurrence rate. Meanwhile, long-term follow-up is also necessary for patients after surgery. In addition, primary intracranial EMC should also be considered when diagnosing and distinguishing a lesion in the cavernous sinus.
- Citation: Zhu ZY, Wang YB, Li HY, Wu XM. Primary intracranial extraskeletal myxoid chondrosarcoma: A case report and review of literature. World J Clin Cases 2022; 10(13): 4301-4313
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4301.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4301
Extraskeletal myxoid chondrosarcoma (EMC) is an ultrarare type of low- to intermediate-grade malignant soft tissue tumor (STS) comprising small round monomorphic cells and has a low incidence — less than 1/1000000 people are diagnosed annually[1]. It mainly occurs in deep soft tissues of the proximal lower extremities and limb girdles, especially in the thigh and popliteal fossa[1,2]. Minor cases have been found in the distal extremities, thorax, enterocoelia, trunk, head and neck region, retroperitoneum and paraspinal soft tissue, and even in bone[3-5]. Primary intracranial EMC is extremely rare, with only 15 cases reported in the literature. While EMC is considered to be a low-grade malignant neoplasm with a prolonged clinical course and indolent growth pattern, long-term follow-up demonstrated high local recurrence and metastasis rates after surgery (35%-50% and 25%-50%, respectively)[1]. Herein, we present a case of primary intracranial EMC located in the left cavernous sinus of a 52-year-old male diagnosed through histopathological and immunohistochemical examination. To the best of our knowledge, this is the first case of primary intracranial EMC arising in the cavernous sinus. Furthermore, we collected information on the existing 15 cases and the present case, summarized the radiographic, histopathological and clinical features of this extraordinarily rare tumor, and reviewed and discussed current research on the histological origin, genetic mutations, diagnosis, treatment strategies and prognosis of primary intracranial EMC to provide greater clinical understanding of this disease.
A 52-year-old male patient was admitted to our department with complaints of a moderate intermittent headache and dizziness for more than 1 mo.
The patient’s symptoms started from more than 1 mo with a moderate intermitted headache and dizziness, and his health status deteriorated during the last 7 d. There was no obvious nausea, vomiting, blurred vision or disturbance of consciousness.
The patient had a normal and healthy condition in the past; no past history of chronic heart, liver, kidney, lung diseases or infectious diseases; and no past history of head trauma or surgery.
The patient had a past history of smoking and alcohol consumption for more than 30 years, had already quit smoking for 5 years and had quit drinking for 1 year. There was no special family history.
During the neurological examination, we found that the patient had mild abducent paralysis on his left eyeball with limited eye movement. No abnormities were found in other physical exams.
No abnormities were found in the laboratory examinations.
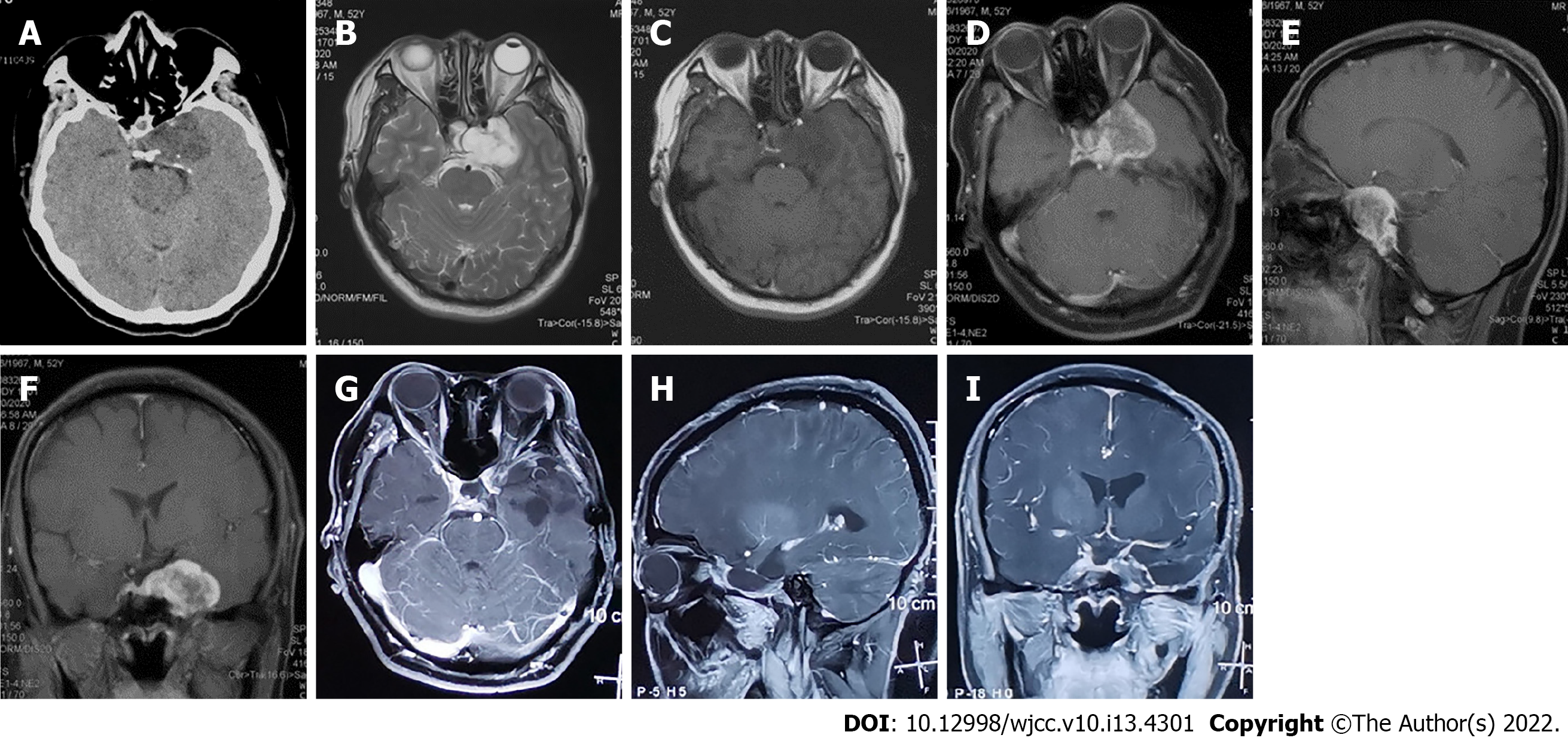
A plain computed tomography (CT) scan of the head revealed a homogeneous low-density and round shape-occupying lesion in his left cavernous sinus. No hemorrhage, calcification or bone destructive lesions were noted on CT (Figure 1A). The magnetic resonance imaging (MRI) scan of the head revealed a 3.4 × 3.0 cm sized, well-defined mass with an irregularly round shape located in the left cavernous sinus and simultaneously involving the sellar region and right cavernous sinus. The tumor was homogeneously hypointense on T1-weighted imaging (T1WI) and heterogeneously hyperintense on T2-weighted imaging (T2WI) with septal or stripe-like iso-hypointensity in the central area and significant hyperintensity in the paracentral area (Figure 1B and C). A gadolinium injection-enhanced MRI scan of the head also revealed a well-defined and heterogeneous well-enhanced tumor with an irregular round shape that was mainly located in the left cavernous sinus and involved the sellar area and right cavernous sinus. In addition, the tumor was not or slightly enhanced in the central part but was significantly enhanced in the remaining part (Figure 1D-F). No abnormities were found in plain chest and abdominal CT scans.
According to the features of CT and MRI scans of the head described above, the preoperative diagnosis was cavernous sinus hemangioma or meningioma; however, the nature and diagnosis of the lesion is hard to determine.
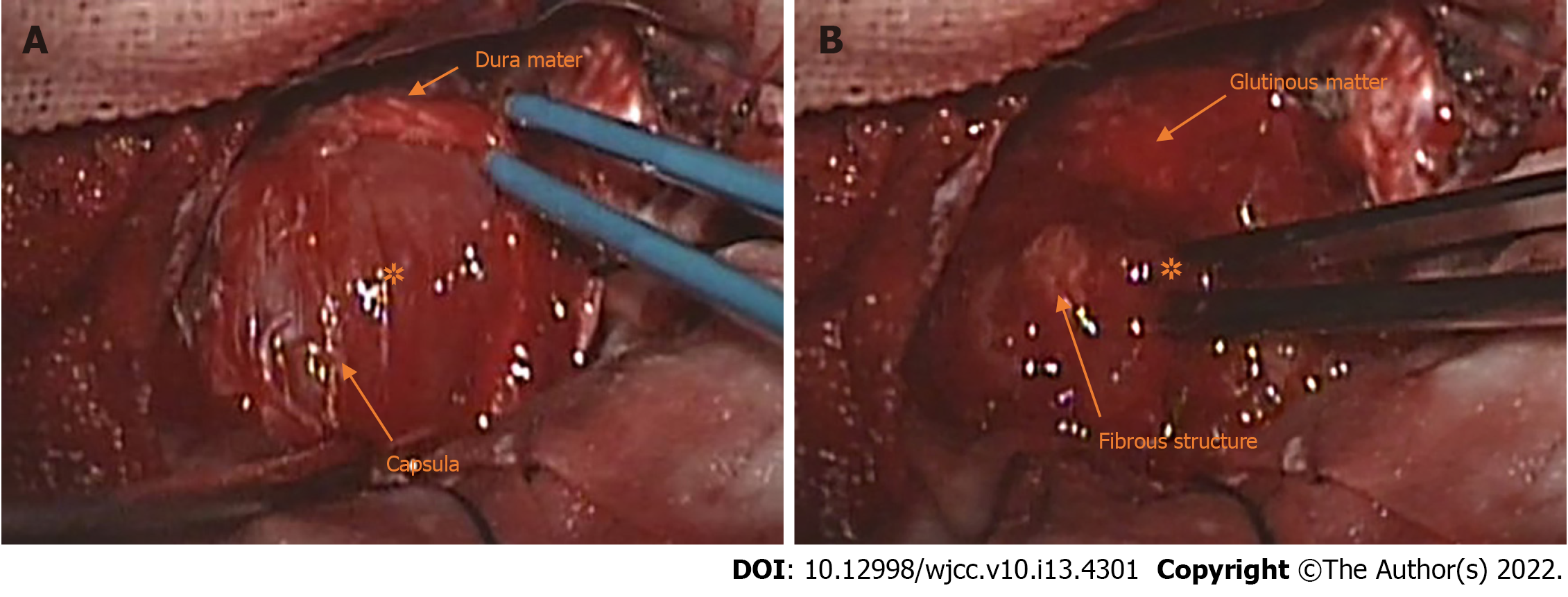
The patient underwent surgery, and the tumor was totally removed from his left cavernous sinus through a supratentorial pterion approach craniotomy and microsurgery. After the surface dura mater of the left cavernous sinus was dissected, we observed that the mass was wrapped by gray–white capsula that adhered tightly to the adjacent dura mater (Figure 2A). The tumor was gray–red in color, with a soft gelatinous texture and moderate vascularity. Notably, it contained abundant glutinous matter. Intratumor gray–white fibrous structures were also observed (Figure 2B). The tumor was grossly totally resected, yet persistent abducent paralysis existed in the patient's left eyes accompanied by diplopia and blurred vision after surgery. The patient had an uneventful recovery after surgery and was discharged 10 days later; shortly thereafter, he was transferred to the oncology department for 45Gy/15F X-ray radiotherapy. The postoperative management conformed to the multidisciplinary treatment (MDT) modality and was made by our surgeons, pathologists, radiologists and oncologists after evaluating and discussing the results of the postoperative pathological exam, the patient’s brain MRI, which was performed before discharge, and the general condition of the patient.
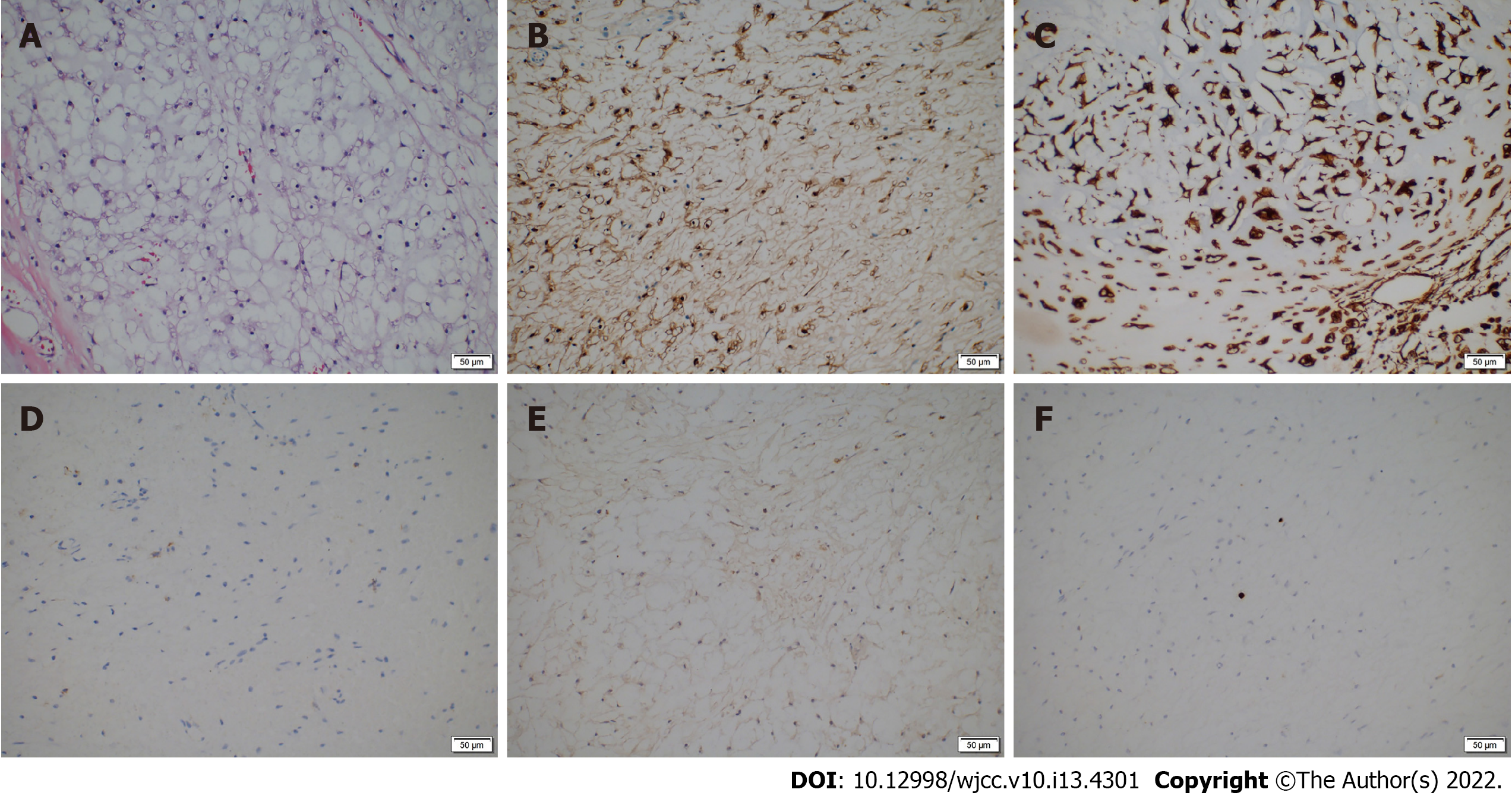
The tumor tissue was fixed in 10% formalin for histological examination. Immunohistochemical analysis was also undertaken and indicated that the tumor was positive for Vimentin, S-100 protein, and lysozyme and negative for epithelial membrane antigen (EMA), CK-pan, CK-7, CK-5, CK-19, and CK-20; the Ki-67 index was less than 1% (Figure 3A-F). According to the results of the histological and immunohistochemical analyses, the Director of Pathology Department of The First Hospital affiliated with Jilin University and her colleagues discussed the pathological features of this lesion and finally diagnosed it as primary intracranial EMC. We performed regular follow-up by phone call contact and informed patients to have regular MRI exams every 3-6 mo after surgery. To date, no radiographic evidence or clinical symptoms have indicated tumor recurrence or metastasis (Figure 1G-I). Meanwhile, the diplopia and blurred vision caused by abducent paralysis in the left eye of the patient showed good improvement. The recovery of this patient 12 mo after the operation was smooth, and the patient returned to his normal life. Regular follow-up will be continued.
Primary intracranial extraskeletal myxoid chondrosarcoma (EMC) is extremely rare, and only 15 cases have been reported in the literature to date. The case presented herein is of a 52-year-old male patient with primary intracranial EMC that occurred in the left cavernous sinus. To the best of our knowledge, this is the first case of primary intracranial EMC arising in this area. We also collected information on 15 previously reported cases of primary intracranial EMC and the present case (shown in Table 1 and Table 2)[6-20] with the aim of performing a systematic review of this tumor type, and we discussed the epidemiological and radiographic features, diagnosis, treatment strategies and prognosis of this rare disease.
| Ref. | Gender/ages | Nation | Clinical symptoms | Size of tumor (cm) | Locations of tumor | Dura mater adhersion | Skull involvement | Treatment | Recurrence | Metastasis | Follow-up |
| Scott et al[6], 1976 | M/39 | United Kingdom | Headache, nausea, vomit | NA | 4th Ventricle | N | N | PR | N | N | 13 d |
| Smith and Davidson[7], 1981 | M/12 | United States | Headache, nausea, vomit, difficulty ambulating | 3.5 × 1.0 × 0.5 | Left cerebellum | Y | N | GTR | N | N | 13 mo |
| Salcman et al[8], 1992 | F/28 | United States | Headache, slow speech, right limb weakness | 7.0 × 5.0 × 4.0 | Left parafacine region | N | N | GTR | Y | N | 10 mo |
| Sato et al[9], 1993 | F/43 | Japan | Blurred vision, gait disturbance | NA | Pineal region | Y | N | PR, RT (60 Gy), chemotherapy | Y | Y | 36 mo |
| Chaskis et al[10], 2002 | F/17 | Spain | Headache, status epilepticus | NA | Right frontal-parietal lobe | Y | N | GTR | Y | N | 16 mo |
| González-Lois et al[11], 2002 | M/69 | Belgium | Headache, dizzy, behavior change | NA | Right frontal lobe | N | N | GTR | N | N | 1 mo |
| Im et al[12], 2003 | M/43 | South Korea | Headache, nausea, vomit | 2.0 | Left parietal lobe | N | N | GTR, RT (59.4 Gy) | N | N | 36 mo |
| Cummings et al[13], 2004 | M/63 | NA | Hearing loss, gait disturbance | 2.4 × 1.8 × 2.4 | Right jugular foramen, right CPA | N | NA | GTR | NA | NA | NA |
| Sorimachi et al[15], 2008 | F/37 | Japan | Headache, nausea, vomit, upward gaze palsy | NA | Pineal region | N | N | GTR, | Y | N | NA |
| O'Brien et al[14], 2008 | F/26 | Ireland | Headache, nausea, seizure | 2.5 | Left CPA | N | N | STR proton therapy | N | N | 12 mo |
| Arpino et al[16], 2011 | F/54 | Ukraine | Headache, left ophthalmopegia | NA | Sellar and parasellar area | NA | Y | GTR | NA | NA | 12 mo |
| Dulou et al[17], 2012 | F/70 | France | Behavior change, difficult in walk | NA | Left frontal lobe | N | N | GTR, preoperative RT (60 Gy) | Y | N | 10 mo |
| Park et al[18], 2012 | F/21 | South Korea | Headache, right limb weakness, bilateral hearing loss, bilateral vision loss | 3.2 × 6.3 × 4.9 | Left lateral ventricle | N | N | GTR, RT (60.8 Gy) | NA | NA | 6 mo |
| Qin et al[19], 2017 | F/41 | China | Headache, vomit | 3.0 × 3.0 × 3.0 | Left cerebellum | Y | Y | GTR, two stage of RT (56 Gy/50 Gy), chemotherapy | N | N | 20 mo |
| Akakin et al[20], 2018 | F/75 | United States | Right limb weakness | NA | Left parafacine region | Y | N | GTR | Y | N | 2 mo |
| Present case | M/52 | China | Headache, dizzy, nausea | 3.4 × 3.0 | Left cavernous sinus | Y | N | GTR, RT (45 Gy) | N | N | 12 mo |
Our study included 16 primary intracranial EMC cases (including the present case) consisting of 6 male patients (6/16, 37.5%) and 10 female patients (10/16, 62.5%) with a median first-onset age of 42 years (range 12-75 years), two of which were juveniles (2/16, 12.5%). The tumor size ranged from 2.0 cm to 7.0 cm (mean diameter was 3.2 cm). The sex ratio is nearly 1:1.7 for primary intracranial EMC, which is different from extracranial EMC in that the male/female ratio is nearly 2:1, with a significant predisposition for males. The median first-onset age of patients with extracranial EMC is 50-60 years[1,5]. The reason for the difference in the male/female incidence rate and median first-onset age between intracranial and extracranial EMC is unclear; we suspected it might result from the scarcity of primary intracranial EMC cases due to its exceeding rarity. To determine whether epidemiological differences such as M/F incidence and median first-onset age definitely existed between primary intracranial EMC and extracranial EMC, more cases and clinical data are needed.
The locations from which primary intracranial EMCs arise are varied and include the cerebellar hemisphere (n = 2), cerebellopontine angle (n = 2), pineal area (n = 2), sellar area (n = 1), cavernous sinus (present case), ventricle system (n = 2; one in the lateral ventricle and the other in the 4th ventricle) and cerebral hemisphere (n = 6; 4 in the frontal or parietal lobe and 2 in the parafalcine region). In most cases, the tumor had clear margins from the brain parenchyma and proved to be in an extra-axial lesion during surgery. Dulou et al[17] reported an extra-axial case situated in the left frontal lobe in which the tumor had a less clear margin and a deep location in the brain cortex. Six cases were found to have tight adhesion with adjacent dura mater, including the falx cerebri and tentorium; 2 cases were located in the ventricle system adhered to the choroid plexus; 2 cases were located in the cerebellopontine angle (CPA) tightly adhered to adjacent cranial nerves; and 4 cases in the cerebrum hemisphere had no relation with either the dura mater or brain parenchyma. One case in the pineal area reported by Sorimachi et al[15] adhered to the superior colliculus and connected with the thalamus by a bundle of blood vessels yet had no connection with the dura mater. In the present case, the tumor had a tight connection with the dura mater of the cavernous sinus. Two cases reported by Qin et al[19] and Arpino et al[16] had cranial bone involvement. According to the data, we found that all the cases, including cases in the ventricle system of primary intracranial EMC, were extra-axial lesions that occurred on the surface or shallow region of the brain cortex or in deep sites of the ventricle system, and cases with involvement in cranial bone or brain parenchyma were rare (3/16; 18.8%). Tumors usually had connections with the adjacent dura mater, nerves or choroid plexus (10/16; 62.5%).
The origin and differentiation of primary intracranial EMC remain unclear and controversial[21]. According to recent studies, EMC contains unique and special NR4A3 chimeric gene mutations induced by different chromosomal translocations and has been reconsidered as a new entity that is different from any other sarcoma. It was also categorized as a mesenchymal tumor with uncertain differentiation in the most recent version of the World Health Organization (WHO) classification of soft tissue and bone tumors[22]. Some researchers have pointed out that a neuroendocrine origin might be possible[21,23-25]. Different ideas about the origin of primary intracranial EMC have also been proposed by researchers. The main speculation is that primary intracranial EMC may originate from multifunctional mesenchymal cells situated in the dura mater, pia-arachnoid, choroid plexus, leptomeninges sheaths around blood vessels and walls of vessels in sulci[6,10,17]. In the present case, we presume that the tumor arose from multifunctional mesenchymal cells in the cavernous sinus.
The main examination modalities adopted for diagnosing primary intracranial EMC include CT of the head and brain MRI. The manifestations of primary intracranial EMC in CT exams vary; although tumors typically show iso/Low density, in some cases with intratumor hemorrhage or calcification, the density could be high or mixed. Intratumor hemorrhage and peritumor edema can be observed, yet calcification is rare (2/16, 12.5%)[6,20], which is different from previous studies reporting that calcification could be seen in more than 50% of cases of extracranial EMC and most cases of intracranial EMC[14,18]. On contrast CT, tumors usually show heterogeneous enhancement, while some could be homogeneous or not well enhanced. The manifestations of primary intracranial EMC in MRI exams are more consistent, as shown in Table 2. Tumors usually exhibit homogeneous hypointensity in T1WI, but the signal can be heterogeneous in cases with intratumor hemorrhage. Tumors commonly show heterogeneous hyperintensity on T2WI, and homogeneous signals can be observed in a few cases. In gadolinium injection-enhanced MRI, most cases were heterogeneously well enhanced, and in some cases, the enhancement pattern can be lobulated or rim/ring-like. Few cases show homogeneous enhancement.
| Ref. | T1WI | T2WI | Enhanced MRI or other exams |
| Scott et al[6], 1976 | NA | NA | NA |
| Smith and Davidson[7], 1981 | NA | NA | NA |
| Salcman et al[8], 1992 | Well-defined, hyperintensity | Homogeneous hyperintensity | NA |
| Sato et al[9], 1993 | NA | NA | NA |
| Chaskis et al[10], 2002 | Hypointensity | NA | Heterogeneous enhancement |
| González-Lois et al[11], 2002 | NA | NA | Significantly homogeneous enhancement |
| Im et al[12], 2003 | Unclear-defined, hypointensity | Hyperintensity | Homogeneously well enhanced |
| Cummings et al[13], 2004 | NA | NA | Heterogeneous enhancement |
| Sorimachi et al[15], 2008 | Mixed signal intensity, hyperintensity (hemorrhage) | NA | Heterogeneous enhancement |
| O'Brien et al[14], 2008 | Hypointensity | Hyperintensity | NA |
| Arpino et al[16], 2011 | Hypointensity | Hyperintensity | Heterogeneously peripheral enhancement |
| Dulou et al[17], 2012 | NA | Hyperintensity, peritumor edema | Heterogeneously ring-like enhancement |
| Park et al[18], 2012 | Homogeneous iso-intensity | Heterogeneous hyperintensity, Peritumor edema | Heterogeneously lobulated enhancement |
| Qin et al[19], 2017 | NA | NA | NA |
| Akakin et al[20], 2018 | NA | Heterogeneous hyperintensity | Heterogeneously rim-like enhancement, DWI showed intratumor calcification |
| Present case | Homogeneous hypointensity | Heterogeneous hyperintensity | Heterogeneously well enhanced |
The clinical manifestations of primary intracranial EMC are diverse and nonspecific, including tumor-related increases in intracranial pressure and the associated symptoms of headache, nausea, and vomiting as well as nervous system dysfunction, which manifest as epilepsy[11,14], vision or hearing disturbances, behavioral changes, limb weakness and difficulty walking and speaking. Tumors located in the ventricle system or near the brain stem could also cause hydrocephalus[9,14,18]. In the present case, the patient suffered from abducent paralysis in the left eye, which was due to tumor compression of the abduct nerve in the cavernous sinus.
The prevalent methods for diagnosing primary intracranial EMC rely on histopathology and immunohistochemistry analyses. EMC shows distinctive histological features: under light microscopy with hematoxylin-eosin (H/E) staining, the tumor has a multilobulated pattern with fibrous septa extending into the deep part of the tumor, and the tumor is composed of uniformly shaped small, oval, spindle or round-like cells that have eosinophilic cytoplasm and small round nuclei and are immersed in abundant myxoid extracellular stroma. The formation of mature hyaline cartilage is rare. Tumor cells commonly interconnect and arrange in cords or nests. Small clusters and complex trabecular or cribriform arrays have also been observed in some cases, and the mitotic activity is usually low. However, features such as high mitotic activity, cellular density, dedifferentiated rhabdoid or pleomorphic epithelioid tumor cells have been observed in some postoperative recurrent cases, indicating a more aggressive and higher grade of recurrent neoplasm. The main differential diagnosis of EMC includes sarcomas, which have morphological or histological features similar to those of EMC in histopathological exams, such as epithelioid leiomyosarcoma, epithelioid angiosarcoma, chordoma, parachordoma, myoepithelioma and rhabdoid tumor[5,26-28]. Relying only on histological examination for diagnosis can be challenging due to the wide histological spectrum and diverse morphological characteristics of EMC; thus, immunohistochemistry should also be employed to further diagnose and differentiate sarcomas that have histological features similar to those of EMC[25,29].
The immunohistochemistry results of primary intracranial EMC have indicated that the cases are positive for vimentin, with some cases expressing EMA and S-100 protein, and negative for CK series such as CK-5/6, CK-7, CK-19, CK-20, CK-pan, and GFAP. Synaptophysin (3/3, 100%) and NSE (2/2, 100%) negativity was found in 3 and 2 intracranial cases, respectively[11,14,15,20], yet they have been reported to be positive in some extracranial EMC cases and have been thought to reflect the neuroendocrine origin of EMC[21,23,24,30]. In addition, tumors were reported to be negative for chromogranin (2/2,100%) in two cases[15,20] yet positive in some extracranial EMC cases and have been thought to be related to neuroendocrine origin[23,31].
Because the pathological features of EMC are diverse and varied, making a precise diagnosis by pathology can be difficult in some cases[25]. In 1995, Stenman et al[32] found a unique NR4A3-related gene rearrangement mutation that existed only in EMC; subsequently, Noguchi et al[33] developed and proposed the use of NR4A3 and EWSR probes for fluorescence in situ hybridization to detect whether tumor cells contain NR4A3 gene rearrangement mutations to diagnose EMC more accurately. The most common type of genetic mutation in EMC is the ESWR1-NR4A3 gene (over 70%), which is caused by reciprocal chromosomal translocation—t (9;22) (q31.1; q12.2); second, the TAF15-NR4A3 gene (approximately 20%) and rare variants of NR4A3 fusion partners (less than 5%), including FUS, TCF12, TGF and HSPA8[1,34]. Other potential diagnostic markers, such as NMB and INSM-1, have also been reported[25,29]. Genetic mutation detection is considered to be the most precise method for diagnosing EMC and distinguishing EMC from other tumors with similar histopathological features[35]. However, it has been limited in its application in regular clinics and hospitals due to its expense and need for high levels of clinical expertise[29]; therefore, of the 16 cases of primary intracranial EMC (including the present case), only 1 patient underwent molecular detection and was found to be positive for EWSR1-NR4A3 gene mutation[13]. In the present case, the patient and his family refused molecular testing due to its cost. Thus, identifying and developing cheaper, more available and precise diagnostic approaches are necessary. The effects of molecular tests on diagnosing primary intracranial EMC still need more data and research for verification.
Soft tissue sarcoma (STS) is a rare mesenchymal neoplasm that, nevertheless, contains more than 70 subtypes, and the management and prognosis of patients can vary significantly between different subtypes[36,37]. Only relying on preoperative radiographic exams and empirical diagnosis sometimes causes misdiagnosis[38]. Pathological examination is the gold standard of diagnosis of STS and is an indispensable method that accurately indicates the pathological natures of intracranial lesions, such as neoplastic or nonneoplastic, benign or malignant, degree of malignancy, progression, pathological subtype and molecular features, and is also the core method that provides crucial and valuable guidance for surgeons, radiologists and oncologists to make proper and beneficial treatments of STS. Thus, accurate diagnosis with the basis of pathological examination is critical for the management of STS and should be diagnosed by expert pathologists due to the various and complicated pathological features of STS[37,39]. Meanwhile, management should be discussed and performed by a multidisciplinary tumor board (MTB) once the lesion is preoperatively suspected to be STS[37,39,40]. Thus, all of the deep and superficial lesions in soft tissue that have diameters over 5 cm should undergo preoperative biopsy and pathological examination, and biopsy is also considered mandatory before treatment[37,39,41]. With regard to intracranial lesions, especially to suspected malignancies, stereotactic frame-based or frameless brain biopsy is recommended to increase the accuracy of preoperative diagnosis and provide guidance for appropriate treatments, including lesion resection, adjuvant radiotherapy and chemotherapy[42,43]. With the guidance of CT, MRI and positron emission tomography (PET) technologies, stereotactic brain biopsy is considered to be a safe, less aggressive and effective means to obtain tissue from intracranial lesions and is generally suitable for patients with the following conditions: (1) Multiple intracranial lesions; (2) The lesion is in the deep locations of the brain, such as the brainstem, thalamus, callosum and basal ganglia, or functional cortical or subcortical areas; (3) The tumor cannot be totally removed by open microsurgery; (4) The general condition of patients is not tolerant to anesthesia, open craniotomy and microsurgery; (5) Patients who have risk factors such as advanced age, systematic disease, severe cardiac disease, etc.; and (6) Based on radiological and clinical manifestations, the preoperative diagnosis of lesions is intricate, ambiguous and unclear[38,42,44,45]. Therefore, if one intracranial lesion is an extra-axial neoplasm and suspected to be STS based on radiographic features, clinical manifestations, history of disease, etc., preoperative biopsy is necessary. In our present case, because the lesion was located in the left cavernous sinus and adjoined the internal carotid artery and cranial nerves, the risks of operating stereotactic brain biopsy in this area were evaluated to be high by surgeons. Thus, we performed open craniotomy and tumor resection on the patient and obtained the whole lesion tissue for further pathological exams.
Currently, the standard and crucial treatment modality for soft tissue sarcoma is multidisciplinary treatment (MDT), including surgery, adjuvant radiotherapy and systematic chemotherapy[39,46,47]. Surgery is considered to be the basic and standard treatment for local lesions of STS. Wide tumor resection with negative margins is recommended on the contrast that positive margins can cause increasing recurrence and metastasis rates and impact the progression-free survival (PFS) and distant metastasis-free survival (DMFS) of patients[37,39,47,48]. Adjuvant radiotherapy is recommended to improve local control and reduce the recurrence of STS[37,39,41,47]. EMSO suggests that postoperative radiotherapy should be applied in patients who have a deep tumor, a tumor size over 5 cm or a high degree of malignancy (grades 2-3)[39,49]. In advanced disease, stereotactic radiotherapy or stereotactic surgery is adoptable for patients who lose the chance for surgery or are in poor condition and cannot tolerate the operation[39]. Given the occurrence of distant metastasis of STS, systematic chemotherapy is also recommended, although the efficacy is still debatable, and the primary first-line chemotherapeutic agents are anthracyclines such as doxorubicin, ifosfamide and gemcitabine[37,39,41]. Other novel treatments, such as targeted therapy, immunotherapy, and antiangiogenic agents, such as pazopanib, are promising, and further research is needed[37,50].
Due to the extreme rarity of primary intracranial EMC, standard and optimal treatment strategies for this disease remain undefined. In our research of the 16 primary intracranial EMC cases available in the literature, 3 did not have any information on the prognosis of patients, and in 2 cases, the patients died from non-EMC-related factors after surgery. Of the remaining 11 cases, 9 patients underwent gross total resection (GTR), 1 underwent partial resection (PR), and the last underwent subtotal resection (STR). Four of the 9 patients who underwent GTR also underwent postoperative RT, and the remaining 5 patients underwent GTR only without postoperative RT. The recurrence rate of the single GTR surgery group was 80% (n = 4, 4/5), and the median progression-free survival (PFS) was 10 mo, yet that in the GTR combined with RT group was 25% (n = 1, 1/4), and the PFS was 36 mo. No case in which GTR was accepted reported metastasis. Only 1 out of the 11 patients who underwent PR died from local recurrence and spinal metastasis of primary intracranial EMC at 36 mo after surgery[9]. Interestingly, metastasis occurred through cerebrospinal fluid (CSF) circulation. One of the 11 patients underwent STR and postoperative proton therapy, and no recurrence or metastasis was found after surgery[14]. According to these data, patients who undergo GTR with postoperative RT seem to have a lower recurrence rate and longer PFS than those who undergo surgery only. Due to the scarcity of data, cases and long-term follow-up, further studies are needed to verify whether GTR combined with RT is better for reducing the recurrence rate and prolonging PFS than single surgery therapy.
To date, the most effective approach for treating EMC is surgery, and wide local resection with a negative microscopic margin is considered to be the standard method and recommended for patients with local lesions, since inadequate initial surgery has been reported by Satoshi Kawaguchi[51] to be a significant risk factor for local recurrence[23,24,52,53]. However, even if patients undergo wide resection, the postoperative recurrence rate still reaches 35%-50% at 5 years, and the metastasis rate is also 25%-50% after radical surgery. Bishop[54] pointed out that indolent biological characteristics and low-grade histological classification might cause combined modality therapy (CMT), such as surgery and radiotherapy (RT), to not be widely used in patients with local EMC, which could result in a high recurrence and metastasis rate. They performed a retrospective study of 41 patients with local EMC and found that patients treated with surgery combined with RT had better local control than those treated with surgery alone (100% and 63%, respectively, local control rate in 10 years). They also found that local recurrence was the only risk factor that led to a high metastasis rate and worse distant metastasis-free survival (DMFS). According to previous research, surgery combined with radiotherapy is beneficial to patients; thus, they recommend CMT for EMC to reduce local recurrence and distal metastasis. Another retrospective study of 87 patients with EMC also reported that surgery with RT combined with CMT could obtain better local control than surgery alone[55]. Data from the Surveillance, Epidemiology and End Result (SEER) database were used to perform a population-based analysis of 156 patients with local EMC, and the results revealed that surgery combined with RT could be considered for patients with local lesions, especially large tumors[56].
In addition to surgery and RT for local lesions, systematic therapy and antiangiogenic therapy have also been investigated. In recent studies, EMCs were found to be more sensitive to anthracycline-based agents than previously expected, and trabectedin could be a suitable alternative for patients with metastatic EMC who are unsensitive or intolerable to anthracycline-based agents[57,58]. Antiangiogenic agents such as sunitinib and pazopanib also showed certain positive effects on EMC[59-61]. Because a special NR4A3 rearrangement mutation exists in EMC and studies have shown that the products of NR4A3-associated fusion genes play an important role in the growth and differentiation of tumor cells and might be related to tumorigenesis, progression and metastasis[62-64], revealing the precise functions and mechanism of these mutations in the pathogenesis and progression of EMC might help us discover potential therapeutic targets for targeted treatment and biomarkers for diagnosis. In addition, the influence of the blood–brain barrier (BBB) on permeability and the effects of chemotherapeutic, antiangiogenic and targeted drugs should also be taken into account when treating primary intracranial EMC. More research on pharmacotherapies for primary intracranial EMC treatment is still needed. In summary, we believe that a radical resection approach, such as GTR combined with RT, is the most beneficial treatment strategy for patients with primary intracranial EMC, but the effects of chemotherapy and antiangiogenic therapy still need to be verified by further research due to the scarcity of data and limited number of studies.
EMC is a low- to intermediate-grade malignant soft tissue sarcoma with indolent biological characteristics, but it has high potential for postoperative recurrence and metastasis even if patients undergo wide resection. However, EMC is considered to have a favorable prognosis due to its protracted clinical course and long survival period, even when local recurrence or distal metastasis occurs[26]. Many studies have investigated the prognostic factors of EMC related to recurrence and metastasis. A large tumor size, older age, proximal location, refusal of postoperative RT, histological characteristics such as high mitotic activity, high Ki-67 index, atypia including anaplasia or rhabdoid cell features, high cellularity and metastasis occurrence have been found to be significantly associated with poor prognosis[1,5,23]. Moreover, different types of NR4A3 translocation events could affect the prognosis of EMC. Patients carrying an EWSR1-NR4A3 chimeric gene showed a better prognosis with longer disease-free survival (DFS) and distal metastasis-free survival (DMFS) than those carrying TAF15-NR4A3 and other variant NR4A3-related chimeric genes[51,65]. Due to the high propensity of recurrence and metastasis of EMC accompanied by a protracted clinical course, long-term follow-up is absolutely indispensable for these patients.
Primary intracranial extraskeletal myxoid chondrosarcoma is an extremely rare disease. To the best of our knowledge, only 15 cases have been reported to date. We herein report the case of a 52-year-old male patient with EMC and review the literature. We believe that our report can enrich the clinical data on primary intracranial EMC and provide a better understanding for clinicians and radiologists who diagnose and manage this rare disease.
The author would like to thank to all the specialists who providing available help for this article, and thank to the patient and his family provide medical history.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Improta L, Italy; Malekzadegan A, Iran S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Stacchiotti S, Baldi GG, Morosi C, Gronchi A, Maestro R. Extraskeletal Myxoid Chondrosarcoma: State of the Art and Current Research on Biology and Clinical Management. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Enzinger FM, Shiraki M. Extraskeletal myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol. 1972;3:421-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 241] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Fukuda T, Ishikawa H, Ohnishi Y, Tachikawa S, Onizuka S, Sakashita I. Extraskeletal myxoid chondrosarcoma arising from the retroperitoneum. Am J Clin Pathol. 1986;85:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Demicco EG, Wang WL, Madewell JE, Huang D, Bui MM, Bridge JA, Meis JM. Osseous myxochondroid sarcoma: a detailed study of 5 cases of extraskeletal myxoid chondrosarcoma of the bone. Am J Surg Pathol. 2013;37:752-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Meis-Kindblom JM, Bergh P, Gunterberg B, Kindblom LG. Extraskeletal myxoid chondrosarcoma: a reappraisal of its morphologic spectrum and prognostic factors based on 117 cases. Am J Surg Pathol. 1999;23:636-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Scott RM, Dickersin R, Wolpert SM, Twitchell T. Myxochondrosarcoma of the fourth ventricle. Case report. J Neurosurg. 1976;44:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Smith TW, Davidson RI. Primary meningeal myxochondrosarcoma presenting as a cerebellar mass: case report. Neurosurgery. 1981;8:577-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Salcman M, Scholtz H, Kristt D, Numaguchi Y. Extraskeletal myxoid chondrosarcoma of the falx. Neurosurgery. 1992;31:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Sato K, Kubota T, Yoshida K, Murata H. Intracranial extraskeletal myxoid chondrosarcoma with special reference to lamellar inclusions in the rough endoplasmic reticulum. Acta Neuropathol. 1993;86:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Chaskis C, Michotte A, Goossens A, Stadnik T, Koerts G, D'Haens J. Primary intracerebral myxoid chondrosarcoma. Case illustration. J Neurosurg. 2002;97:228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | González-Lois C, Cuevas C, Abdullah O, Ricoy JR. Intracranial extraskeletal myxoid chondrosarcoma: case report and review of the literature. Acta Neurochir (Wien). 2002;144:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Im SH, Kim DG, Park IA, Chi JG. Primary intracranial myxoid chondrosarcoma: report of a case and review of the literature. J Korean Med Sci. 2003;18:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Cummings TJ, Bridge JA, Fukushima T. Extraskeletal myxoid chondrosarcoma of the jugular foramen. Clin Neuropathol. 2004;23:232-237. [PubMed] |
| 14. | O'Brien J, Thornton J, Cawley D, Farrell M, Keohane K, Kaar G, McEvoy L, O'Brien DF. Extraskeletal myxoid chondrosarcoma of the cerebellopontine angle presenting during pregnancy. Br J Neurosurg. 2008;22:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Sorimachi T, Sasaki O, Nakazato S, Koike T, Shibuya H. Myxoid chondrosarcoma in the pineal region. J Neurosurg. 2008;109:904-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Arpino L, Capuano C, Gravina M, Franco A. Parasellar myxoid chondrosarcoma: a rare variant of cranial chondrosarcoma. J Neurosurg Sci. 2011;55:387-389. [PubMed] |
| 17. | Dulou R, Chargari C, Dagain A, Teriitehau C, Goasguen O, Jeanjean O, Védrine L. Primary intracranial extraskeletal myxoid chondrosarcoma. Neurol Neurochir Pol. 2012;46:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Park JH, Kim MJ, Kim CJ, Kim JH. Intracranial extraskeletal myxoid chondrosarcoma : case report and literature review. J Korean Neurosurg Soc. 2012;52:246-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Qin Y, Zhang HB, Ke CS, Huang J, Wu B, Wan C, Yang CS, Yang KY. Primary extraskeletal myxoid chondrosarcoma in cerebellum: A case report with literature review. Medicine (Baltimore). 2017;96:e8684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Akakın A, Urgun K, Ekşi MŞ, Yılmaz B, Yapıcıer Ö, Mestanoğlu M, Toktaş ZO, Demir MK, Kılıç T. Falcine Myxoid Chondrosarcoma: A Rare Aggressive Case. Asian J Neurosurg. 2018;13:68-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Goh YW, Spagnolo DV, Platten M, Caterina P, Fisher C, Oliveira AM, Nascimento AG. Extraskeletal myxoid chondrosarcoma: a light microscopic, immunohistochemical, ultrastructural and immuno-ultrastructural study indicating neuroendocrine differentiation. Histopathology. 2001;39:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Horvai A, Agaram N, Lucas D. Extraskeletal myxoid condrosarcoma. World Health Organization (WHO) Classification of Soft Tissue and Bone Tumours, 2020: 303-305. |
| 23. | Oliveira AM, Sebo TJ, McGrory JE, Gaffey TA, Rock MG, Nascimento AG. Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and ploidy analysis of 23 cases. Mod Pathol. 2000;13:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Okamoto S, Hisaoka M, Ishida T, Imamura T, Kanda H, Shimajiri S, Hashimoto H. Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and molecular analysis of 18 cases. Hum Pathol. 2001;32:1116-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Subramanian S, West RB, Marinelli RJ, Nielsen TO, Rubin BP, Goldblum JR, Patel RM, Zhu S, Montgomery K, Ng TL, Corless CL, Heinrich MC, van de Rijn M. The gene expression profile of extraskeletal myxoid chondrosarcoma. J Pathol. 2005;206:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Lucas DR, Fletcher CD, Adsay NV, Zalupski MM. High-grade extraskeletal myxoid chondrosarcoma: a high-grade epithelioid malignancy. Histopathology. 1999;35:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Folpe AL, Agoff SN, Willis J, Weiss SW. Parachordoma is immunohistochemically and cytogenetically distinct from axial chordoma and extraskeletal myxoid chondrosarcoma. Am J Surg Pathol. 1999;23:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Flucke U, Tops BB, Verdijk MA, van Cleef PJ, van Zwam PH, Slootweg PJ, Bovée JV, Riedl RG, Creytens DH, Suurmeijer AJ, Mentzel T. NR4A3 rearrangement reliably distinguishes between the clinicopathologically overlapping entities myoepithelial carcinoma of soft tissue and cellular extraskeletal myxoid chondrosarcoma. Virchows Arch. 2012;460:621-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Yoshida A, Makise N, Wakai S, Kawai A, Hiraoka N. INSM1 expression and its diagnostic significance in extraskeletal myxoid chondrosarcoma. Mod Pathol. 2018;31:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Shao R, Lao IW, Wang L, Yu L, Wang J, Fan Q. Clinicopathologic and radiologic features of extraskeletal myxoid chondrosarcoma: a retrospective study of 40 Chinese cases with literature review. Ann Diagn Pathol. 2016;23:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Patel SR, Burgess MA, Papadopoulos NE, Linke KA, Benjamin RS. Extraskeletal myxoid chondrosarcoma. Long-term experience with chemotherapy. Am J Clin Oncol. 1995;18:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Stenman G, Andersson H, Mandahl N, Meis-Kindblom JM, Kindblom LG. Translocation t(9;22)(q22;q12) is a primary cytogenetic abnormality in extraskeletal myxoid chondrosarcoma. Int J Cancer. 1995;62:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Noguchi H, Mitsuhashi T, Seki K, Tochigi N, Tsuji M, Shimoda T, Hasegawa T. Fluorescence in situ hybridization analysis of extraskeletal myxoid chondrosarcomas using EWSR1 and NR4A3 probes. Hum Pathol. 2010;41:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Urbini M, Astolfi A, Pantaleo MA, Serravalle S, Dei Tos AP, Picci P, Indio V, Sbaraglia M, Benini S, Righi A, Gambarotti M, Gronchi A, Colombo C, Dagrada GP, Pilotti S, Maestro R, Polano M, Saponara M, Tarantino G, Pession A, Biasco G, Casali PG, Stacchiotti S. HSPA8 as a novel fusion partner of NR4A3 in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2017;56:582-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Paioli A, Stacchiotti S, Campanacci D, Palmerini E, Frezza AM, Longhi A, Radaelli S, Donati DM, Beltrami G, Bianchi G, Barisella M, Righi A, Benini S, Fiore M, Picci P, Gronchi A. Extraskeletal Myxoid Chondrosarcoma with Molecularly Confirmed Diagnosis: A Multicenter Retrospective Study Within the Italian Sarcoma Group. Ann Surg Oncol. 2021;28:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Hui JY. Epidemiology and Etiology of Sarcomas. Surg Clin North Am. 2016;96:901-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 37. | Bourcier K, Le Cesne A, Tselikas L, Adam J, Mir O, Honore C, de Baere T. Basic Knowledge in Soft Tissue Sarcoma. Cardiovasc Intervent Radiol. 2019;42:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 38. | Callovini GM, Telera S, Sherkat S, Sperduti I, Callovini T, Carapella CM. How is stereotactic brain biopsy evolving? Clin Neurol Neurosurg. 2018;174:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | de Juan Ferré A, Álvarez Álvarez R, Casado Herráez A, Cruz Jurado J, Estival González A, Martín-Broto J, Martínez Marín V, Moreno Vega A, Sebio García A, Valverde Morales C. SEOM Clinical Guideline of management of soft-tissue sarcoma (2020). Clin Transl Oncol. 2021;23:922-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Blay JY, Soibinet P, Penel N, Bompas E, Duffaud F, Stoeckle E, Mir O, Adam J, Chevreau C, Bonvalot S, Rios M, Kerbrat P, Cupissol D, Anract P, Gouin F, Kurtz JE, Lebbe C, Isambert N, Bertucci F, Toumonde M, Thyss A, Piperno-Neumann S, Dubray-Longeras P, Meeus P, Ducimetière F, Giraud A, Coindre JM, Ray-Coquard I, Italiano A, Le Cesne A. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol. 2017;28:2852-2859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 41. | Gilbert NF, Cannon CP, Lin PP, Lewis VO. Soft-tissue sarcoma. J Am Acad Orthop Surg. 2009;17:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Ersahin M, Karaaslan N, Gurbuz MS, Hakan T, Berkman MZ, Ekinci O, Denizli N, Aker FV. The safety and diagnostic value of frame-based and CT-guided stereotactic brain biopsy technique. Turk Neurosurg. 2011;21:582-590. [PubMed] |
| 43. | Krieger MD, Chandrasoma PT, Zee CS, Apuzzo ML. Role of stereotactic biopsy in the diagnosis and management of brain tumors. Semin Surg Oncol. 1998;14:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Hildebrand J. Indications for stereotactically-aided differential diagnosis: the neurologist's view. Acta Neurochir (Wien). 1993;124:23-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Apuzzo ML, Sabshin JK. Computed tomographic guidance stereotaxis in the management of intracranial mass lesions. Neurosurgery. 1983;12:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 181] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Andritsch E, Beishon M, Bielack S, Bonvalot S, Casali P, Crul M, Delgado Bolton R, Donati DM, Douis H, Haas R, Hogendoorn P, Kozhaeva O, Lavender V, Lovey J, Negrouk A, Pereira P, Roca P, de Lempdes GR, Saarto T, van Berck B, Vassal G, Wartenberg M, Yared W, Costa A, Naredi P. ECCO Essential Requirements for Quality Cancer Care: Soft Tissue Sarcoma in Adults and Bone Sarcoma. A critical review. Crit Rev Oncol Hematol. 2017;110:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 47. | Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70:200-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 48. | von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, Keedy V, Kim E, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, Morris ZS, O'Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Ruo B, Schuetze S, Tap WD, Wayne JD, Bergman MA, Scavone JL. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:536-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (1)] |
| 49. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv51-iv67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 459] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 50. | Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11:187-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 51. | Kawaguchi S, Wada T, Nagoya S, Ikeda T, Isu K, Yamashiro K, Kawai A, Ishii T, Araki N, Myoui A, Matsumoto S, Umeda T, Yoshikawa H, Hasegawa T; Multi-Institutional Study of 42 Cases in Japan. Extraskeletal myxoid chondrosarcoma: a Multi-Institutional Study of 42 Cases in Japan. Cancer. 2003;97:1285-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | McGrory JE, Rock MG, Nascimento AG, Oliveira AM. Extraskeletal myxoid chondrosarcoma. Clin Orthop Relat Res. 2001;185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Chiusole B, Le Cesne A, Rastrelli M, Maruzzo M, Lorenzi M, Cappellesso R, Del Fiore P, Imbevaro S, Sbaraglia M, Terrier P, Ruggieri P, Dei Tos AP, Rossi CR, Zagonel V, Brunello A. Extraskeletal Myxoid Chondrosarcoma: Clinical and Molecular Characteristics and Outcomes of Patients Treated at Two Institutions. Front Oncol. 2020;10:828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Bishop AJ, Bird JE, Conley AP, Roland CL, Moon BS, Satcher RL, Livingston JA, Patel S, Wang WL, Lazar AJ, Lewis VO, Lin PP, Guadagnolo BA. Extraskeletal Myxoid Chondrosarcomas: Combined Modality Therapy With Both Radiation and Surgery Improves Local Control. Am J Clin Oncol. 2019;42:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Drilon AD, Popat S, Bhuchar G, D'Adamo DR, Keohan ML, Fisher C, Antonescu CR, Singer S, Brennan MF, Judson I, Maki RG. Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer. 2008;113:3364-3371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Kemmerer EJ, Gleeson E, Poli J, Ownbey RT, Brady LW, Bowne WB. Benefit of Radiotherapy in Extraskeletal Myxoid Chondrosarcoma: A Propensity Score Weighted Population-based Analysis of the SEER Database. Am J Clin Oncol. 2018;41:674-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Morioka H, Takahashi S, Araki N, Sugiura H, Ueda T, Takahashi M, Yonemoto T, Hiraga H, Hiruma T, Kunisada T, Matsumine A, Susa M, Nakayama R, Nishimoto K, Kikuta K, Horiuchi K, Kawai A. Results of sub-analysis of a phase 2 study on trabectedin treatment for extraskeletal myxoid chondrosarcoma and mesenchymal chondrosarcoma. BMC Cancer. 2016;16:479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Stacchiotti S, Dagrada GP, Sanfilippo R, Negri T, Vittimberga I, Ferrari S, Grosso F, Apice G, Tricomi M, Colombo C, Gronchi A, Dei Tos AP, Pilotti S, Casali PG. Anthracycline-based chemotherapy in extraskeletal myxoid chondrosarcoma: a retrospective study. Clin Sarcoma Res. 2013;3:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Stacchiotti S, Pantaleo MA, Astolfi A, Dagrada GP, Negri T, Dei Tos AP, Indio V, Morosi C, Gronchi A, Colombo C, Conca E, Toffolatti L, Tazzari M, Crippa F, Maestro R, Pilotti S, Casali PG. Activity of sunitinib in extraskeletal myxoid chondrosarcoma. Eur J Cancer. 2014;50:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Stacchiotti S, Ferrari S, Redondo A, Hindi N, Palmerini E, Vaz Salgado MA, Frezza AM, Casali PG, Gutierrez A, Lopez-Pousa A, Grignani G, Italiano A, LeCesne A, Dumont S, Blay JY, Penel N, Bernabeu D, de Alava E, Karanian M, Morosi C, Brich S, Dagrada GP, Vallacchi V, Castelli C, Brenca M, Racanelli D, Maestro R, Collini P, Cruz J, Martin-Broto J. Pazopanib for treatment of advanced extraskeletal myxoid chondrosarcoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019;20:1252-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Stacchiotti S, Dagrada GP, Morosi C, Negri T, Romanini A, Pilotti S, Gronchi A, Casali PG. Extraskeletal myxoid chondrosarcoma: tumor response to sunitinib. Clin Sarcoma Res. 2012;2:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Filion C, Motoi T, Olshen AB, Laé M, Emnett RJ, Gutmann DH, Perry A, Ladanyi M, Labelle Y. The EWSR1/NR4A3 fusion protein of extraskeletal myxoid chondrosarcoma activates the PPARG nuclear receptor gene. J Pathol. 2009;217:83-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Brenca M, Stacchiotti S, Fassetta K, Sbaraglia M, Janjusevic M, Racanelli D, Polano M, Rossi S, Brich S, Dagrada GP, Collini P, Colombo C, Gronchi A, Astolfi A, Indio V, Pantaleo MA, Picci P, Casali PG, Dei Tos AP, Pilotti S, Maestro R. NR4A3 fusion proteins trigger an axon guidance switch that marks the difference between EWSR1 and TAF15 translocated extraskeletal myxoid chondrosarcomas. J Pathol. 2019;249:90-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Filion C, Labelle Y. The oncogenic fusion protein EWS/NOR-1 induces transformation of CFK2 chondrogenic cells. Exp Cell Res. 2004;297:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Agaram NP, Zhang L, Sung YS, Singer S, Antonescu CR. Extraskeletal myxoid chondrosarcoma with non-EWSR1-NR4A3 variant fusions correlate with rhabdoid phenotype and high-grade morphology. Hum Pathol. 2014;45:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |