Published online Aug 19, 2025. doi: 10.5498/wjp.v15.i8.107342
Revised: April 24, 2025
Accepted: June 18, 2025
Published online: August 19, 2025
Processing time: 140 Days and 22.3 Hours
Visceral hypersensitivity is the core pathogenesis of irritable bowel syndrome (IBS) and is often accompanied by negative emotions such as anxiety or depre
To investigate the pathological mechanisms visceral hypersensitivity and negative emotions in IBS, as well as the effect mechanism of EA.
A model of diarrhoeal IBS (IBS-D) with negative emotions was prepared by chronic restraint combined with glacial acetic acid enema. The effect of EA was verified by abdominal withdrawal reflex and open-field test. PVN CRF-colonic mast cell (MC)/transient potential receptor vanilloid type 1 (TRPV1) pathway was detected by immunofluorescence, Western blot, ELISA, and toluidine blue staining. Moreover, PVN CRFergic neurons were activated or inhibited by chemogenetical technique to observe the changes of effect indicator.
In the model group, IBS-D symptoms and negative emotions were successfully induced. Notably, the combination of Baihui (GV20) with Tianshu (ST25) and Dachangshu (BL25) acupoints showed the greatest efficacy in improving the negative emotions and visceral hypersensitivity in model mice. Furthermore, we found that EA inhibited overactivated PVN CRFergic neurons and the overexpression of serum CRF, colonic CRF, CRF-receptor 1 (CRF-R1), mast cell tryptase (MCT), protease-activated receptor 2 and TRPV1 in model mice. Moreover, we found that activating PVN CRFergic neurons induced negative emotions and visceral hypersensitivity in normal mice; however, inhibiting PVN CRFergic neurons alleviated negative emotions and intestinal symptoms in model mice and decreased the expression of colonic CRF-R1, MCT, and TRPV1.
This research highlights the key role of PVN CRF-MC CRF-R1 and the downstream MC/TRPV1 pathway in the pathological process of IBS-D and the mechanism of the effect of EA.
Core Tip: An important feature of functional gastrointestinal disorders (FGIDs) is accompanied with emotional disorders, such as anxiety and depression. Clinically, acupuncture not only improves physical symptoms, but also has a unique psychological effect in treatment of FGIDs. However, the underlying mechanisms remain unclear. Brain-gut crosstalk may mediatepathological process of FGIDs, corticotropin releasing factor (CRF) is an important mediator in brain-gut crosstalk. So, our study focused on the CRF of brain-gut, we hypothesized that electroacupuncture improved mood disorders and visceral pain in irritable bowel syndrome, which may be achieved through paraventricular nucleus CRF-colonic CRF-receptor 1 and downstream pathway.
- Citation: Xu JG, Yuan Y, Ma HK, Huang S, Zhu SL, Li TT, Wang XY, Shen GM, Wang H. Electroacupuncture ameliorates visceral hypersensitivity and negative emotions by regulating paraventricular hypothalamic nucleus and colonic corticotropin-releasing factor signaling. World J Psychiatry 2025; 15(8): 107342
- URL: https://www.wjgnet.com/2220-3206/full/v15/i8/107342.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i8.107342
Irritable bowel syndrome (IBS) is a functional gastrointestinal disease that is closely related to mood[1]. Visceral hypersensitivity is one of the core pathogenic mechanisms of IBS. Main clinical manifestation of IBS is a decrease in the patient's sensory threshold for gastrointestinal filling, dilation, and contraction, which ultimately results in abdominal pain and distension. The development of visceral hypersensitivity is closely related to psychological/physiological stress, early adverse events, and chronic stress[2,3] and is often accompanied by anxiety (15%-45%), depression (20%-30%) and other types (40%-60%) of negative emotions[4]. Studies have shown that IBS patients have a greater probability of anxiety and depression and that activity in the brain, such as the anterior cingulate cortex, hypothalamus, insula, amygdala, and hippocampus, is positively correlated with colonic dilatation[5,6]. These regions are closely related to emotional-visceral regulation.
The brain-gut axis may provide a partial explanation for the interaction between gastrointestinal dysfunction and mood disorders. The brain-gut axis suggests an intimate and complex contact between the central nervous system (CNS) and enteric nervous system (ENS), and brain-gut peptides such as neurotransmitters, hormones, and immune factors play important roles in communication between the CNS and the ENS. Oversecretion of brain-gut peptides can influence ENS, neuroendocrine and immune functions, thus increasing the risk of visceral hypersensitivity in IBS patients[7]. Among the brain-gut peptides, corticotropin-releasing factor (CRF, also called as corticotropin-releasing hormone) is a key factor in regulating negative emotions-related gastrointestinal dysfunction and is mainly released from the paraventricular hypothalamic nucleus (PVN)[8]. Central CRF acts on the gastrointestinal tract through the brain-gut axis and is involved in coordinating the gastrointestinal response to stress, which includes the regulation of gastric acid secretion and gastric emptying, colon-related hormone secretion and motor function, and intestinal permeability and visceral hypersensitivity[9].
How PVN CRF affects intestinal symptoms remains unclear. Studies have shown that colonic mast cell (MC) activation is positively correlated with the intestinal symptoms of IBS and that CRF can act on the high-affinity receptors of colonic MCs[10]. When colonic MCs are activated, they can degranulate and release histamine, MC tryptase (MCT), and leukotrienes. MCT can act on the protease-activated receptor 2 (PAR2) in intestine to promote the expression of the transient potential receptor vanilloid type 1 (TRPV1) through the intracellular PKC pathway, thus mediating the development of visceral hypersensitivity[11]. Whether the PVN CRF affects the colonic MC/TRPV1 pathway to regulate the visceral hypersensitivity and negative emotions in IBS is not clear.
It is important to explore the interaction between the CNS and the ENS in functional gastrointestinal disorders accompanied by negative emotions. The latest Rome IV guidelines classify IBS as diarrhoeal (IBS-D), constipated and mixed[12]. In our study, we prepared a model of IBS-D accompanied by negative emotions via chronic restraint[13-17] combined with a glacial acetic acid enema[18,19]. Electroacupuncture (EA) has unique advantages in the treatment of IBS and negative emotions. Animal studies have shown that EA at ST25 and BL25 acupoints can relieve visceral sensitivity by affecting the sensory neurons in the spinal cord and intestinal cannabinoid receptor 2, and ameliorate intestinal diarrhoea symptoms by modulating 5-HT and tryptophan hydroxylase[20-22]. EA at GV20 may be involved in improving negative emotions in mice through regulation of hippocampal brain-derived neurotrophic factor/tyrosine receptor kinase B proteins, the toll-like receptor 4 signaling pathway and other mechanisms[23,24]. Therefore, we chose ST25, BL25, and GV20 to treat IBS-D accompanied by negative emotions model mice and further explored the effect mechanism of EA.
In our study, we used chronic restraint combined with glacial acetic acid enema to induce IBS-D accompanied by negative emotions in mice. The PVN CRF-colonic MC/TRPV1 pathway was hypothesized to elucidate how chronic restraint combined with glacial acetic acid enema induced negative emotions and visceral hypersensitivity in IBS-D, as well as the effect mechanism of EA. This study provides evidence for EA in the treatment of IBS-D.
Specific pathogen-free male C57BL/6J mice (22 ± 2 g) aged 6-8 weeks were used for all the experiments. All the animals were purchased from Hangzhou Ziyuan Experimental Animal Science and Technology Co., Ltd. The mice were maintained under standard laboratory conditions, with an ambient temperature of 23-25 °C and 50% humidity, a normal 12-hour light/dark cycle, and free access to water and food unless they were undergoing chronic stress. All experimental animal procedures were conducted in accordance with the Anhui University of Chinese Medicine Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Anhui University of Chinese Medicine (AHUCM-mouse-2024148).
There is no uniform model for IBS-D accompanied by negative emotions. Through preliminary experimental exploration and reference to the relevant literature, we decided to use male mice subjected to restraint stress using a 50 mL injection tube for 6 hours per day for 28 days and gave the mice 3% glacial acetic acid (150 ± 20 μL/each) injected into the colon 2 cm from the anus at two-day intervals starting on day 22, followed by an immediate saline enema (150 ± 20 μL/each) to replenish electrolytes and reduce mortality of the mice, then the mice were inverted to prevent the solution from leaking out. Control mice were allowed to move freely with access to food and were given saline enemas synchronized with the model mice.
The mice in the EA group were maintained under anaesthesia by continuous inhalation of 1%-2% isoflurane. The handles of two sterile acupuncture needles (0.25 mm × 13 mm) were wrapped side by side with medical tape to expose the end of the handles, and, while keeping the two needles 1 mm apart, both tips were inserted into the acupuncture points: Unilateral Tianshu (ST25) (located 5 mm lateral to the intersection of the upper 2/3 and lower 1/3 of the line between the xiphoid process and the superior border of the pubic symphysis and inserted obliquely into the abdominal musculature by 3-4 mm); Unilateral Dachangshu (BL25) (located just below the spinous process of the fourth lumbar vertebra and 5 mm to the sides and inserted 3 mm vertically); and Baihui (GV20) (located in the middle of the parietal bone and inserted 2-3 mm vertically). The needling was alternated left and right ST25 with BL25 every day. The end of the needle handle was connected to a Huatuo brand EA instrument (SH-I, Suzhou, China), and a sparse-close waveform (2/15 Hz) with a current intensity of 0.5 mA was selected for needling (20 minutes once a day for 7 consecutive days).
The chemogenetically activated virus rAAV-CRH-hM3D(Gq)-mCherry-WPREs (AAV2/9, 5.54E + 12 vg/mL; Brain VTA Co., Ltd.) or the chemogenetically inhibited virus rAAV-CRH-hM4D(Gi)-mCherry-WPREs (AAV2/9, 5.57E + 12 vg/mL; Brain VTA Co., Ltd.) was injected into the bilateral PVN of C57/B6J mice. The virus was specifically expressed on CRFergic neurons in mice, and when the virus was expressed for three weeks, it was combined with an intraperitoneal injection of clozapine-N-oxide (CNO) (5 mg/kg), which specifically activated/inhibited CRFergic neurons (effective within 30 minutes to 4 hours after injection).
The mice were maintained under anaesthesia by continuous inhalation of 1%-2% isoflurane. The head was then fixed in a stereotaxic device (RWD Co., Ltd., China). A 36 °C heating pad was used to maintain body temperature of the mice. The skull was then exposed, and a drawn glass microelectrode was used and levelled to ensure that the skull was straight in all planes. Cranial perforations were made at the injection site using a cranial drill, and 200 nL of virus was injected into the PVN at a rate of 35 nL/minute through a glass microelectrode connected to an infusion pump (Micro 4, WPI Co., Ltd., United States). After the injection was complete, leave the pipette in place for 10 minutes to prevent transmission of the virus. The coordinates of the PVN are as follows: Medial-lateral: ± 0.25 mm, anterior-posterior: -0.62 mm, and dorsal-ventral: -4.55 mm.
Evaluation of animal models: A model of IBS-D accompanied by negative emotions was prepared by chronic restraint combined with glacial acetic acid enema, then this model was evaluated by behavioural tests, visceral sensitivity tests, and the degree of diarrhoea.
Analysis of the effects and mechanism of EA: To investigate the effects of EA at different acupoints in mice, we prepared a new batch of model mice. The mice in the EA group were synchronized with the mice in the model group for model preparation, and the mice in the EA group were treated with additional EA in addition to chronic restraint and glacial acetic acid enemas starting on the 22nd day. The control and model mice were synchronized with inhaled anaesthesia during EA in the EA group.
The EA groups were randomly assigned as the GV20 group, the ST25 + BL25 group, and the GV20 + ST25 + BL25 group, and the therapeutic effects of EA at different acupoints were assessed by visceral sensitivity measurements and behavioural tests. The EA group with the best efficacy was then selected to assess the mechanism associated with the brain-gut axis in the mice with IBS-D accompanied by negative emotions.
Further analysis of the mechanism with chemogenetics: The chemogenetically activated virus was injected into the PVN of C57/B6J mice and allowed to be expressed for three weeks, after which the mice were randomly assigned as the hM3Dq + saline group, hM3Dq + CNO group, and hM3Dq + CNO + EA group. Three weeks later, the hM3Dq + saline group was injected with saline intraperitoneally for one week, the hM3Dq + CNO group was injected with CNO intraperitoneally for one week, and the hM3Dq + CNO + EA group was given CNO intraperitoneally once a day and ST25, BL25 or GV20 EA was given on the basis of the treatment. The hM3Dq + saline group and hM3Dq + CNO group mice were synchronized with inhalation anaesthesia during EA in the EA group. By testing the corresponding indicators, further studies were performed to determine whether EA has therapeutic effects on negative emotions and visceral hypersensitivity in IBS-D by modulating PVN CRFergic neurons.
After injection of chemogenetically inhibited virus into the PVN of C57/B6J mice, the mice were randomly assigned as normal + hM4Di + saline, model + hM4Di + saline, and model + hM4Di + CNO groups. The model + hM4Di + saline/CNO groups were subjected to chronic restraint combined with glacial acetic acid enema. After three weeks, one week of saline was injected intraperitoneally into the normal + hM4Di + saline group, and one week of saline/CNO was injected intraperitoneally into the model + hM4Di + saline group and the model + hM4Di + CNO group in combination with chronic restraint and glacial acetic acid enemas. The role of PVN CRFergic neurons in IBS-D was investigated by testing the corresponding indices.
Open-field test (OFT) were conducted in a dimly lit test chamber, the mice were habituated for more than half a day prior to testing. The test was videotaped using a video tracking system. A white plexiglas case (50 cm long, 50 cm wide and 40 cm high) was used, and free exploration for 5 minutes was recorded via SmartIOv2.0 software. The distance in center and time in center were calculated. The interior of the device was cleaned by 75% ethanol and water at the end of each trial to remove olfactory residues.
The degree of visceral sensitization in the mice was determined by the abdominal withdrawal reflex (AWR) evoked by colorectal dilatation (CRD). The mice were fasted for 12 hours (with free access to water) prior to testing and then placed on a custom-made immobilizer. The mice were first lightly anaesthetized, and then an uninflated balloon catheter (6-Fr, 2 mm OD) coated with paraffin oil was slowly placed in the colon 2 cm from the anus. The catheter was secured to the root of the tail with medical tape, and the other end was connected to a 1 mL syringe filled with saline. When the mice were awake and acclimatized, CRD was performed by expanding the catheter balloon via the injection of saline (which was injected rapidly and maintained for 20 seconds). The AWR scoring criteria were as follows: Score 0, no response to CRD stimulation; score 1, the mice briefly moved their head and then stopped; score 2, the mice contracted their abdominal muscles; score 3, the mice contracted and lifted their abdomen; and score 4, the mice arched their backs and lifted their pelvis. Using 3 as the pressure threshold for visceral pain, the amount of saline injected when the AWR of the mice was a 3-minute response was recorded, and the measurements were repeated five times per mouse with a 3-minute interval between measurements. Finally, the average value was taken.
Fresh faeces excreted by the mice for 3 hours were collected in containers for weighing, then dried for 24 hours at 60 °C. The rate of faecal moisture content = (mass before drying - mass after drying)/mass before drying × 100%.
Clean and dry trays were prepared and lined with clean filter paper, and fresh faeces were collected from the mice for 3 hours. The total number of faecal pellets, percentage of loose stool, and diameter of the faecal-contaminated filter paper were calculated. Diarrhoea index was calculated as loose stool rate × average loose stool level. The loose stool rate is the ratio of the number of loose stools to the total number of stools, and the average loose stool level is the ratio of the sum of all loose stool levels to the total number of loose stools. The loose stool level was calculated according to the diameter of loose stool contaminated filter paper, which was divided into 4 levels: Less than 1 cm for level 1, 1 to 1.9 cm for level 2, 2 to 3 cm for level 3, and more than 3 cm for level 4. If the shape of the faeces was approximately circular, the diameter was measured; if not, the length of the longest and the approximate circle was measured, and the average was taken. If the faeces could not be separated into grains, a pile was defaulted to one grain.
Blood was collected from abdominal aorta of mice and centrifuged (3000 rpm, 15 minutes) to extract the upper layer of serum after standing for 1 hour at room temperature. According to the instructions of the CRF ELISA kit (SBJ-M0891, SenBeiJia Biological Technology Co., Ltd.) to complete all steps, and the standard curve was plotted with the concentration of the standard vs the absorbance value. The expression level of CRF was calculated from the standard curve and the absorbance value corresponding to mouse serum CRF.
A 2 cm length of colon 3 cm from the anus was removed and stored at -80 °C. Colon was homogenized by RIPA lysis buffer (P0013B, Beyotime) supplemented with PMSF (ST506, Beyotime) at a ratio of 1000:10. Lysed tissues were thoroughly mixed with 5 × SDS-PAGE protein sampling buffer (P0015 L, Beyotime) and then placed in boiling water for 5-8 minutes. The proteins were separated on 10% or 12% SDS-polyacrylamide gels, PVDF membranes (IPVH00010, Millipore, 0.45 μm) were activated using methanol and the proteins were subsequently transferred onto the PVDF membranes. The samples were then placed in 5% skim milk in TBST (Tris-buffered saline containing Tween-20) and incubated for 2 hours at room temperature. The samples were then incubated overnight at 4 °C with the following antibodies: CRF-receptor 1 (CRF-R1) (rabbit polyclonal antibody, YT5490, ImmunoWay, 1:1000), PAR2 (rabbit polyclonal antibody, YN2681, ImmunoWay, 1:1000), MCT (rabbit polyclonal antibody, 13343-1-AP, Proteintech, 1:1000), TRPV1 (rabbit polyclonal antibody, DF10320, Affinity, 1:1000), and GAPDH (mouse monoclonal antibody, 60004-1-Ig, Proteintech, 1:5000). After washing, the PVDF membrane was incubated with secondary antibodies, namely, goat anti-rabbit (RS0002, ImmunoWay, 1:10000) and goat anti-mouse (RS0001, ImmunoWay, 1:10000) at room temperature for 1.5 hours. Exposure development was performed using an exposure machine (Tanon 5200). Finally, the results of the quantitative analysis were analysed using ImageJ.
The mice were anaesthetized by isoflurane (3%-4%) then perfused sequentially with saline and paraformaldehyde. Brain and colon were subsequently removed. After dehydration, the brain was wrapped in optimal cutting temperature compound (BL557A, Biosharp) and cut into 40 μm coronal slices, and the colon was wrapped in dehydrated paraffin wax and cut into 5 μm coronal slices.
The slices were blocked with 5% goat serum, 5% BSA and 0.5% Triton for 1 hour. The slices were incubated with the following antibodies at 4 °C overnight: CRF (rabbit monoclonal antibody, ab272391, Abcam, 1:100), c-fos (guinea pig monoclonal antibody, 226308, SYSY, 1:200), CD117 (rabbit polyclonal antibody, 18696-1-AP, Proteintech, 1:100), CRF-R1 (rabbit polyclonal antibody, YT5490, ImmunoWay, 1:200) and TRPV1 (rabbit polyclonal antibody, DF10320, Affinity, 1:200). Then slices were incubated with a secondary antibody (Alexa Fluor® 488-conjugated AffiniPure goat anti-rabbit IgG (H + L), 111-545-003, Jackson, 1:200; Alexa Fluor® 594-conjugated AffiniPure goat anti guinea pig IgG (H + L), 111-585-003, Jackson 1:200; HPR-labeled goat anti-rabbit IgG IF555-Tyramide, G1233-50UL, Servicebio, 1:200 and HPR-labeled goat anti-rabbit IgG IF488-Tyramide, G1231-50UL, Servicebio, 1:200) at room temperature for 1.5 hours. Then fluorescent slices were stained by an antifluorescent quencher containing 4,6-diamidino-2-phenylindole, and slices were subse
The colon were fixed in paraformaldehyde, dehydrated in gradient ethanol, wrapped in paraffin wax, and sectioned using a slicer (thickness of 5 μm); after slicing, the sections were spread out with a spreading machine and dried with a roaster. Sections 5-μm thick were stained with Haematoxylin and eosin (HE), differentiated with ethanol hydrochloride, and sealed by neutral gum. Observations were made via an optical microscope at a magnification of × 200.
After the tissues were dehydrated, embedded, sectioned, and deparaffinized, they were stained with toluidine blue solution, differentiated with glacial acetic acid until the nuclei and granules were clear, and sealed by neutral gum. The degranulation of MCs were observed under a 400 × optical microscope, and the MCs were round, oval, and irregularly shaped.
Colon sections approximately 1 mm3 were removed, washed three times in PBS, and placed in electron microscope fixative for 24 hours. Gradient ethanol dehydration followed by resin embedding and sectioning in a microtome (60-nm thickness). Staining was performed by means of 2% uranium acetate in a saturated alcohol solution, followed by three rinses each with 70% ethanol and ultrapure water. The samples were then stained with lead citrate (2.6%) as a CO2-free solution and washed three times by ultrapure water. The ultrastructure of the colon was visualized via projection electron microscopy (8000 ×).
We analysed the data using GraphPad Prism 9.5.0, and the data are expressed as the means ± SEM. Unpaired t-tests were used to compare two groups. Comparisons between multiple groups were made using one-way ANOVA followed by Tukey's post hoc multiple comparisons. Significance levels are expressed as aP < 0.05, bP < 0.01.
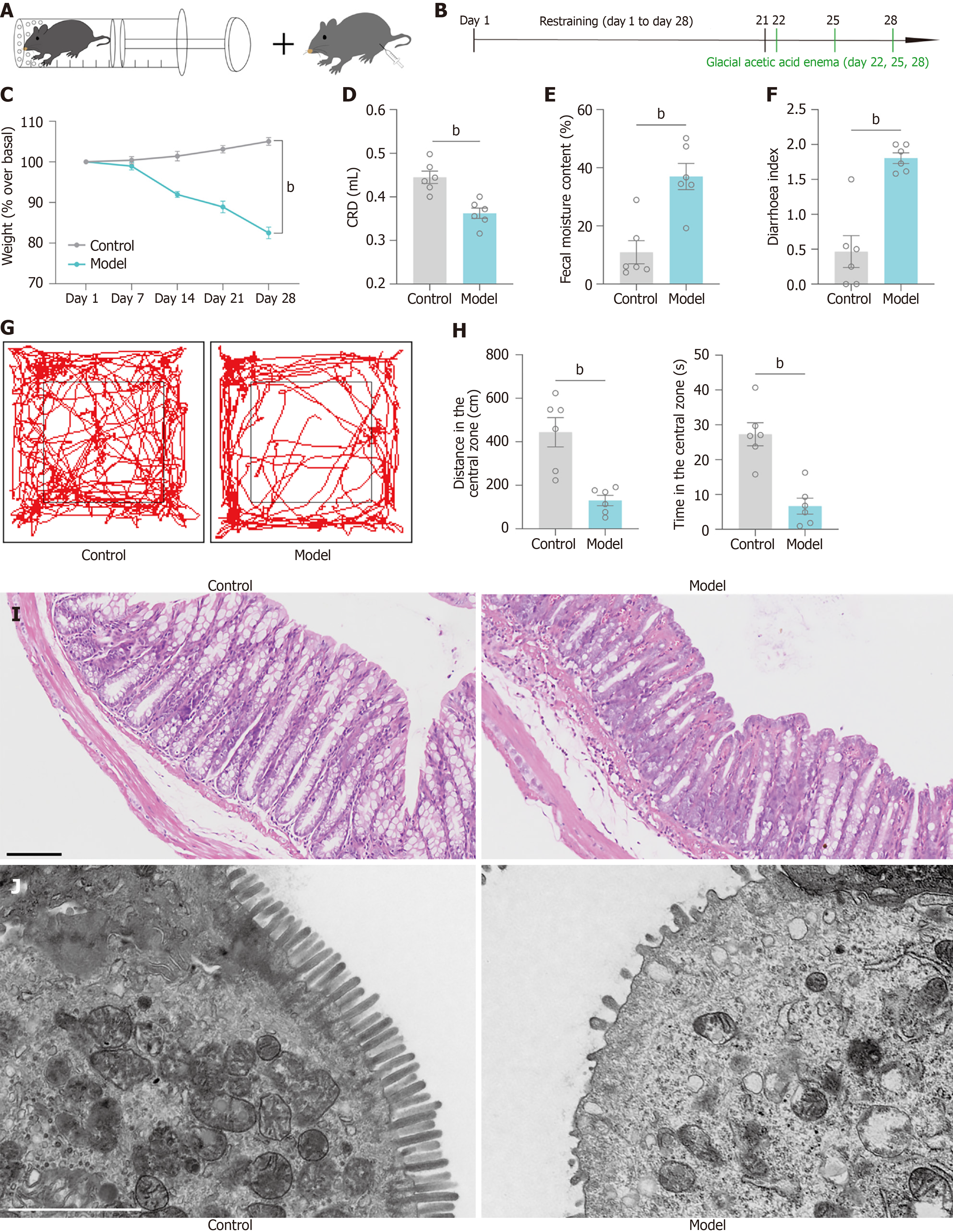
We prepared an IBS-D model accompanied by negative emotions via chronic restraint combined with glacial acetic acid enema (Figure 1A and B) and evaluated whether the model was successfully established using various tests to provide a foundation for subsequent experiments.
The results have shown that the weights of the mice in the model group gradually decreased and were significantly different to those of the normal controls (Figure 1C). We tested the visceral sensitivity of the mice by the AWR induced by CRD, and the results revealed that the amount of saline injected into the mice in the model group was decreased, i.e., the visceral sensitivity was increased (Figure 1D). Furthermore, the faecal moisture content (Figure 1E) and diarrhoea index (Figure 1F) tests revealed that the mice in the model group experienced diarrhoea. The OFT revealed significant decreases in distance in center and time in center (Figure 1G and H). HE staining of mouse colonic tissues showed that the colonic mucosa of mice in the model group exhibited damage, disorganized cell arrangement, and impaired crypt structure (Figure 1I). Observation of colonic tissues by transmission electron microscopy revealed a disorganized arrangement of colonic villi, a reduced number of villi and a reduced number of organelles in the model group mice (Figure 1J).
Thus, chronic restraint combined with glacial acetic acid enemas was effective in inducing a model of IBS-D accompanied by negative emotions.
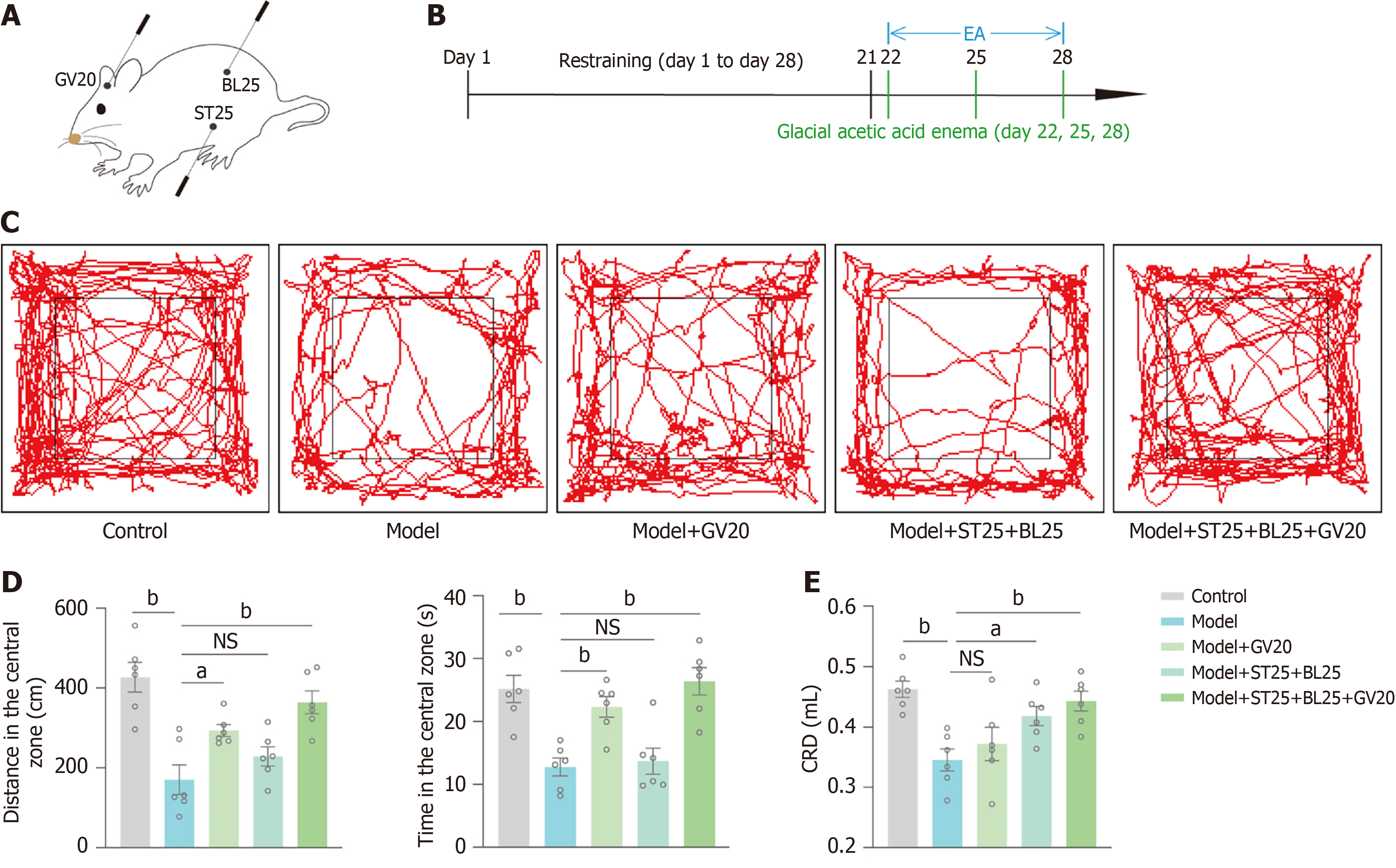
To investigate the role of EA at different acupoints in mice with IBS-D accompanied by negative emotions, we randomized the EA group into GV20 group, ST25 + BL25 group, and GV20 + ST25 + BL25 group. One week of EA was performed from day 22 (Figure 2A and B). The OFT revealed that distance in center and time in center were lower in the model group than in the control group. Compared with the model group, GV20 significantly improved distance in center and time in center. EA at GV20 effectively improved the negative emotions of the model mice. ST25 in combination with BL25 increased the above indices in the model mice but did not significantly differ from those in the model group of mice. The combination of three acupoints, GV20, ST25 & BL25, had a better effect on the negative emotions of the model mice (Figure 2C and D). The degree of visceral sensitivity of the mice was examined by CRD-induced AWR, and the results revealed that visceral sensitivity of mice in the model group was increased, whereas the combination of ST25 with BL25 significantly reduced the visceral sensitivity of the model mice. The visceral sensitivity of GV20 was reduced but did not significantly differ from that of the model group, and the use of a combination of the three acupoints had the best effect to improve visceral hypersensitivity in mice modelling IBS-D accompanied by negative emotions (Figure 2E). Therefore, different acupoints have their own therapeutic specificity, and the combination of GV20 with the ST25 and BL25 acupoints is more helpful in the treatment of negative emotions and visceral pain in IBS-D mice.
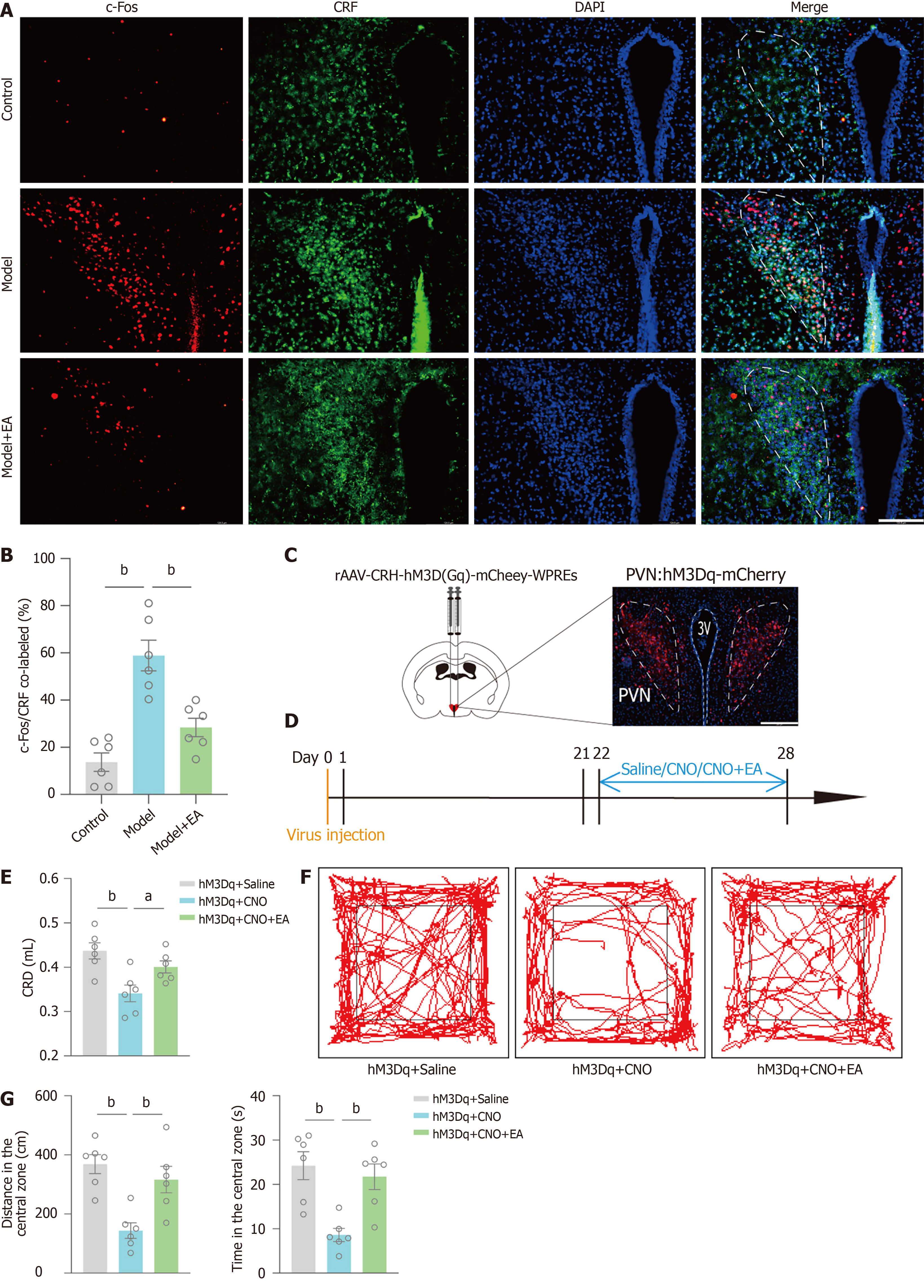
PVN CRFergic neurons play important roles in modulating stress-related behaviour and gastrointestinal responses. Therefore, in our study, we explored the mechanism by which EA regulates negative emotions and visceral hypersensitivity in IBS-D mice via PVN CRFergic neurons.
The PVN immunofluorescence results showed an increase in the CRF/c-fos co-labelling rate in the model group compared with the control group, i.e., an increase in the activity of PVN CRFergic neurons and a decrease in the CRF/c-fos co-labelling rate in the EA group compared with the model group (Figure 3A and B). Next, we specifically activated PVN CRFergic neurons by chemogenetic activation via the injection of chemogenetically activated viruses into the PVN of C57/B6J mice and the intraperitoneal injection of CNO-specific activation of PVN CRFergic neurons in mice after three weeks of viral expression (as hM3Dq + CNO group). The hM3Dq + saline group was injected with saline intraperitoneally for one week, and the hM3Dq + CNO + EA group was given CNO intraperitoneally and underwent EA stimulation for one week. Chemogenetically activated viruses were observed to be expressed in the PVN by PVN coronal sectioning (Figure 3C and D). The degree of visceral sensitization in mice was examined by CRD-induced AWR, and the results revealed that the amount of saline injected into the mice in the hM3Dq + CNO group was lower than that in the hM3Dq + saline group, and the amount of saline injected into the mice in the hM3Dq + CNO + EA group was greater than that of the mice in the hM3Dq + CNO group (Figure 3E). The distance in center and time in center were significantly lower in the hM3Dq + CNO group than in the hM3Dq + saline group, as shown by the OFT. Indicators above were significantly greater in the hM3Dq + CNO + EA group than in the hM3Dq + CNO group (Figure 3F and G). Thus, specific activation of PVN CRFergic neurons mediates negative emotions and promotes the onset of visceral hypersensitivity, and EA is able to reverse this effect. These findings demonstrate that EA has therapeutic effects on negative emotions and visceral hypersensitivity in IBS-D mice via the modulation of PVN CRFergic neurons.
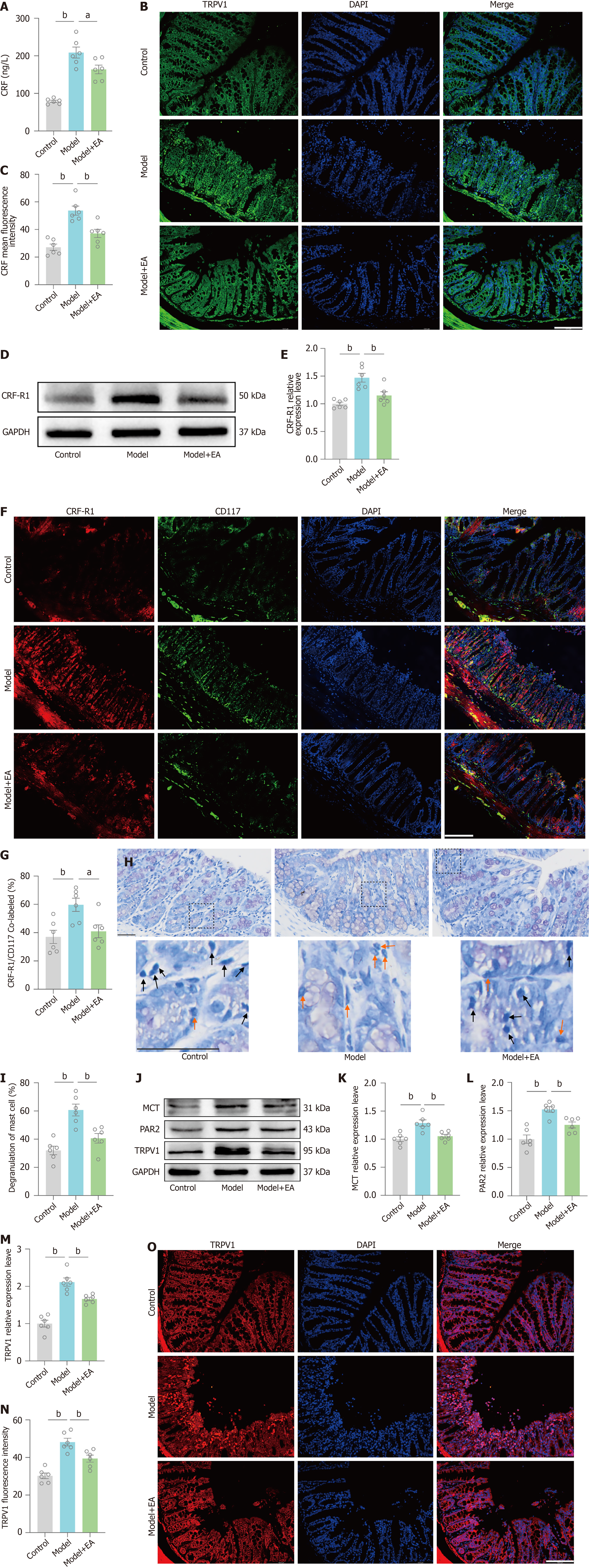
We further explored how PVN CRFergic neuronal activation induces visceral hypersensitivity. We assayed serum CRF by ELISA, and the results showed an increase in the CRF concentration in the model group compared with the control group and a decrease in the CRF concentration in the EA group compared with the model group (Figure 4A). Immunofluorescence of the colonic mucosa showed an increase in CRF expression in the model group compared with the control group and a decrease in CRF expression in the EA group compared with the model group (Figure 4B and C). Colonic CRF-R1 expression was detected by Western blot, and the results showed that the relative expression level of colonic CRF-R1 was significantly higher in the model group than in the control group and that the relative expression level of CRF-R1 was significantly less in the EA group than in the model group (Figure 4D and E). CD117 is a specific marker of MCs, immunofluorescence co-labeling of colonic CRF-R1 with CD117 showed that the CRF-R1/CD117 co-labelling rate was increased in the model group compared with the control group, and the CRF-R1/CD117 co-labelling rate was reduced in the EA group compared with the model group (Figure 4F and G). We observed MC degranulation in the colonic mucosa of the mice by toluidine blue staining and found that, compared with that in the control group, the MC degranulation rate in the model group was greater, and the MC degranulation rate in the EA group was less than that in the model group (Figure 4H and I). The relative expression of MCT, PAR2, and TRPV1 was examined by Western blot to detect downstream pathways after MC activation. The results revealed an increase in the relative expression of MCT, PAR2, and TRPV1 in the model group compared with the control group and a decrease in the relative expression levels of MCT, PAR2, and TRPV1 in the EA group compared with the model group (Figure 4J-M). Immunofluorescence detection of the intestinal mucosa showed an increase in TRPV1 expression in the model group compared with the control group and a decrease in TRPV1 expression in the EA group compared with the model group (Figure 4N and O).
These results directly demonstrate that chronic restraint combined with glacial acetic acid enema-induced central CRF acts on colonic CRF-R1 via the brain-gut axis to initiate the MC-MCT-PAR2-TRPV1 pathway, which mediates visceral hypersensitivity in IBS-D. Furthermore, EA can ameliorate brain-gut disturbances by modulating the PVN CRF, thereby inhibiting the MC/TRPV1 pathway and ultimately ameliorating visceral hypersensitivity in IBS-D mice.
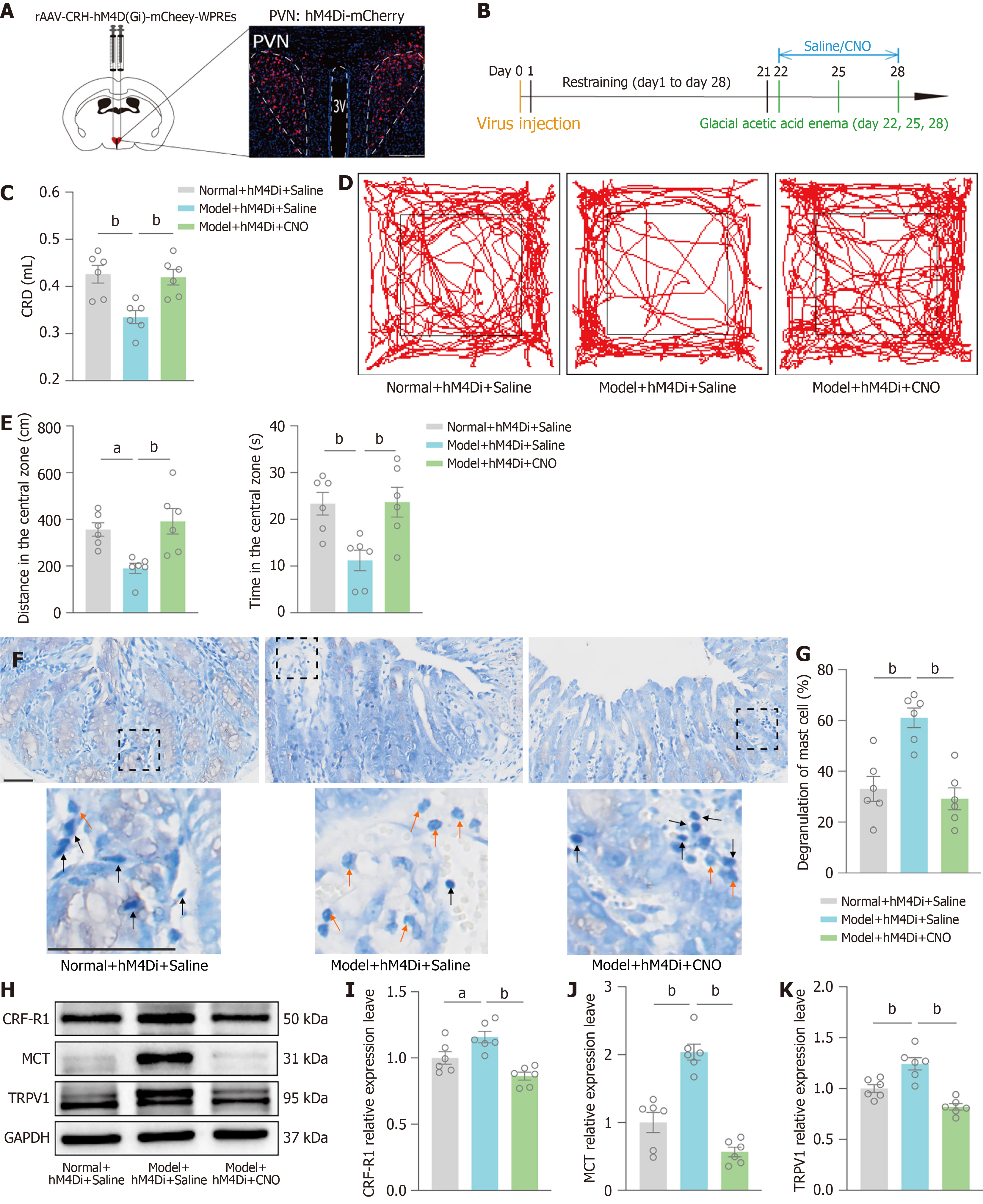
In this study, we found that PVN CRFergic neurons and the MC/TRPV1 pathway participate in regulation of the negative emotions and visceral hypersensitivity by EA. However, whether PVN CRFergic neurons, through the colonic MC/TRPV1 pathway, control visceral hypersensitivity in IBS mice is not clear. Therefore, we inhibited PVN CRFergic neurons by chemogenetics to observe changes in the MC/TRPV1 pathway, negative emotions and visceral hypersensitivity.
The model + hM4Di + saline/CNO group was subjected to model preparation, and after three weeks, the normal + hM4Di + saline group was injected with saline intraperitoneally for one week with the model + hM4Di + saline group, and CNO was injected intraperitoneally for one week with the model + hM4Di + CNO group. Chemogenetically inhibited virus expression in the PVN was observed by PVN coronal sectioning (Figure 5A and B). The degree of visceral sensitivity of the mice was examined by CRD-induced AWR, and the results revealed that mice in the model + hM4Di + saline group had a lower saline injection volume than those in the normal + hM4Di + saline group. The saline injection volume was higher in the model + hM4Di + CNO group than in the model + hM4Di + saline group, i.e., visceral hypersensitivity was alleviated (Figure 5C). The OFT revealed that mice in the model + hM4Di + saline group exhibited a reduction in distance in center and time in center compared to the normal + hM4Di + saline group. The model + hM4Di + CNO group exhibited a reversal of this result compared to the model + hM4Di + saline group (Figure 5D and E). MC degranulation was detected via toluidine blue staining of the colonic mucosa, and the results showed an increase in the MC degranulation rate in the model + hM4Di + saline group compared with the normal + hM4Di + saline group and a decrease in the degranulation rate in the model + hM4Di + CNO group compared with the model + hM4Di + saline group (Figure 5F and G). The relative expression of CRF-R1, MCT, and TRPV1 were tested by Western blot, and the results showed that the relative expression of CRF-R1, MCT, and TRPV1 were greater in the model + hM4Di + saline group than in the normal + hM4Di + saline group and that the relative expression of CRF-R1, MCT, and TRPV1 were lower in the model + hM4Di + CNO group than in the model + hM4Di + saline group (Figure 5H-K).
These results further confirm that the inhibition of PVN CRFergic neurons can effectively improve brain-gut interactions and inhibit the colonic MC/TRPV1 pathway, ultimately ameliorating chronic restraint combined with glacial acetic acid enema-induced negative emotions and visceral hypersensitivity.
Our study revealed that the activation of PVN CRFergic neurons and the intestinal CRF- CRF-R1-MCT-PAR2-TRPV1 pathway may be responsible for chronic restraint combined with glacial acetic acid enema-induced negative emotions and visceral hypersensitivity. However, EA is capable of inhibiting PVN CRFergic neurons and the CRF-CRF-R1-MC-MCT-PAR2-TRPV1 pathway to relieve negative emotions and visceral hypersensitivity in IBS-D, partially revealing the mechanism of EA.
The IBS-D model was constructed to account for mood, visceral hypersensitivity and diarrhoea. Studies have shown that stress, such as acute/chronic restraint, tail pinching, and forced swimming, can induce negative emotions and visceral hypersensitivity in rats and mice[25-27]. Therefore, we used chronic restraint stress to cause mood changes and increased visceral sensitivity in mice and combined with glacial acetic acid enemas to cause diarrhoea in mice. However, high doses and continuous enemas increase mortality in mice; therefore, on the third week of chronic restraint, we used 3% glacial acetic acid enemas every 2 days, followed by isodose saline enemas to decrease mortality. Our study confirmed that chronic restraint combined with glacial acetic acid enema induced IBS-D model, which included negative emotions, visceral hypersensitivity, diarrhoea, and morphological changes in the colonic mucosa and villi. These results suggest that chronic restraint combined with glacial acetic acid enemas can be used to build model of IBS-D accompanied by negative emotions.
IBS is known as a psychosomatic disorder, and acupuncture has uniquely advantageous in treatment of IBS. Acupuncture can improve visceral pain of IBS, which can be divided into central and peripheral mechanisms. For example, acupuncture can inhibit the hypothalamic-pituitary-adrenal (HPA) axis, which modulates stress in IBS patients, thus improving visceral hypersensitivity. Moreover, acupuncture with Tianshu (ST25), Zusanli (ST36), and Taichong (LR3) can reduce visceral hypersensitivity, diarrhoea, and negative emotions by decreasing serum CRF and CRF-R1 levels and increasing the expression of the tight junction proteins occludin, claudin-1 and ZO-1 in rats[28]. In addition, EA at ST25, ST36, Sanyinjiao (SP6), and LR3 decreases the levels of CRF and CRF-R1 in the hypothalamus and colonic mucosa, which has dual therapeutic effects on relieving negative emotions and repairing the intestinal mucosal barrier[29]. EA can also relieve visceral pain by decreasing intestinal chromaffin cell 5-HT levels, inhibiting 5-HT3 receptors in sensory endings and increasing peripheral 5-HT4 receptors[30]. EA reduces visceral hypersensitivity by inhibiting the intestinal Epac1-Piezo2-5-HT pathway[31] or the expression of P2X3 receptors in the anterior cingulate cortex, intestinal interneurons, dorsal root ganglia, and spinal cord[32]. A systematic review of clinical trials reveal that GV20, Yintang (GV29), ST25, Zhongwan (CV12), ST36, Shangjuxu (ST37), LR3 and SP6 are commonly used acupoints for the treatment of IBS[33]. Acupuncture can relieve the anxiety/depression status[34-36] and intestinal symptoms of IBS patients, thus improving the quality of life in IBS patients[37]. Clinical evidence suggests that EA at GV20, GV29, Shenmen (HT7), SP6 and other mood-related acupoints is effective in improving anxiety and depression, and has long-term stable therapeutic effects[38,39]. EA at gastrointestinal related acupoints, such as ST25, ST36, ST37, CV12, Guanyuan (CV4), and Da
CRF is widely distributed in the CNS and peripheral nervous systems, with a particularly high concentration in the PVN. Exposure to chronic stress could involve PVN CRFergic neurons to modulate behavioural and gastrointestinal responses. Studies have shown that pharmacological activation of PVN CRFergic neurons increases depressive-like behaviours in animal experiments[44], and patients with depression have excessive CRF in the PVN and an overactivated CRF-initiated HPA axis[45]. Activation of PVN CRFergic neurons can increase visceral sensitivity; in contrast, inhibition of this activity can suppress visceral hypersensitivity[46,47]. CRF receptors also play important roles in the CNS and peripheral nervous system. The binding of CRF with CRF-R1 may be involved in mediating negative emotions. In a clinical trial, 20 patients with major depressive disorder showed significant improvement in their depressive symptoms with a selective blocker of CRF-R1[48]. In the gastrointestinal tract, increased levels of CRF-R1 cause colonic motility and mediate visceral nociception, whereas increased levels of CRF-receptor 2 inhibit gastric emptying and decrease visceral nociception[49]. In our study, we found that PVN CRFergic neuronal activity was increased in mice subjected to chronic restraint combined with glacial acetic acid enemas and that PVN CRFergic neuronal activity decreased with EA at GV20 combined with ST25 and BL25. To further verify whether PVN CRFergic neurons participate in the effect of EA, we activated PVN CRFergic neurons by chemogenetics and found that EA significantly ameliorated the negative emotions and visceral hypersensitivity in specifically activated PVN CRFergic mice. These results demonstrate that PVN CRFergic neurons participate in regulation of the negative emotions and visceral hypersensitivity by EA. However, how PVN CRFergic neurons modulate visceral hypersensitivity in IBS mice remains unclear.
In negative emotions such as anxiety and depression, the CNS secretes the brain-gut peptide CRF, which can affect the intestinal enteric neuroendocrine system via the brain-gut axis. Intestinal mucosal MCs are closely linked to the ENS and are important links in the enteric neuroendocrine connection. MC infiltration and the degree of degranulation are positively correlated with abdominal pain in IBS[50]. The basis of this connection is that most intestinal mucosal MCs are directly connected to enteric neurons through synapse-like connections. When the gut produces a stress response, intestinal inflammatory factors, calcitonin-associated gene peptide, substance P and CRF can combine with high-affinity receptors on the surface of MCs[51,52]; thus, promoting the activation and degranulation of MCs to release histamine, tryptase, and other substances[53,54]. TRPV1 is a nonselective cation channel protein and a common ion channel that integrates receptors of pain or injury in the nervous system. The activation of MCs results in the release of tryptase, which combines with PAR2 in enterosensory nerve endings, followed by the upregulation of TRPV1 via the PKC pathway to induce visceral hypersensitivity[55]. Therefore, chronic restraint combined with glacial acetic acid enema-activated CRFergic neurons may act through the brain-gut axis to initiate the colonic MC/TRPV1 pathway, ultimately leading to the development of visceral hypersensitivity. Our study demonstrated that chronic restraint combined with glacial acetic acid enema increased serum CRF and colonic CRF and expression of the CRF-R1 receptor in the MCs, further increasing the release of MCT and the expression of PAR2 and TRPV1, thus inducing visceral hypersensitivity in IBS-D mice. EA inhibited the CRF-MC/TRPV1 pathway to ameliorate visceral hypersensitivity in IBS-D mice. To further demonstrate the role of PVN CRFergic neurons in controlling the CRF-R1-MC/TRPV1 pathway, we inhibited PVN CRFergic neurons by chemogenetics in model mice. When PVN CRFergic neurons were inhibited, overactivation of the colonic CRF-R1-MC/TRPV1 pathway in model mice was decreased, and negative emotions and visceral hypersensitivity were also relieved. These results indicate that PVN CRFergic neurons may participate in the regulation of negative mood and visceral hypersensitivity in IBS-D mice by EA through the colonic MC/TRPV1 pathway.
However, there are some limitations in this study, the study only focus on the PVN, in the following studies, we can focus on the neural circuits of PVN, such as PVN-central nucleus of the amygdala, PVN-dorsal nucleus of vagus motor nerve.
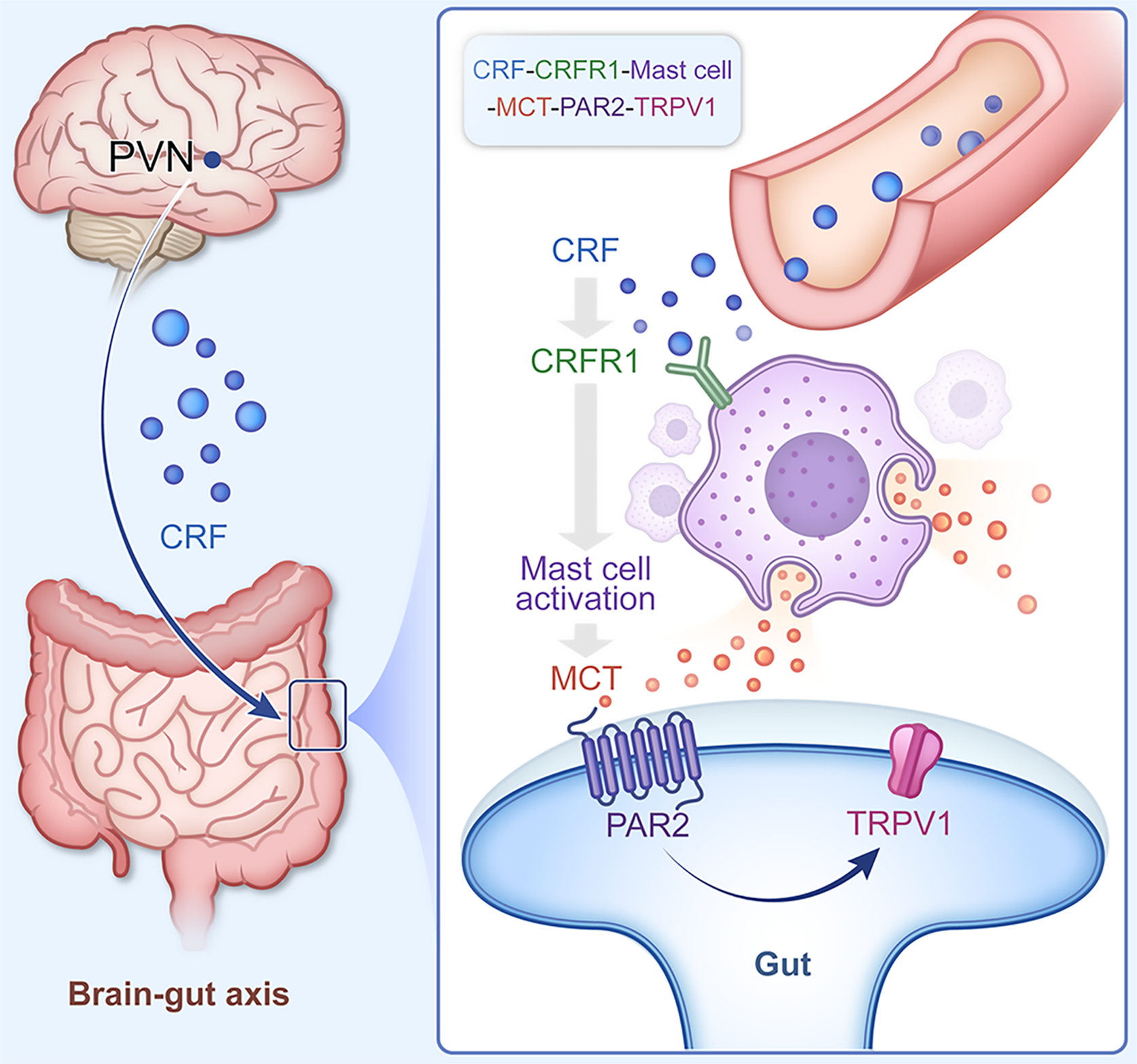
We revealed that EA may control the PVNCRF-MC/TRPV1 pathway to improve negative emotions and visceral hypersensitivity in IBS-D model. Our study provides an experimental evidence for understanding the mechanism of the effect of EA and provides a new interpretation of the somato-visceral connection via the brain-gut axis.
Chronic restraint combined with glacial acetic acid enema activated PVN CRFergic neurons and mediated negative emotions in IBS-D mice. CRF combined with CRF-R1 activated the colonic MC/TRPV1 pathway, which regulated visceral hypersensitivity in IBS-D mice. EA may relieve IBS-D through the PVNCRF-MC/TRPV1 pathway (Figure 6). Our study provides new insight into the mechanisms the overall regulatory effect of EA on brain-gut crosstalk.
We thank all the experimenters of Anhui University of Chinese Medicine for their support of this study.
| 1. | Guo Q, Lin H, Chen P, Tan S, Wen Z, Lin L, He J, Wen J, Lu S. Dynamic changes of intestinal flora in patients with irritable bowel syndrome combined with anxiety and depression after oral administration of enterobacteria capsules. Bioengineered. 2021;12:11885-11897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Labanski A, Langhorst J, Engler H, Elsenbruch S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology. 2020;111:104501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 3. | Lu Y, Huang J, Zhang Y, Huang Z, Yan W, Zhou T, Wang Z, Liao L, Cao H, Tan B. Therapeutic Effects of Berberine Hydrochloride on Stress-Induced Diarrhea-Predominant Irritable Bowel Syndrome Rats by Inhibiting Neurotransmission in Colonic Smooth Muscle. Front Pharmacol. 2021;12:596686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Orock A, Yuan T, Greenwood-Van Meerveld B. Importance of Non-pharmacological Approaches for Treating Irritable Bowel Syndrome: Mechanisms and Clinical Relevance. Front Pain Res (Lausanne). 2020;1:609292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Wang D, Zhang X, Zhang X, Huang Z, Song Y. Magnetic resonance imaging analysis of brain function in patients with irritable bowel syndrome. BMC Gastroenterol. 2017;17:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Wang Z, Ocampo MA, Pang RD, Bota M, Bradesi S, Mayer EA, Holschneider DP. Alterations in prefrontal-limbic functional activation and connectivity in chronic stress-induced visceral hyperalgesia. PLoS One. 2013;8:e59138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Pellissier S, Bonaz B. The Place of Stress and Emotions in the Irritable Bowel Syndrome. Vitam Horm. 2017;103:327-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Jiang Z, Rajamanickam S, Justice NJ. CRF signaling between neurons in the paraventricular nucleus of the hypothalamus (PVN) coordinates stress responses. Neurobiol Stress. 2019;11:100192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Tache Y, Larauche M, Yuan PQ, Million M. Brain and Gut CRF Signaling: Biological Actions and Role in the Gastrointestinal Tract. Curr Mol Pharmacol. 2018;11:51-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Hamilton MJ. Mast Cell Activation Syndrome and Gut Dysfunction: Diagnosis and Management. Curr Gastroenterol Rep. 2024;26:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Li Y-J, Li J, Dai C. Butyrate promotes visceral hypersensitivity in IBS model via mast cell-derived DRG neuron lincRNA-01028-PKC-TRPV1 pathway. mBio. 2024;15:e0153324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Pereyra F, Bustos Fernández LM, Schlottmann F, Zamora R, Marconi A, Steinberg L, Pereyra L. Prevalence of extra-intestinal symptoms according to irritable bowel syndrome subtype. Neurogastroenterol Motil. 2024;36:e14796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Sun Y, Liu FL, Song GQ, Qian W, Hou XH. Effects of acute and chronic restraint stress on visceral sensitivity and neuroendocrine hormones in rats. Chin J Dig Dis. 2006;7:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Chen Q, Zhang H, Sun CY, He QY, Zhang RR, Luo BF, Zhou ZH, Chen XF. Evaluation of two laboratory model methods for diarrheal irritable bowel syndrome. Mol Med. 2023;29:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 15. | Chen W, Liao L, Huang Z, Lu Y, Lin Y, Pei Y, Yi S, Huang C, Cao H, Tan B. Patchouli alcohol improved diarrhea-predominant irritable bowel syndrome by regulating excitatory neurotransmission in the myenteric plexus of rats. Front Pharmacol. 2022;13:943119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Tang M, Chen M, Li Q. Paeoniflorin ameliorates chronic stress-induced depression-like behavior in mice model by affecting ERK1/2 pathway. Bioengineered. 2021;12:11329-11341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Shen J, Yang L, Wei W. Role of Fto on CaMKII/CREB signaling pathway of hippocampus in depressive-like behaviors induced by chronic restraint stress mice. Behav Brain Res. 2021;406:113227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Wu H, Zhan K, Rao K, Zheng H, Qin S, Tang X, Huang S. Comparison of five diarrhea-predominant irritable bowel syndrome (IBS-D) rat models in the brain-gut-microbiota axis. Biomed Pharmacother. 2022;149:112811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Hou Q, Zhu S, Zhang C, Huang Y, Guo Y, Li P, Chen X, Wen Y, Han Q, Liu F. Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. Biomed Pharmacother. 2019;118:109206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Zhang L, Yu C, Chen B, Chao Y, Zhang H, Zhao Q, Yang K, Zhang Y, Chen S. Modulation of colonic function in irritable bowel syndrome rats by electroacupuncture at ST25 and the neurobiological links between ST25 and the colon. Front Neurosci. 2022;16:930489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Zhang H, He W, Hu XF, Li YZ, Liu YM, Ge WQ, Zhanmu OY, Chen C, Lan YY, Su YS, Jing XH, Zhu B, Pan HL, Yu LL, Li M. Electroacupuncture Reduces Visceral Pain Via Cannabinoid CB2 Receptors in a Mouse Model of Inflammatory Bowel Disease. Front Pharmacol. 2022;13:861799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Zhu X, Liu Z, Niu W, Wang Y, Zhang A, Qu H, Zhou J, Bai L, Yang Y, Li J. Effects of electroacupuncture at ST25 and BL25 in a Sennae-induced rat model of diarrhoea-predominant irritable bowel syndrome. Acupunct Med. 2017;35:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Xu X, Zheng P, Zhao H, Song B, Wang F. Effect of Electroacupuncture at GV20 on Sleep Deprivation-Induced Depression-Like Behavior in Mice. Evid Based Complement Alternat Med. 2020;2020:7481813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Jiang H, Long X, Wang Y, Zhang X, Chen L, Yang X, Zhao B, Zhang Y, Chai Y, Bao T. Acupuncture Ameliorates Depression-Like Behaviors Through Modulating the Neuroinflammation Mediated by TLR4 Signaling Pathway in Rats Exposed to Chronic Restraint Stress. Mol Neurobiol. 2024;61:2606-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 25. | Greenwood-Van Meerveld B, Johnson AC. Stress-Induced Chronic Visceral Pain of Gastrointestinal Origin. Front Syst Neurosci. 2017;11:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Deng Y, Zhou M, Wang J, Yao J, Yu J, Liu W, Wu L, Wang J, Gao R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes. 2021;13:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 198] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 27. | Lin S, Li Q, Jiang S, Xu Z, Jiang Y, Liu L, Jiang J, Tong Y, Wang P. Crocetin ameliorates chronic restraint stress-induced depression-like behaviors in mice by regulating MEK/ERK pathways and gut microbiota. J Ethnopharmacol. 2021;268:113608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Wang K, Hou YJ, Wang L, Liao CX, Song W, Chen Y, Zhou SY. Mechanisms of Peitu Yimu acupuncture in repairing intestinal mucosal barrier by regulating CRF/CRFR1 pathway in diarrhea-predominant irritable bowel syndrome rats. Zhen Ci Yan Jiu. 2024;49:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Chen Y, Zhao Y, Luo DN, Zheng H, Li Y, Zhou SY. Electroacupuncture Regulates Disorders of Gut-Brain Interaction by Decreasing Corticotropin-Releasing Factor in a Rat Model of IBS. Gastroenterol Res Pract. 2019;2019:1759842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Yang Y, Wang J, Zhang C, Guo Y, Zhao M, Zhang M, Li Z, Gao F, Luo Y, Wang Y, Cao J, Du M, Wang Y, Lin X, Xu Z. The efficacy and neural mechanism of acupuncture therapy in the treatment of visceral hypersensitivity in irritable bowel syndrome. Front Neurosci. 2023;17:1251470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | Guo J, Chen L, Wang YH, Song YF, Zhao ZH, Zhao TT, Lin ZY, Gu DM, Liu YQ, Peng YJ, Pei LX, Sun JH. Electroacupuncture Attenuates Post-Inflammatory IBS-Associated Visceral and Somatic Hypersensitivity and Correlates With the Regulatory Mechanism of Epac1-Piezo2 Axis. Front Endocrinol (Lausanne). 2022;13:918652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Weng ZJ, Wu LY, Zhou CL, Dou CZ, Shi Y, Liu HR, Wu HG. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015;11:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Zhang B, Shi H, Cao S, Xie L, Ren P, Wang J, Shi B. Revealing the magic of acupuncture based on biological mechanisms: A literature review. Biosci Trends. 2022;16:73-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 34. | Wang X, Shi X, Lv J, Zhang J, Huo Y, Zuo G, Lu G, Liu C, She Y. Acupuncture and related therapies for the anxiety and depression in irritable bowel syndrome with diarrhea (IBS-D): A network meta-analysis of randomized controlled trials. Front Psychiatry. 2022;13:1067329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Hou Y, Chang X, Liu N, Wang Z, Wang Z, Chen S. Different acupuncture and moxibustion therapies in the treatment of IBS-D with anxiety and depression: A network meta-analysis. Medicine (Baltimore). 2024;103:e37982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Wang Z, Hou Y, Sun H, Wang Z, Zhang H. Efficacy of acupuncture treatment for diarrhea-predominant irritable bowel syndrome with comorbid anxiety and depression: A meta-analysis and systematic review. Medicine (Baltimore). 2024;103:e40207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Zhang G, Zhang T, Cao Z, Tao Z, Wan T, Yao M, Su X, Wei W. Effects and Mechanisms of Acupuncture on Diarrhea-Predominant Irritable Bowel Syndrome: A Systematic Review. Front Neurosci. 2022;16:918701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Liu C, Zhao Y, Qin S, Wang X, Jiang Y, Wu W. Randomized controlled trial of acupuncture for anxiety and depression in patients with chronic insomnia. Ann Transl Med. 2021;9:1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Wei XY, Chen H, Guo C, Tan WL, Zhan SH. The Instant and Sustained Effect of Electroacupuncture in Postgraduate Students with Depression: An fMRI Study. Neuropsychiatr Dis Treat. 2021;17:873-883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Zheng H, Li Y, Zhang W, Zeng F, Zhou SY, Zheng HB, Zhu WZ, Jing XH, Rong PJ, Tang CZ, Wang FC, Liu ZB, Wang SJ, Zhou MQ, Liu ZS, Zhu B. Electroacupuncture for patients with diarrhea-predominant irritable bowel syndrome or functional diarrhea: A randomized controlled trial. Medicine (Baltimore). 2016;95:e3884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Zhenzhong L, Xiaojun Y, Weijun T, Yuehua C, Jie S, Jimeng Z, Anqi W, Chunhui B, Yin S. Comparative effect of electroacupuncture and moxibustion on the expression of substance P and vasoactive intestinal peptide in patients with irritable bowel syndrome. J Tradit Chin Med. 2015;35:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Qi LY, Yang JW, Yan SY, Tu JF, She YF, Li Y, Chi LL, Wu BQ, Liu CZ. Acupuncture for the Treatment of Diarrhea-Predominant Irritable Bowel Syndrome: A Pilot Randomized Clinical Trial. JAMA Netw Open. 2022;5:e2248817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 43. | Zhao T, Pei L, Ning H, Guo J, Song Y, Zhou J, Chen L, Sun J, Mi Z. Networks Are Associated With Acupuncture Treatment in Patients With Diarrhea-Predominant Irritable Bowel Syndrome: A Resting-State Imaging Study. Front Hum Neurosci. 2021;15:736512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 44. | Qin XY, Shan QH, Fang H, Wang Y, Chen P, Xiong ZQ, Swaab DF, Zhou JN. PSD-93 up-regulates the synaptic activity of corticotropin-releasing hormone neurons in the paraventricular nucleus in depression. Acta Neuropathol. 2021;142:1045-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Montgomery KR, Bridi MS, Folts LM, Marx-Rattner R, Zierden HC, Wulff AB, Kodjo EA, Thompson SM, Bale TL. Chemogenetic activation of CRF neurons as a model of chronic stress produces sex-specific physiological and behavioral effects. Neuropsychopharmacology. 2024;49:443-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 46. | Zhang G, Yu L, Chen ZY, Zhu JS, Hua R, Qin X, Cao JL, Zhang YM. Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav Immun. 2016;55:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Huang ST, Song ZJ, Liu Y, Luo WC, Yin Q, Zhang YM. BNST(AV) (GABA)-PVN(CRF) Circuit Regulates Visceral Hypersensitivity Induced by Maternal Separation in Vgat-Cre Mice. Front Pharmacol. 2021;12:615202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Todorovic C, Jahn O, Tezval H, Hippel C, Spiess J. The role of CRF receptors in anxiety and depression: implications of the novel CRF1 agonist cortagine. Neurosci Biobehav Rev. 2005;29:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42 Suppl 17:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 581] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 51. | Cheng L, Luo QQ, Chen SL. The role of intestinal mast cell infiltration in irritable bowel syndrome. J Dig Dis. 2021;22:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Zhang H, Wang Z, Wang G, Song X, Qian Y, Liao Z, Sui L, Ai L, Xia Y. Understanding the Connection between Gut Homeostasis and Psychological Stress. J Nutr. 2023;153:924-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 53. | Hasler WL, Grabauskas G, Singh P, Owyang C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol Motil. 2022;34:e14339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 54. | Jia J, Zeng M, Zhu D, Jiao X, Zhang B, Yang R, Feng W, Zheng X. An Amide Alkaloid Isolated from Ephedra sinica Ameliorates OVA-Induced Allergic Asthma by Inhibiting Mast Cell Activation and Dendritic Cell Maturation. Int J Mol Sci. 2022;23:13541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Li H, Zhang W, Ma F, Zhang X, Wang Y, Wang J. Abdominal Massage Improves the Symptoms of Irritable Bowel Syndrome by Regulating Mast Cells via the Trypase-PAR2-PKCε Pathway in Rats. Pain Res Manag. 2022;2022:8331439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |