Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3164
Peer-review started: July 17, 2021
First decision: October 16, 2021
Revised: October 25, 2021
Accepted: February 22, 2022
Article in press: February 22, 2022
Published online: April 6, 2022
Processing time: 255 Days and 2.6 Hours
Neuroendocrine carcinoma (NEC) of the esophagus is rare and highly aggressive, and lacks biological features. Currently, there are no established standard treatments for this cancer. In this report, we describe a patient with large-cell NEC of the esophagus who was successfully treated using endoscopic submucosal dissection (ESD) combined with adjuvant chemotherapy.
A 55-year-old woman presented with intermittent mild dysphagia for 2 mo. Gastroscopy revealed a disc-shaped protruding lesion about 18 mm × 18 mm in size on the upper esophagus. Endoscopic ultrasonography demonstrated that the bulged lesion originated from the muscularis mucosa. We assessed en bloc resections using ESD for therapeutic diagnosis to devise a safe and appropriate treatment. Histopathological examination revealed a poorly differentiated neoplasm comprising of large cells with marked nuclear atypia and multifocal necrosis. In addition, the specimens had a negative horizontal margin and vertical margins. Depth of invasion was classified as submucosa 2 (SM2) without lymphovascular invasion. These histopathological results were consistent with a diagnosis of esophageal NEC, large cell type. Adjuvant therapy has been considered for ESD patients with SM2/SM3 lesions and patients with poorly differentiated lesions. After comprehensive consideration, we initiated combination treatment, i.e., ESD plus adjuvant chemotherapy. The patient remained disease-free at the 2-year follow-up.
En bloc resection approach using ESD may play a vital role as a diagnostic and therapeutic modality for esophageal NEC.
Core Tip: We describe a patient with large-cell neuroendocrine carcinoma (NEC) of the esophagus who was successfully treated using endoscopic submucosal dissection (ESD) combined with adjuvant chemotherapy. No standard treatment strategies are available for esophageal NEC due to a lack of clinical studies and its rarity. En bloc resection approach using ESD may play a vital role as a diagnostic and therapeutic modality for esophageal NEC.
- Citation: Tang N, Feng Z. Endoscopic submucosal dissection combined with adjuvant chemotherapy for early-stage neuroendocrine carcinoma of the esophagus: A case report. World J Clin Cases 2022; 10(10): 3164-3169
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3164.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3164
Neuroendocrine carcinoma (NEC) of the esophagus is extremely rare with an incidence ranging from 0.01 to 0.08 cases per 100000/year[1]. This is an aggressive tumor that lacks biological features. Poorly differentiated NECs are of either small cell or large cell cytology. The former is the dominant histological type and only 10% of NECs are classified as large cell cytology[2].
Currently, there is a lack of clinical epidemiological studies that have been performed with no established standard treatment[3,4]. The early diagnosis of esophageal NEC is difficult due to the absence or late onset of symptoms. Hence, most cases are diagnosed at late stages (III and IV) based on the AJCC 8th edition. This accounts for the low survival rates, with the median overall survival of about 11.3 mo[5]; however, cases with long-term survival after surgical resection were not excluded[6]. Diagnosis usually relies on endoscopy and pathology, with histological diagnosis made via immunohistochemical staining for common neuroendocrine markers, i.e., Ki67, chromogranin A (CgA), synaptophysin (Syn), cytokeratin (CK), and lymphocyte antigen 56 (CD56)[7-9]. Patient survival would be improved if early diagnosis and histological complete resection could be achieved[10]. Herein, we report a case of large-cell NEC of the esophagus treated using endoscopic submucosal dissection (ESD) at its early stage.
A 55-year-old woman presented with intermittent mild dysphagia for 2 mo.
The patient had a normal appetite, no weight loss, and no nausea or vomiting. Her symptoms often occurred when consuming dry food that induced mild dysphagia. The symptoms were initially noticed 2 mo before evaluation.
The patient had no prior history of esophageal surgery. There was no history of diabetes mellitus, hypertension, stroke, or coronary artery disease.
The patient acknowledged no relevant family history of cancer.
No obvious abnormalities were found on physical examination.
Laboratory data were within normal ranges, including total bilirubin, direct bilirubin, C-reactive protein (CRP), carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA199), and neuron-specific enolase (NSE).
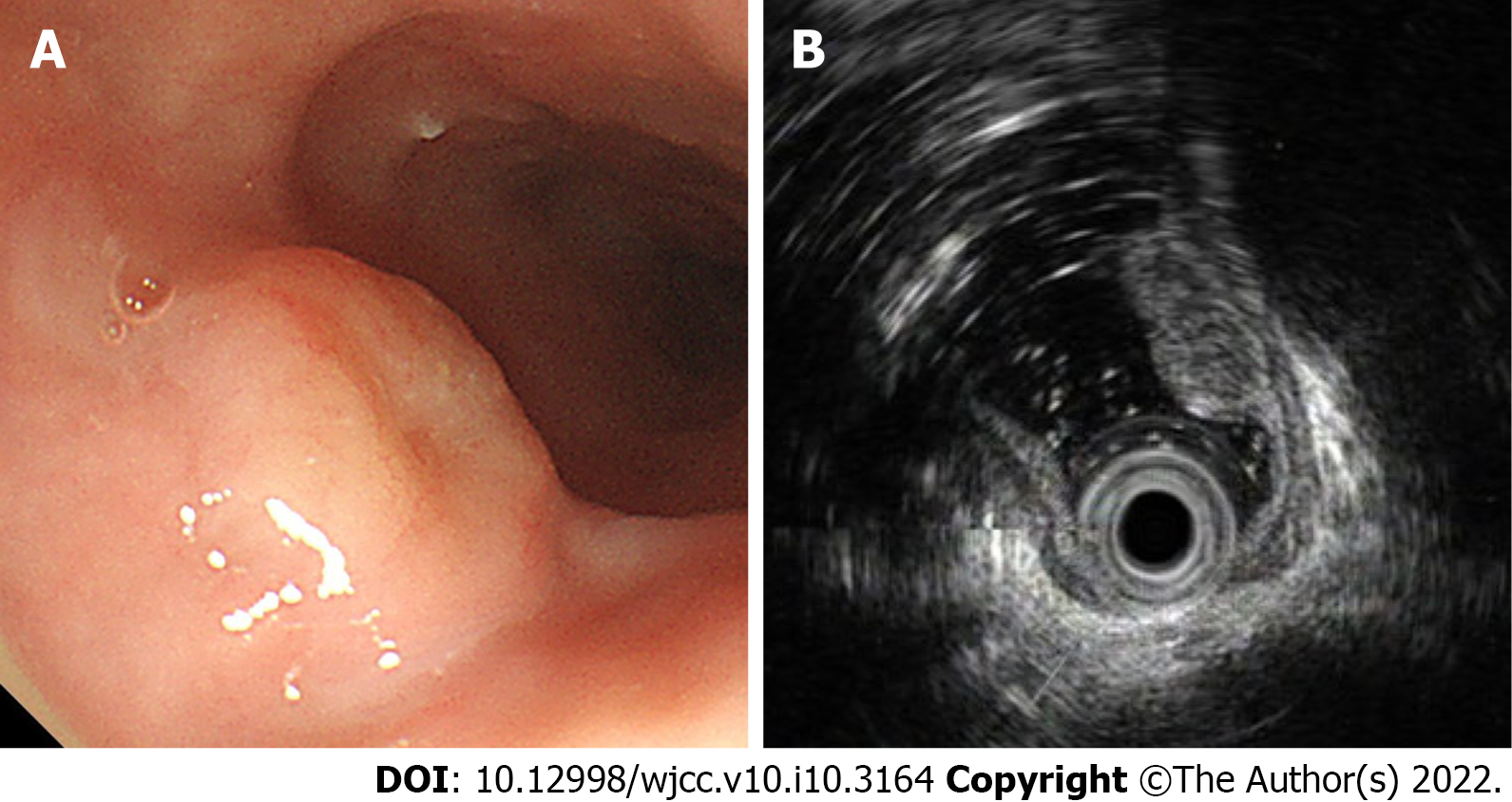
Chest enhanced computed tomography was normal, while abdominal computed tomography revealed a hepatic cyst and renal calculus. There was no evidence of distant metastases. Gastroscopy revealed a disc-shaped protruding lesion about 18 mm × 18 mm in size on the upper esophagus, 25 cm from the incisors (Figure 1A). Endoscopic ultrasonography (EUS) with a 20 MHz catheter probe showed that the bulged lesion was highly echoic and homogeneous, originating from the muscularis mucosa (Figure 1B).
The final diagnosis of the presented case was NEC of the esophagus.
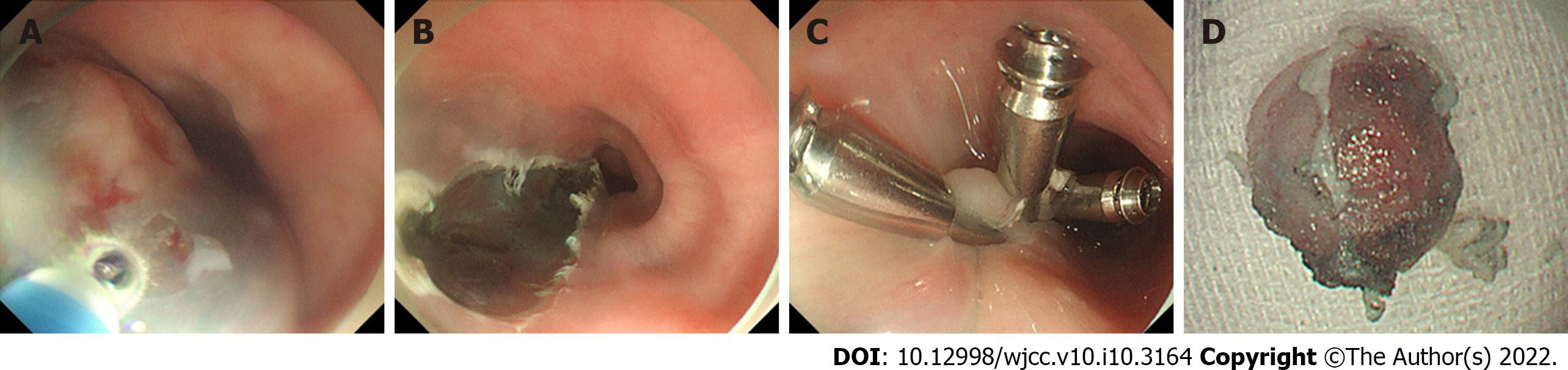
Gastroscopy and EUS showed that the tumor was confined to the muscularis mucosae. No radiographic evidence of lymph node enlargement and distant metastasis was found. Hence, we assessed en bloc resection using ESD for confirmation and determine the appropriate treatment. The edge of the protruding lesion was marked intraoperatively. We proceeded with a submucosal injection of indigo-carmine diluted with normal saline solution to create a submucosal lifting (Figure 2A). The lesion was completely removed using a dual knife with step-by-step electrocauterization (Figure 2B). Afterward, metal clips were used to close the mucosal gap (Figure 2C). Tumor samples were sent for pathological examination. Dissection time was 14 min (Video). The patient was started on a liquid diet 72 h post dissection. After monitoring for complications, the patient was discharged from hospital 5 d later.
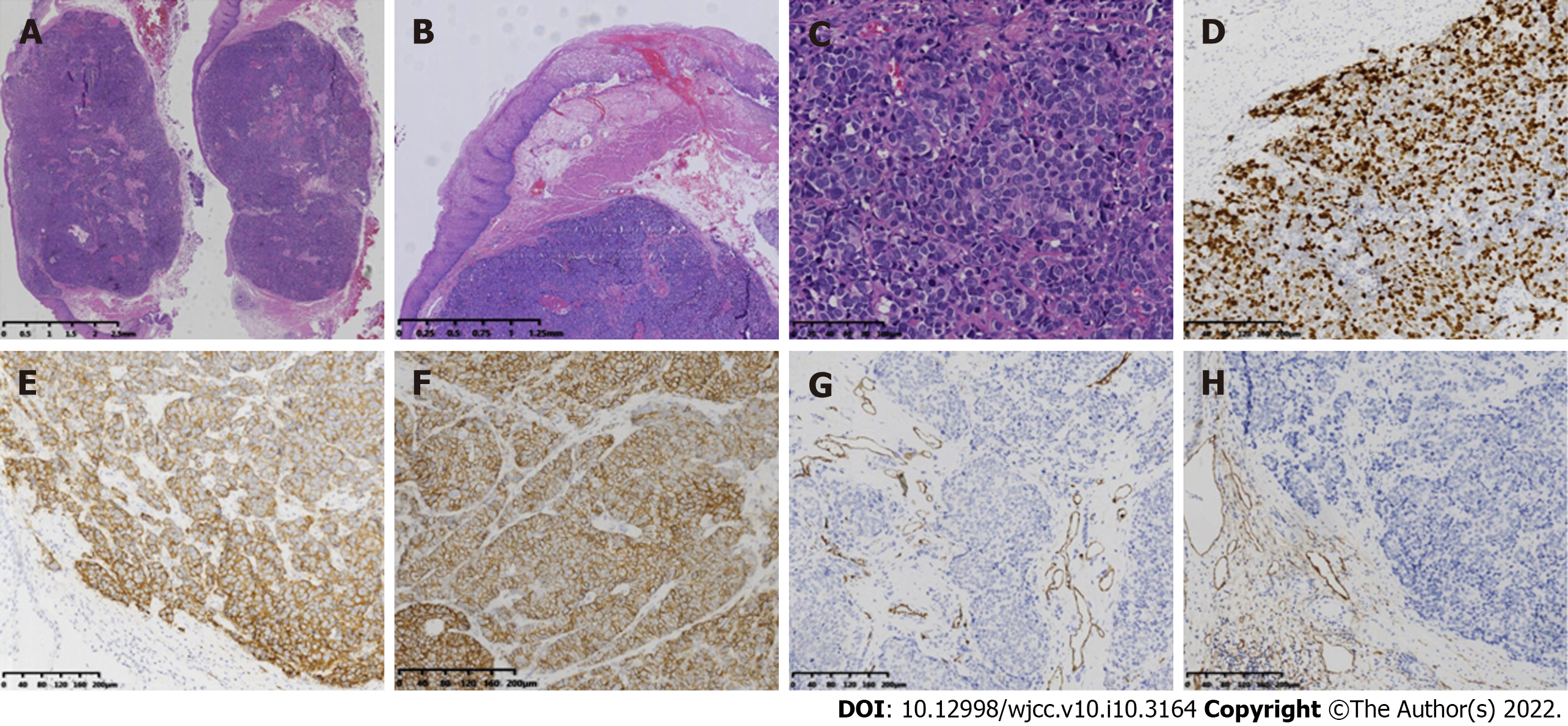
Macroscopically, the resected specimen was a gray and white mucosal tissue, with a nodule of 9 mm × 8 mm × 8 mm observed under the mucosa and having a tough texture (Figure 2D). Histopathological examination revealed a poorly differentiated neoplasm comprising of large cells with marked nuclear atypia, multifocal necrosis, and 20 mitotic figures per ten high-power fields. Both horizontal and vertical margins of resection were negative (Figure 3A and C). Depth of invasion was classified as submucosa 2 (SM2, submucosal invasion ≥ 200 µm) (Figure 3B). Immunohistochemistry staining revealed tumor tissue that was positive for the following markers: Ki67 (80%+), CgA, Syn, CD56, and cytokeratin (AE1/AE3), and negative for P40, P63, and S-100 protein (Figure 3D-F). Lymphovascular invasion was also ruled out using immunohistochemical staining for CD31 and CD240 (Figure 3G and H).
Based on the immunohistochemistry staining results, the tumor was classified as large cell esophageal carcinoma (LCEC), Grade 3, and limited disease (LD)/T1aN0M0 stage. Currently, the therapeutic strategy for NEC of the esophagus has still not been established. The National Comprehensive Cancer Network (NCCN) guidelines Version 3.2018 recommend that resectable large cell NECs should be treated by resection and chemotherapy with or without radiotherapy[11]. Based on the type, grade, and stage of this tumor, ESD may be considered as the primary treatment modality for mucosal lesions. Adjuvant therapy has been considered for ESD patients with SM2/SM3 lesions and patients with poorly differentiated lesions[12,13]. Based on careful consideration, we decided to include adjuvant EP regimen chemotherapy (Etoposide 60 mg/m2 days 1-5 + Cisplatin 25 mg/m2 days 1-3, repeat cycle every 3 wk for 6 cycles) after ESD surgery. The patient was closely followed every 3 mo for 2 years. CT scans did not identify regional lymphadenopathy or distant metastasis during follow-up.
Esophageal NEC is a poorly differentiated, highly malignant tumor consisting of small or medium to large cells with significant nuclear atypia, multifocal necrosis, and mass mitosis (> 20/10 high power field)[14,15].
Gross examinations of esophageal NECs have shown a wide variety of features, including submucosal polypoid infiltrating growths that are often covered by a normal epithelium, or ulcerative nodules on the surface of the esophagus mucosa. Microscopically, neuroendocrine tumor cells are arranged in nests with trabecular growths surrounded by palisades and rosettes. The frequency of lymphatic, venous, and peripheral nerve invasions is high[16].
Immunohistochemistry staining for NETS are usually positive for Syn, CgA, and NSE, where Syn is the most sensitive marker. NETs usually have a Ki-67 index > 20%. In addition, AE1/AE3, cytokeratin 34bE12 (CK34bE12), CD56, thyroid transcription factor-1 (TTF-1), and cytokeratin 10/13 staining was also observed in NETs[17,18].
Although the incidence is low, primary esophageal NETs are still relatively easy to detect using routine gastroscopy. At present, an increasing number of esophageal NETs are diagnosed early by endoscopy (tumor size less than 11 mm-20 mm and confined to the mucosa/submucosa). Due to the low frequency of lymph node and distant metastasis, local resection (including endoscopic treatment) is the treatment option. Endoscopic resection is a minimally invasive treatment for patients with esophageal NETs. ESD has an excellent resection rate and can provide histological grading to guide subsequent treatment decisions. If the tumor cannot be completely resected, additional surgery or systemic chemoradiotherapy will be required[19]. For our patient, we performed ESD resection of the esophageal NEC. Although the specimens had a negative horizontal margin and vertical margins without lymphovascular invasion, depth of invasion was classified as SM2. Adjuvant therapy has been considered for ESD patients with SM2/SM3 lesions and patients with poorly differentiated lesions. To a certain extent, complete resection of the tumor could prevent excessive treatment burden. However, endoscopic treatment for relapsing remnant tumors may be difficult due to the presence of fibrotic tissue hampering the separation of tumor from the intrinsic muscularis. Studies have demonstrated that ESD treatment could reduce the incidence of this outcome due to complete histological resection[20].
In conclusion, no standard treatment strategies are available for esophageal NEC due to a lack of clinical studies and its rarity. En bloc resection approach using ESD may play a vital role as a diagnostic and therapeutic modality for esophageal NEC.
The authors sincerely thank the entire staff of our department for offering their assistance with patient treatment and manuscript writing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Costache RS, Wong YJ S-Editor: Zhang YL L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 322] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 2. | MCKEOWN F. Oat-cell carcinoma of the oesophagus. J Pathol Bacteriol. 1952;64:889-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Hou X, Wei JC, Wu JX, Wang X, Fu JH, Lin P, Yang HX. Multidisciplinary modalities achieve encouraging long-term survival in resectable limited-disease esophageal small cell carcinoma. PLoS One. 2013;8:e69259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Joseph S, Wang YZ, Boudreaux JP, Anthony LB, Campeau R, Raines D, O'Dorisio T, Go VL, Vinik AI, Cundiff J, Woltering EA. Neuroendocrine tumors: current recommendations for diagnosis and surgical management. Endocrinol Metab Clin North Am. 2011;40:205-231, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Savva C, Kaye P, Soomro I, Parsons SL, James E, Madhusudan S. Primary Esophagogastric Neuroendocrine Carcinoma: a Retrospective Study from the Nottingham Upper Gastrointestinal Cancer Center. J Gastrointest Cancer. 2018;49:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Hong L, Zhang Y, Liu Z. Neuroendocrine carcinoma of esophageal and gastric cardia: clinicopathologic and immunohistochemistry study of 80 cases. Oncotarget. 2018;9:10754-10764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Hudson E, Powell J, Mukherjee S, Crosby TD, Brewster AE, Maughan TS, Bailey H, Lester JF. Small cell oesophageal carcinoma: an institutional experience and review of the literature. Br J Cancer. 2007;96:708-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Lu XJ, Luo JD, Ling Y, Kong YZ, Feng LL, Zhou J, Wang F. Management of small cell carcinoma of esophagus in China. J Gastrointest Surg. 2013;17:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Kukar M, Groman A, Malhotra U, Warren GW, Bogner P, Nwogu CE, Demmy TL, Yendamuri S. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol. 2013;20:4239-4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Tustumi F, Takeda FR, Uema RH, Pereira GL, Sallum RA, Cecconello I. Primary neuroendocrine neoplasm of the esophagus - Report of 14 cases from a single institute and review of the literature. Arq Gastroenterol. 2017;54:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Goetz MP, Gradishar WJ, Anderson BO, Abraham J, Aft R, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Farrar WB, Giordano SH, Goldstein LJ, Isakoff SJ, Lyons J, Marcom PK, Mayer IA, Moran MS, Mortimer J, O'Regan RM, Patel SA, Pierce LJ, Reed EC, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Telli ML, Ward JH, Young JS, Shead DA, Kumar R. NCCN Guidelines Insights: Breast Cancer, Version 3.2018. J Natl Compr Canc Netw. 2019;17:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, Toh Y, Doki Y, Naomoto Y, Nemoto K, Booka E, Matsubara H, Miyazaki T, Muto M, Yanagisawa A, Yoshida M. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 390] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 13. | Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, Toh Y, Doki Y, Naomoto Y, Nemoto K, Booka E, Matsubara H, Miyazaki T, Muto M, Yanagisawa A, Yoshida M. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16:25-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 14. | Babu Kanakasetty G, Dasappa L, Lakshmaiah KC, Kamath M, Jacob LA, Mallekavu SB, Rajeev LK, Haleshappa RA, Kadabur Nagendrappa L, Saldanha SC, Kumar RV. Clinicopathological Profile of Pure Neuroendocrine Neoplasms of the Esophagus: A South Indian Center Experience. J Oncol. 2016;2016:2402417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Egashira A, Morita M, Kumagai R, Taguchi KI, Ueda M, Yamaguchi S, Yamamoto M, Minami K, Ikeda Y, Toh Y. Neuroendocrine carcinoma of the esophagus: Clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017;12:e0173501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, Sun Q, Yang J, Huang L, Ye Q. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37:467-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Lu J, Xue LY, Lu N, Zou SM, Liu XY, Wen P. Superficial primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical analysis of 15 cases. Dis Esophagus. 2010;23:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Chen I, Zhang D, Velez M, Kovar S, Liao X. Poorly differentiated neuroendocrine carcinomas of the gastrointestinal tract: A single-institute study of 43 cases. Pathol Res Pract. 2021;226:153614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Morita M, Taguchi K, Kagawa M, Nakanoko T, Uehara H, Sugiyama M, Ota M, Ikebe M, Sugimachi K, Esaki T, Toh Y. Treatment strategies for neuroendocrine carcinoma of the upper digestive tract. Int J Clin Oncol. 2020;25:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Lee DS, Jeon SW, Park SY, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy. 2010;42:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |