Published online Aug 19, 2025. doi: 10.5498/wjp.v15.i8.106971
Revised: June 2, 2025
Accepted: June 24, 2025
Published online: August 19, 2025
Processing time: 139 Days and 2.9 Hours
Diamine oxidase (DAO) is secreted by epithelial cells in the intestinal villi, and its serum levels are elevated after intestinal mucosal damage. d-lactate (D-LA) is a gut microbial metabolite that can enter the systemic circulation if intestinal barrier function is impaired. Both DAO and D-LA are serum markers of small bowel mucosal integrity, and can be valuable biomarkers of intestinal barrier damage in inflammatory bowel disease (IBD). Intestinal barrier dysfunction was recently found to contribute to psychological symptoms in IBD patients. However, the correlations among DAO, D-LA, psychological symptoms, and disease activity in IBD remain unexplored.
To explore the correlations between serum markers of intestinal barrier dysfun
We enrolled of 126 participants in this study. Psychological symptom questionnaires (depression, patient health questionnaire-9; anxiety, generalized anxiety disorder-7; and stress, perceived stress scale) and a quality of life (QOL) questionnaire (IBD questionnaire 32) were collected at the baseline. Serum DAO and D-LA levels were measured to assess intestinal barrier integrity. Receiver operating characteristic (ROC) curves were used to identify candidate markers of psychological symptoms and disease activity in IBD patients. Logistic regression was applied, with DAO as an independent variable for predicting psychological symptoms in IBD.
Serum DAO levels were significantly higher in IBD patients with moderate-to-severe psychological symptoms than in patients with mild or no psychological symptoms. DAO was positively correlated with depression and negatively correlated with QOL in IBD patients. ROC curves revealed that DAO was independently associated with psychological symptoms and clinical activity in patients with IBD. Additionally, logistic regression analysis revealed that each 1-ng/mL increase in DAO levels was significantly associated with an increased risk of psychological symptoms in IBD patients (OR: 1.019, 95%CI: 1.002-1.037). These results highlight the potential of DAO as a novel biomarker for both depression and disease activity in IBD patients.
This study indicates that DAO may be associated with depression and disease activity in IBD patients; however, prospective studies are required to validate its causal relationship.
Core Tip: Few studies have explored the association between markers of intestinal barrier dysfunction and psychological symptoms in patients with inflammatory bowel disease (IBD). Our study revealed that serum diamine oxidase (DAO) levels were significantly elevated in IBD patients with moderate-to-severe psychological symptoms as compared to those with no or mild psychological symptoms. Furthermore, correlation analysis indicated that DAO was positively associated with depression and negatively associated with quality of life in IBD patients. Additionally, receiver operating characteristic curves confirmed that DAO is associated with clinical disease activity in patients with IBD. This study suggests the potential of DAO as a novel biomarker for predicting psychological symptoms and disease activity in IBD.
- Citation: Lyu SC, Zhong GQ, Shi RJ, Sun Y, Li J, Li MS, Chen Y. Diamine oxidase as a biomarker for depression and disease activity in inflammatory bowel disease: A cross-sectional observational study. World J Psychiatry 2025; 15(8): 106971
- URL: https://www.wjgnet.com/2220-3206/full/v15/i8/106971.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i8.106971
Inflammatory bowel disease (IBD) is a chronic relapsing inflammatory condition that includes ulcerative colitis (UC) and Crohn’s disease (CD). With industrialization and the adoption of Westernized diets and lifestyles, the incidence and prevalence rates of IBD are increasing worldwide[1]. Owing to its chronic nature, low mortality and high disability rates, IBD imposes great social and economic burdens[2]. The psychological symptoms of IBD also contribute to its global disease burden[3]. Both anxiety and depression are found more frequently in IBD patients than in the general population, and the successful treatment of psychological illnesses can enhance the quality of life (QOL) of IBD patients and improve disease outcomes[4]. Additionally, patients with severe disease, especially those with active gastrointestinal symptoms, have increased rates of stress, anxiety, depression, and impaired QOL[5,6]. These results indicate a mutual causal relationship between disease severity and psychological symptoms. The interaction between the brain and the gut, involving psychological, neurological, endocrine, and immune factors, is probably critical for the development of IBD. Animal experiments utilizing pharmacological, nutritional, or neurostimulation methods indicate that targeting the cholinergic anti-inflammatory pathway via the anti-tumor necrosis factor (TNF) effects of the efferent vagus nerve could serve as a therapeutic strategy for IBD[7]. The communication between the gut and brain involves intricate processes that include inflammatory cytokines, immune cells, autonomic nerves, and intestinal microbiota, and plays an important role in the development of certain gut and brain disorders[8].
The gut–brain axis has been implicated in many neuropsychiatric disorders, including anxiety and depression[9]. Psychological stress is associated with exacerbated inflammatory response in patients with IBD[10]. Acute stress can alter the gut microbial composition and increase patients’ susceptibility to neuropsychiatric disorders, whereas chronic stress can impair intestinal integrity, leading to low-grade inflammation that may be functionally associated with neuropsychiatric disorders[9]. Depression and anxiety-related disorders are affected by the composition of the gut microbiota, while markers of gut integrity and inflammation correlate with the severity of psychological symptoms[11]. These above reports indicate that makers of intestinal integrity can potentially aid in the diagnosis of psychological symptoms.
The enzyme diamine oxidase (DAO) is produced by the epithelial cells of the intestinal villi, and its activity increases successively from the duodenum to the ileum[12]. Impaired intestinal integrity leads to DAO release, and increases its serum concentration. Owing to the stability of DAO in the blood, its concentration in the blood can serve as an indicator of reduced intestinal integrity[13]. The bacterial metabolite d-lactate (D-LA) is released within the gastrointestinal tract. Typically, D-LA levels are low in serum; however, in the presence of intestinal barrier damage, D-LA can enter the bloodstream. Consequently, elevated serum D-LA levels indicate increased intestinal mucosal permeability and bacterial overgrowth[12].
DAO and D-LA are commonly used to assess intestinal barrier function, and are effective in evaluating disease activity in IBD[14,15]. Despite the growing interest in the relationship between DAO and psychological symptoms in patients with IBD, limited research has systematically explored this association. Consequently, it remains unclear whether DAO or D-LA can serve as potential indicators of psychological symptoms in this patient population. In light of this knowledge gap, the present study aimed to investigate the associations among DAO and D-LA levels, disease activity, and psychological symptoms in patients with IBD.
In all, 126 subjects, including 114 patients with IBD and 12 healthy controls, were enrolled between January 1, 2022 and January 1, 2023, at the IBD Center and Medical Examination Center of The Third Affiliated Hospital of Guangzhou Medical University. IBD was diagnosed using the criteria outlined in the Chinese consensus on diagnosis and treatment in IBD[16]. The inclusion criteria were as follows: Patients who were diagnosed with IBD, were aged 18 years or above, were capable of reading and writing, and consented to participate in this study. Patients meeting any of the following criteria were excluded from the study: Refusal to consent, pregnancy, concomitant colon cancer or other malignant tumors, use of antibiotics or probiotics within the past 3 months, use of anti-anxiety or anti-depression medication within the past month (the washout period prior to the administration of the psychological questionnaires was determined in accordance with the type and half-life of the medication used), presence of any mental illness other than anxiety or depression, and occurrence of major traumatic events in the recent past. The study protocol was approved by the Ethics Committee and Clinical Trial Registry of the Third Affiliated Hospital of Guangzhou Medical University (approval No: LCYJ-2022-015). Written informed consent was obtained from all the participants in this study.
This is a cross-sectional observational study. Data on demographics (age, sex, body mass index, occupation, education level, marital status, medication use, and previous bowel resections), Montreal classification of IBD, clinical disease activity, laboratory parameters, and endoscopic disease activity were collected at the baseline. In patients with CD, clinical disease activity was assessed using the CD activity index (CDAI), with CDAI < 150 indicating clinical remission. Endoscopic activity was evaluated using the simple endoscopic score for CD (SES-CD), with SES-CD ≤ 2 indicating endoscopic remission. In patients who had undergone ileocolonic resection, endoscopic activity was assessed using the Rutgeerts score, with scores ≤ i1 indicating endoscopic remission. In patients with UC, the Mayo clinic score (MCS) was used to judge clinical activity, with MCS ≤ 2 indicating clinical remission; the Mayo endoscopic sub-score (MES) was used to evaluate endoscopic activity, and MES < 2 indicated endoscopic remission[17].
We used the patient health questionnaire-9 (PHQ-9), generalized anxiety disorder-7 (GAD-7) questionnaire, and perceived stress scale (PSS-10) to evaluate the participants for symptoms of depression, anxiety, and stress, respectively[18-20]. A PHQ-9 score ≥ 10, GAD-7 score ≥ 10 or PSS-10 score ≥ 14 was considered to be indicative of moderate-to-severe psychological symptoms, whereas a PHQ-9 score < 10, GAD-7 score < 10 and PSS-10 score < 14 indicated no or mild psychological symptoms. The IBD questionnaire 32 (IBDQ-32)[21] was used to assess the QOL of IBD patients, and IBDQ-32 scores ≥ 170 indicated a better QOL[22].
Serological samples were collected from all recruited participants for biomarker analysis, and the participants were required to fast for at least 12 hours before blood collection. After a 1-hour clotting period at room temperature, the 2-mL blood samples were centrifuged at 3500 g for 10 minutes. The resultant serum samples were collected and kept frozen at -80 °C until they were analyzed. The serum concentrations of DAO and D-LA were measured using enzyme-linked immunosorbent assay kits from Elabscience Biotechnology Co. Ltd. (Wuhan, China; product code for DAO: E-EL-H1241, product code for D-LA: E-BC-K002-M; intra- and inter-assay coefficients of variation, < 10%), according to the manu
The distribution of variables was assessed using the Shapiro-Wilk test. Continuous variables were evaluated using the student t test, Mann-Whitney U test or Kruskal-Wallis test, as appropriate, and presented as mean ± SD or median and interquartile range. Categorical variables were assessed using the χ2 test, and presented as percentages or proportions. Spearman rank test was used to examine whether the correlation between 2 variables was significant. The diagnostic performance of the biomarkers was evaluated using receiver operating characteristic (ROC) curves, and optimal cut-off values of the biomarkers were calculated using the Youden index. The Delong test was used to assess the statistical significance of differences between the area under the curve (AUC) of different biomarkers. Spearman correlation analysis was applied to identify the correlation of DAO and D-LA levels with various factors. Logistic regression analysis was employed to investigate the association between DAO and psychological symptoms in IBD. Values of P < 0.05 (2-tailed) indicated statistical significance. GraphPad Prism software (version 8.0.2, GraphPad Software, San Diego, CA, United States) was used for statistical analyses.
We initially enrolled 183 participants in this study, of whom, 56 were excluded due to a lack of blood samples, and 1 was excluded due to missing baseline data. Thus, finally, 126 participants were included in this analysis, including 114 IBD patients (CD, 99 patients; UC, 15 patients) and 12 healthy controls. The demographic and clinical characteristics of the study participants are presented in Table 1. Moderate-to-severe psychological symptoms were present in 56 patients, while 58 patients had no or mild psychological symptoms. Patients in the no-or-mild psychological symptoms group were significantly younger than the healthy controls [median age: 30.00 years (22.75-40.00 years) vs. 46.00 years (30.75-63.25 years), P = 0.004]. Age, sex, and body mass index did not significantly differ between the groups with moderate-to-severe and no-or-mild psychological symptoms. In addition, the CDAI, MCS, SES-CD, MES, and Rutgeert scores did not differ between the above 2 groups. The median DAO level was higher in IBD patients with moderate-to-severe psychological symptoms than in patients with no or mild psychological symptoms (33.80 vs 20.00 ng/mL, P = 0.039). The median D-LA level was higher in IBD patients with no or mild psychological symptoms than in the healthy controls (0.49 vs 0.24 mmol/L, P = 0.007). The IBDQ-32 score was significantly lower in patients with moderate-to-severe psychological symptoms than in patients with no or mild psychological symptoms (174.0 vs 197.0, P < 0.001). However, other laboratory parameters such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and albumin (ALB) levels did not significantly differ among the 3 study groups.
| Variable | Moderate-to-severe psychological symptoms | P valuec | No or mild psychological symptoms | P valued | Healthy controls |
| Sample size, n | 56 | 58 | 12 | ||
| Age, years, (median, IQR) | 31.5 (25.0-41.0) | 0.376 | 30.0 (22.75-40.0) | 0.004b | 46.0 (30.75-63.25) |
| Female, n (%) | 15 (26.8) | 0.682 | 18 (31.0) | 1.000 | 4 (33.3) |
| BMI (median, IQR) | 19.9 (18.0-22.0) | 0.392 | 20.8 (18.3-22.4) | 0.338 | 20.9 (19.8-23.6) |
| CD location, n (%) | 0.586 | - | |||
| Ileal (L1) | 12 (21.4) | 8 (13.8) | - | ||
| Colonic (L2) | 6 (10.7) | 6 (10.3) | - | ||
| Ileocolonic (L3) | 31 (55.4) | 36 (62.1) | - | ||
| Upper gastrointestinal involvement (L4) | 0 (0) | 0 (0) | - | ||
| UC extent, n (%) | 0.713 | - | |||
| Proctitis (E1) | 0 (0) | 2 (0.25) | - | ||
| Left-sided (E2) | 1 (14.3) | 1 (0.13) | - | ||
| Extensive (E3) | 6 (85.7) | 5 (0.62) | - | ||
| Perianal disease, n (%) | 5 (8.90) | 7 (12.10) | - | ||
| Clinical remission, n (%) | - | ||||
| CDAI, median (IQR) | 154.5 (102.9-177.7) | 0.320 | 131.7 (102.4-160.3) | - | |
| MCS, median (IQR) | 10.0 (2.0-11) | 0.069 | 4.0 (1.0-6.0) | - | |
| Endoscopic remission, n (%) | - | ||||
| SES-CD, median (IQR) | 3.0 (2.006.0) | 0.223 | 3.0 (2.006.0) | - | |
| MES, median (IQR) | 2.0 (003.0) | 0.765 | 1.0 (0.3002.80) | - | |
| Rutgeerts score | 2.0 (2.0-2.0) | 0.876 | 1.5 (1.0-3.0) | - | |
| Anti-TNF use, n (%) | 18 (32.1) | 0.840 | 17 (29.3) | - | |
| DAO, ng/mL, median (IQR) | 33.8 (14.7-48.8) | 0.039a | 20.0 (6.8-44.4) | 0.119 | 7.6 (3.7–28.2) |
| D-LA, mmol/L, median (IQR) | 0.41 (0.23-0.61) | 0.309 | 0.49 (0.29-0.71) | 0.007b | 0.24 (0.08–0.39) |
| IBDQ-32, median (IQR) | 174.00 (155.75-192.75) | 0.001b | 197.00 (187.75-209.00) | - | - |
| CRP, median (IQR) | 2.35 (0.65-10.07) | 0.718 | 1.98 (0.55-6.80) | 0.787 | 2.43 (1.21-6.24) |
| ESR, median (IQR) | 11.00 (7.75-18.25) | 0.695 | 10.50 (8.00-18.50) | 0.543 | 3.50 (1.00-8.25) |
| ALB, mean ± SD | 37.46 ± 4.95 | 0.666 | 37.88 ± 5.60 | 0.875 | 37.58 ± 4.02 |
| Marital status, n (%) | 0.320 | 0.019a | |||
| Single | 31 (55.4) | 32 (55.2) | 2 (16.7) | ||
| Married | 25 (44.5) | 23 (39.7) | 10 (83.3) | ||
| Divorced | 0 (0) | 3 (5.2) | 0 (0) | ||
| Education level, n (%) | 0.840 | 0.189 | |||
| College and above | 38 (67.9) | 41 (70.7) | 6 (50.0) | ||
| High school and below | 18 (32.1) | 17 (29.3) | 6 (50.0) | ||
| Occupational status, n (%) | 0.499 | 0.721 | |||
| Full-time employee | 32 (57.1) | 32 (55.2) | 7 (58.3) | ||
| Freelancer | 7 (12.5) | 9 (15.5) | 2 (16.7) | ||
| Unemployed | 3 (5.4) | 7 (12.1) | 0 (0) | ||
| Retired or student | 14 (25.0) | 10 (17.2) | 3 (25.0) |
The median PHQ-9 score of the IBD patients was 3.0 (1.0-7.0); their median GAD-7 score was 3.0 (0-6.0), and their median PSS-10 score was 13.0 (7.0-17.0). Among the 114 IBD patients, 16 (14%) had moderate-to-severe symptoms of depression, 10 (8.7%) had moderate-to-severe symptoms of anxiety, and 53 (46.5%) experienced moderate-to-high stress. The median IBDQ-32 score was 190.0 (168.5-204.0).
In this study, 58 (46.0%) participants were married; 65 (51.6%) were unmarried; and 3 (2.4%) were divorced. Additionally, 67.5% of the participants had graduated from college, and 32.5% had graduated from high school or below. Finally, 89 participants were full-time employees or freelancers, and 37 benefited from social or family support (Table 1).
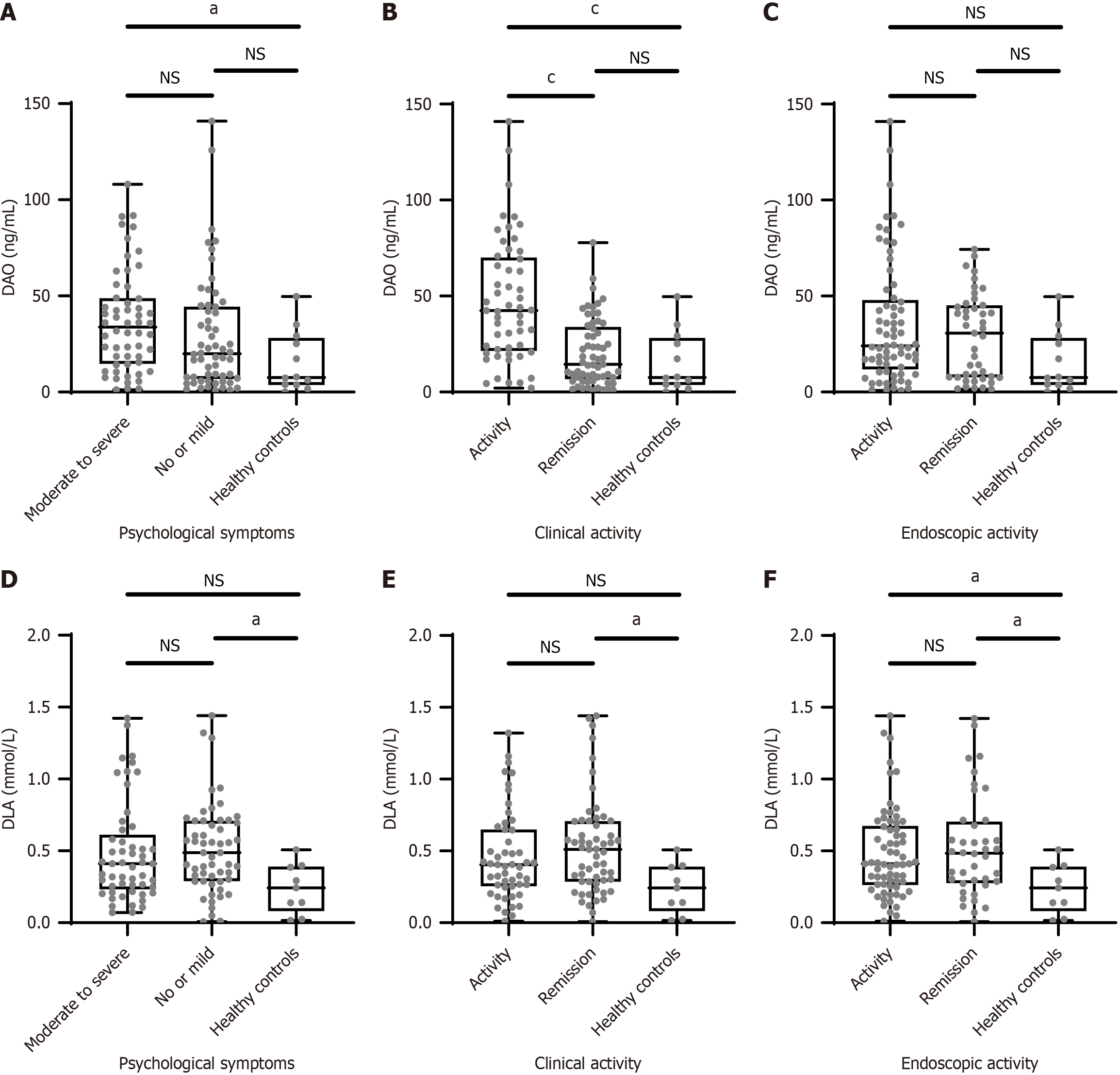
The median serum DAO level was significantly higher in IBD patients with moderate-to-severe psychological symptoms [33.81 ng/mL (14.72-48.81 ng/mL)] than in the healthy controls [7.60 ng/mL (3.70-28.20 ng/mL), P < 0.05]. Although the median DAO level was higher in IBD patients with moderate-to-severe psychological symptoms than in patients with no or mild psychological symptoms, the difference was not statistically significant (Figure 1A). Furthermore, the median DAO concentration in IBD patients with clinically active disease was 42.38 ng/mL (21.50-70.09 ng/mL), which was significantly higher than the DAO concentration in IBD patients with clinical remission [14.48 ng/mL (6.67-33.95 ng/mL), P < 0.01; Figure 1B). Patients who achieved endoscopic remission had a median DAO level of 31.92 ng/mL (7.81-45.39 ng/mL), whereas those with endoscopic disease activity had a DAO concentration of 24.25 ng/mL (13.34-48.39 ng/mL). The difference in DAO levels between the endoscopic activity and endoscopic remission groups did not achieve statistical significance (P > 0.05; Figure 1C).
Serum D-LA levels were relatively low in all participants (detection range: 0.06-8.00 mmol/L). IBD patients had higher serum D-LA levels than healthy controls (Figure 1D-F); however, the D-LA levels did not significantly differ between different IBD patient groups based on psychological symptoms, clinical disease activity, and endoscopic disease activity.
Compared to patients in clinical remission, those experiencing clinical disease activity more frequently exhibited psychological symptoms (58.5% vs 41.0%, P > 0.05). Psychological symptoms manifested more often as depression (P < 0.01) in IBD patients with clinical disease activity than in those with clinical remission, and the QOL was lower in the former group than in the latter group (P < 0.001). Compared to the healthy controls, a higher percentage of patients with clinical disease activity experienced stress (P < 0.01; Supplementary Figure 1A-D).
The endoscopic activity group showed a lower percentage of individuals with psychological symptoms than the endoscopic remission group (46.38% vs 51.16%, P > 0.05), and no significant differences were noted in the PHQ-9, GAD-7, and PSS-10 scores between the endoscopic activity and endoscopic remission groups (all P > 0.05; Supplementary Figure 1E-G). However, QOL was better in those with endoscopic remission than in those with endoscopic disease activity (P < 0.05; Supplementary Figure 1H).
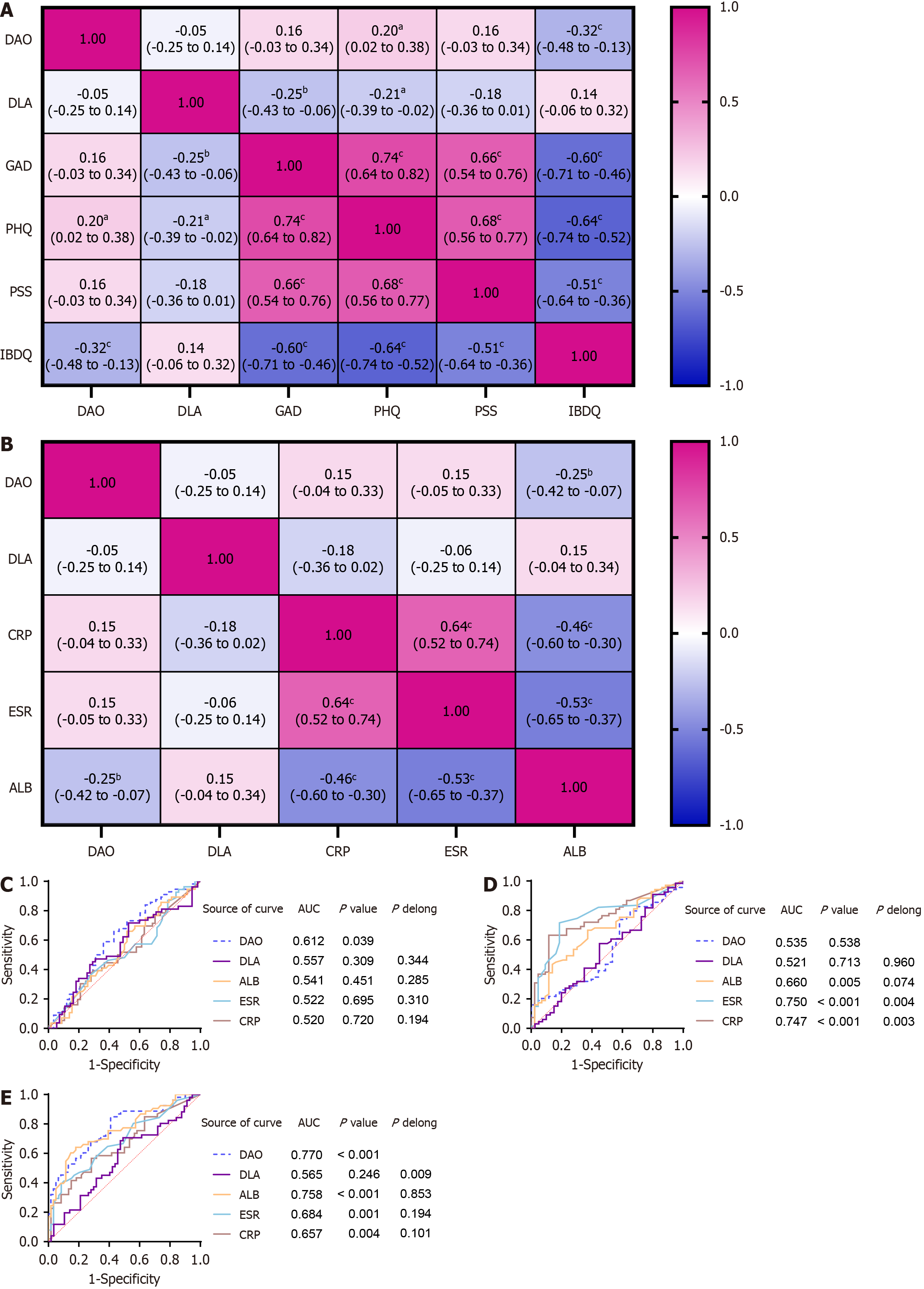
Spearman’s correlation analysis was performed to identify correlations among DAO levels, D-LA levels, psychological scores, QOL, and laboratory parameters. There was a weak yet statistically significant positive correlation between DAO levels (ng/mL) and PHQ-9 scores (r = 0.20, P < 0.05). Additionally, we observed a moderate, statistically significant negative correlation between DAO (ng/mL) and IBDQ-32 scores. (r = -0.32, P < 0.001). Furthermore, strong negative correlations were detected among the PHQ-9, GAD-7, PSS-10 scores, and QOL of IBD patients (Figure 2A). In addition, we found a weak, negative correlation between DAO (ng/mL) and ALB (g/L; r = -0.25, P < 0.01; Figure 2B). In contrast to DAO, D-LA was negatively correlated with PHQ-9 and GAD-7 scores. In addition, we found that laboratory biomarkers were not correlated with D-LA levels.
Only DAO had diagnostic value in distinguishing psychological symptoms (AUC: 0.612, P < 0.05; Figure 2C); however, it was not effective in differentiating endoscopic activity vs. endoscopic remission (P > 0.05) in patients with IBD (Figure 2D). In contrast, CRP, ESR, and ALB were capable of distinguishing endoscopic disease activity, but failed to differentiate psychological symptoms. All 4 biomarkers (DAO, CRP, ESR, and ALB) had diagnostic value for clinical disease activity (all P < 0.05; Figure 2E). To further evaluate the diagnostic efficacy of DAO, we performed the DeLong test. The results demonstrated that DAO did not exhibit a statistically significant difference in predicting psychological symptoms and clinical activity compared to other classic serum biomarkers (ALB, ESR, CRP) in patients with IBD (P > 0.05; Figure 2E).
We employed logistic regression analysis to predict psychological symptoms in patients with IBD. The findings indicated that every 1-ng/mL increase in DAO levels was significantly correlated with a higher risk of psychological symptoms in IBD patients (OR = 1.019, 95%CI: 1.002-1.037). Using multivariable logistic regression analysis, we incorporated multiple variables, including DAO, D-LA, gender, age, body mass index, educational level, CRP, ESR, marital status, and endoscopic remission, to develop a predictive model. The model is as follows:
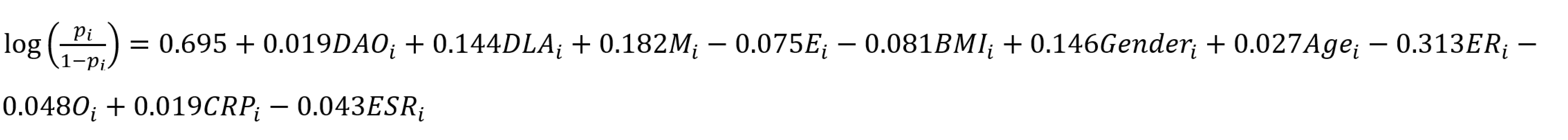
pi: Probability; i: Individuals with IBD with moderate-to-severe psychological symptoms; M: Marital status; E: Education level; ER: Endoscopic remission; O: Occupational status.
Our study is the first in China to analyze the associations between biomarkers of intestinal permeability in IBD patients and a range of psychological symptoms, including depression, anxiety, stress, and QOL. The findings reveal that increased intestinal permeability is associated with clinical disease activity as well as depression in IBD patients. However, due to the cross-sectional study design, we could not establish a causal relationship between DAO and psychological symptoms in IBD.
Diet constitutes one of the crucial factors influencing the intestinal microbiota. Various dietary patterns (such as high-fiber diets, Western diets, etc.) will result in remarkable alterations in the composition and functionality of the intestinal microbiota, thereby influencing signal transduction along the gut–brain axis. For example, a high-fiber diet can enhance the generation of short-chain fatty acids (SCFAs), and these metabolites are capable of affecting brain function through channels such as the vagus nerve[23]. The influence of diverse drugs on the intestinal microbiota are discrepant, which might give rise to the contradictory conclusions witnessed in some studies. For example, some drugs may augment the abundance of certain beneficial microorganisms, while others might lead to dysregulation of the microbiota[24]. The functionality and composition of the gut microbiota are subject to the impact of multiple factors, such as age, lifestyle, and environment. These variations might give rise to changes in signal transduction along the gut–brain axis, thereby influencing the consistency of research outcomes[25]. The impact of the aforementioned confounding factors may serve as one of the potential explanations for the lack of significant differences in serum D-LA levels among IBD patients across different disease activity and psychological symptom groups.
The gut–brain axis is directly related to psychiatric disorders and has garnered widespread research interest. Although the precise mechanisms are not yet fully understood, gastrointestinal microbiota may contribute to the regulation of brain function[9]. Damage to the intestinal barrier is characteristic of IBD, and enables the translocation of toxins, antigens, and bacteria from the intestinal lumen to the bloodstream[26]. Alterations in the composition of the gut microbiota have been observed in IBD patients, and structural changes in the composition of commensal bacteria can disrupt the intestinal barrier and increase intestinal permeability[26]. It has recently been postulated that depressive symptoms may be mechanistically linked to excess intestinal inflammation and dysregulation of the gut–brain axis[27]. Therefore, we focused on utilizing the objective indicators, DAO and D-LA, as risk factors for the diagnosis of disease activity and psychological symptoms in IBD patients.
Studies have reported that certain plasma biomarkers of intestinal barrier permeability are associated with depression and anxiety disorders. Stevens et al[28] found that levels of zonulin, fatty acid binding protein 2 (FABP2), and lipopolysaccharide (LPS) were higher in patients with depressive or anxiety disorders than in control subjects. In contrast, Iordache et al[29] discovered that calprotectin and LPS-binding protein, but not zonulin or intestinal fatty acid binding protein (IFABP)/FABP2, were correlated with PHQ-9 scores. Liśkiewicz et al[11] found that plasma IFABP/FABP2 levels were negatively correlated with major depression, while calprotectin levels were positively correlated with major depressive disorder; they did not observe any correlation between serum zonulin, TNF-alpha, LPS, and depressive disorder severity. However, no study has as yet examined the potential correlations of DAO levels with depression, anxiety, and stress. In this study, we demonstrated that patients with active gastrointestinal symptoms were more likely to have psychological symptoms than those with clinical remission, as those in the clinical activity group had higher PHQ-9 scores and lower IBDQ-32 scores. Moreover, serum DAO levels were notably elevated in the clinical activity group compared to the clinical remission group, yet they showed no significant difference between the endoscopic activity and endoscopic remission groups. These results are consistent with an earlier study that reported that gastrointestinal symptoms, rather than active intestinal inflammation, were associated with anxiety, depression, and perceived stress[5].
The influence of DAO on depression through intestinal barrier dysfunction can be elucidated through several key mechanisms.
Intestinal barrier dysfunction and the release of inflammatory cytokines: Injury to the intestinal mucosal barrier correlates with elevated DAO levels. DAO is an acritical enzyme that catalyzes histamine oxidation, mainly produced by villous epithelial cells in the small intestine. DAO activity reflects the function of the small intestine, and a large amount of DAO will be released when the small intestinal villous epithelial cells are damaged. Under normal circumstances, the serum DAO level is stable, but this level will increase when the intestinal mucosal barrier is damaged[30].
The gut–brain axis and depressive disorder: Gut dysbiosis increases intestinal barrier permeability, causing a leaky gut with decreased levels of tight and adherent junction proteins, loss of goblet cells, increased LPS (an endotoxin derived from the cell walls of Gram-negative bacteria), and increased blood-brain barrier permeability. These changes trigger the production of pro-inflammatory cytokines by altering the hypothalamic-pituitary-adrenal axis and increasing cortisol levels, which in turn lead to decreased levels of 5-hydroxytryptamine and dopamine, brain-derived neurotrophic factor, indoleamine 2,3-dioxygenase, kynurenine, and tryptophan 2,3-dioxygenase, resulting in depression[31].
Microbial metabolite translocation: Metabolic products of the gut microbiota (such as tryptophan metabolites) are fundamental in regulating intestinal barrier function and immune system functions[32]. For instance, indole-3-propionic acid, a metabolite of tryptophan, can exert a protective effect by regulating intestinal barrier function and inhibiting neuroinflammation. However, disruption of the balance of these metabolic products may lead to the aggravation of neuroinflammation and depressive symptoms[33]. Although there is no direct metabolic link between SCFAs and DAO, they are indirectly related in regulating intestinal health. SCFAs indirectly influence the activity of DAO by strengthening intestinal barrier function and modulating immune responses. For example, the enhancement of intestinal barrier function can reduce the translocation of inflammatory mediators such as histamine, thus alleviating the burden on DAO[34]. In addition, the regulation of intestinal microbiota by SCFAs might impact serum DAO levels[35].
All the pathways discussed above through which DAO affects psychological symptoms have not yet been explored. In our research, serum DAO levels were markedly increased in IBD patients with moderate-to-severe psychological symptoms than in those with no or mild psychological symptoms. Additionally, ROC curve analysis revealed that DAO was valuable as a predictor of psychological symptoms in IBD patients, with an optimal cut-off value of 22.94 ng/mL. Although CRP, ESR, and ALB are effective biomarkers of inflammatory status, including clinical and endoscopic activity, they cannot be used to differentiate IBD patients with psychological symptoms. Therefore, we consider that serum DAO levels may aid the diagnosis of the psychological symptoms of IBD, especially depression.
Cai et al[14] have demonstrated that DAO and D-LA are both associated with disease activity and possess prognostic value in patients with CD. In the current study, we noted that serum DAO levels were associated with clinical activity but were unable to distinguish between endoscopic remission and endoscopic activity in patients with IBD. Additionally, serum D-LA levels failed to differentiate both clinical and endoscopic remission from clinical and endoscopic activity in IBD patients. This discrepancy can be attributed to 2 primary factors. First, the differences in the primary research populations are notable; the study by Cai et al[14] focused exclusively on patients with CD, whereas our investigation centered on a broader population of individuals with IBD. Second, the scope of analysis differs significantly between the 2 studies. Cai et al[14] concentrated solely on clinical activity, while our study adopted a comprehensive approach, examining clinical activity, endoscopic activity, and psychological symptoms in IBD.
In our study, DAO showed limited effectiveness in differentiating endoscopic activity among patients with IBD. This limitation can be explained by the fact that the majority of patients in the endoscopic activity group exhibited mild disease activity. This is evidenced by a median SES-CD score of 6 in CD patients. Among UC patients, 37.5% had an MES score of 2, and 72.7% of postoperative CD patients demonstrated a Rutgeerts score of i2. Additionally, the lack of significant differences may be attributable to the relatively small sample size, inherent disease heterogeneity, and variations in disease stages. Uncontrolled confounding factors, such as diet and medications, should be considered as important directions for future research. Although our study applied exclusion criteria such as the use of antibiotics, probiotics, anti-anxiety medications, and antidepressants, diet, as a potential confounder was not accounted for in this study. We anticipate that in the future, dietary intake will be analyzed using direct methods, such as direct observation, duplicate diets, and nutritional biomarkers. Finally, large sample sizes, multi-center designs, standardized enrollment procedures, and randomized controlled trials are essential for minimizing the influence of confounding factors on study outcomes.
In addition, we found that the serum levels of D-LA were higher in IBD patients with psychological symptoms than in healthy controls. However, no statistically significant differences in D-LA levels were noted between groups based on psychological symptoms, clinical activity, or endoscopic activity. This could be attributed to insufficient detection sensitivity (detection range: 0.06-8.00 mmol/L) and the relatively low concentration of D-LA in all participants. Therefore, further research is necessary to determine whether D-LA is an effective biomarker for predicting psychological symptoms and disease activity in IBD patients.
We found that the scores for depression, anxiety, and perceived stress were negatively correlated with QOL. Stress has been found to increase vulnerability to psychiatric disorders, such as depression and anxiety[4]. This may explain why patients with IBD often experience these psychological symptoms concurrently. Functional gastrointestinal symptoms are common among individuals with both IBD and psychological disorders, and can greatly affect their CDAI or Mayo scores. Severe gastrointestinal symptoms can lead to a lower QOL, and the causes of IBD symptoms are often complex and multifactorial. Stress can affect gastrointestinal symptoms through the gut–brain axis in various ways, including nonspecific effects, increased intestinal permeability, immune cell activation, and indirect effects. Anxiety and depression can also stimulate the production of pro-inflammatory cytokines, which adversely affect IBD symptoms and lead to a lower QOL[36]. Therefore, clinicians must consider the presence of underlying psychological illnesses in patients with IBD, particularly when objective measurements of inflammation are ruled out[5].
Correlation analyses revealed that DAO was not only associated with higher clinical disease activity but also negatively correlated with lower serum ALB levels in IBD patients. The serum ALB-to-globulin ratio is significantly lower among IBD patients than among non-IBD controls, according to a report by Wang et al[37]. Thus, we speculate that a dysfunctional intestinal barrier may allow translocated bacteria and their metabolites to pass through the bloodstream and activate immune cells, which then produce pro-inflammatory cytokines and ultimately affect liver function, resulting in lower ALB levels in IBD patients. Psychological symptoms can also affect appetite in patients with IBD, leading to hypoproteinemia.
Our study has certain limitations. First, the cross-sectional study design precludes the establishment of causal relationships. Second, the relatively small sample size, particularly the limited number of UC patients (n = 15), may restrict the generalizability of our findings. Third, uncontrolled confounding factors, such as diet and medications, should be considered in future research. Fourth, only 2 biomarkers were assessed in our study, potentially resulting in an incomplete evaluation of intestinal barrier function. Finally, the specific mechanisms underlying the alterations in psychological symptoms and intestinal barrier function in IBD patients were not investigated in our study, and deserve further exploration.
In summary, the current results demonstrate a correlation between serum DAO levels, psychological symptoms, and clinical disease activity, which may contribute to the development of a potential biomarker for distinguishing psychological symptoms, particularly depression, in patients with IBD. Further prospective studies are warranted to establish its causal relationship.
Serum DAO levels were found to be significantly higher in IBD patients with moderate-to-severe psychological symptoms than in those with no or mild psychological symptoms. In addition, the limited data in this study revealed that DAO was positively associated with PHQ-9 scores and negatively associated with QOL in IBD patients. Additionally, ROC curve analysis confirmed that DAO is a potential biomarker for predicting clinical activity in patients with IBD, highlighting the potential of DAO as a novel biomarker for both depressive symptoms and disease activity. Further prospective cohort studies of high quality are essential to confirm the indications and applications of DAO in IBD.
The authors would like to express their gratitude to all members of the research group for their valuable support. Special thanks are extended to MPH Pei-He Xia for assisting in reviewing the statistical analysis, and to Shan-Ping Wang for patiently and meticulously explaining the pathophysiological mechanisms.
| 1. | Zhao M, Gönczi L, Lakatos PL, Burisch J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J Crohns Colitis. 2021;15:1573-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 256] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 2. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1455] [Article Influence: 291.0] [Reference Citation Analysis (0)] |
| 3. | Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2149] [Cited by in RCA: 2073] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 4. | Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 281] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 5. | Mules TC, Swaminathan A, Hirschfeld E, Borichevsky G, Frampton C, Day AS, Gearry RB. The impact of disease activity on psychological symptoms and quality of life in patients with inflammatory bowel disease-results from the Stress, Anxiety and Depression with Disease Activity (SADD) Study. Aliment Pharmacol Ther. 2022;55:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Swaminathan A, Fan D, Borichevsky GM, Mules TC, Hirschfeld E, Frampton CM, Day AS, Siegel CA, Gearry RB. The disease severity index for inflammatory bowel disease is associated with psychological symptoms and quality of life, and predicts a more complicated disease course. Aliment Pharmacol Ther. 2022;56:664-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 488] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 8. | Fukasawa N, Tsunoda J, Sunaga S, Kiyohara H, Nakamoto N, Teratani T, Mikami Y, Kanai T. The gut-organ axis: Clinical aspects and immune mechanisms. Allergol Int. 2025;74:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Naveed M, Zhou QG, Xu C, Taleb A, Meng F, Ahmed B, Zhang Y, Fukunaga K, Han F. Gut-brain axis: A matter of concern in neuropsychiatric disorders…! Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Schneider KM, Blank N, Alvarez Y, Thum K, Lundgren P, Litichevskiy L, Sleeman M, Bahnsen K, Kim J, Kardo S, Patel S, Dohnalová L, Uhr GT, Descamps HC, Kircher S, McSween AM, Ardabili AR, Nemec KM, Jimenez MT, Glotfelty LG, Eisenberg JD, Furth EE, Henao-Mejia J, Bennett FC, Pierik MJ, Romberg-Camps M, Mujagic Z, Prinz M, Schneider CV, Wherry EJ, Bewtra M, Heuckeroth RO, Levy M, Thaiss CA. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186:2823-2838.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 152] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 11. | Liśkiewicz P, Kaczmarczyk M, Misiak B, Wroński M, Bąba-Kubiś A, Skonieczna-Żydecka K, Marlicz W, Bieńkowski P, Misera A, Pełka-Wysiecka J, Kucharska-Mazur J, Konopka A, Łoniewski I, Samochowiec J. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106:110076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Linsalata M, Riezzo G, Clemente C, D'Attoma B, Russo F. Noninvasive Biomarkers of Gut Barrier Function in Patients Suffering from Diarrhea Predominant-IBS: An Update. Dis Markers. 2020;2020:2886268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Aschenbach JR, Ahrens F, Schwelberger HG, Fürll B, Roesler U, Hensel A, Gäbel G. Functional characteristics of the porcine colonic epithelium following transportation stress and Salmonella infection. Scand J Gastroenterol. 2007;42:708-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Cai J, Chen H, Weng M, Jiang S, Gao J. Diagnostic and Clinical Significance of Serum Levels of D-Lactate and Diamine Oxidase in Patients with Crohn's Disease. Gastroenterol Res Pract. 2019;2019:8536952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Zhang Q, Gao X, Wu J, Chen M. The Correlation between Endotoxin, D-Lactate, and Diamine Oxidase with Endoscopic Activity in Inflammatory Bowel Disease. Dis Markers. 2022;2022:9171436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Inflammatory Bowel Disease Group; Chinese Society of Gastroenterology; Chinese Medical Association. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J Dig Dis. 2021;22:298-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 17. | Limdi JK, Picco M, Farraye FA. A review of endoscopic scoring systems and their importance in a treat-to-target approach in inflammatory bowel disease (with videos). Gastrointest Endosc. 2020;91:733-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6086] [Cited by in RCA: 6847] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 19. | Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11947] [Cited by in RCA: 18882] [Article Influence: 993.8] [Reference Citation Analysis (0)] |
| 20. | Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385-396. [PubMed] |
| 21. | Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, Tompkins C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804-810. [PubMed] |
| 22. | D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P, Schölmerich J, Sutherland LR. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 795] [Article Influence: 44.2] [Reference Citation Analysis (1)] |
| 23. | Ribeiro G, Ferri A, Clarke G, Cryan JF. Diet and the microbiota - gut - brain-axis: a primer for clinical nutrition. Curr Opin Clin Nutr Metab Care. 2022;25:443-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 561] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 25. | Petrut SM, Bragaru AM, Munteanu AE, Moldovan AD, Moldovan CA, Rusu E. Gut over Mind: Exploring the Powerful Gut-Brain Axis. Nutrients. 2025;17:842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Chen Y, Cui W, Li X, Yang H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front Immunol. 2021;12:761981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 27. | Moulton CD, Pavlidis P, Norton C, Norton S, Pariante C, Hayee B, Powell N. Depressive symptoms in inflammatory bowel disease: an extraintestinal manifestation of inflammation? Clin Exp Immunol. 2019;197:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, Raizada MK. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018;67:1555-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 340] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 29. | Iordache MM, Tocia C, Aschie M, Dumitru A, Manea M, Cozaru GC, Petcu L, Vlad SE, Dumitru E, Chisoi A. Intestinal Permeability and Depression in Patients with Inflammatory Bowel Disease. J Clin Med. 2022;11:5121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 30. | Shi L, Li Y, Liu Y, Jia H. Alterations of gut microbiota and cytokines in elevated serum diamine oxidase disorder. Medicine (Baltimore). 2022;101:e31966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Patel RA, Panche AN, Harke SN. Gut microbiome-gut brain axis-depression: interconnection. World J Biol Psychiatry. 2025;26:1-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Fu Y, Lyu J, Wang S. The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front Immunol. 2023;14:1277102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 33. | Konopelski P, Mogilnicka I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals' Health and Disease. Int J Mol Sci. 2022;23:1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 105] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 34. | Miyoshi M, Ueno M, Matsuo M, Hamada Y, Takahashi M, Yamamoto M, Yamamoto I, Mikajiri R, Tabuchi S, Wakida K, Yamanishi M, Hirai M, Usami M. Effect of dietary fatty acid and micronutrient intake/energy ratio on serum diamine oxidase activity in healthy women. Nutrition. 2017;39-40:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Akhtar M, Chen Y, Ma Z, Zhang X, Shi D, Khan JA, Liu H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim Nutr. 2022;8:350-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 165] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 36. | Sajadinejad MS, Asgari K, Molavi H, Kalantari M, Adibi P. Psychological issues in inflammatory bowel disease: an overview. Gastroenterol Res Pract. 2012;2012:106502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Wang Y, Li C, Wang W, Wang J, Li J, Qian S, Cai C, Liu Y. Serum Albumin to Globulin Ratio is Associated with the Presence and Severity of Inflammatory Bowel Disease. J Inflamm Res. 2022;15:1907-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |