Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3222
Peer-review started: October 3, 2021
First decision: January 11, 2022
Revised: January 25, 2022
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: April 6, 2022
Processing time: 177 Days and 7.4 Hours
Cases of severe pneumonia complicated with acute myocardial infarction (AMI) with good prognosis after percutaneous coronary intervention (PCI) are rare, especially those with postoperative pericarditis and intestinal obstruction.
A 53-year-old male patient was admitted to the emergency department of our hospital because of paroxysmal chest tightness for 4 d, aggravated with chest pain for 12 h. The symptoms, electrocardiography, biochemical parameters, echocardiography and chest computed tomography confirmed the diagnosis of severe pneumonia complicated with AMI. The patient was treated with antiplatelet aggregation, anticoagulation, lipid regulation, vasodilation, anti-infective agents and direct PCI. The patient was discharged after 3 wk of treatment. Follow-up showed that the patient was asymptomatic without recurrence.
For patients with severe pneumonia complicated with AMI, PCI and antibiotic therapy is a life-saving strategy.
Core Tip: Severe pneumonia complicated with acute myocardial infarction (AMI) is a critical and rare disease in clinic. Patients with good prognosis after emergency percutaneous coronary intervention (PCI) combined with antibiotic treatment have been rarely reported. We here report a case of severe pneumonia and AMI complicated with pericarditis and intestinal obstruction after direct PCI. The patient had a good prognosis after anti-infective and symptomatic treatment.
- Citation: Liu WC, Li SB, Zhang CF, Cui XH. Severe pneumonia and acute myocardial infarction complicated with pericarditis after percutaneous coronary intervention: A case report . World J Clin Cases 2022; 10(10): 3222-3231
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3222.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3222
The cause of death of patients with severe pneumonia is not only pneumonia itself, but also the complications of pneumonia[1,2]. There is evidence showing that acute pulmonary infection can increase the incidence of cardiovascular adverse events[3].
Successful percutaneous coronary intervention (PCI) for severe pneumonia complicated with acute myocardial infarction (AMI) is rare. Here, we report a case of severe pneumonia complicated with AMI successfully treated with PCI and antibiotics. Written informed consent was obtained from the patient for publication of this case report.
A 53-year-old male patient was admitted to the emergency department of our hospital because of intermittent chest tightness for 4 d, aggravated with chest pain for12 h.
Four days before admission, the patient developed intermittent chest tightness and cough after catching a cold, which was relieved after a few minutes. Twelve hours before admission, the patient had persistent chest tightness with chest and back pain, cough, dyspnea and difficulty lying flat, and the symptoms could not be relieved. The patient had no fever, yellow sputum, hemoptysis, abdominal pain or diarrhea.
The patient had a history of type 2 diabetes and chronic cerebral infarction without any sequelae.
The patient’s temperature was 36.8℃, pulse rate was 82 beats/min, respiratory rate was 16 breaths/min and blood pressure was 134/86 mmHg. The respiratory sound of both lungs was reduced and a small amount of dry and wet rales could be heard in the middle and lower lungs. The heart rate was 82 beats/min, the heart rhythm was uniform, and there was no obvious murmur in the auscultation area of each valve or pericardial friction sound. There was no edema in either lower limbs. The pathological signs were negative.
Routine blood tests showed that the leukocyte count was 14.36 × 109/L (reference range: 3.5 × 109-9.5 × 109/L), neutrophil count 11.45 × 109/L (reference range: 1.8 × 109-6.3 × 109/L) and neutrophil ratio 79.70% (reference range: 40.0%-75.0%). C-reactive protein (CRP) was 106.0 mg/L (reference range: 0-6.0 mg/L). Procalcitonin (PCT) was 0.30 ng/mL (reference range: 0-0.25 ng/mL). The markers of myocardial necrosis showed that creatine kinase isozyme (CK-MB) was 223.85 U/L (reference range: 0-24.0 U/L), myoglobin (Myo) 3091.0 ng/mL (reference range: 28 - 72 ng/mL) and high-sensitivity troponin T (hs-TNT) was 4963.0 pg/mL (reference range: 12.70-24.90 pg/mL). The precursor of amino terminal-B-type natriuretic peptide (NT-proBNP) was 717.0 pg/mL (reference range: ≤ 125 pg/mL). D-dimer was 0.45 mg/L (reference range: 0-0.55 mg/L).
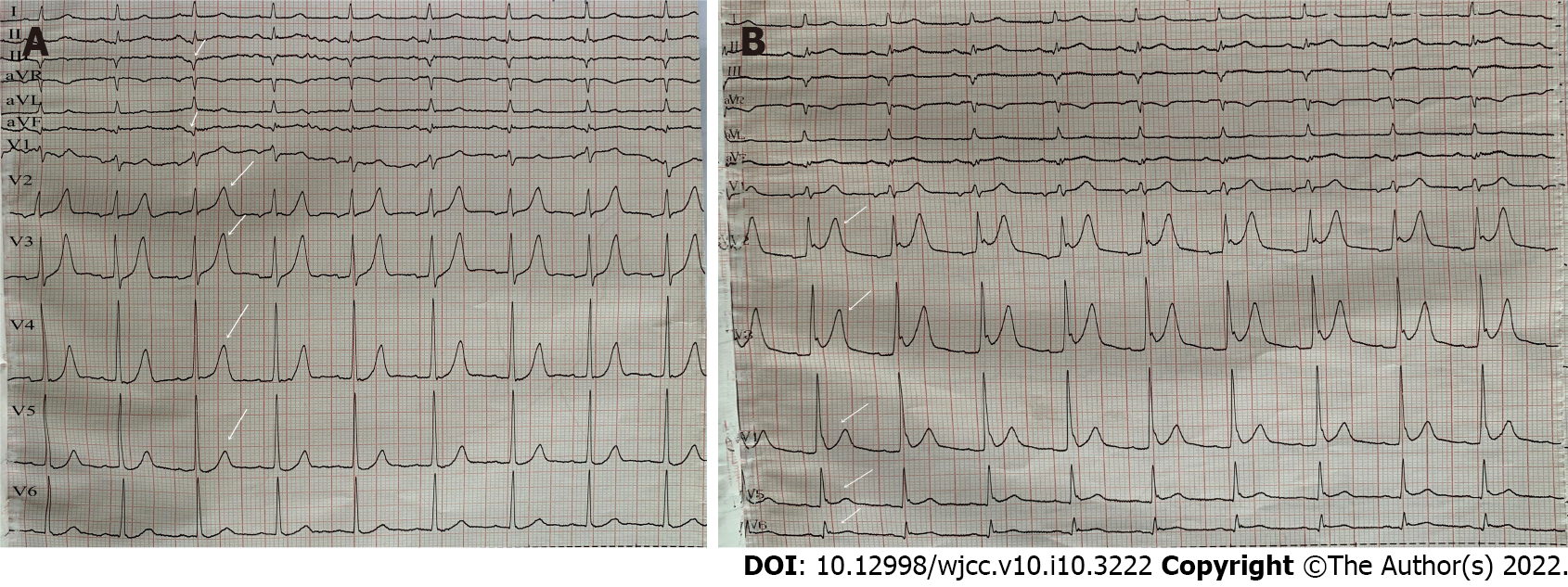
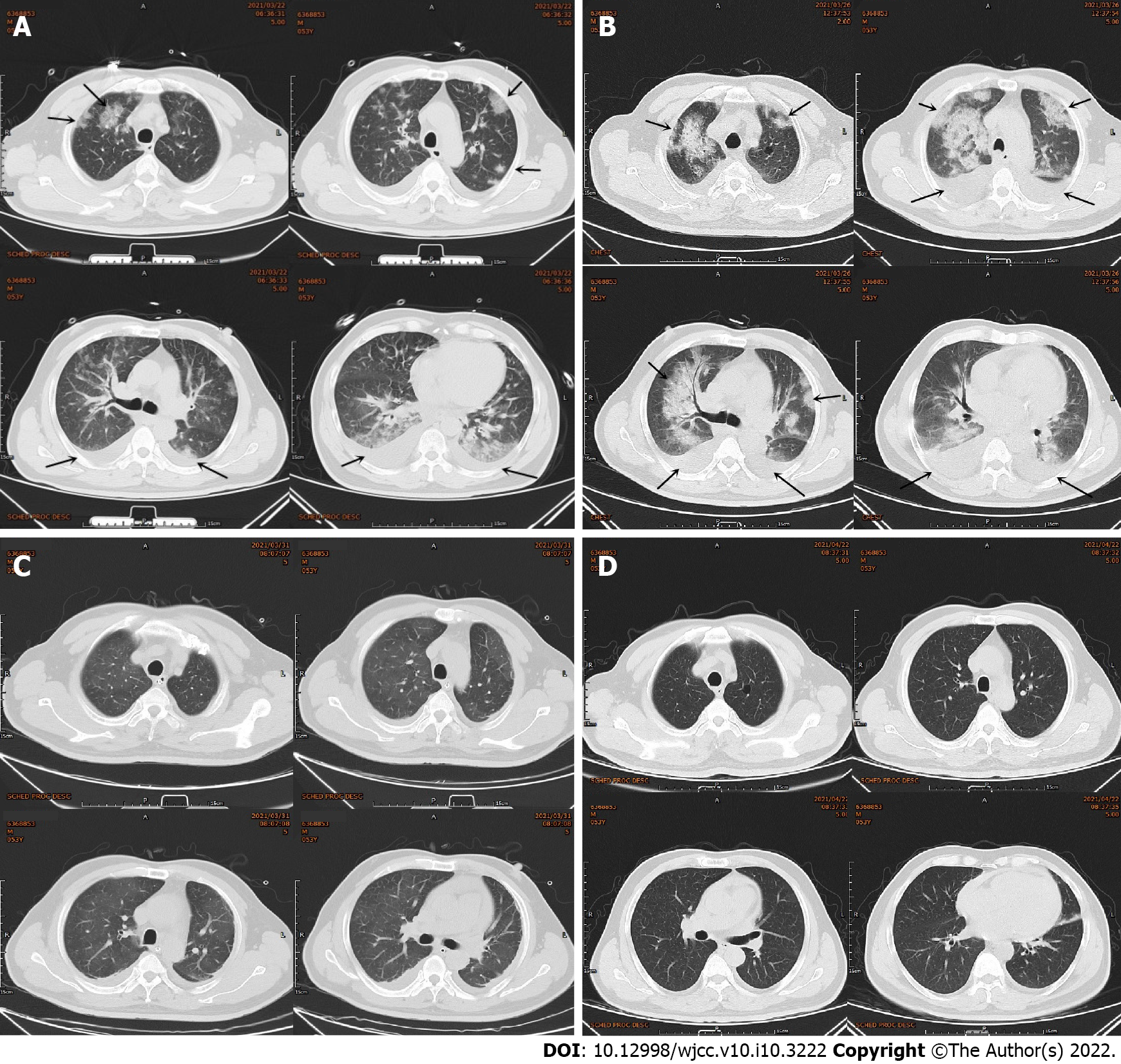
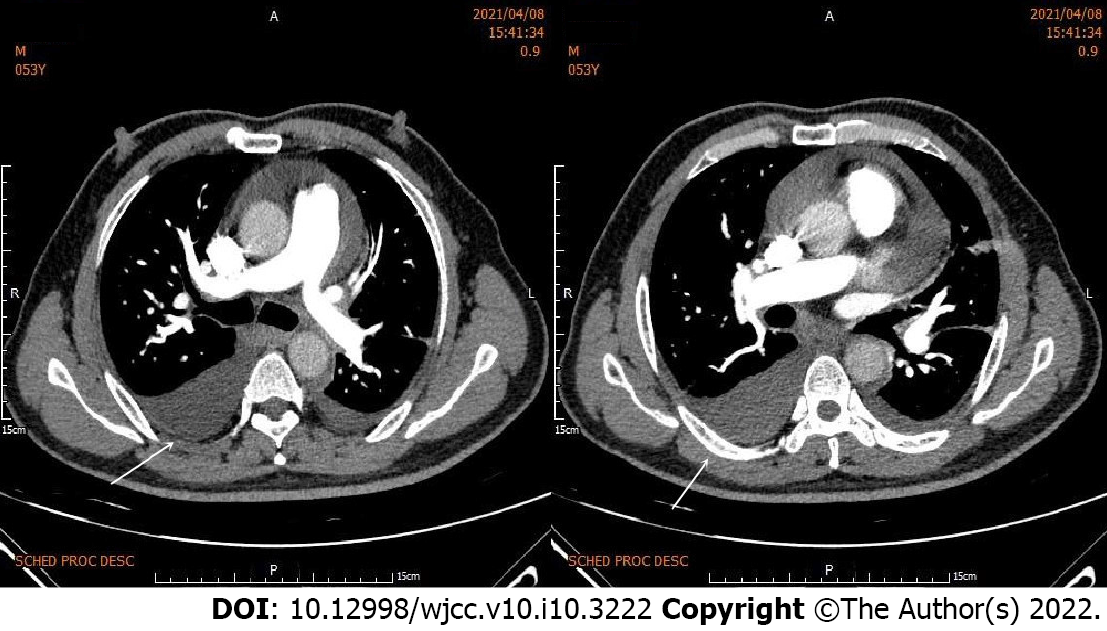
Electrocardiography (ECG) showed sinus rhythm, pathological Q waves on III, aVF leads and high and sharp T waves on V1-V5 Leads (Figure 1A). Chest computed tomography (CT) showed multiple patchy high-density shadows in both lungs, bilateral pleural effusion and enlarged hilar shadows (Figure 2A). Color Doppler echocardiography showed left atrial enlargement, a small amount of aortic regurgitation and tricuspid regurgitation, decreased left ventricular diastolic function (grade II), and normal left ventricular systolic function.
The patient was finally diagnosed with severe pneumonia complicated with non- ST-segment elevation myocardial infarction (NSTEMI).
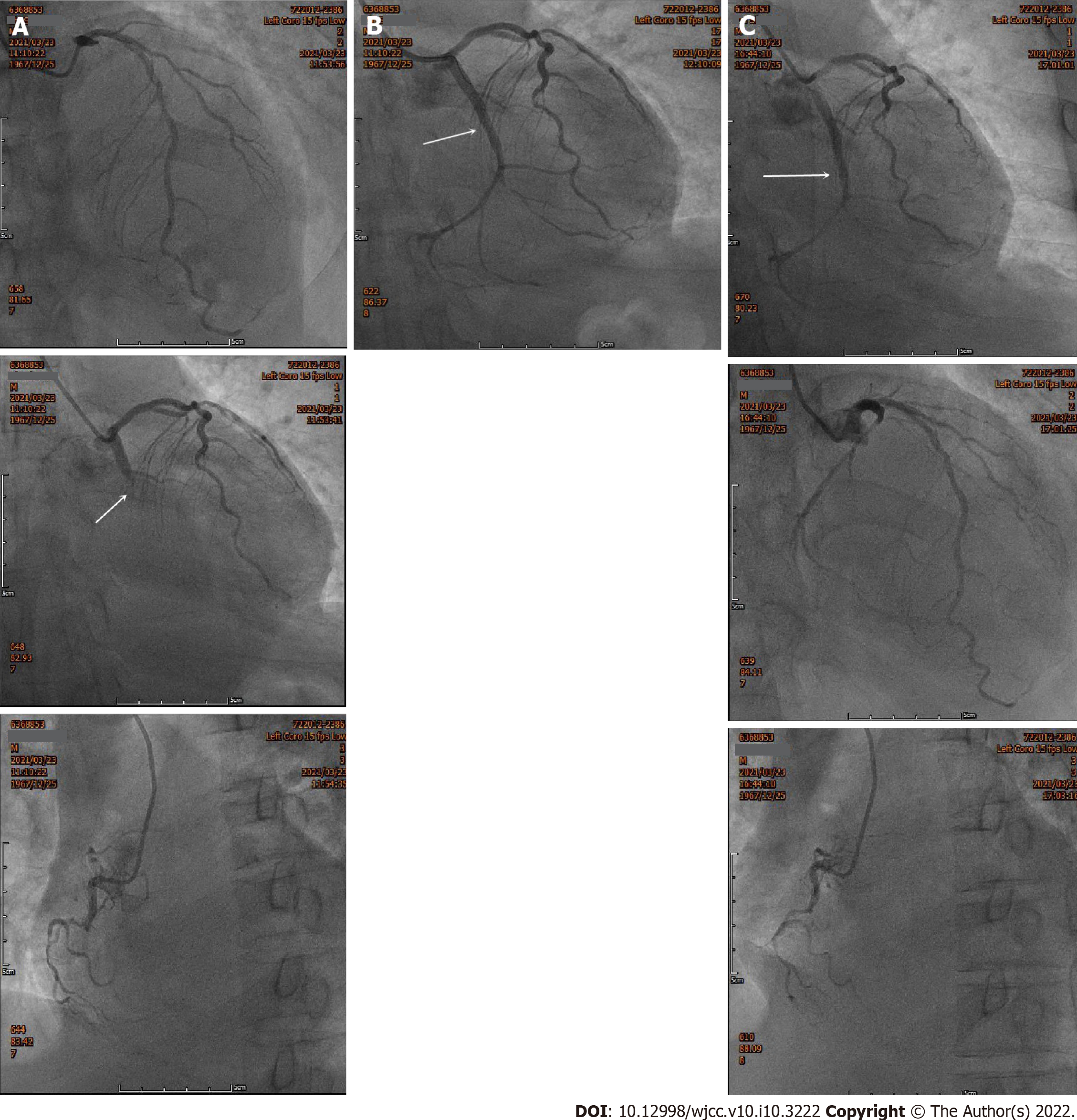
The patient was treated with antiplatelet aggregation, anticoagulation, lipid regulation, plaque stabilization, coronary artery expansion, anti-infective agents and noninvasive ventilator-assisted respiration. The next day, the chest pain worsened without significant remission. Emergency coronary angiography (CAG) was performed, and showed that the coronary arteries were left dominant with localized mild stenosis at the proximal left anterior descending branch (LAD), complete occlusion at the proximal circumflex branch (LCX) and diffuse stenosis at the distal right coronary artery (RCA), the heaviest of which was about 90% (Figure 3A). Considering that the LCX was the offending vessel, interventional therapy was administered. A Resolute 3.0 mm × 22 mm stent was implanted at the proximal LCX. After reperfusion therapy, there was no residual stenosis or dissection of the LCX, and the blood flow of thrombolysis in myocardial infarction (TIMI) was grade 3 (Figure 3B).
However, the patient still complained of chest pain and shortness of breath after the operation. Postoperative ECG showed sinus rhythm and ST-segment elevation on leads V2-V6 of the anterior wall (Figure 1B). Because acute thrombosis or reinfarction was not excluded, emergency CAG was performed again after explaining the condition to the patient’s family. The second CAG showed that the original stent of the LCX was unobstructed and there were no significant dynamic changes or thrombus in the LAD and the RCA (Figure 3C). The patient complained of left chest pain after the operation. The pain worsened when turning the body and breathing, accompanied by chest tightness, shortness of breath, inability to lie flat, without expectoration or fever. On physical examination, wet rales could be heard in both lungs, the heart rate was 90 beats/min, the rhythm was uniform and pericardial friction sound appeared. ECG showed that the ST-segment of V2-V6 Leads increased with arch back downward, which was consistent with the characteristics of pericarditis. Repeated blood tests showed that the leukocyte count was 12.64 × 109/L and neutrophil ratio was 79.30%. CRP increased to 360.0 mg/L. PCT increased to 0.99 ng/mL. CK-MB was 34.8 U/L, Myo was 59.1 ng/mL and hs-TNT was 3860.0 pg/mL. NT-proBNP increased to 3801.0 pg/mL. D-dimer was 0.84 mg/L. Repeated chest CT showed multiple patchy high-density shadows in both lungs and bilateral pleural effusion, which was significantly worse than before (Figure 2B). Echocardiography showed left ventricular enlargement, segmental wall motion abnormality, aortic valve calcification with a small amount of regurgitation and tricuspid regurgitation, and reduced left ventricular diastolic function (grade II) and left ventricular systolic function. According to the symptoms of chest pain, new pericardial friction sound, ST-segment arch back downward elevation on ECG, elevated CRP and PCT, reduced markers of myocardial necrosis, and findings of chest CT and echocardiography, it was considered that pneumonia was aggravated and AMI was complicated with pericarditis.
Considering the aggravation of pulmonary infection and heart failure, the antibiotics were upgraded from levofloxacin to biapenem combined with moxifloxacin, and the recombinant human brain natriuretic peptide was pumped intravenously to treat heart failure. Six days later, the patient’s symptoms of chest pain and shortness of breath were significantly relieved. ECG showed sinus rhythm and the elevation of ST-segment on leads V2-V6 decreased obviously. Chest CT showed multiple patchy high-density shadows and the pleural effusion was obviously absorbed (Figure 2C). Blood test showed that the leukocyte count decreased to 5.68 × 109/L, neutrophil count decreased to 3.63 × 109/L, neutrophil ratio decreased to 63.9 %, CRP decreased to 138.00 mg/L, PCT decreased to 0.23 ng/mL, CK-MB decreased to 26.8 U/L and NT-proBNP decreased to 710.70 pg/mL. D-dimer increased to 4.4 mg/L. Based on the disappearance of chest pain and pericardial friction sound, the ST-segment depression on ECG, inflammatory absorption on chest CT and the decreased inflammatory indexes, the patient’s pneumonia and pericarditis were considered significantly improved.
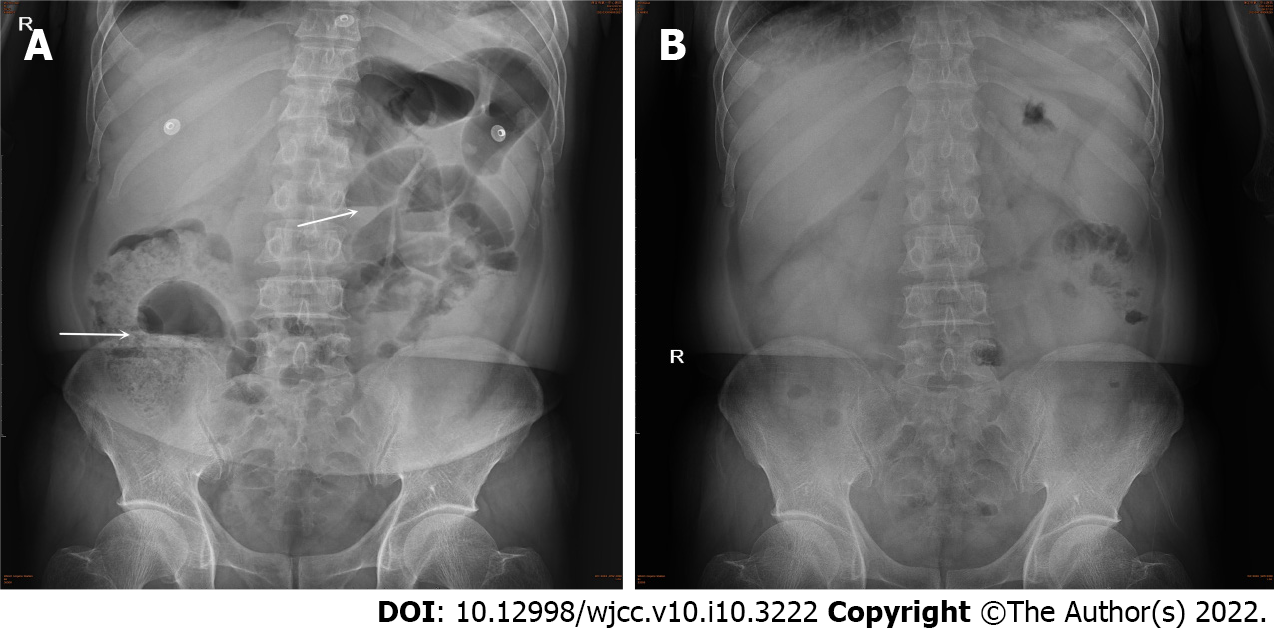
However, the patient complained of abdominal pain, abdominal distention and inability to defecate. Blood tests showed that the leukocyte count, the neutrophil count, the neutrophil ratio and the D-dimer increased again. Bedside abdominal X-ray showed intestinal dilatation and gas-fluid levels, which supported the diagnosis of intestinal obstruction (Figure 4A). The patient was given an enema, gastrointestinal motility, gastrointestinal decompression and anti-infective treatment. Three days later, the patient could defecate and exhaust on his own and the symptoms of abdominal pain and distention disappeared. Abdominal X-ray showed that the intestinal obstruction disappeared (Figure 4B).
Four days later, no chest pain, shortness of breath or any other discomfort were observed. Blood tests showed that the leukocyte count, the neutrophil count, neutrophil ratio, CRP and PCT all decreased. But the D-dimer increased to 7.39 mg/L, and deep venous thrombosis and pulmonary embolism were excluded. Color Doppler ultrasound of deep veins of both lower limbs showed no obvious thrombosis. Pulmonary embolism was not seen on enhanced pulmonary CT, but the pleural effusion was more than before (Figure 5). Paracentesis of the pleura was carried out. It was found that the color of pleural effusion was red and turbid, the Rivalta test was positive, the leukocyte count was 1077 × 106/L (reference range: < 8 × 106/L), red blood cell counts 149784 × 106/L (reference range: < 5000 × 106/L), monocytes accounted for 86% and the multinucleated cells accounted for 14% in leukocyte classification. The pleural effusion biochemistry showed that albumin was 18.17 g/L, globulin 19.86 g/L, lactate dehydrogenase 688.96 U/L, chlorine 103.55 mmol/L, glucose 10.12 mmol/L and adenosine deaminase 12.85 U/L. No cancer cells were found by pathological examination of the hydrothorax. The tumor markers and antinuclear antibody spectrum were negative. Finally, it was considered that the increased D-dimer was related to the bloody pleural effusion, which was secondary to severe pneumonia. After thoracic drainage and effective anti-infective treatment, the symptoms of chest pain and shortness of breath disappeared completely and the condition improved significantly. Blood tests showed that the leukocyte count, the neutrophil count, the neutrophil ratio and PCT were all decreased to the normal range. CRP decreased to 18.6 mg/L and D-dimer decreased to 5.33 mg/L. The patient’s condition improved and he was discharged from hospital.
Two weeks later, the patient went to the clinic for follow-up. The patient had no symptoms. Routine blood tests showed that the leukocyte count was 5.88 × 109/L, neutrophil count 3.23 × 109/L, and neutrophil ratio 54.9%. CRP decreased to 6.83 mg/L. D-dimer decreased to 2.19 mg/L. NT-proBNP decreased to 679.8 pg/mL. Chest CT showed that the patchy high-density shadows and pleural effusion were almost absorbed (Figure 2D). Six weeks later, follow-up examinations showed that the leukocyte count was 6.07 × 109/L, neutrophil count 3.66 × 109/L, and neutrophil ratio 60.2%. CRP decreased to 1.76 mg/L. D-dimer decreased to 0.24 mg/L, which was normal (Table 1). The prognosis of the patient was good.
| Blood test | Mar 22nd | Mar 23rd | Mar 25th | Mar 26th | Mar 27th | Mar 28th | Mar 29th | Mar 31st | Apr 1st | Apr 2nd | Apr 4th | Apr 6th | Apr 8th | Apr 13th | Apr 22nd | Apr 29th | Jun 3rd |
| Leukocyte count (3.5 × 109-9.5 × 109/L) | 14.36 | 12.29 | 12.64 | 8.82 | 5.92 | 6.13 | 5.68 | 10.11 | 10.78 | 9.73 | 6.28 | 5.75 | 4.52 | 5.96 | 5.19 | 5.88 | 6.07 |
| Neutrophil count (1.8 × 109-6.3 × 109/L) | 11.45 | 10.23 | 10.02 | 7.21 | 4.13 | 3.97 | 3.63 | 7.90 | 8.68 | 7.72 | 4.66 | 4.19 | 2.92 | 3.84 | 3.21 | 3.23 | 3.66 |
| Lymphocyte count (1.1 × 109-3.2 × 109/L) | 2.16 | 1.24 | 1.32 | 1.06 | 0.98 | 1.42 | 1.31 | 1.49 | 1.44 | 1.44 | 1.15 | 1.13 | 1.03 | 1.38 | 1.43 | 2.07 | 1.80 |
| Monocyte count (0.1 × 109-3.6 × 109/ L) | 0.64 | 1.02 | 1.10 | 0.64 | 0.32 | 0.62 | 0.53 | 0.64 | 0.49 | 0.40 | 0.35 | 0.32 | 0.38 | 0.52 | 0.34 | 0.37 | 0.37 |
| Neutrophil ratio (40.0%-75.0%) | 79.7 | 83.3 | 79.3 | 81.8 | 69.7 | 64.7 | 63.9 | 78.2 | 80.5 | 79.4 | 74.2 | 72.8 | 64.7 | 64.4 | 61.7 | 54.9 | 60.2 |
| CK-MB (0-24 U/L) | 223.8 | 129.4 | 49.0 | 34.8 | 33.6 | 13.96 | 26.8 | 34.0 | 25.9 | 39.0 | 23.6 | 17.2 | 13.9 | - | 11.1 | 14.6 | - |
| NT-proBNP (≤ 125 pg/mL) | 717.0 | 1266 | 3801 | 3638 | 1686 | 1364 | 710.7 | 1319 | 801.9 | 848.8 | - | - | - | 1168 | 718.2 | 679.8 | - |
| CRP (0.00-6.00 mg/L) | 8.86 | 106.0 | 360.0 | - | - | - | 138.0 | - | - | 73.9 | 27.4 | 15.5 | 12.9 | 18.6 | 9.42 | 6.83 | 1.76 |
| PCT (0-0.25 ng/mL) | 0.08 | 0.30 | 0.99 | 0.68 | 0.52 | - | 0.23 | 0.19 | - | 0.10 | 0.05 | 0.05 | 0.01 | 0.01 | - | - | - |
| D-dimer (0-0.55 mg/L) | 0.45 | 0.31 | 0.84 | - | 2.91 | 2.54 | 4.40 | 5.72 | 6.44 | 7.72 | 7.99 | 7.39 | 7.31 | 5.33 | 6.99 | 2.19 | 0.24 |
Some studies have shown that there is a correlation between community-acquired pneumonia (CAP) and AMI[4]. AMI is found in 15% of patients with severe pneumonia and there is a significant association between AMI and the pneumonia severity index score. AMI is the possible cause of death in patients with severe pneumonia[5]. Other studies have suggested that the incidence of CAP complicated with acute coronary syndrome is 0.7% to 11%[6,7]. A total of 50 119 patients were included in the study of Perry et al[8], which showed that about 1.5% of patients with pneumonia had AMI within 90 d, which was the largest number of cases in previous studies about cardiovascular events after pneumonia. Rae’s study suggested that the cause of death of most patients with severe pneumonia was cardiac arrest, arrhythmia, AMI and heart failure. About one third of pneumonia patients have cardiovascular complications during hospitalization. The risk of AMI is highest in the first few days of CAP and decreases over time. One year after CAP, the risk of AMI remains increased[9]. Another study showed that the incidence of CAP complicated with AMI was about 3.1% in patients aged > 65 years, while the incidence of AMI was about 1.0% in those under 65 years[10]. In our case report, the patient was only 53 years old, but after severe pneumonia, he was complicated with AMI, pericarditis, intestinal obstruction and pleural effusion which is rare.
Studies have shown that plaque rupture or in situ thrombosis formation caused by acute pneumonia is the main etiology of pneumonia complicated with AMI[11]. Acute pneumonia can cause an increase of proinflammatory factors, which can then lead to atherosclerotic inflammatory changes, endothelial dysfunction, atheroma instability, rupture of the atheromatous plaque, increased fibrinogen levels, and prothrombotic vascular conditions[12-16]. Another etiology of AMI caused by pneumonia is platelet activation. The elevated neutrophils adhere to the surface of vascular endothelial cells, then promote expression of inflammatory factors and adhesion molecules, which leads to an increase of thromboxane synthesis, platelet aggregation and vasoconstriction[9,17]. Ventilation-perfusion mismatching and intrapulmonary shunt caused by severe pneumonia can aggravate hypoxia. Systemic inflammatory response can lead to organ hypoperfusion and multiple organ failure. The heart is one of the most vulnerable organs.
The main clinical manifestations of pneumonia are cough, expectoration, fever and dyspnea. In the present case, the patient had cough and dyspnea, but there was no fever in the whole disease course. Due to different types of pneumonia, different severity of pneumonia, different incidence groups and other factors, some patients with pneumonia may not have fever. The causes of pneumonia without fever maybe explained as follows. First, in the early stage of pneumonia, because of its short infection time, small number of pathogens and relatively weak pathogenicity, it can- not stimulate the body to produce fever. Second, some elderly patients may not have fever in the event of lung infection due to their aging and weakened nerve reflex. Third, in case of severe pneumonia, due to the extremely poor state of the body, it is impossible to induce fever. In the present case, the patient had severe pneumonia and AMI at the same time. The body was in a serious state of stress. The stress state can increase the secretion of pituitary and adrenocortical hormones. Excessive glucocorticoids inhibit the immune response, resulting in weakening of the response to pathogens, thus reducing the body’s resistance ability. In addition, in the acute phase of AMI, echocardiography showed only diastolic dysfunction and no systolic dysfunction, which may be related to the early myocardial ischemia, but the infarct area was not large enough to affect the systolic function. Moreover, many patients with acute NSTEMI were confirmed by CAG that most of the infarct related vessels were circumflex and intermediate branches, and a few were left main artery lesions.
For patients with severe pneumonia complicated with AMI, prompt reperfusion combined with targeted anti-infective therapy is still preferred. According to the 2020 European Society of Cardiology (ESC) guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation, early interventional therapy (< 24 h) should be adopted for patients with established diagnosis of NSTEMI, with new continuous ST/T dynamic changes (with or without symptoms), cardiac arrest and resuscitation without ST-segment elevation or cardiogenic shock, and GRACE score > 140[18]. Timely and effective opening of the infarct-related vessels can effectively prevent the occurrence of complications such as heart failure, arrhythmia, cardiac arrest and cardiac rupture, so as to improve the survival rate of patients. Prompt and appropriate antimicrobial therapy is still a crucial treatment for severe pneumonia. The therapeutic effect of antibiotics can be optimized by adjusting the drug according to their pharmacodynamic and pharmacokinetic characteristics[19]. Multidisciplinary clinical guideline research shows that the combined application of anti-coccal and anti-bacilli drugs has a good therapeutic effect in severe pneumonia or pneumonia caused by atypical pathogens. In addition, some studies have shown that immunomodulators, glucocorticoids, macrolides and statins have certain adjuvant therapeutic effects in severe pneumonia[20]. For patients with acute hypoxemic respiratory failure, noninvasive or invasive ventilation is needed to correct hypoxic respiratory failure[21].
For patients with severe pneumonia complicated with AMI, there is no definite evidence proving that emergency PCI can improve the long-term prognosis until now. A prospective cohort study showed that for patients with AMI after infection, the mortality during hospitalization was 13% in the PCI group vs 8% in the non-PCI group, and the 1-year mortality was 24% in the PCI group vs 19% in the non-PCI group. This study indicates that PCI might not improve short-term and long-term prognosis in patients with angiography-confirmed coronary stenosis[22]. However, our patient who had severe pneumonia complicated with AMI had good prognosis after emergency PCI combined with effective anti-infective treatment.
Severe pneumonia complicated with AMI, pericarditis and intestinal obstruction is rare clinically. Our case report shows that emergency PCI combined with effective antibiotic treatment can save the lives of patients with severe pneumonia complicated with AMI, pericarditis and intestinal obstruction.
We thank all of our colleagues at the Department of Cardiology and the Department of Medical Imaging, Baoding No. 1 Central Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and Cardiovascular Systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anandan H, Fujimori S, Teragawa H S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Mortensen EM, Coley CM, Singer DE, Marrie TJ, Obrosky DS, Kapoor WN, Fine MJ. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 262] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Chalmers JD. ICU admission and severity assessment in community-acquired pneumonia. Crit Care. 2009;13:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Singanayagam A, Singanayagam A, Elder DH, Chalmers JD. Is community-acquired pneumonia an independent risk factor for cardiovascular disease? Eur Respir J. 2012;39:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Viasus D, Garcia-Vidal C, Manresa F, Dorca J, Gudiol F, Carratalà J. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J Infect. 2013;66:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Ramirez J, Aliberti S, Mirsaeidi M, Peyrani P, Filardo G, Amir A, Moffett B, Gordon J, Blasi F, Bordon J. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin Infect Dis. 2008;47:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Cangemi R, Calvieri C, Falcone M, Bucci T, Bertazzoni G, Scarpellini MG, Barillà F, Taliani G, Violi F; SIXTUS Study Group. Relation of Cardiac Complications in the Early Phase of Community-Acquired Pneumonia to Long-Term Mortality and Cardiovascular Events. Am J Cardiol. 2015;116:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Postma DF, Spitoni C, van Werkhoven CH, van Elden LJR, Oosterheert JJ, Bonten MJM. Cardiac events after macrolides or fluoroquinolones in patients hospitalized for community-acquired pneumonia: post-hoc analysis of a cluster-randomized trial. BMC Infect Dis. 2019;19:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Perry TW, Pugh MJ, Waterer GW, Nakashima B, Orihuela CJ, Copeland LA, Restrepo MI, Anzueto A, Mortensen EM. Incidence of cardiovascular events after hospital admission for pneumonia. Am J Med. 2011;124:244-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Rae N, Finch S, Chalmers JD. Cardiovascular disease as a complication of community-acquired pneumonia. Curr Opin Pulm Med. 2016;22:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kang Y, Fang XY, Wang D, Wang XJ. Factors associated with acute myocardial infarction in older patients after hospitalization with community-acquired pneumonia: a cross-sectional study. BMC Geriatr. 2021;21:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Aliberti S, Ramirez JA. Cardiac diseases complicating community-acquired pneumonia. Curr Opin Infect Dis. 2014;27:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 13. | Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Peyrani P, Ramirez J. What is the association of cardiovascular events with clinical failure in patients with community-acquired pneumonia? Infect Dis Clin North Am. 2013;27:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Crawford VL, McNerlan SE, Stout RW. Seasonal changes in platelets, fibrinogen and factor VII in elderly people. Age Ageing. 2003;32:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Woodhouse PR, Khaw KT, Plummer M, Foley A, Meade TW. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: winter infections and death from cardiovascular disease. Lancet. 1994;343:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 404] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Feldman C, Anderson R. Community-Acquired Pneumonia: Pathogenesis of Acute Cardiac Events and Potential Adjunctive Therapies. Chest. 2015;148:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2569] [Cited by in RCA: 3146] [Article Influence: 786.5] [Reference Citation Analysis (0)] |
| 19. | De Pascale G, Bello G, Tumbarello M, Antonelli M. Severe pneumonia in intensive care: cause, diagnosis, treatment and management: a review of the literature. Curr Opin Pulm Med. 2012;18:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Sibila O, Restrepo MI, Anzueto A. What is the best antimicrobial treatment for severe community-acquired pneumonia (including the role of steroids and statins and other immunomodulatory agents). Infect Dis Clin North Am. 2013;27:133-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 22. | Putot A, Chagué F, Manckoundia P, Brunel P, Beer JC, Cottin Y, Zeller M. Post-Infectious Myocardial Infarction: Does Percutaneous Coronary Intervention Improve Outcomes? J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |