Published online Jul 19, 2025. doi: 10.5498/wjp.v15.i7.103684
Revised: April 21, 2025
Accepted: June 4, 2025
Published online: July 19, 2025
Processing time: 124 Days and 20 Hours
Perinatal depression affects 10%-20% of pregnant women and subsequently influences maternal health and fetal development. Concerns over the safety of antidepressants during pregnancy have prompted the exploration of nutritional interventions as adjunct therapies. This study evaluated the impact of combining preconception and prenatal supplementation with myo-inositol, probiotics, and trace elements on mood, quality of life, and fetal development in depressed mothers.
To identify a novel therapeutic approach to reduce pregnancy complications and improve maternal-fetal health outcomes.
This retrospective cohort study included 314 pregnant women who were diag
The intervention group presented significant reductions in gestational diabetes mellitus (13.04% vs 23.53%, P = 0.016) and gestational hypertension (3.73% vs 9.15%, P = 0.049). Higher levels of inositol, iron, zinc, and probiotics were observed near term in the intervention group. Postpartum mood assessments indicated lower anxiety and depression scores for the intervention group, with significant improvements in the positive and negative affect schedule-now (P = 0.002), trait anxiety (P = 0.002), and Patient Health Questionnaire-8 (P = 0.018) scores. The World Health Organization Quality of life Assessment: Brief Version scores improved in the psychological (P = 0.041) and environmental (P = 0.009) domains postpartum. Fetal biparietal diameter and femoral length were greater in the intervention group alongside better neonatal body length and reduced neonatal unit admissions (2.48% vs 7.84%, P = 0.031).
Combined supplementation with myo-inositol, probiotics, and trace elements from preconception through pregnancy may reduce pregnancy-related complications, enhance mood and quality of life, and improve fetal growth metrics.
Core Tip: This study assessed the effects of combined myo-inositol, probiotics, and trace elements on maternal and fetal health. Results showed reduced gestational diabetes mellitus and hypertension rates, improved postpartum mood, and enhanced quality of life scores. Fetal growth parameters and neonatal outcomes also improved. These findings highlight a promising strategy to reduce complications and improve maternal-fetal health.
- Citation: Qi SF, Sun HX, Zhao J. Effects of combined preconception and prenatal myo-inositol, probiotics, and trace element supplementation on the outcomes of depressed mothers. World J Psychiatry 2025; 15(7): 103684
- URL: https://www.wjgnet.com/2220-3206/full/v15/i7/103684.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i7.103684
The incidence of perinatal depression is a growing public health concern, affecting approximately 10%-20% of women during pregnancy and the postpartum period[1]. This condition not only affects maternal mental and emotional health but also poses significant risks to fetal development and birth outcomes[2]. In recent years research has focused on the potential role of nutritional interventions as adjunct therapies for managing perinatal depression[3]. Although pharmacotherapy remains a primary treatment strategy, concerns about the safety and efficacy of antidepressant use during pregnancy have led to increased interest in alternative or complementary approaches[4].
Myo-inositol, a naturally occurring compound in the vitamin B complex, has gained attention for its potential benefits in modulating mood and metabolic parameters[5]. As a structural component of cell membranes and a precursor to inositol phosphates and other biomolecules, myo-inositol is involved in several biological processes, including insulin signal transduction, cellular osmoregulation, and neurotransmitter regulation[6]. Its role in improving insulin sensitivity and reducing the incidence of gestational diabetes mellitus (GDM) makes it a promising candidate for prenatal supplementation[7]. Research indicates that myo-inositol may alleviate depressive symptoms by modulating neurotransmitter pathways[8].
In addition, the gut–brain axis is increasingly recognized as a pivotal factor in mental health, with probiotics playing a crucial role in this connection[9]. Probiotic supplementation, particularly with strains such as Lactobacillus rhamnosus, has demonstrated efficacy in influencing the gut microbiome, which can affect mood by modulating inflammatory responses and neurotransmitter production[10]. Probiotics may contribute to maintaining a balanced gut microbiota, enhancing the production of neuroactive compounds such as serotonin, thereby exerting potential anxiolytic and antidepressant effects[11].
Trace elements such as zinc and iron are essential for numerous biological functions, including the immune response and oxygen transport, which are vital during pregnancy for maternal health and fetal development[12]. Deficiencies in these micronutrients have been associated with adverse pregnancy outcomes and a heightened risk of mood disorders[13]. Consequently, supplementation with zinc and iron is not only beneficial for preventing deficiencies but also holds promise for alleviating mood disturbances associated with perinatal depression[14].
The integration of myo-inositol, probiotics, and trace elements into a combined supplementation strategy offers a novel, holistic approach to enhancing maternal mood, quality of life, and fetal development[15]. However, the evidence supporting this approach remains limited, and comprehensive studies evaluating the synergistic effects of such a regimen are sparse. Our retrospective cohort study aimed to address this gap by examining the impact of combined preconception and prenatal supplementation on a cohort of pregnant women diagnosed with mild to moderate depression. The purpose of this study was to evaluate the effects of combined preconception and prenatal supplementation with myo-inositol, probiotics, and trace elements on mood, quality of life, and fetal development among depressed mothers.
We conducted a retrospective analysis of 314 pregnant women who were diagnosed with depression and treated at our hospital between January 2019 and December 2021. Patient data were obtained from the medical recording system. The participants were divided into two groups on the basis of whether they received combined supplementation with myo-inositol, probiotics, or trace elements before pregnancy. The intervention group comprised 161 women who received the supplements, whereas the control group included 153 women who did not.
The Institutional Review Board and Ethics Committee of the Second Children and Women’s Healthcare of Jinan City approved this study. Given that the research was retrospective and exclusively utilized de-identified patient data, which did not affect patient care or pose any harm, informed consent was unnecessary. This waiver of consent was granted following the regulatory and ethical guidelines governing retrospective research studies.
Inclusion criteria: Participants were eligible if they were over 18 years old, delivered at our hospital between 2019 and 2021, and had a diagnosis of mild or moderate perinatal depression, as indicated by a Zung self-rating depression scale score of less than 69 (Cronbach’s α = 0.8727)[16]. Additionally, participants had to have received psychological therapy, could read and understand independently, demonstrated good compliance by consuming at least 60% of the prescribed formulations, and had complete case records.
Exclusion criteria: Participants were excluded if they had severe depression requiring medication, were lost to follow-up within 2 years postpartum, had a lifetime diagnosis of bipolar disorder, or currently suffered from alcohol or substance dependence. Other exclusions included cognitive impairment; receiving treatment for HIV, hepatitis B, or hepatitis C; and having an educational level of elementary school or lower.
Three months prior to planning conception, participants began taking the supplements, which continued until the end of pregnancy. Both the intervention and control formulations were packaged identically and stored at temperatures between 2-6 °C. These compounds were administered twice daily and presented similar sensory characteristics. Both formulations contained common ingredients: Folic acid (400 μg/day, lot number H12020215, Tianjin Lisheng Pharmaceutical Co., Ltd., China), vitamin D (10 μg/day, lot number H35021450, Sinopharm Holding Xingsha Pharmaceutical (Xiamen) Co., Ltd., China), vitamin B2 (1.8 mg/day, lot number H42020612, Central China Pharmaceutical Co., Ltd.), vitamin B6 (2.6 mg/day, lot number H42020613, Central China Pharmaceutical Co., Ltd.), and vitamin B12 (5.2 μg/day, lot number H14023321, Shanxi Yunpeng Pharmaceutical Co., Ltd.). The intervention formulation also included myo-inositol (4 g/day, lot number H32026275, Jiangsu Suhua Pharmaceutical Group Co., Ltd.), calcium iron zinc tablets (1 g/day, lot number SC10736098210111, Jiangxi Shangshang Industrial Co., Ltd.), and probiotics [Lactobacillus rhamnosus NCC 4007 (CGMCC 1.3724), with a dosage of 5 × 109 CFU/day]. This dosage was chosen based on previous studies indicating that a dose of 5 × 109 CFU/day is effective in improving gut health and modulating the gut-brain axis[17]. The average viable count of refrigerated probiotic products remained within the target range throughout their shelf life. Prenatal, perinatal, and neonatal outcomes were assessed via medical records, and compliance with the trial formulation was monitored by counting the remaining sachets.
Maternal biomarkers were assessed at two key timepoints: At the time of planning pregnancy (prior to intervention) and1 week before delivery. After 8 h of fasting, 4 mL of venous blood was collected from each participant. Blood samples were immediately centrifuged at 3000 rpm for 10 min at 4 °C to separate plasma, which was then stored at -80 °C until analysis. The plasma concentrations of folate, homocysteine (indicative of 1-carbon status and other physiological conditions and deficiencies in folate and B-vitamins), riboflavin, flavin mononucleotide (FMN) (indicative of riboflavin status), and myo-inositol were measured in the control and intervention groups via a targeted method based on liquid chromatography-tandem mass spectrometry provided by Bevital, Bergen, Norway. Additionally, blood concentrations of calcium, iron, and zinc were determined via an atomic absorption spectrophotometer (AA800, Jingke Ruida Technology Co., Ltd., Beijing, China). Fecal samples were collected in sterile containers and immediately frozen at -80 °C to preserve microbial DNA integrity. These samples were later used to amplify the genetic sequences of the probiotics via an Applied Biosystems Polymerase Chain Reaction instrument (Thermo Fisher Scientific, United States), and the probiotics were counted.
At the time of planning pregnancy (prior to intervention) and 2 days postpartum, women completed a questionnaire designed to collect data on maternal mood. This process followed an explanation provided by a physician. Two physicians recorded and statistically analyzed the collected data. The positive affect (PA) and negative affect (NA) schedule was employed to evaluate participants’ current mood, achieving an interclass correlation of 0.93[18]. The scores for PA and NA were calculated on a scale of 20 to 80, with higher scores indicating greater levels of PA or NA.
The participants also completed the state-trait anxiety inventory, which includes 40 items and has a Cronbach’s α of 0.837[19]. The state-trait anxiety inventory addresses “state” anxiety (SA), which refers to the presence and severity of current anxiety symptoms, through 20 items. All the items were rated on a four-point Likert scale. Responses for SA items ranged from “not at all” to “very much so”, reflecting participants’ feelings at that moment. For “trait” anxiety (TA) items, responses ranged from “almost never” to “almost always”, indicating the frequency of feelings in general. Items were appropriately reverse-coded and summed to yield SA and TA scores, each ranging from 20 to 80. Higher SA and TA scores indicated higher SA and TA levels, respectively.
The Patient Health Questionnaire-8 (PHQ-8), which has a Cronbach’s alpha of 0.922[20], was used to assess depressive symptoms. The PHQ-8 includes eight items corresponding to the Diagnostic and Statistical Manual criteria for depression, excluding suicidal ideation. The participants rated the frequency of being bothered by each item over the prior 2 weeks via a four-point Likert scale, with responses ranging from “0: Not at all” to “3: Nearly every day”. An overall score was calculated by summing the responses across all the items, resulting in a total score ranging from 0 to 24. Cutoff scores were used to categorize the severity of depressive symptoms: Scores of 5 indicated mild depression; 10 indicated moderate depression; 15 indicated moderately severe depression; and 20 or more indicated severe depression.
At the time of planning pregnancy (prior to intervention) and 2 days postpartum, participants completed the World Health Organization Quality of life Assessment: Brief Version questionnaire, which has a Cronbach’s alpha of 0.90[21]. This questionnaire includes one item from each of the 24 facets of the World Health Organization Quality of life-100 that pertain to perceived quality of life. These facets cover areas such as energy, fatigue, sleep, rest, body image, self-esteem, personal relationships, social support, financial resources, health and social care, and the home environment, along with two additional items addressing overall quality of life and general health.
The questionnaire items were rated on five-point Likert scales, where “1” denotes “very poor; very dissatisfied; not at all; and never” and “5” represents “very good; very satisfied; an extreme amount; extremely; completely; and always”. The items were summed and standardized across four domains: Physical health; psychological; social relationships; and environment. Higher scores indicated greater perceptions of quality or aptitude within each domain.
Ultrasound measurements of fetal biparietal diameter (BPD), femoral length (FL), transverse abdominal diameter, anterior-posterior abdominal diameter, and abdominal circumference (AC) were collected and recorded within 1 week prior to delivery. These measurements were conducted and analyzed by professional ultrasound technicians to ensure data quality.
The BPD was assessed from the outer edge of the parietal bone nearest the probe to the inner edge of the parietal bone on the opposite side via a cross-section of the fetal brain that included key anatomical landmarks such as the hyaline membrane cavity, thalamus, third ventricle, and accumbens compartment. Head circumference was measured on the same plane as the BPD via the ellipse function of the ultrasonic instrument. The AC was obtained perpendicular to the fetal umbilical plexus level, encompassing the spine, gastric vesicles, liver, umbilical vein, skin, and subcutaneous fat. The FL was measured from the greater trochanter to the lateral condyle, ensuring clear visibility of the bones at both ends during the scan.
After birth, two physicians recorded various neonatal data, including gestational age at birth, the number of admissions to the neonatal ward, cases of sepsis, placental weight, placental area, and umbilical cord length. Neonatal length, weight, and head circumference were measured via a neonatal measurement device (Holtain Ltd., Crymych, United Kingdom), with a precision of 0.01 cm.
The data were analyzed via SPSS 29.0 statistical software (SPSS Inc., Chicago, IL, United States). For categorical data, n (%) was used for representation. For continuous data with a normal distribution, the mean ± SD format was adopted. P < 0.05 was considered statistically significant.
This retrospective cohort study compared the effects of preconception and prenatal supplementation with myo-inositol, probiotics, and trace elements on mood, quality of life, and fetal development among depressed mothers. Table 1 shows highly similar baseline characteristics between the control group (n = 153) and the intervention group (n = 161). Age, body mass index, marital status, educational level, smoking status, household income, compliance, parity, infant sex, breastfeeding rates, incidence of preterm deliveries, preterm premature rupture of membranes, major postpartum hemorrhage, severe nausea and vomiting, preeclampsia, chronic hypertension with superimposed preeclampsia, and scores on the Zung self-rating depression scale did not significantly differ between the groups (P > 0.05). However, significant differences were observed in the prevalence of GDM, which was lower in the intervention group than in the control group (13.04% vs 23.53%, P = 0.016), and in the occurrence of gestational hypertension, which was also lower in the intervention group (3.73% vs 9.15%, P = 0.049). These results suggest that the intervention regimen may have beneficial effects on reducing certain pregnancy-related complications.
| Parameters | Control group (n = 153) | Intervention group (n = 161) | t/χ2 | P value |
| Age (year), mean ± SD | 27.25 ± 2.17 | 27.36 ± 2.61 | 0.416 | 0.678 |
| BMI (kg/m2), mean ± SD | 23.68 ± 2.74 | 23.71 ± 2.83 | 0.084 | 0.933 |
| Marital status (married/other) | 149 (97.39)/4 (2.61) | 151 (93.79)/10 (6.21) | 2.382 | 0.123 |
| Educational level | 0.555 | 0.758 | ||
| Junior high school | 51 (33.33) | 48 (29.81) | ||
| High school and vocational school | 42 (27.45) | 49 (30.43) | ||
| College and above | 60 (39.22) | 64 (39.75) | ||
| Smoker | 6 (3.92) | 8 (4.97) | 0.202 | 0.653 |
| Household income (monthly) | 0.071 | 0.965 | ||
| < 10000 RMB | 38 (24.84) | 39 (24.22) | ||
| 10000-20000 RMB | 78 (50.98) | 81 (50.31) | ||
| > 20000 RMB | 37 (24.18) | 41 (25.47) | ||
| Compliance | 122 (79.74) | 133 (82.61) | 0.424 | 0.515 |
| Nulliparous | 105 (68.63) | 115 (71.43) | 0.293 | 0.588 |
| Infant sex (male/female) | 70 (45.75) | 77 (47.83) | 0.136 | 0.713 |
| Any breastfeeding | 146 (95.42) | 157 (97.52) | 1.014 | 0.314 |
| All preterm deliveries (< 37 weeks) | 13 (8.50) | 9 (5.59) | 1.017 | 0.313 |
| PPROM | 9 (5.88) | 4 (2.48) | 2.282 | 0.131 |
| Major postpartum hemorrhage | 8 (5.23) | 4 (2.48) | 1.607 | 0.205 |
| GDM | 36 (23.53) | 21 (13.04) | 5.806 | 0.016 |
| Severe nausea and vomiting | 24 (15.69) | 19 (11.80) | 1.002 | 0.317 |
| Gestational hypertension | 14 (9.15) | 6 (3.73) | 3.869 | 0.049 |
| Preeclampsia | 6 (3.92) | 4 (2.48) | 0.163 | 0.687 |
| Chronic hypertension with superimposed preeclampsia | 5 (3.27) | 2 (1.24) | 0.694 | 0.405 |
| Zung self-rating depression scale | 45.75 ± 4.26 | 45.37 ± 4.65 | 0.75 | 0.454 |
Table 2 shows comparable values between the two groups in terms of the levels of folate (25.37 ± 3.12 nmol/L vs 24.79 ± 3.46 nmol/L, P = 0.12), homocysteine (7.31 ± 1.17 μmol/L vs 7.28 ± 1.34 μmol/L, P = 0.865), riboflavin (8.78 ± 2.04 nmol/L vs 8.52 ± 1.97 nmol/L, P = 0.241), FMN (12.08 ± 2.57 nmol/L vs 12.41 ± 2.39 nmol/L, P = 0.245), inositol (16.27 ± 3.24 μmol/L vs 15.98 ± 3.61 μmol/L, P = 0.449), calcium (9.12 ± 1.06 mg/dL vs 9.23 ± 1.12 mg/dL, P = 0.352), iron (92.17 ± 10.25 μg/dL vs 93.74 ± 9.76 μg/dL, P = 0.165), zinc (86.33 ± 6.19 μg/dL vs 86.04 ± 7.18 μg/dL, P = 0.706), and probiotics measured as colony-forming units (92.37 ± 16.15 CFU × 108/g vs 93.25 ± 17.24 CFU × 108/g, P = 0.642). These findings indicate that both cohorts were similar with respect to biomarkers prior to the beginning of the supplementation intervention.
| Variable | Control group (n = 153) | Intervention group (n = 161) | t | P value |
| Folate (nmol/L) | 25.37 ± 3.12 | 24.79 ± 3.46 | 1.558 | 0.12 |
| Homocysteine (μmol/L) | 7.31 ± 1.17 | 7.28 ± 1.34 | 0.170 | 0.865 |
| Riboflavin (nmol/L) | 8.78 ± 2.04 | 8.52 ± 1.97 | 1.176 | 0.241 |
| FMN (nmol/L) | 12.08 ± 2.57 | 12.41 ± 2.39 | 1.164 | 0.245 |
| Inositol (μmol/L) | 16.27 ± 3.24 | 15.98 ± 3.61 | 0.757 | 0.449 |
| Calcium (mg/dL) | 9.12 ± 1.06 | 9.23 ± 1.12 | 0.932 | 0.352 |
| Iron (μg/dL) | 92.17 ± 10.25 | 93.74 ± 9.76 | 1.391 | 0.165 |
| Zinc (μg/dL) | 86.33 ± 6.19 | 86.04 ± 7.18 | 0.378 | 0.706 |
| Probiotics (CFU × 108/g) | 92.37 ± 16.15 | 93.25 ± 17.24 | 0.465 | 0.642 |
Table 3 shows that compared with the control group, the intervention group had higher levels of inositol (22.37 ± 3.45 μmol/L vs 21.28 ± 4.71 μmol/L, P = 0.021), iron (111.35 ± 10.28 μg/dL vs 107.34 ± 12.28 μg/dL, P = 0.002), zinc (95.34 ± 10.81 μg/dL vs 91.74 ± 9.34 μg/dL, P = 0.002), and probiotics. These indices were measured as colony-forming units (99.64 ± 16.27 CFU × 108/g vs 95.34 ± 15.27 CFU × 108/g, P = 0.016). For biomarkers such as calcium levels, which were marginally greater in the intervention group (10.15 ± 2.27 mg/dL vs 9.66 ± 2.14 mg/dL, P = 0.051), although these differences did not reach statistical significance, they may still hold clinical relevance. The observed trend suggests a potential benefit of supplementation on maternal calcium metabolism, which warrants further investigation in larger studies. The effect size for this comparison was small (Cohen’s d = 0.22), indicating a minor but potentially meaningful difference. Other variables, including folate, homocysteine, riboflavin, and FMN, did not differ significantly between the groups (P > 0.05). These significant differences suggest that the supplementation regimen positively influenced certain biomarkers in the intervention group.
| Variable | Control group (n = 153) | Intervention group (n = 161) | t | P value |
| Folate (nmol/L) | 42.28 ± 5.37 | 42.69 ± 3.46 | 0.801 | 0.424 |
| Homocysteine (μmol/L) | 3.95 ± 1.03 | 3.94 ± 1.14 | 0.063 | 0.95 |
| Riboflavin (nmol/L) | 16.31 ± 3.67 | 17.08 ± 4.28 | 1.709 | 0.088 |
| FMN (nmol/L) | 10.35 ± 2.07 | 10.39 ± 2.34 | 0.154 | 0.877 |
| Inositol (μmol/L) | 21.28 ± 4.71 | 22.37 ± 3.45 | 2.326 | 0.021 |
| Calcium (mg/dL) | 9.66 ± 2.14 | 10.15 ± 2.27 | 1.962 | 0.051 |
| Iron (μg/dL) | 107.34 ± 12.28 | 111.35 ± 10.28 | 3.130 | 0.002 |
| Zinc (μg/dL) | 91.74 ± 9.34 | 95.34 ± 10.81 | 3.156 | 0.002 |
| Probiotics (CFU × 108/g) | 95.34 ± 15.27 | 99.64 ± 16.27 | 2.413 | 0.016 |
Table 4 shows comparable scores between the two groups in terms of PA (27.36 ± 3.15 vs 27.42 ± 3.62, P = 0.866), NA (67.65 ± 10.25 vs 69.47 ± 9.71, P = 0.107), SA (9.44 ± 2.88 vs 9.52 ± 3.01, P = 0.806), TA (12.35 ± 2.36 vs 12.64 ± 2.61, P = 0.302), and PHQ-8 (27.36 ± 3.15 vs 27.42 ± 3.62, P = 0.866). These findings indicate that the participants had similar mood-related characteristics at baseline in both cohorts.
| Variable | Control group (n = 153) | Intervention group (n = 161) | t | P value |
| PANAS-NOW | ||||
| PA | 12.35 ± 2.36 | 12.64 ± 2.61 | 1.048 | 0.296 |
| NA | 27.36 ± 3.15 | 27.42 ± 3.62 | 0.166 | 0.868 |
| STAI | ||||
| State anxiety | 67.65 ± 10.25 | 69.47 ± 9.71 | 1.614 | 0.108 |
| Trait anxiety | 71.34 ± 8.18 | 72.35 ± 8.02 | 1.112 | 0.267 |
| PHQ-8 | 9.44 ± 2.88 | 9.52 ± 3.01 | 0.241 | 0.81 |
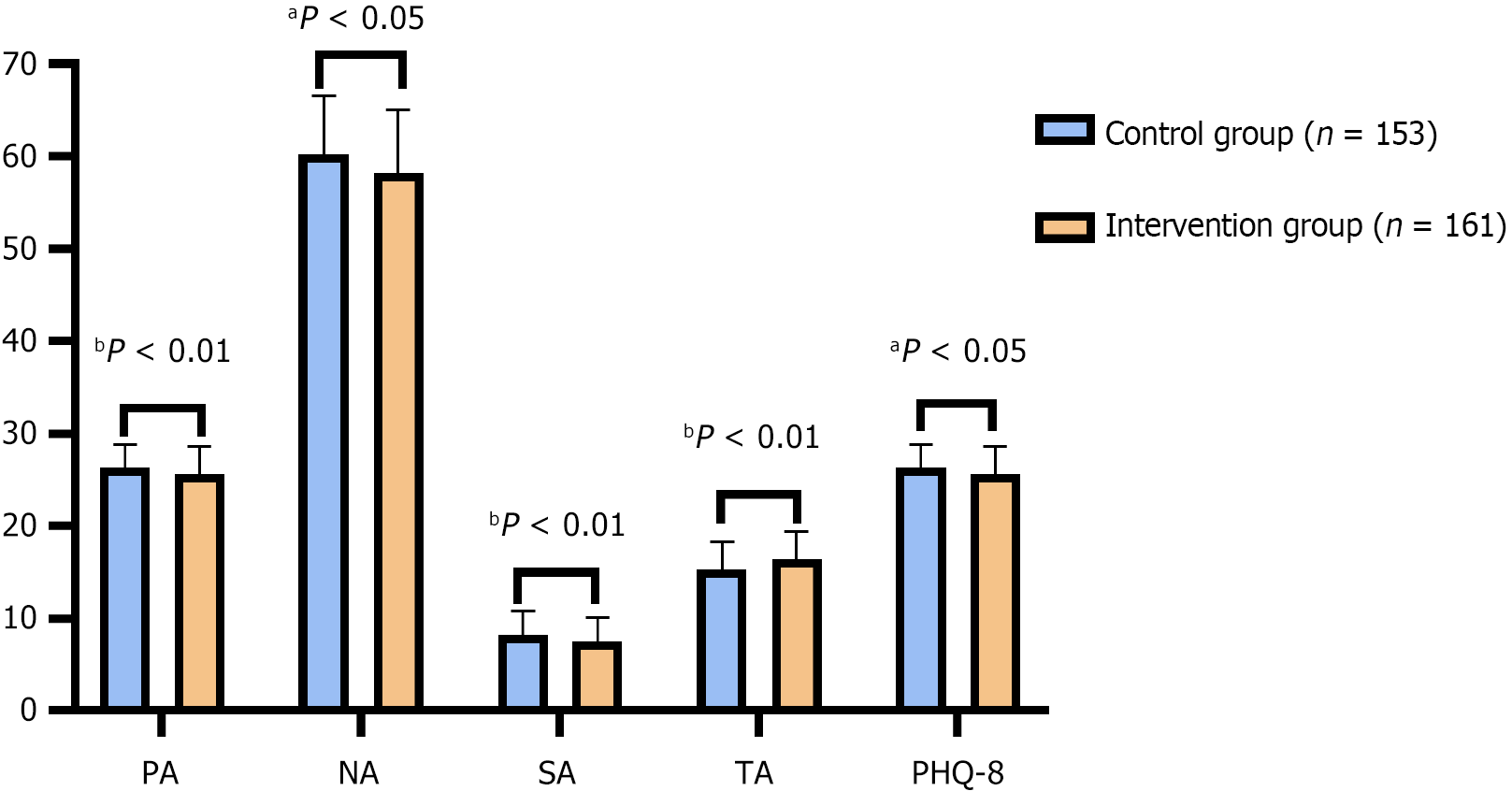
The intervention group presented higher scores for TA (16.34 ± 3.14 vs 15.26 ± 3.02, P = 0.002), suggesting improved mood states (Figure 1). Conversely, the intervention group presented lower scores for PA (25.57 ± 3.12 vs 26.33 ± 2.55, P = 0.018), NA (58.22 ± 6.82 vs 60.21 ± 6.37, P = 0.008), SA (7.56 ± 2.53 vs 8.24 ± 2.62, P = 0.02), and PHQ-8 scores (25.57 ± 3.12 vs 26.33 ± 2.55, P = 0.018), indicating lower anxiety and depressive symptoms in the intervention group than in the control group. These results further suggest that the supplementation regimen may contribute positively to mood and anxiety levels in postpartum individuals.
Table 5 shows comparable scores between the two groups in terms of physical health scores (45.68 ± 6.84 vs 46.12 ± 6.72, P = 0.57), psychological scores (33.13 ± 6.77 vs 33.65 ± 7.11, P = 0.509), social relationship scores (42.25 ± 7.12 vs 41.98 ± 7.62, P = 0.748), and environment scores (43.66 ± 7.87 vs 42.97 ± 8.12, P = 0.446). These findings indicate that participants in both groups had similar quality-of-life profiles at baseline prior to any intervention.
| Variable | Control group (n = 153) | Intervention group (n = 161) | t | P value |
| Physical health | 45.68 ± 6.84 | 46.12 ± 6.72 | 0.569 | 0.570 |
| Psychological | 33.13 ± 6.77 | 33.65 ± 7.11 | 0.661 | 0.509 |
| Social relationships | 42.25 ± 7.12 | 41.98 ± 7.62 | 0.322 | 0.748 |
| Environment | 43.66 ± 7.87 | 42.97 ± 8.12 | 0.762 | 0.446 |
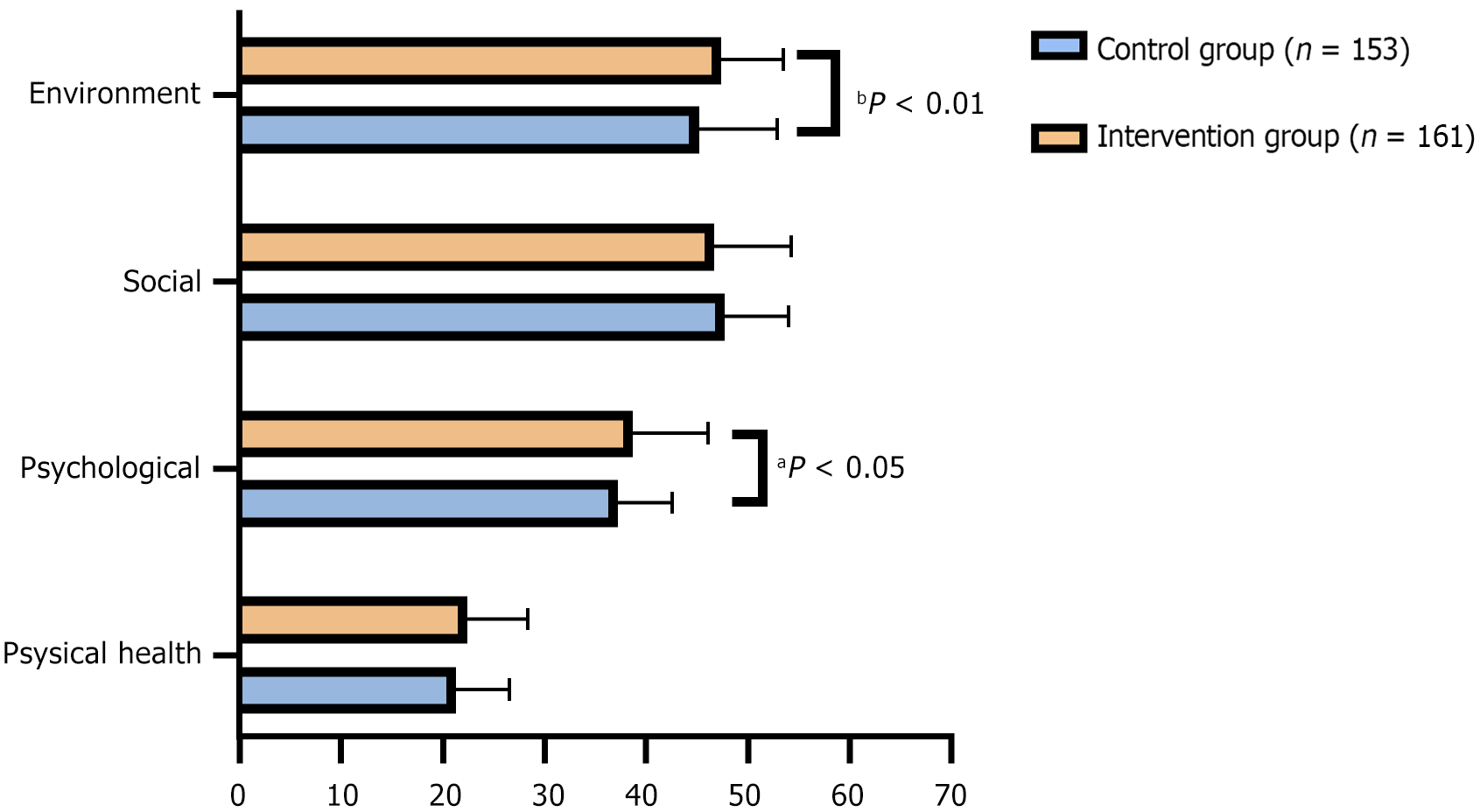
The intervention group had significantly higher scores in the psychological domain (38.75 ± 7.36 vs 37.26 ± 5.34, P = 0.041) and the environment domain (47.31 ± 6.21 vs 45.22 ± 7.65, P = 0.009), suggesting improvements in psychological well-being and environmental perception with supplementation (Figure 2). Although the physical health scores were higher in the intervention group (22.36 ± 6.07 vs 21.25 ± 5.32), this difference did not reach statistical significance (P = 0.086). The scores for social relationships were not significantly different between the groups (47.65 ± 6.41 vs 46.62 ± 7.64, P = 0.199). These findings suggest a potential benefit of supplementation in enhancing the psychological and environmental aspects of quality of life in postpartum mothers.
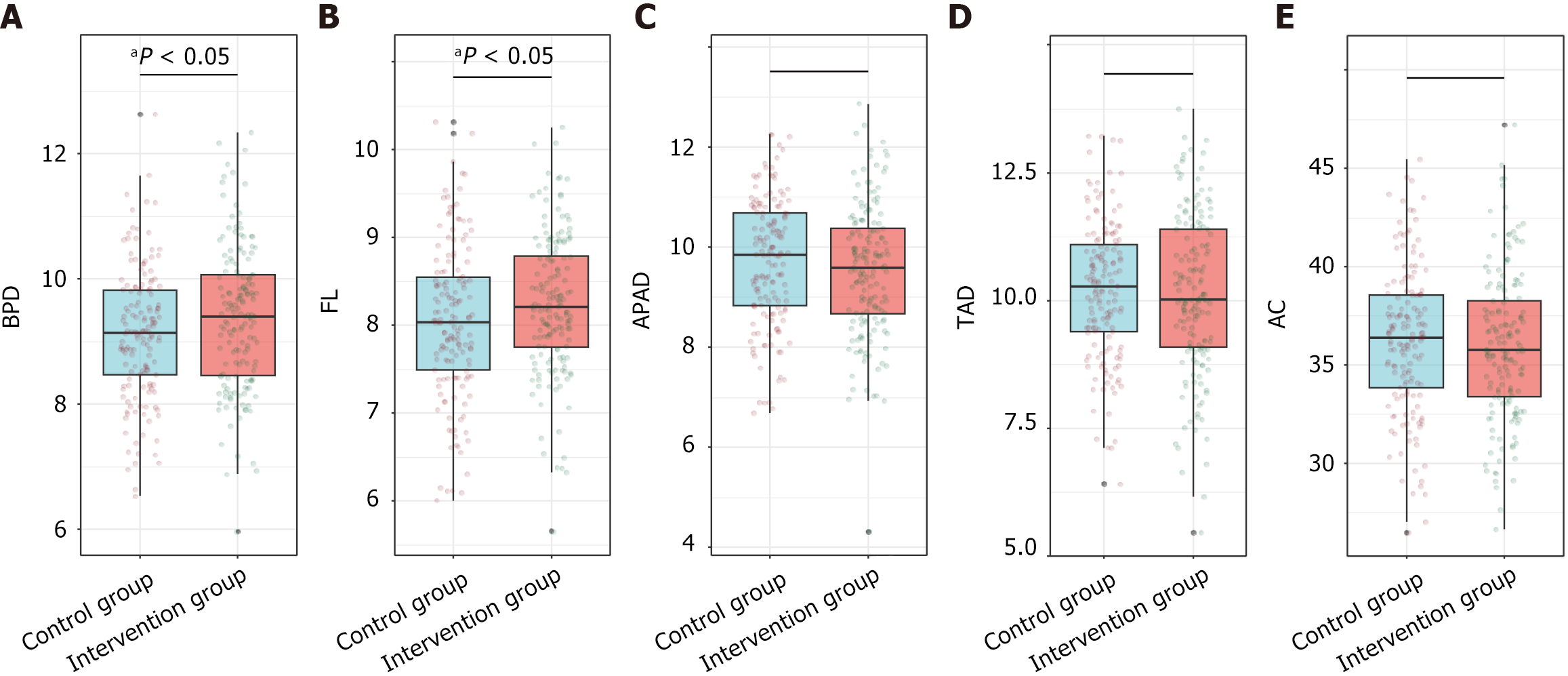
Ultrasound examination conducted 1 week before delivery revealed a significantly greater BPD of 9.38 ± 1.13 cm in the intervention group than in the control group (9.12 ± 1.05 cm; P = 0.042) and a longer FL of 8.24 ± 0.79 cm vs 8.04 ± 0.87 cm (P = 0.036) (Figure 3). Other parameters, including the abdominal anteroposterior diameter, thoracic anteroposterior diameter, and AC, were not significantly different between the groups, with P values of 0.205, 0.622, and 0.501, respectively. These findings suggest that combined preconception and prenatal supplementation may positively influence certain aspects of fetal growth.
Table 6 shows the assessment scores for basic neonatal conditions at birth. The neonates in the intervention group were born at a slightly earlier gestational age (39.15 ± 1.09 weeks vs 39.42 ± 1.23 weeks, P = 0.036) and had a significantly greater body length at birth (49.71 ± 1.56 cm vs 49.25 ± 1.35 cm, P = 0.005). Although the birth weight was greater in the intervention group (3.28 ± 0.52 kg vs 3.18 ± 0.51 kg), this difference did not reach statistical significance (P = 0.088; Cohen’s d = 0.20). Despite the lack of statistical significance, the moderate effect size suggests potential clinical relevance. The head circumference was also slightly smaller in the intervention group (34.57 ± 2.81 cm vs 35.14 ± 2.36 cm, P = 0.053; Cohen’s d = 0.21), which is close to reaching statistical significance and may warrant further investigation. Placental weight, placental area, and umbilical cord length were not significantly different between the groups (P > 0.05 for all variables). Notably, the rate of admission to the neonatal unit was significantly lower in the intervention group (2.48% vs 7.84%, P = 0.031). The incidence of neonatal septicemia was not significantly different between the groups (P = 0.456). These findings suggest that the supplementation regimen may contribute to positive neonatal outcomes, including reduced neonatal unit admissions.
| Variable | Control group (n = 153) | Intervention group (n = 161) | t/χ2 | P value |
| Gestational age at birth | 39.42 ± 1.23 | 39.15 ± 1.09 | 2.111 | 0.036 |
| Birth weight (kg) | 3.18 ± 0.51 | 3.28 ± 0.52 | 1.714 | 0.088 |
| Body length (cm) | 49.25 ± 1.35 | 49.71 ± 1.56 | 2.820 | 0.005 |
| Head circumference | 35.14 ± 2.36 | 34.57 ± 2.81 | 1.945 | 0.053 |
| Placental weight (g) | 541.55 ± 15.82 | 540.19 ± 17.52 | 0.721 | 0.472 |
| Placental area (cm2) | 175.69 ± 17.64 | 174.74 ± 16.78 | 0.492 | 0.623 |
| Umbilical cord length (cm) | 55.12 ± 17.85 | 57.36 ± 18.94 | 1.074 | 0.284 |
| Admission to neonatal unit, n (%) | 12 (7.84) | 4 (2.48) | 4.658 | 0.031 |
| Neonatal septicemia, n (%) | 2 (1.31) | 0 (0) | 0.556 | 0.456 |
The selection of biomarkers in this study was guided by their established roles in perinatal health and potential interactions with the administered supplements. Myo-inositol has been shown to enhance insulin sensitivity and improve glucose metabolism, crucial for preventing GDM. Elevated levels of myo-inositol have also been linked to improved neuroplasticity and neurotransmitter signaling, potentially alleviating depressive symptoms. Zinc, an essential trace element, plays a vital role in immune function and oxidative stress reduction, both important for maternal and fetal well-being. Additionally, probiotics such as Lactobacillus rhamnosus have been associated with reduced inflammation and improved gut-brain axis communication, contributing to mood stabilization.
This retrospective cohort study examined the effects of combined preconception and prenatal supplementation with myo-inositol, probiotics, and trace elements on mood, quality of life, and fetal development among pregnant women diagnosed with mild to moderate depression. One of the most intriguing insights from this study is the significant reduction in pregnancy-related complications observed in the intervention group, specifically the lower prevalence of GDM and gestational hypertension. The inclusion of myo-inositol, which has been implicated in improved insulin signaling and carbohydrate metabolism, likely contributed to these findings[22]. Myo-inositol plays a key role as a precursor to inositol phosphates that mediate insulin action, and its supplementation has been demonstrated to modulate insulin resistance during pregnancy, which could explain the reduced incidence of GDM in our study cohort[23]. Additionally, the trace element zinc could contribute to improved insulin function and the regulation of blood pressure, helping mitigate the risks associated with gestational hypertension[24].
The observed increase in certain blood indices in the intervention group provides further insights into the biological underpinnings of these outcomes. The elevated levels of inositol, iron, and zinc, along with increased probiotic concentrations observed near term, are worth mentioning. The role of myo-inositol in cellular signaling and osmoregulation may positively affect fetal and maternal tissue responses to stress and nutrient transfer[7,22]. Furthermore, elevated iron and zinc levels can ensure optimal oxygen transport and immune function for both mothers and fetuses[25,26]. Probiotics, specifically Lactobacillus rhamnosus, may contribute to maintaining a healthy gut microbiome, inhibiting pathogenic bacteria, and potentially lowering inflammation, a factor linked to depressive symptoms[27,28].
In terms of maternal mood, the results indicated a notable improvement among those receiving the supplementation, as evidenced by lower anxiety and depression scores postpartum than in the controls. The reduction in the NA and TA scores emphasized the potential mood-stabilizing benefits of this supplement combination[29]. This could be partially attributed to the role of probiotics in the gut-brain axis where they have been postulated to synthesize or influence neurotransmitters such as serotonin and gamma-aminobutyric acid, which are crucial for mood regulation[30,31]. Additionally, normalizing inositol levels could improve neuroplasticity and neurotransmitter signaling, thereby reducing depressive symptoms[5]. The enriched nutritional environment, supported by optimized mineral and vitamin status, likely facilitates biochemical processes essential for neurotransmitter function, potentially reducing mood disturbances associated with depression[32].
The quality of life enhancements in the psychological and environmental domains observed in the intervention group could be attributed to interconnected improvements in mood and physiological well-being[33]. A robust relationship between mood and quality of life in postpartum women has been noted previously. In our study supplementation likely strengthened resilience against stress and promoted a positive maternal psychological state[34]. Probiotics, which modulate the gut microbiota, might reduce systemic inflammation, a contributor to mood disorders and a diminished quality of life[35]. Furthermore, maternal empowerment from improved metabolic and nutritional status during pregnancy could promote self-esteem and environmental control, contributing to perceived improvements in quality of life[36].
Assessments of fetal development indicated that the intervention group exhibited favorable outcomes, such as increased BPD and FL measured by ultrasound, along with increased neonatal body length at birth. These outcomes may be directly influenced by the role of myo-inositol in cell growth and differentiation, trace element contributions to fetal development, and probiotics ensuring maternal health and lowering adverse outcomes[37,38]. Myo-inositol might support placental function and fetal growth; furthermore, zinc and iron ensure critical cellular proliferation and hemoglobin synthesis[7]. Such nutritional enrichment can create a conducive intrauterine environment, promoting optimal fetal development[6]. The significant reduction in neonatal unit admissions from 7.84% in the control group to 2.48% in the intervention group (P = 0.031) represents a 68.4% relative risk reduction. This decrease suggests fewer infants needing immediate postnatal care, potentially lowering healthcare costs and improving long-term outcomes for both infants and their families.
Recent randomized controlled trials on myo-inositol during pregnancy have shown benefits in reducing GDM and other complications[39]. However, our study extended these findings by demonstrating additional benefits, including improved maternal mood, quality of life, and reduced neonatal morbidity. The inclusion of probiotics and trace elements alongside myo-inositol is a novel approach that addresses multiple aspects of maternal and fetal health. Compared with previous studies, our research provided a more comprehensive evaluation of the synergistic effects of combined supplementation. For example, while Santamaria et al[40] focused on GDM reduction, our findings indicated that adding probiotics and trace elements can also mitigate gestational hypertension and neonatal unit admissions. These results highlight the potential of a holistic nutritional strategy to optimize both maternal and neonatal outcomes.
Although certain variables such as calcium levels, neonatal birth weight, and head circumference did not reach traditional statistical significance, they exhibited small to moderate effect sizes, suggesting potential clinical relevance. Specifically, despite the non-significant difference in calcium levels, considering the increased demand for calcium during pregnancy and its importance for fetal bone development, these trends warrant further exploration. Additionally, changes in neonatal birth weight and head circumference may reflect the impact of the intervention on fetal growth. Future studies should aim to verify these trends through larger sample sizes or extended follow-up periods to determine if these variables indeed hold clinical significance.
This study revealed multiple practical implications and important directions for future research. Although retrospective analyses cannot confirm causality, the observed correlations encourage further exploration of combining nutritional supplements with traditional therapies for pregnant women with mild to moderate depression. Particularly, in situations where pharmacological interventions face challenges, supplement regimens can serve as an adjunctive strategy to promote maternal and neonatal health. For instance, tailoring supplement formulations based on regional dietary habits, genetic backgrounds, and socioeconomic statuses may enhance their efficacy and applicability.
However, this study had certain limitations, such as potential selection bias and inherent constraints from the retrospective design. These issues should be addressed in future prospective and randomized controlled trials. Expanding the socioeconomic backgrounds, geographic locations, and cultural differences of study participants can validate the generalizability and external validity of the findings. Extending the follow-up period can help assess the impact on postnatal development and maternal mental health, particularly at different stages of child growth (e.g., preschool, adolescence), to comprehensively understand the long-term benefits of interventions.
Future studies should optimize sample selection and increase sample sizes to include populations from diverse socioeconomic backgrounds and regions, ensuring broad applicability of the findings. Measurement indicators should incorporate new biomarkers reflecting maternal-fetal health or use advanced imaging techniques to monitor fetal growth, thereby obtaining more comprehensive data support. Adjusting supplement dosages and combinations based on individual differences is crucial. Genetic testing can identify which populations benefit most from specific supplements, and micronutrient ratios can be adjusted based on regional dietary habits to maximize intervention effects and minimize adverse reactions. Conducting multicenter collaborative studies will further enhance the representativeness and reliability of the data, providing scientific evidence for developing personalized nutritional interventions.
Our study suggested that combined preconception and prenatal supplementation with myo-inositol, probiotics, and trace elements may confer significant benefits in improving pregnancy outcomes, mood, and quality of life in depressed mothers. The findings underscored the value of such nutritional interventions, providing an integrative approach to address complex physiological and psychological demands during pregnancy. As research progresses, these insights can shape guidelines for enhancing prenatal care, contributing to healthier pregnancies and neonatal development.
| 1. | Yu H, Shen Q, Bränn E, Yang Y, Oberg AS, Valdimarsdóttir UA, Lu D. Perinatal Depression and Risk of Suicidal Behavior. JAMA Netw Open. 2024;7:e2350897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 2. | Xie H, Cong S, Wang R, Sun X, Han J, Ni S, Zhang A. Effect of eHealth interventions on perinatal depression: A meta-analysis. J Affect Disord. 2024;354:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Wong S, Le GH, Kwan ATH, Teopiz KM, Rhee TG, Ho R, Rosenblat JD, Mansur R, McIntyre RS. Efficacy of esketamine for perinatal depression: a systematic review and meta-analysis. CNS Spectr. 2024;1-9. [PubMed] [DOI] [Full Text] |
| 4. | Voelker R. What Is Perinatal Depression? JAMA. 2024;331:1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Castillo P, Palou M, Yau-Qiu ZX, Rodríguez AM, Palou A, Picó C. Myo-Inositol Supplementation in Suckling Rats Protects against Adverse Programming Outcomes on Hypothalamic Structure Caused by Mild Gestational Calorie Restriction, Partially Comparable to Leptin Effects. Nutrients. 2021;13:3257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Chang HF, Yong HEJ, Zhang H, Wong JT, Barton SJ, Titcombe P, Albert BB, El-Heis S, Nield H, Ong J, Lavelle L, Ramos-Nieves JM, Godin JP, Silva-Zolezzi I, Cutfield WS, Godfrey KM, Chan SY; The NiPPeR Study Group. Higher Plasma Myo-Inositol in Pregnancy Associated with Reduced Postpartum Blood Loss: Secondary Analyses of the NiPPeR Trial. Nutrients. 2024;16:2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Chu AHY, Tint MT, Chang HF, Wong G, Yuan WL, Tull D, Nijagal B, Narayana VK, Meikle PJ, Chang KTE, Lewis RM, Chi C, Yap FKP, Tan KH, Shek LP, Chong YS, Gluckman PD, Lee YS, Fortier MV, Godfrey KM, Eriksson JG, Karnani N, Chan SY. High placental inositol content associated with suppressed pro-adipogenic effects of maternal glycaemia in offspring: the GUSTO cohort. Int J Obes (Lond). 2021;45:247-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Ibrahim I, Abdullahi H, Fagier Y, Ortashi O, Terrangera A, Okunoye G. Effect of antenatal dietary myo-inositol supplementation on the incidence of gestational diabetes mellitus and fetal outcome: protocol for a double-blind randomised controlled trial. BMJ Open. 2022;12:e055314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Adıgüzel E, Çiçek B, Ünal G, Aydın MF, Barlak-Keti D. Probiotics and prebiotics alleviate behavioral deficits, inflammatory response, and gut dysbiosis in prenatal VPA-induced rodent model of autism. Physiol Behav. 2022;256:113961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 10. | Browne PD, Bolte AC, Besseling-van der Vaart I, Claassen E, de Weerth C. Probiotics as a treatment for prenatal maternal anxiety and depression: a double-blind randomized pilot trial. Sci Rep. 2021;11:3051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Colquitt AS, Miles EA, Calder PC. Do Probiotics in Pregnancy Reduce Allergies and Asthma in Infancy and Childhood? A Systematic Review. Nutrients. 2022;14:1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Bulka CM, Eaves LA, Gardner AJ, Parsons PJ, Galusha AL, Roell KR, Smeester L, O'Shea TM, Fry RC. Prenatal exposure to multiple metallic and metalloid trace elements and the risk of bacterial sepsis in extremely low gestational age newborns: A prospective cohort study. Front Epidemiol. 2022;2:958389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Dettwiler M, Flynn AC, Rigutto-Farebrother J. Effects of Non-Essential "Toxic" Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses. Int J Environ Res Public Health. 2023;20:5536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Lu Y, Zhang Y, Guan Q, Xu L, Zhao S, Duan J, Wang Y, Xia Y, Xu Q. Exposure to multiple trace elements and miscarriage during early pregnancy: A mixtures approach. Environ Int. 2022;162:107161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Qiu Y, Liu Y, Gan M, Wang W, Jiang T, Jiang Y, Lv H, Lu Q, Qin R, Tao S, Huang L, Xu X, Liu C, Dou Y, Ke K, Sun T, Jiang Y, Xu B, Jin G, Ma H, Shen H, Hu Z, Lin Y, Du J; China National Birth Cohort (CNBC) Study Group. Association of prenatal multiple metal exposures with child neurodevelopment at 3 years of age: A prospective birth cohort study. Sci Total Environ. 2024;942:173812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Mammadova F, Sultanov M, Hajiyeva A, Aichberger M, Heinz A. Translation and adaptation of the Zung Self- Rating Depression Scale for application in the bilingual Azerbaijani population. Eur Psychiatry. 2012;27 Suppl 2:S27-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Barthow C, Wickens K, Stanley T, Mitchell EA, Maude R, Abels P, Purdie G, Murphy R, Stone P, Kang J, Hood F, Rowden J, Barnes P, Fitzharris P, Craig J, Slykerman RF, Crane J. The Probiotics in Pregnancy Study (PiP Study): rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth. 2016;16:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Ostir GV, Smith PM, Smith D, Ottenbacher KJ. Reliability of the positive and negative affect schedule (PANAS) in medical rehabilitation. Clin Rehabil. 2005;19:767-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Döner S, Efe YS, Elmalı F. Turkish adaptation of the state-trait anxiety inventory short version (STAIS-5, STAIT-5). Int J Nurs Pract. 2024;30:e13304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Pavlov C, Egan K, Limbers C. Reliability and validity of the PHQ-8 in first-time mothers who used assisted reproductive technology. Hum Reprod Open. 2022;2022:hoac019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Rondung E, Oliveira S, Esteves F. Validity and reliability of the WHOQOL-BREF in a pregnant population. Health Qual Life Outcomes. 2023;21:96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Rawlins LE, Maroofian R, Cannon SJ, Daana M, Zamani M, Ghani S, Leslie JS, Ubeyratna N, Khan N, Khan H, Scardamaglia A, Cloarec R, Khan SA, Umair M, Sadeghian S, Galehdari H, Al-Maawali A, Al-Kindi A, Azizimalamiri R, Shariati G, Ahmad F, Al-Futaisi A, Rodriguez Cruz PM, Salazar-Villacorta A, Ndiaye M, Diop AG, Sedaghat A, Saberi A, Hamid M, Zaki MS, Vona B, Owrang D, Alhashem AM, Obeid M, Khan A, Beydoun A, Najjar M, Tajsharghi H, Zifarelli G, Bauer P, Hakami WS, Al Hashem AM, Boustany RN, Burglen L, Alavi S, Gunning AC, Owens M, Karimiani EG, Gleeson JG, Milh M, Salah S, Khan J, Haucke V, Wright CF, McGavin L, Elpeleg O, Shabbir MI, Houlden H, Ebner M, Baple EL, Crosby AH. Elucidating the clinical and genetic spectrum of inositol polyphosphate phosphatase INPP4A-related neurodevelopmental disorder. Genet Med. 2025;27:101278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Saha S, Saha S. The effects of prenatal dietary supplements on blood glucose and lipid metabolism in gestational diabetes mellitus patients: A systematic review and network meta-analysis protocol of randomized controlled trials. PLoS One. 2022;17:e0267854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Merced-Nieves FM, Chelonis J, Pantic I, Schnass L, Téllez-Rojo MM, Braun JM, Paule MG, Wright RJ, Wright RO, Curtin P. Prenatal trace elements mixture is associated with learning deficits on a behavioral acquisition task among young children. New Dir Child Adolesc Dev. 2022;2022:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Wei L, Huang H, Chen X, Wang X, Zhang R, Su L, Duan W, Rahman M, Golam Mostofa M, Qamruzzaman Q, Shen H, Hu Z, Wei Y, Christiani DC, Chen F. Umbilical cord serum elementomics of 52 trace elements and early childhood neurodevelopment: Evidence from a prospective birth cohort in rural Bangladesh. Environ Int. 2022;166:107370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Dou Y, Guo W, Lin Y, Jiang Y, Jiang T, Qin R, Lv H, Lu Q, Jin G, Ma H, Hu Z, Liu H, Du J; China National Birth Cohort (CNBC Study Group). Association between prenatal exposure to trace elements mixture and visual acuity in infants: A prospective birth cohort study. Chemosphere. 2023;333:138905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Perna A, Venditti N, Merolla F, Fusco S, Guerra G, Zoroddu S, De Luca A, Bagella L. Nutraceuticals in Pregnancy: A Special Focus on Probiotics. Int J Mol Sci. 2024;25:9688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Martín-Peláez S, Cano-Ibáñez N, Pinto-Gallardo M, Amezcua-Prieto C. The Impact of Probiotics, Prebiotics, and Synbiotics during Pregnancy or Lactation on the Intestinal Microbiota of Children Born by Cesarean Section: A Systematic Review. Nutrients. 2022;14:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Scrandis DA, Scrandis KS. Perinatal depression and anxiety. Nurse Pract. 2024;49:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Schalla MA, Stengel A. The role of stress in perinatal depression and anxiety - A systematic review. Front Neuroendocrinol. 2024;72:101117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Lu D, Valdimarsdóttir UA, Wei D, Chen Y, Andreassen OA, Fang F, László KD, Bränn E. Perinatal depression and risk of maternal cardiovascular disease: a Swedish nationwide study. Eur Heart J. 2024;45:2865-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Miyazaki J, Ikehara S, Tanigawa K, Kimura T, Ueda K, Ozono K, Kimura T, Kobayashi Y, Yamazaki S, Kamijima M, Sobue T, Iso H; Japan Environment, Children's Study JECS Group. Prenatal exposure to selenium, mercury, and manganese during pregnancy and allergic diseases in early childhood: The Japan Environment and Children's study. Environ Int. 2023;179:108123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Dama MH, Van Lieshout RJ. Perinatal Depression: A Guide to Detection and Management in Primary Care. J Am Board Fam Med. 2024;36:1071-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Hagatulah N, Bränn E, Oberg AS, Valdimarsdóttir UA, Shen Q, Lu D. Perinatal depression and risk of mortality: nationwide, register based study in Sweden. BMJ. 2024;384:e075462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 35. | Ji M, Li R, Xu Y. Meta-analysis of the effect of different exercise modalities in the prevention and treatment of perinatal depression. J Affect Disord. 2024;350:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Kendall-Tackett KA. Screening for Perinatal Depression: Barriers, Guidelines, and Measurement Scales. J Clin Med. 2024;13:6511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Sheyholislami H, Connor KL. Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients. 2021;13:2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Seif El Dahan K, Bejjani J, Nasrallah AA, Youssef L, Mladenovic A, Dosch L, Leone A, Jurjus A. Probiotics Properties: A Focus on Pregnancy Outcomes. Eur J Obstet Gynecol Reprod Biol. 2022;272:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Zhang H, Lv Y, Li Z, Sun L, Guo W. The efficacy of myo-inositol supplementation to prevent gestational diabetes onset: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:2249-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Santamaria A, Alibrandi A, Di Benedetto A, Pintaudi B, Corrado F, Facchinetti F, D'Anna R. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo-inositol: a secondary analysis from 3 RCTs. Am J Obstet Gynecol. 2018;219:300.e1-300.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |