Published online Dec 12, 2015. doi: 10.5528/wjtm.v4.i3.78
Peer-review started: July 29, 2015
First decision: September 21, 2015
Revised: November 13, 2015
Accepted: December 1, 2015
Article in press: December 2, 2015
Published online: December 12, 2015
Processing time: 143 Days and 17.1 Hours
AIM: To determine the role for the intermediate filament protein nestin in glioma invasion.
METHODS: We examined the expression and function of nestin in gliomas (Grades II-IV as defined by the World Health Organization). We determined nestin expression using Immunohistochemical methods. To elucidate nestin’s biological function(s), we reduced mRNA levels by 61% and 87% in two glioblastoma-derived neurosphere lines using short hairpin RNAs and determined the effect of reduced nestin expression on glioma cell proliferation and invasion using MTS and matrigel migration assays, respectively. We also utilized quantitative real time polymerase chain reaction assays to determine the effect of reduced nestin expression on the expression of other markers associated with glioma stem cells and their differentiated progenies.
RESULTS: We found a significant correlation between nestin immunoreactivity and astrocytoma tumor grade, with 36% of grade II, 75% of grade III, and 100% of grade IV tumors expressing significant levels of the protein when assessed using immunohistochemistry. Reduction in nestin expression had no effect on cell growth in culture, but did retard the capacity of one line to migrate in-vitro on matrigel. Interestingly, in the line whose migration was not affected, mRNA levels of a second intermediate filament, synemin (also knowns as desmuslin), were elevated following introduction of shRNA targeting nestin. As synemin was not induced in the line which required nestin for migration, it is a possibility that synemin may compensate for the loss of nestin in this process.
CONCLUSION: Nestin expression is prominent in high-grade astrocytomas. Nestin is not required for cell growth but it may, however, be required for cell motility.
Core tip: Despite its common use as a marker of poorly differentiated stem and progenitor cells, the functional role of nestin in normal and neoplastic cells is poorly understood. Here we show that in gliomas, there is a significant positive correlation between nestin protein expression and increasing pathological grade. However, when nestin expression was inhibited, we found no significant effects on cell growth, expression of stem-cell markers, and the ability to initiate intracranial xenografts. Our data suggest that the functional role of nestin is limited, even though the migratory potential of some glioblastoma neurospheres is reduced by nestin knockdown.
- Citation: Lin A, Marchionni L, Sosnowski J, Berman D, Eberhart CG, Bar EE. Role of nestin in glioma invasion. World J Transl Med 2015; 4(3): 78-87
- URL: https://www.wjgnet.com/2220-6132/full/v4/i3/78.htm
- DOI: https://dx.doi.org/10.5528/wjtm.v4.i3.78
Glioblastoma is the most common malignant primary brain tumor in adults. Despite some therapeutic advances in recent years, the prognosis for patients with glioblastoma remains dismal, and most patients die within 2 years of diagnosis. Several reports have suggested that the inability to cure glioblastoma is due to persistence of cancer stem cells, which have been shown to sustain the growth of many human tumors (reviewed in[1]). Cancer stem cells in brain tumors are thought to share many characteristics with other neural stem cells. For example, they contain proteins preferentially expressed in neural stem and progenitor cells such as CD133[2], OLIG2[3] and nestin[4,5]. The origin of stem-like cells in glioblastoma and other brain tumors is not yet clear, but they may arise from non-neoplastic adult stem cells, or from better-differentiated cells which reacquire stem-like properties via mutations and/or epigenetic modifications[1].
Nestin is a class VI intermediate filament protein[6], normally expressed in neuroepithelial stem and progenitor cells of the developing mammalian central nervous system (CNS). It is also expressed in adult neural stem cells lining the ventricular system of the subventricular zone and the dentate gyrus of the hippocampus, but in differentiated adult cells nestin is replaced by other intermediate filaments such as neurofilaments and glial-fibrillary-acidic-protein (GFAP)[4]. Re-expression of nestin in the adult CNS usually accompanies pathological conditions such as brain injury, ischemia, inflammation, or neoplastic transformation, and its presence in these processes may indicate a role in reactive and/or regenerative processes[7].
Nestin expression has been found in many types of brain tumors[5,8-10]. However, its functional role in tumor formation, proliferation, and migration is still not well understood. Recently, nestin has been shown to be required for prostate cancer cell migration in-vitro and in-vivo[11]. In the present study, we investigate the expression of nestin in astrocytic tumors and its functional role in glioblastoma. The latter experiments were performed in glioblastoma neurosphere lines containing stem-like cancer cells. Our results suggest that nestin is not required for proliferation in-vitro or engraftment in-vivo, but that it may be required for in-vitro migration in some glioblastoma cell lines.
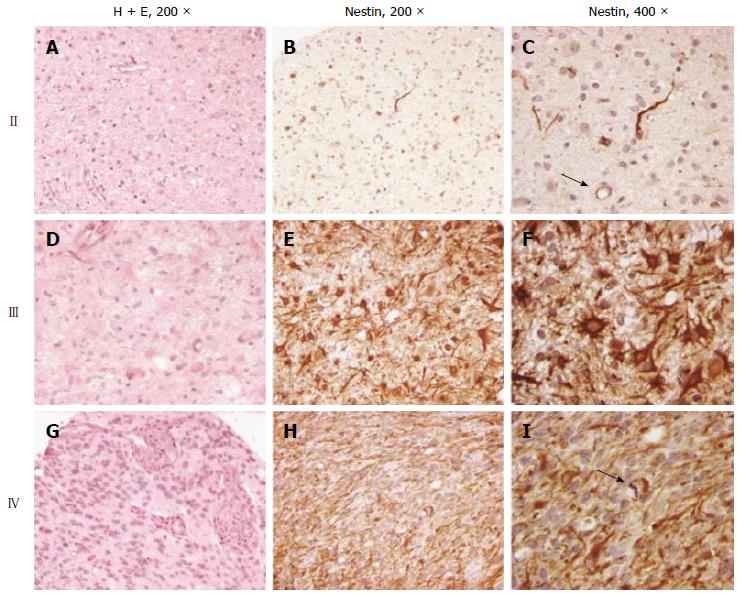
The tissue microarray, containing four 0.6 mm cores per tumor from a number of grade II fibrillary astrocytomas, grade III anaplastic astrocytomas, and grade IV glioblastoma multiforme, was created as previously described using samples obtained from the Department of Pathology, Johns Hopkins University School of Medicine, with Institutional Review Board approval[12]. A minimum of two of the four tissue cores had to be evaluable, and contain at least 10% nestin-immunopositive tumor cells, for a case to be scored as a positive. The glioblastoma neurosphere lines HSR-GBM1 and HSR040622, were a kind gift from Dr. Angelo Vescovi and were maintained as previously described[13].
Slides were deparaffinized and endogenous peroxidase activity was blocked by incubation in a hydrogen peroxide/methanol buffer. Unless stated otherwise, all of the following incubation steps were carried out at room temperature. First, antigen retrieval was performed by incubation of the slides in 10 mmol/L citrate buffer (pH 6.0) at 90 °C for 20 min. Incubation with the primary mouse anti-nestin antibody (1:5000; Chemicon, Billerica, MA) for 60 min was preceded by blocking with serum-free Protein Block (Dako Cytomation, Carpinteria, CA) for 20 min. Negative controls were performed by substitution of PBS for the primary antibody. After washing in PBS, HRP-conjugated anti-mouse IgG (1:500; Dako Cytomation) was applied to the slides for 30 min. For tyramide-based signal amplification the slides were subsequently treated using the TSA Biotin System according to the manufacturer’s protocol (PerkinElmer Life Sciences, Waltham, MA). Finally, slides were incubated for 8 min with a DAB solution (Sigma, St. Louis, MO), counterstained with hematoxylin, dehydrated, cleared, and mounted.
For immunocytochemistry, neurospheres were spun onto Superfrost plus slides (Fisher Scientific, Pittsburgh, PA) using a Shandon Cytospin3 (Thermo Shandon, Waltham, MA) for 5 min at 1500 rpm. Cells were then washed once for 5 min with PBS and then fixed in freshly prepared 4% para-formaldehyde (in PBS, pH 7.4) at room temperature for 30 min. After three washes with PBS for 5 min each, cells were permeabilized using PBST (PBS + 0.2% Triton × 100) for 5 min. Blocking was performed for 45 min at room temperature with PBST containing 5% normal horse or goat serum. The primary antibodies used were rabbit anti-Ki67 1:1000 (Novocastra Laboratories, United Kingdom), and mouse anti-nestin MAB5326 1:2500 (Chemicon, Temecula, CA). Nuclei were stained with DAPI (Pierce, Rockford, IL) for 3 min in PBS. Quantitation of Ki-67 immunostaining was made by counting separately the positively and negatively stained nuclei. At least ten high-power fields containing a minimum of one hundred cells each were counted per slide using a 63X objective. Only moderate to strong staining intensity was scored as positive. Ki-67 index was expressed as the percentage of positively stained nuclei to all nuclei.
Lentiviruses were generated essentially as previously described[11]. Briefly, 5 μg of lentiviral vector (either pSicoR or pSicoR/shNestin) and 2.5 μg of each packaging vector were cotransfected in 293T cells using the FuGENE 6 reagent (Roche Diagnostics, Indianapolis, IN). Oligos targeting the human nestin RNA sequence: 5’ TGCTGTTGACAGTGAGCGCGGCTAGTCCCTGCCTGAATAATAGTGAAGCCACAGATGTATTATTCAGGCAGGGACTAGCCATGCCTACTGCCTCGGA-3’ (human nestin shRNA1). Twenty four hours after transfection, growth medium was replaced with DMEM containing 2% FBS. Supernatants were collected 48 h and 72 h post medium change, filtered through a 0.45-μm filter, and used directly to infect neurospheres. One round of infection was usually sufficient to infect enough cells for subsequent drug selection. Virus was allowed to infect cells for 4 h and then the cells were washed once with PBS and plated back into Neurocult medium. Seventy-two hours later, positive cells were selected in puromycin (5 μg/mL). Puromycin resistant neurospheres were expanded for 3 wk before assayed for nestin expression.
RNA extraction and quantitative real time polymerase chain reaction (RT-PCR). For RNA extraction from cultured cells, 3.75 × 105 cells were plated in 75 cm2 tissue culture flasks each containing 10 mL of NeuroCult medium (Stem Cell Technologies, Canada) supplemented with hEGF and hFGF-b (Peprotech, Rocky Hill, NJ). Cultures were grown in the presence of 10 ng/mL puromycin (Sigma, St. Louis, MO) and incubated for 5 d in a humidified incubator. Cells were spun at 276 × g for 10 min followed by a rinse with ice-cold PBS. Cell pellets were processed for RNA extraction using the Qiagen RNeasy kits. Reverse transcription was performed as previously described[14].
mRNA levels were analyzed by RT-PCR analysis performed in triplicate with SYBR Green reagents (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions on an I-Cycler IQ real-time detection system (BioRad, Hercules, CA). To minimize contaminating genomic DNA, a thirty minutes on-column DNase step was included during RNA extraction. The standard curve method was used to determine expression levels, and all values were normalized to actin. Oligo sequences were as follows: Nestin forward: 5’CCAGGAGCCACTGAAGACTC; nestin reverse: 5’ CCTTTCCCAGGTTCTCTTCC; actin beta forward: 5’ CCCAGCACAATGAAGATCAAT; actin beta reverse: 5’ GATCCACACGGAGTACTTG. CD133 forward: 5’ CATCCACAGATGCTCCTAAGG; CD133 reverse: 5’ AAGAGAATGCCAATGGGTCCA; OLIG2 forward: 5’ GGACAAGCTAGGAGGCAGTG; OLIG2 reverse: 5’ ATGGCGATGTTGAGGTCGTG; GFAP forward: 5’ AACTGAGGCACGAGCAAAGT; GFAP reverse: 5’ GCAGTGCCCTGAAGATTAGC; MAP2 forward: 5’ CCATCTTGGTGCCGAGTGAG; MAP2 reverse: 5’ TGGGAGTCGCAGGAGATTTTG; vimentin forward: 5’ TACCGGAGACAGGTGCAGTCCCTCA; vimentin reverse: 5’ TCACGAAGGTGACGAGCCATTTCCT; Synemin transcript variants M/H (AJ310521.1, AJ310522.1, respectively) forward: 5’ ACAGGTGCTGGAGGATGTG; Synemin transcript variants M/H reverse: 5’ CGGATCGCCTCTACGTTACT; Synemin transcript variants M/H/L (AJ310521.1, AJ310522.1, AJ697971.1, respectively) forward: 5’ GGCCTCAGTCTGGAGGTGG; Synemin transcript variants M/H/L reverse: 5’ CCCAGATCACTATCTGTGGATTACT; To ensure that measurements of gene expression changes reflected a direct effect of nestin mRNA levels, we considered a significant expression level change only in cases where un-infected and scrambled control (shC) infected cells each showed significant level change as compared with shNestin (shN) infected cells.
Cells were plated in triplicates at 2500 cells in 96-well culture plates and incubated in a humidified incubator for a total period of seven days. MTS (Promega, Madison, WI) reagent was added to selected wells on the first, second, and seventh days before absorbance was read at 490 nm. Fold change in cell mass was calculated as the absorbance on day seven divided by the absorbance on day one.
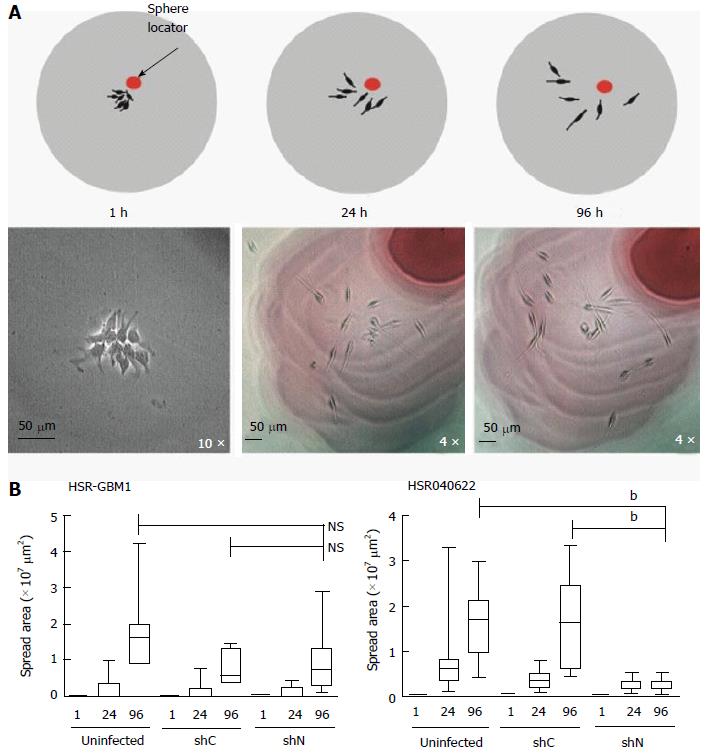
Forty-eight hours before plating neurospheres, single cells were plated at a density of 3.75 × 105 cells/mL. To prepare the matrigel substrate, 10 cm2 culture dishes were coated with low growth factor containing matrigel (BD biosciences, San Jose, CA) at a 1:100 dilution with Neurocult medium and placed in a humidified incubator over-night. The next day, plates were rinsed once with PBS before neurosphere plating. Neurospheres were plated at a very low density in which, on average, a single neurosphere could be visualized per field. Most neurospheres attached to the matrix one hour post plating at which point the medium was replaced to minimize continuous adhesion of any remaining floating neurospheres. The position of 20-25 spheres was noted on the bottom of the plate (sphere locator in Figure 1B) to allow monitoring of cell spreading over time. Sphere area was calculated by multiplying the two longest perpendicular axes extending from the two furthest cells in a sphere. Each experiment was performed at least three times.
VassarStats software (http://faculty.vassar.edu/lowry/VassarStats.html) was used for statistical analyses. The significance of differences in nestin expression in astrocytomas of varying grades was analyzed using the Freeman-Halton extension of the Fisher exact probability test for contingency tables.
Statistical analyses in this study were reviewed by Dr. Luigi Marchionne, who is a trained biostatistician and a co-author.
The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, at libitum access to food and water) for at least two weeks before experimentation. For xenograft studies, 1 × 105 viable cells were diluted with fresh medium and injected over 10 min into the right striatum of athymic (nu/nu) mice (Harlan, Indianapolis, IN, http://www.harlan.com). Mice were monitored daily and sacrificed at the first indication of tumor development (ataxia, seizure, lethargy, or cachexia). Brains were surgically removed and fixed immediately in formalin before submission for histological analysis, as previously described[15].
Animal care and use statement: Discomfort was be minimized by the use of anesthesia during potentially painful procedures (intracranial injections). The anesthetic used was Ketamine-Xylazine in sterile saline injected intraperitoneally in accordance with the Institutional Animal Care and Use Committee.
We assessed a total of 41 astrocytic tumors for nestin immunoreactivity in tissue arrays containing evaluable cores from 11 grade II fibrillary astrocytomas, 16 grade III anaplastic astrocytomas, and 14 grade IV glioblastoma (Figure 2). Immunoreactivity for nestin was identified in all tumor types, with a positive correlation between nestin immunoreactivity and increasing grade of these infiltrating astrocytic tumors. A significant level of nestin immunoreactivity, defined as 10% or more of tumor cells, was identified in 4/11 (36%) grade II fibrillary astrocytoma (Figure 2A-C), 12/16 (75%) grade III anaplastic astrocytoma (Figure 2D-F), and 14/14 (100%) grade IV glioblastoma, (Figure 2G-I). These differences were statistically significant (P < 0.001, Fisher exact test). Nestin immunoreactivity was not exclusively observed in tumor cells, and was also detected in reactive astrocytes, in microglial cells adjacent to neoplastic elements, and in endothelial cells (Figure 2C). We did not quantitate the percentage of nestin-positive cells in each tumor, but there appeared to be an increase in the extent of nestin immunoreactivity with increasing grade.
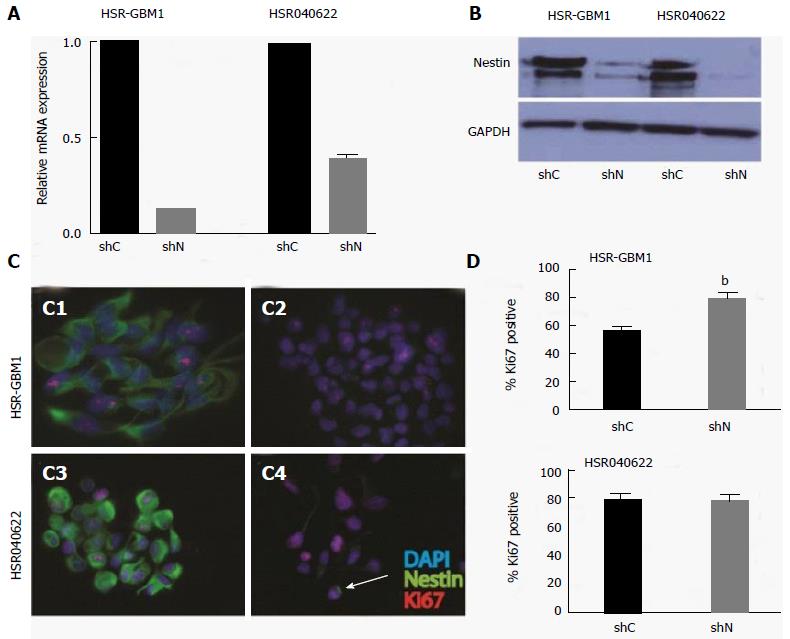
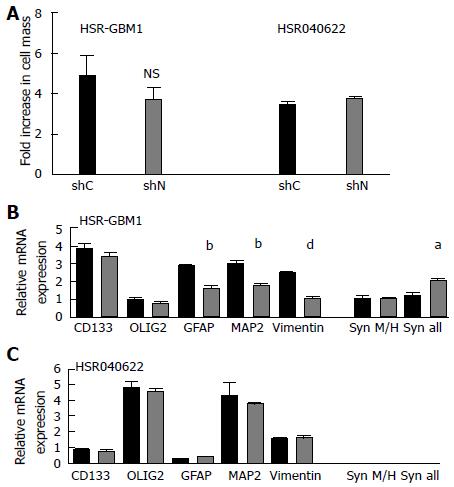
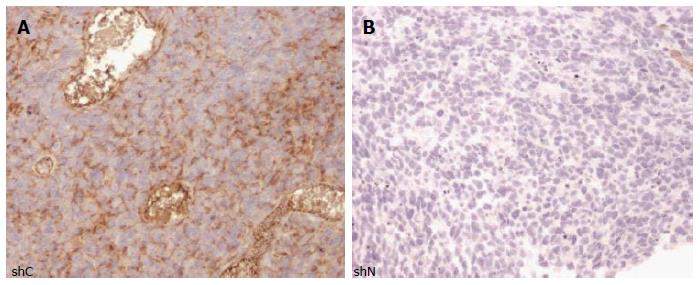
Proliferation and migration are important characteristics of stem and progenitor cells in the developing brain, as well as stem-like brain tumor cells[13,16]. Both processes may be influenced by cytoskeleton dynamics. To test if nestin has a role in cellular proliferation, we stably transduced two human glioblastoma neurosphere lines with lentiviruses encoding short hairpin RNAs (shRNAs) targeting either the nestin transcript (shNestin/shN) or a nonspecific scrambled sequence (shC). Utilizing this system, we achieved 87% and 61% reduction in nestin mRNA levels in HSR-GBM1 and HSR040622, respectively, as compared to shC expressing cells (Figure 3A and B). To further evaluate the effect of reduced nestin mRNA, we examined the expression of nestin protein on a single cell level. Immunocytochemistry analysis confirmed that nestin protein was present in the majority of cells of shC infected lines (Figure 3C1, C3) and in uninfected cells (data not shown). Transduction with shN almost completely eliminated detectable nestin staining in HSR-GBM1 (Figure 3C2). In HSR040622, sporadic residual nestin positive cells could be found (arrow in Figure 3C4). Despite the dramatic reduction in nestin expression, we found no reduction in proliferation of HSR-GBM1 cells, with 56% and 80% Ki67 positive nuclei for shC and shN infected cells, respectively (Figure 3D, top panel). Similar results were observed for HSR040622 with 79% Ki67 positive nuclei for both shC and shN infected cells, respectively (Figure 3D, lower panel). To more directly test the effect of nestin reduction on proliferation, we next performed MTS assays which allow sensitive evaluation of changes in viable cell mass over time. We found no significant differences between the growth rates of HSR-GBM1 with high and low nestin levels, with 4.8 and 3.7 fold increases in cell mass for shC and shN infected cells, respectively. Similar results were observed for HSR040622, with 3.40 and 3.7 fold increases in total cell mass for shC and shNestin infected cells (Figure 4A).
Nestin has been widely used as a marker of neural stem cells. We therefore investigated the possibility that nestin reduction may alter expression of other stem/progenitor or differentiation markers within glioblastoma derived neurospheres. We compared mRNA levels of the neural stem/progenitor cell markers CD133 and OLIG2, as well markers of glial (GFAP) and neuronal (MAP2) differentiation. Levels of the intermediate filaments vimentin and synemin were also measured, as changes in their expression could potentially compensate at least in part for nestin loss. The expression level of synemin was analyzed using two different primer pairs. The first (M/H) amplifies a product which corresponds to the M and H synemin transcript variants, while the second pair (“all”) amplifies a product which represents the M, H, and L transcript variants. We found no significant differences in the mRNA level of CD133, OLIG2, MAP2, and vimentin in HSR040622 (Figure 4C). Interestingly, HSR-GBM1 cells with reduced nestin also express significantly lower levels of GFAP (56%, P < 0.01, two-tailed t-test) and MAP2 (60%, P < 0.01, two tailed t-test) (Figure 4B). In contrast, we observed an 80% increase in the level of synemin when analyzed using primers which anneal to its three transcript variants (M, H, and L; P < 0.05, two-tailed t test), but no significant change in level of the M and H transcript variants alone. We infer from these observations that the increased levels of synemin result from a significant increase in the level of synemin L in HSR-GBM1 (Figure 4B).
Glioma cell motility plays a key role in tumor spread, and in the current inability to cure patients with these malignancies. It has been previously shown that nestin is required for metastasis of the AT6.3 prostate cancer line, suggesting it may be directly involved in cancer cell migration[11]. We therefore explored the possibility that nestin may play a role in glioma cell migration as well. Migration of HSR-GBM1 and HSR040622 was examined in a two-dimensional neurosphere outgrowth assay, which allowed migration from a small, defined number of cells to be assessed over time (Figure 1A). Glioma neurospheres were allowed to form over two days in suspension and then plated onto matrigel-coated plates. One hour after plating, neurospheres had tightly bound, forming small colonies with an average cross-sectional area of 196 μm2 for HSR-GBM1 and 236 μm2 for HSR040622. The growth in cross-sectional area of individual colonies was then measured over time. Only limited cellular proliferation occurred over the time course of the assay, and, as we found no change in proliferation with reduced nestin levels, we believe any differences in area are due to effects on migration. Nestin knockdown dramatically reduced cell spreading in HSR040622 cells (Figure 1B, right panel) from an average area of 1.66 × 107μm2 for shC infected to 2.2 × 106× 105μm2 for shN infected cells (two tailed t test; P < 0.0001). In contrast, we found no significant decrease in migration for HSR-GBM1, with an average area of 7.24 × 106μm2 for shC infected and 9.12 × 106μm2 for shNestin infected cells (Figure 1B, left panel).
An intracranial xenograft model was employed to compare the tumorigenicity and migratory capacity of HSR-GBM1 shC and shN infected cells. We injected 1 × 105 viable tumor cells into the right striatum of athymic nude mice and monitored tumor formation for a period of 16 wk. Xenografts formed in all animals injected with either shC or shN infected HSR-GBM1 cells, but we found no significant difference between these two lines in terms of their in-vivo growth. Both forming large, infiltrative tumors, resulting in death as early as 63 d following injection. Immunostaining of these xenografts confirmed that tumor cells maintained reduced nestin expression over the relatively prolonged period of in-vivo growth (Figure 5).
We examined nestin immunoreactivity in astrocytomas of grades II to IV, and found a significant positive correlation between protein expression and increasing pathological grade. These results are similar to those previously reported in gliomas by several other groups[4]. Dahlstrand et al[4], showed higher nestin expression in malignant tumors such as glioblastoma when compared to lower grade glial tumors. Ehrmann et al[17] reported that in astrocytomas and malignant melanomas, nestin expression could be used as an auxiliary indicator of dedifferentiation and progression. Nestin expression has also been detected in non-glial malignancies, including hemangioblastomas[9], melanoma[18,19], basal epithelial breast tumors[20], prostate cancer[11], and in gastrointestinal stromal tumors[21]. In many of these, immunoreactivity correlated with increasing pathological grade as well[4,8,17,20-25]. The fact that higher grade tumors show increased nestin immunoreactivity suggests that the percentage of cells with a stem/progenitor phenotype may increase during tumor progression. In addition, the fact that nestin expression is associated with increasing grade in a very wide variety of malignancies indicates that a better functional understanding of this protein may lead to insights into how tumors progress.
Despite its common use as a marker of poorly differentiated stem and progenitor cells, the functional role of nestin in normal and neoplastic cells is poorly understood. It has recently been shown that nestin can serve as a scaffold for a number of signal transduction proteins, such as cdk5 and p35/45, thereby regulating their function[26-28]. Intermediate filaments function as cytoskeletal scaffolds in the nucleus and cytoplasm[29] and may be involved in cellular migration and metastatic potential[30-32]. In addition, it has been shown that nestin is required for migration and metastasis of prostate cancer cells[11]. Such studies highlight the need to examine potential functional roles of nestin, in addition to using it as a marker of differentiation status.
We examined the requirement for ongoing nestin expression in growth and migration of two glioblastoma-derived neurosphere lines. Growth was not significantly affected in either line when nestin levels were reduced by 60% or more using shRNA. These findings indicate that as in prostate cancer[11], glial cells appear to grow nicely with severely reduced nestin levels. It has been suggested that nestin may be only one of several intermediate filaments involved in proliferation, and therefore its loss may be compensated for by other members of this large family of proteins[11]. Indeed, we detected about a two fold-increase in synemin mRNA levels following knockdown of nestin in HSR-GBM1, although it appears that only the L transcript variant is induced (Figure 4B).
We also examined the expression of several mRNA expressed in either stem/progenitor or better differentiated cells in lines with varying nestin levels. Loss of nestin did not seem to alter expression of CD133 or Olig2, although the glial marker GFAP and the neuronal marker MAP2 were decreased somewhat following nestin knockdown. The significance of this latter observation is not clear, but the fact that CD133 and Olig2 levels were unchanged, and that cultures could be passaged for over six months with low nestin levels, strongly suggest that it is not required for the maintenance of tumor-propagating stem-like cells.
Understanding the migration of glial tumor cells is of fundamental importance if we are to eventually cure malignant brain tumors. Our results suggest that nestin may be required for such migration in-vitro in a subset of tumors, as the spread of HSR040622 cells was almost completely abolished in-vitro by nestin knockdown. In contrast, the in-vitro spread of the second line examined was unaffected by shRNA targeting nestin, and these cells could still form invasive intracranial xenografts despite prolonged reduction of nestin. The molecular basis for the varying requirements of glioblastoma neurosphere lines for nestin is not clear. It is possible that the increase in synemin levels observed in the HSR-GBM1 cells with nestin-targeting shRNA may compensate for the reduced nestin levels, allowing cells to migrate normally. Indeed, the intermediate filament synemin has previously been shown to contribute to the migratory properties of astrocytoma cells by influencing the dynamics of the actin cytoskeleton[33].
In summary, our studies support the concept that nestin expression is a common feature of astrocytic brain tumors, and that protein levels correlate with tumor grade. However, the functional role of nestin appears to be limited, although the migratory potential of some glioblastoma neurospheres is reduced by nestin knockdown. Further studies will be needed to fully understand the role of nestin in migration, and the heterogeneity between different glioblastoma lines.
The challenges in curing glioblastoma have been partially attributed to the persistence of cancer stem cells following treatment. This subpopulation of cells have been shown to express proteins preferentially expressed in neural stem and progenitor cells such as CD133, OLIG2, and nestin. Nestin is a class VI intermediate filament protein, normally expressed in neuroepithelial stem and progenitor cells of the developing mammalian central nervous system (CNS). In this study, the authors focus on determining if nestin plays a functional role in tumor formation, proliferation, and migration as this is still not well understood.
Previous work has established that nestin is required for prostate cancer cell migration in-vitro and in-vivo. In the present study, the authors investigate the expression of nestin in astrocytic tumors and its functional role in glioblastoma.
This is the first study evaluating nestin’s role in tumor formation, proliferation, and migration utilizing glioma stem cells.
The findings suggest that nestin may be involved in glioblastoma cell invasion in some tumors. Inhibition of nestin expression and/or function may represent a potential therapeutic approach to reduce or inhibit glioblastoma cell spreading throughout the CNS.
All terms used in this paper are described in the main text.
The relationship of nestin and glioma was well discussed in the paper.
P- Reviewer: Hatanpaa KJ, Zhang XL S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5561] [Article Influence: 264.8] [Reference Citation Analysis (0)] |
| 3. | Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, Bachoo RM, Kane M, Louis DN, Depinho RA. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334-5341. [PubMed] |
| 5. | Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303-313. [PubMed] |
| 6. | Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2437] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 7. | Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 499] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 8. | Strojnik T, Røsland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133-143; discussion 143-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Sugawara K, Kurihara H, Negishi M, Saito N, Nakazato Y, Sasaki T, Takeuchi T. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002;82:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Sakurada K, Saino M, Mouri W, Sato A, Kitanaka C, Kayama T. Nestin expression in central nervous system germ cell tumors. Neurosurg Rev. 2008;31:173-176; discussion 173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, Berman DM. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007;67:9199-9206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 13. | Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011-7021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 1931] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 14. | Bar EE, Chaudhry A, Farah MH, Eberhart CG. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol. 2007;170:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Stearns D, Chaudhry A, Abel TW, Burger PC, Dang CV, Eberhart CG. c-myc overexpression causes anaplasia in medulloblastoma. Cancer Res. 2006;66:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295:263-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 436] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 17. | Ehrmann J, Kolár Z, Mokry J. Nestin as a diagnostic and prognostic marker: immunohistochemical analysis of its expression in different tumours. J Clin Pathol. 2005;58:222-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Brychtova S, Fiuraskova M, Hlobilková A, Brychta T, Hirnak J. Nestin expression in cutaneous melanomas and melanocytic nevi. J Cutan Pathol. 2007;34:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Li H, Cherukuri P, Li N, Cowling V, Spinella M, Cole M, Godwin AK, Wells W, DiRenzo J. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 2007;67:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Yang XH, Wu QL, Yu XB, Xu CX, Ma BF, Zhang XM, Li SN, Lahn BT, Xiang AP. Nestin expression in different tumours and its relevance to malignant grade. J Clin Pathol. 2008;61:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Ma YH, Mentlein R, Knerlich F, Kruse ML, Mehdorn HM, Held-Feindt J. Expression of stem cell markers in human astrocytomas of different WHO grades. J Neurooncol. 2008;86:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Maderna E, Salmaggi A, Calatozzolo C, Limido L, Pollo B. Nestin, PDGFRbeta, CXCL12 and VEGF in glioma patients: different profiles of (pro-angiogenic) molecule expression are related with tumor grade and may provide prognostic information. Cancer Biol Ther. 2007;6:1018-1024. [PubMed] |
| 24. | Mao Y, Zhou L, Zhu W, Wang X, Yang G, Xie L, Mao X, Jin K. Proliferative status of tumor stem cells may be correlated with malignancy grade of human astrocytomas. Front Biosci. 2007;12:2252-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Schiffer D, Manazza A, Tamagno I. Nestin expression in neuroepithelial tumors. Neurosci Lett. 2006;400:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Sahlgren CM, Mikhailov A, Vaittinen S, Pallari HM, Kalimo H, Pant HC, Eriksson JE. Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol. 2003;23:5090-5106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE. A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO J. 2006;25:4808-4819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Toivola DM, Tao GZ, Habtezion A, Liao J, Omary MB. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 2005;15:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Alberts B, Alexander J, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell 4th Edition. New York: Garland Science 2002; . |
| 30. | Bellin RM, Huiatt TW, Critchley DR, Robson RM. Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J Biol Chem. 2001;276:32330-32337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P. Vimentin contributes to human mammary epithelial cell migration. J Cell Sci. 1999;112:4615-4625. [PubMed] |
| 32. | Hendrix MJ, Seftor EA, Chu YW, Trevor KT, Seftor RE. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev. 1996;15:507-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 193] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Pan Y, Jing R, Pitre A, Williams BJ, Skalli O. Intermediate filament protein synemin contributes to the migratory properties of astrocytoma cells by influencing the dynamics of the actin cytoskeleton. FASEB J. 2008;22:3196-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |