Published online Mar 19, 2022. doi: 10.5498/wjp.v12.i3.410
Peer-review started: October 11, 2021
First decision: November 17, 2021
Revised: December 15, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: March 19, 2022
Processing time: 158 Days and 1.8 Hours
Oxidative stress results in the production of excess reactive oxygen species (ROS) and triggers hippocampal neuronal damage as well as occupies a key role in the pathological mechanisms of neurodegenerative disorders such as Alzheimer’s disease (AD). A recent study confirmed that magnesium had an inhibitory effect against oxidative stress-related malondialdehyde in vitro. However, whether Magnesium-L-threonate (MgT) is capable of suppressing oxidative stress damage in amyloid β (Aβ)25-35-treated HT22 cells and the AD mouse model still remains to be investigated.
To explore the neuroprotective effect of MgT against oxidative stress injury in vitro and in vivo, and investigate the mechanism.
Aβ25-35-induced HT22 cells were preconditioned with MgT for 12 h. APPswe/PS1dE9 (APP/PS1) mice were orally administered with MgT daily for 3 mo. After MgT treatment, the viability of Aβ25-35-treated HT22 cells was determined via conducting cell counting kit-8 test and the cognition of APP/PS1 mice was measured through the Morris Water Maze. Flow cytometry experiments were applied to assess the ROS levels of HT22 cells and measure the apoptosis rate of HT22 cells or hippocampal neurons. Expression of B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X (Bax), hypoxia-inducible factor (HIF)-1α, NADPH oxidase (NOX) 4, Aβ1-42 and phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) pathway proteins was quantified by Western blot.
In vitro data confirmed that Aβ25–35-induced HT22 cells had a significantly lower cell viability, higher ROS level and higher apoptosis rates compared with those of control cells (all P < 0.001). MgT prevented the Aβ25-35-triggered oxidative stress damage by elevating viability and decreasing ROS formation and apoptosis of HT22 cells (all P < 0.001). APP/PS1 mice exhibited worse cognitive performance and higher apoptosis rate of hippocampal neurons than wild-type (WT) mice (all P < 0.01). Meanwhile, significant higher expression of Aβ1-42 and NOX4 proteins was detected in APP/PS1 mice than those of WT mice (both P < 0.01). MgT also ameliorated the cognitive deficit, suppressed the apoptosis of hippocampal neuron and downregulated the expression of Aβ1-42 and NOX4 proteins in APP/PS1 mouse (all P < 0.05). Moreover, MgT intervention significantly downregulated HIF-1α and Bax, upregulated Bcl-2 and activated the PI3K/Akt pathway both in vitro and in vivo (all P < 0.05).
MgT exhibits neuroprotective effects against oxidative stress and hippocampal neuronal apoptosis in Aβ25-35-treated HT22 cells and APP/PS1 mice.
Core Tip: The dysfunction of oxidative stress is considered to stimulate the production of reactive oxygen species and induce hippocampal neuron damage which are the significant hallmarks of neurodegenerative diseases such as Alzheimer’s disease. Recent studies have explored the in vitro anti-malondialdehyde effect of magnesium. However, the potential neuroprotective effect of Magnesium-L-threonate (MgT) against oxidative stress remains to be explored. Our study demonstrated that MgT exhibited neuroprotective effects on suppressing oxidative stress and hippocampal neuronal apoptosis in vitro and in vivo, suggesting the promising therapeutic potential of MgT in oxidative stress-associated neurodegenerative disorders.
- Citation: Xiong Y, Ruan YT, Zhao J, Yang YW, Chen LP, Mai YR, Yu Q, Cao ZY, Liu FF, Liao W, Liu J. Magnesium-L-threonate exhibited a neuroprotective effect against oxidative stress damage in HT22 cells and Alzheimer’s disease mouse model. World J Psychiatry 2022; 12(3): 410-424
- URL: https://www.wjgnet.com/2220-3206/full/v12/i3/410.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i3.410
As a progressive neurodegenerative disease, Alzheimer’s disease (AD) occupies most cases of dementia, and it is clinically characterized by the deterioration of cognitive ability and brings a massive burden on AD patients’ survival quality and social medical cost[1]. Although the pathological mechanism of AD is still incompletely elucidated, it was reported that oxidative stress occupied a key role in the pathogenic mechanism of this disease[2]. Numerous researches indicated that oxidative stress was a vital issue during the development of the neurodegenerative diseases, including AD, amyotrophic lateral sclerosis and so on. Oxidative stress could also accelerate amyloid β (Aβ) aggregation and induce neuronal apoptosis in the brain tissues, especially in the hippocampus[3-7]. Hence, the exploration of antioxidative stress agents suggests a promising therapeutic option for achieving a neuroprotective effect against neurodegenerative diseases associated with hippocampal neuronal damage.
Magnesium is one of the essential cations in the intracellular environment and is only second to potassium in concentration. Magnesium is involved in the synthesis of many enzymes that are important in various biological processes[8]. The concentration of brain magnesium is decreased in AD patients when compared with control subjects[9]. Based on this finding, recent research has assessed the application of the novel magnesium compound Magnesium-L-threonate (MgT), which increases brain magnesium concentration after oral administration, for ameliorating AD-associated pathological changes[10-12]. Although MgT exhibits a protective effect against synaptic damage in an AD mouse model[11], its effects on oxidative stress and hippocampal neuronal damage remain unexplored. It has been recently confirmed that magnesium has an inhibitory effect against oxidative-stress-related malondialdehyde (MDA) in vitro[13,14]; therefore, it has become of interest to investigate whether MgT is capable of suppressing oxidative stress damage in vivo. Therefore, this research explored the potential protective effects of MgT against oxidative stress and neuronal injury in Aβ25-35-treated HT22 cells and in APPswe/PS1dE9 (APP/PS1) mouse hippocampus.
For the in vitro experiment, in order to evaluate the capacity of MgT against Aβ25-35-triggered oxidative stress and neuronal damage and explore the related mechanism, HT22 cell was chosen as the cell model, and it is well known as the immortalized murine hippocampal neuron[15]. We also explored the in vivo potential neuroprotective effects of MgT against oxidative stress, Aβ production and hippocampal neuronal damage in APP/PS1 mouse, a typical animal model of AD[16].
MgT was acquired from Macklin (Shanghai, China); Aβ25-35 was purchased from MedChemExpress LLC (New Jersey, USA); The cell counting kit-8 (CCK-8) detection kit was provided from APExBIO Technology LLC (Houston, USA); A fluorescein isothiocyanate-annexin V/propidium iodide apoptosis agent was obtained from BD (New Jersey, USA); A reactive oxygen species (ROS) testing kit was supplied from Beyotime Biotechnology (Shanghai, China); The antibodies were purchased from Cell Signaling Technology (Danvers, USA), BioLegend (San Diego, USA) and Abcam (Cambridge, USA); The rest of experimental materials were bought from Thermo Fisher Scientific (Waltham, USA), CWBIO (Beijing, China) and Gibco (New York, USA).
Based on the previously described method, HT22 cell culture and differentiation procedures were carried out[17,18]. Briefly, HT22 cell was cultured in the normal cell culture medium and then differentiated in N2 supplement-containing neurobasal medium for 1 d prior to drug administrations. According to the previous research[19], when it was exposed to 40 μmol/L Aβ25-35 for 1 d, the viability of HT22 cell would significantly decrease. Therefore, this study chose 40 μmol/L as the appropriate concentration of Aβ25-35 administration. Before Aβ25-35 treatment, the dilution of Aβ25-35 was carried out by using sterile saline and then it was kept at 37°C for 7 d for peptide pre-aging, as reported previously[19]. In order to investigate whether MgT could be applied to inhibit the oxidative stress damage triggered by Aβ25-35 administration, HT22 cell was preconditioned with or without 50 μmol/L MgT for 12 h prior to be processed with 40 μmol/L Aβ25-35 for 1 d.
The viability was assessed via the CCK-8 experiment for HT22 cell exposed to Aβ25-35 and MgT. Briefly, after different drug treatments for the three groups, each well of HT22 cells was incubated with 10 μL CCK-8 and the absorbance value was acquired at 450 nm by using an absorbance reader (California, USA).
Total intracellular ROS generation was detected using an oxidation-sensitive fluorogenic dichloro-dihydro-fluorescein diacetate (DCFH-DA) probe and further quantified with flow cytometry, as described previously[20]. Briefly, after drug administration, HT22 cells were washed and reacted with 10 μmol/L DCFH-DA probe during this experiment procedure. The cell samples were collected and finally detected using the flow cytometer (BD, USA). The percentages of DCFH-DA labeled cells represented the intracellular ROS level.
APP/PS1 male mice and wild-type (WT) litter-mate male mice were acquired from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). The animal experiment received the approbation from the local animal ethical and welfare committee. All protocols were designed to minimize discomfort or pain to the mice. The mice were housed in a specific-pathogen-free environment (23 ± 1 °C, 12 h/12 h light/dark, 50% humidity) with free access to water and food.
In the animal experiment procedure, 6-mo-old mice weighing 33-35 g were set as three groups (three mice per group): MgT-treated APP/PS1 mice (registered as ‘TG + MgT group’), control APP/PS1 mice (TG group) and control WT mice (WT group). MgT-treated mice received daily administration of MgT (910 mg/kg/d) via drinking water for 3 mo on the basis of the previously described method[11]. The remaining mice (TG and WT groups) were treated with drinking water. After drug treatment, mice were used for the Morris Water Maze test and then killed under deep anesthesia (intraperitoneal injection, 150 mg/kg pentobarbital sodium) to collect the hippocampal tissues for further biochemical investigations.
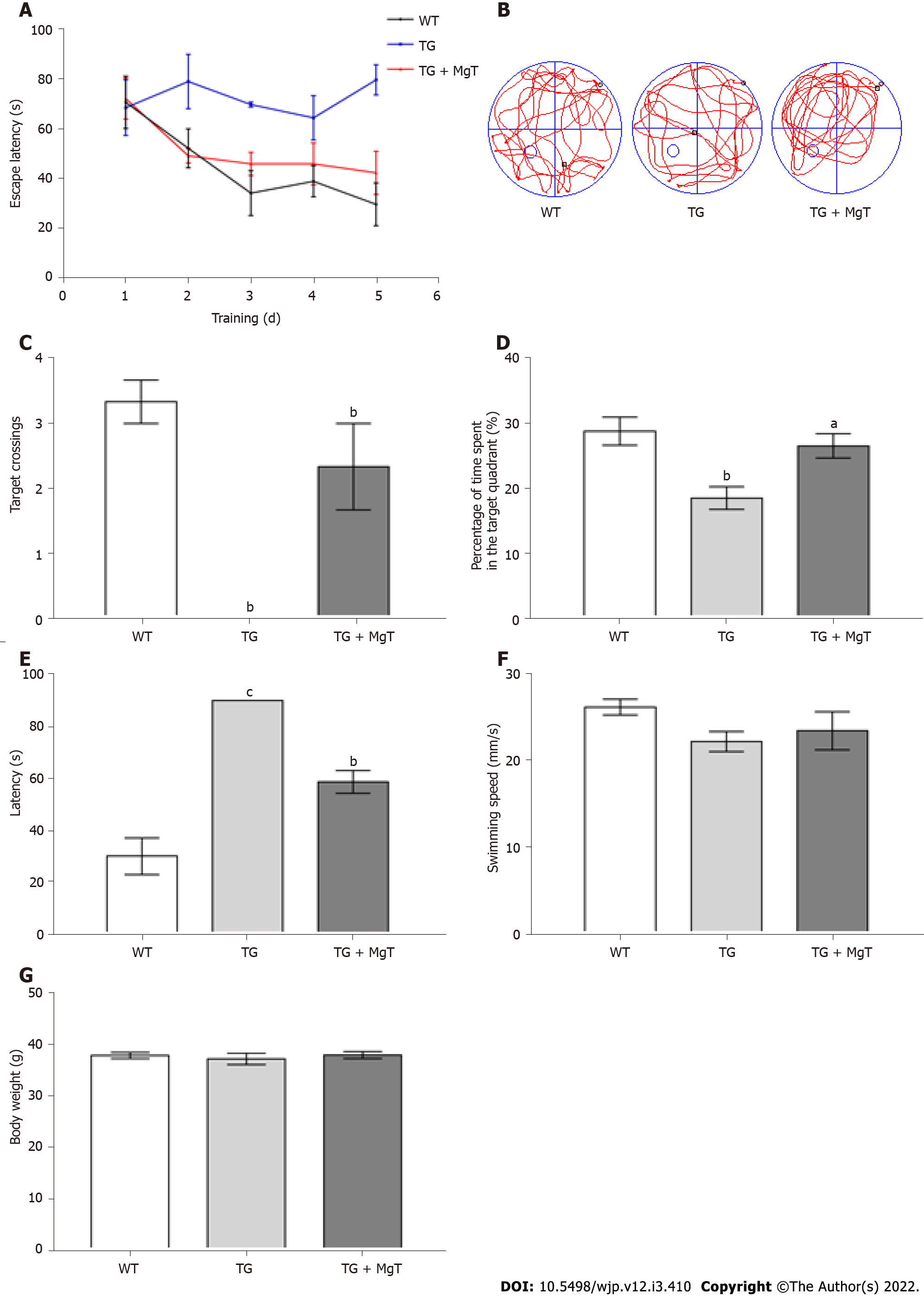
All mice were behaviorally tested for cognitive ability using the Morris water maze after 3 mo of treatments with or without MgT, as previously described[1]. At the beginning, each mouse was pretrained in this water maze with the visible platform for 1 d. Subsequently, all mice received the hidden platform training for 5 d (4 trails per day, 90 s per trial). For each trail, the mice were released from four starting quadrant positions in a different order and swam for 90 s. If the exploration time of mouse was less than 90 s, the trails would stop and the time to find the hidden platform was recognized as escape latency. If the mouse missed the setting time, it would be guided to arrive in the platform and the escape latency of 90 s was recorded. For each mouse, before the statistical analysis was carried out, the escape latencies of four trails were averaged. Finally, the platform was taken out and the mice were tested on a 90 s probe test at 24 h after the hidden platform training. After each trail, mice should be dried with a clean towel and put on an electric blanket to keep their body warm. For each mouse, the latency to arrive in the removed platform, the percentage of the time spent in the target quadrant (the quadrant where the platform was previously settled) and the number of times crossed the target position (the previous location of the platform) were measured during the probe test.
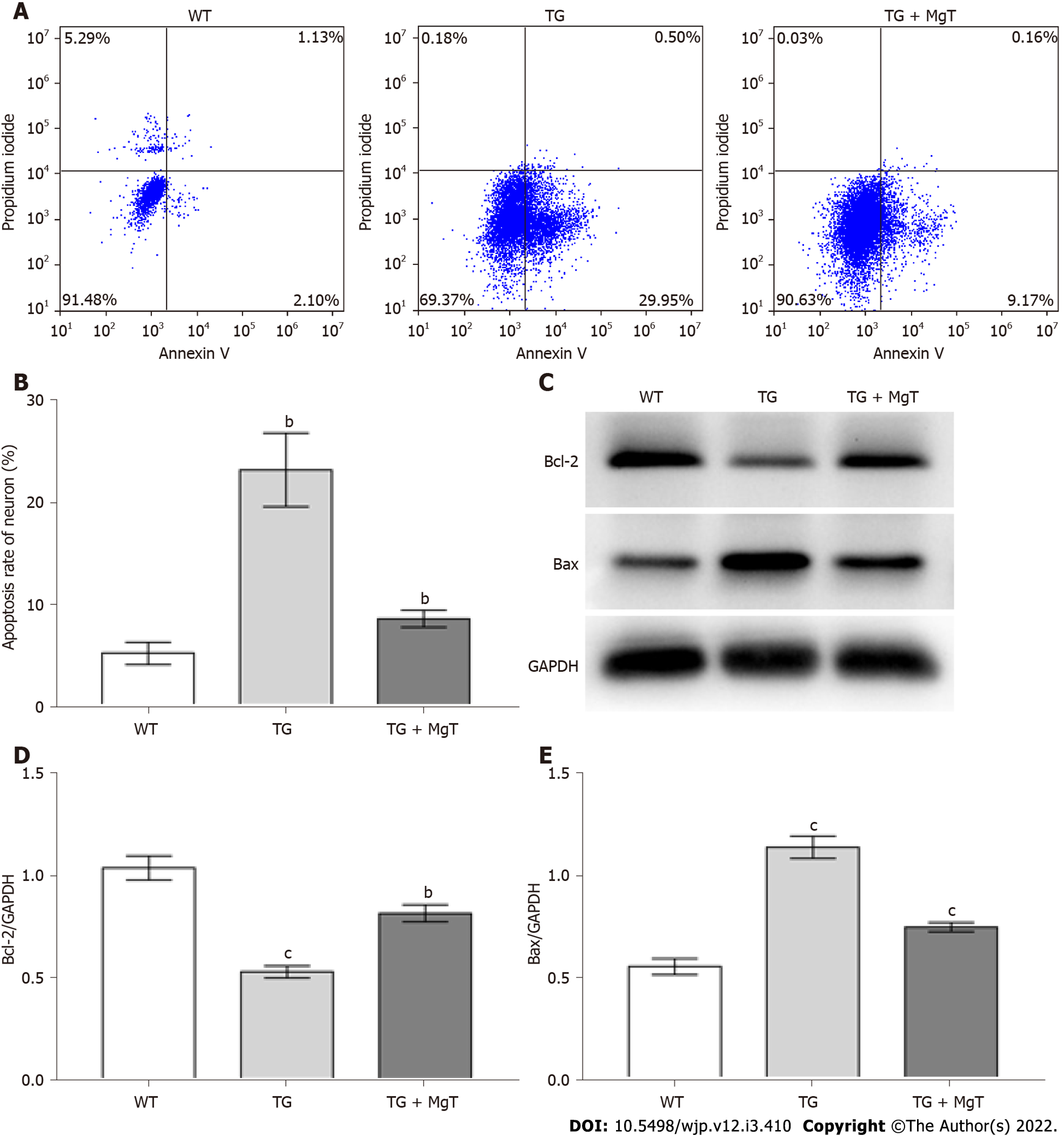
A fluorescein isothiocyanate-annexin V/propidium iodide testing agent was utilized to measure the apoptosis rate of HT22 cells. After drug administration, HT22 cells were washed, trypsin digested and incubated with this testing agent before flow cytometry. The allophycocyanin-annexin V/propidium iodide kit was also applied to assess the apoptosis rate of hippocampal neurons. After isolation of the hippocampal tissue, a single cell suspension was prepared, stained with anti-NeuN antibody, followed by appropriate Alexa-Fluor-488-conjugated secondary antibody, and finally detected with this kit for flow cytometric examination.
The proteins in HT22 cells or hippocampal tissue were quantified, probed with a series of specific primary antibodies and visualized with a Digital Imaging machine (Gel Logic, Rochester, New York, USA). The relative protein density was quantified as previously described[21]. The involved primary antibodies were diluted to 1:1000, except for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000).
Significance was measured using one-way analysis of variance with Fisher’s least significant difference tests for multiple comparison using Prism 6 software (Graphpad, San Diego, CA, USA). For each group, data were shown as mean ± SE and P < 0.05 indicated significant differences.
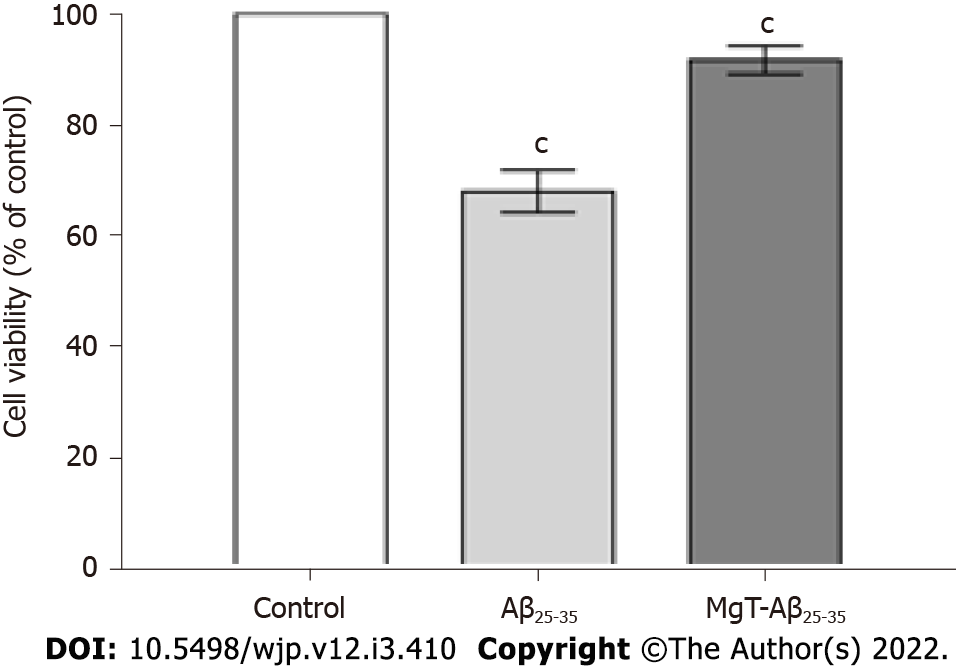
As demonstrated in Figure 1, Aβ25-35-exposed cells showed obvious lower cell viability than control cells (P < 0.001). Compared with Aβ25-35-exposed cells, the viability of MgT-Aβ25-35-exposed cells was obviously elevated (P < 0.001). Thus, all data of the CCK8 test illustrated that the pretreatment with MgT inhibited the cytotoxicity in the Aβ25-35-exposed HT22 cell model.
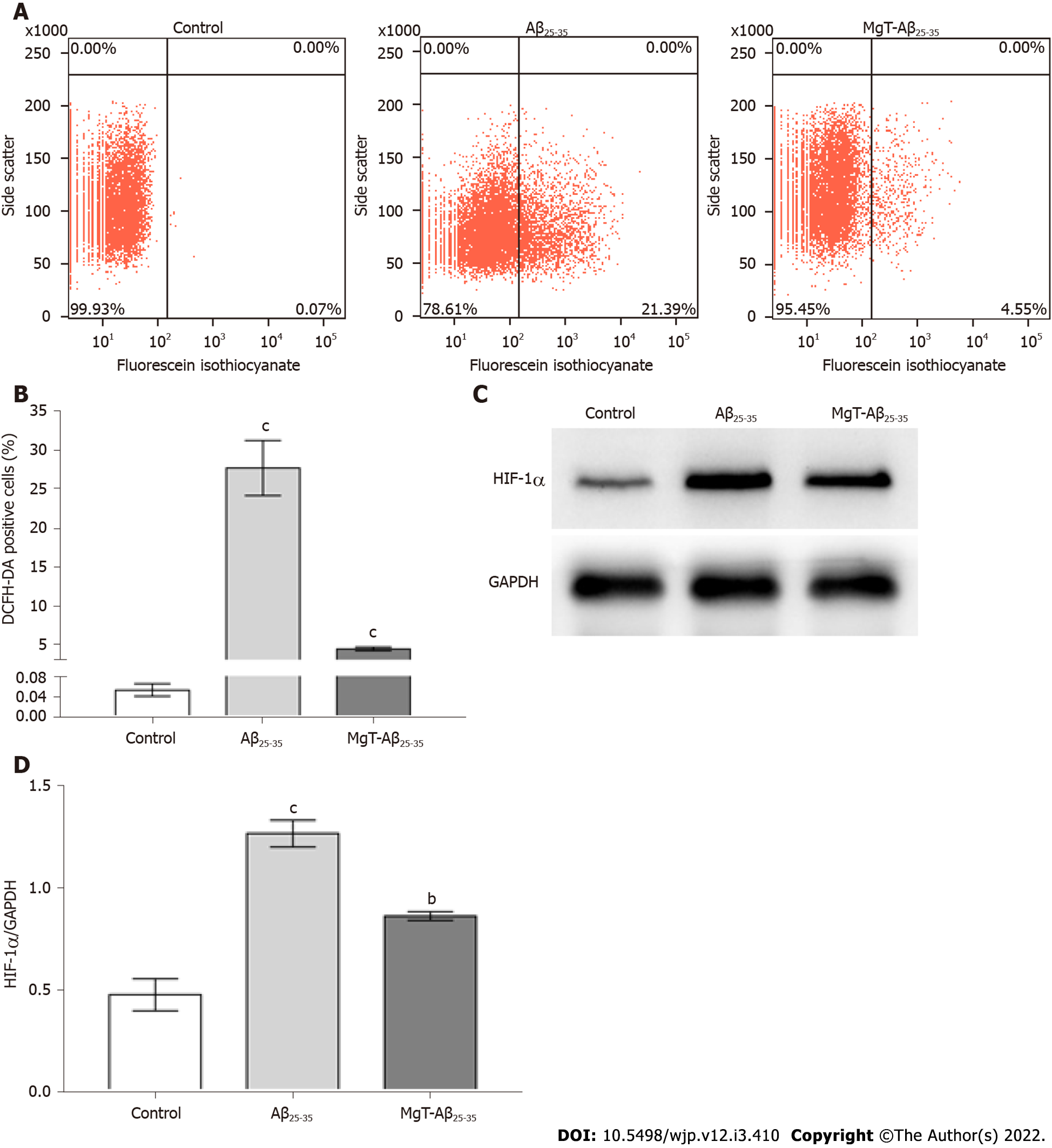
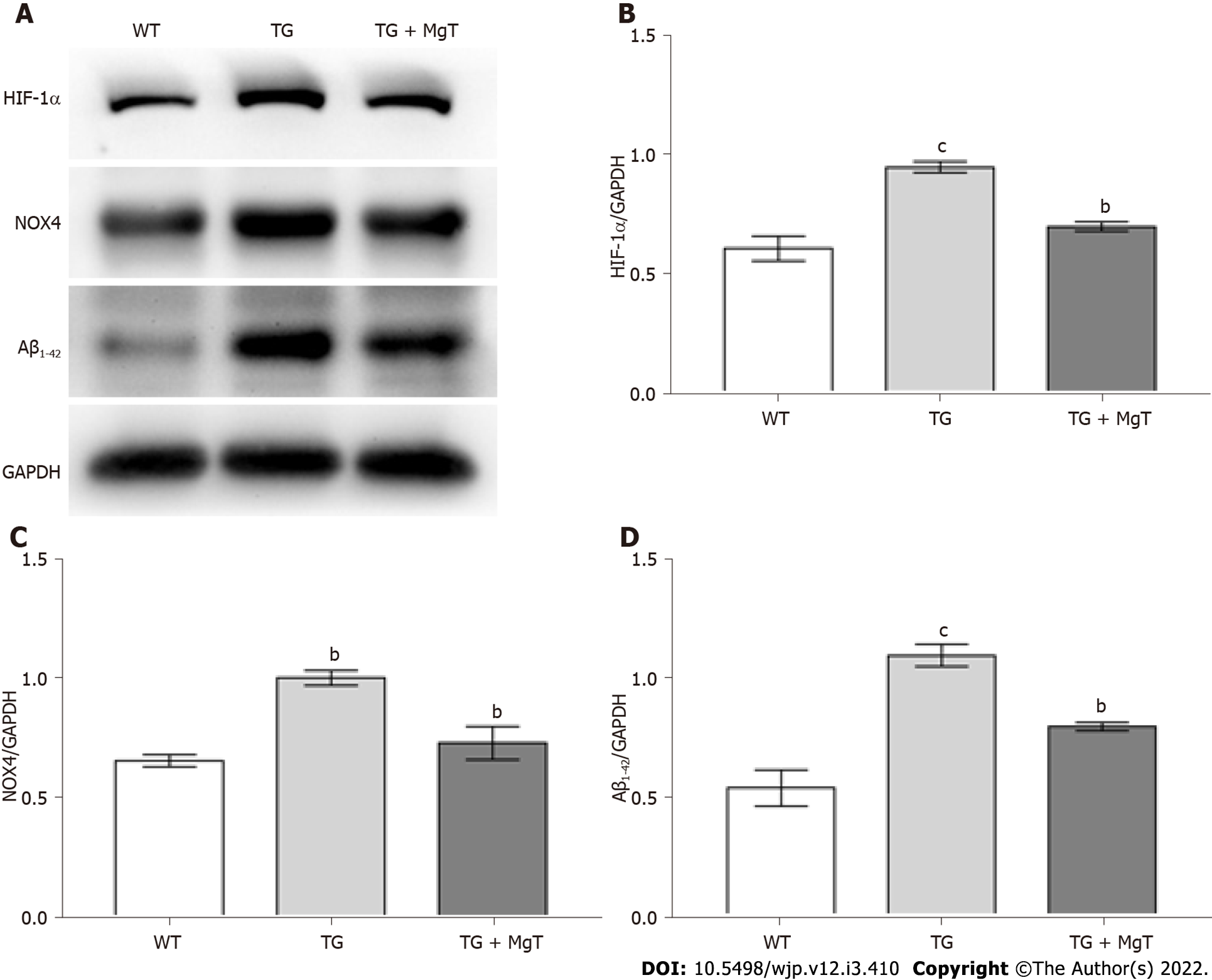
Intracellular ROS level measured by the DCFH-DA test exhibited an obvious increase in Aβ25-35-administrated cells vs control cells (P < 0.001). Compared with Aβ25-35-treated cells, the ROS level was remarkably decreased in MgT-Aβ25-35-treated cells (P < 0.001) (Figure 2A and B). As indicated in Figure 2C and D, hypoxia-inducible factor (HIF)-1α protein expression was increased in the Aβ25-35-exposed HT22 cells (P < 0.001), which was effectively downregulated by MgT treatment (P < 0.01).
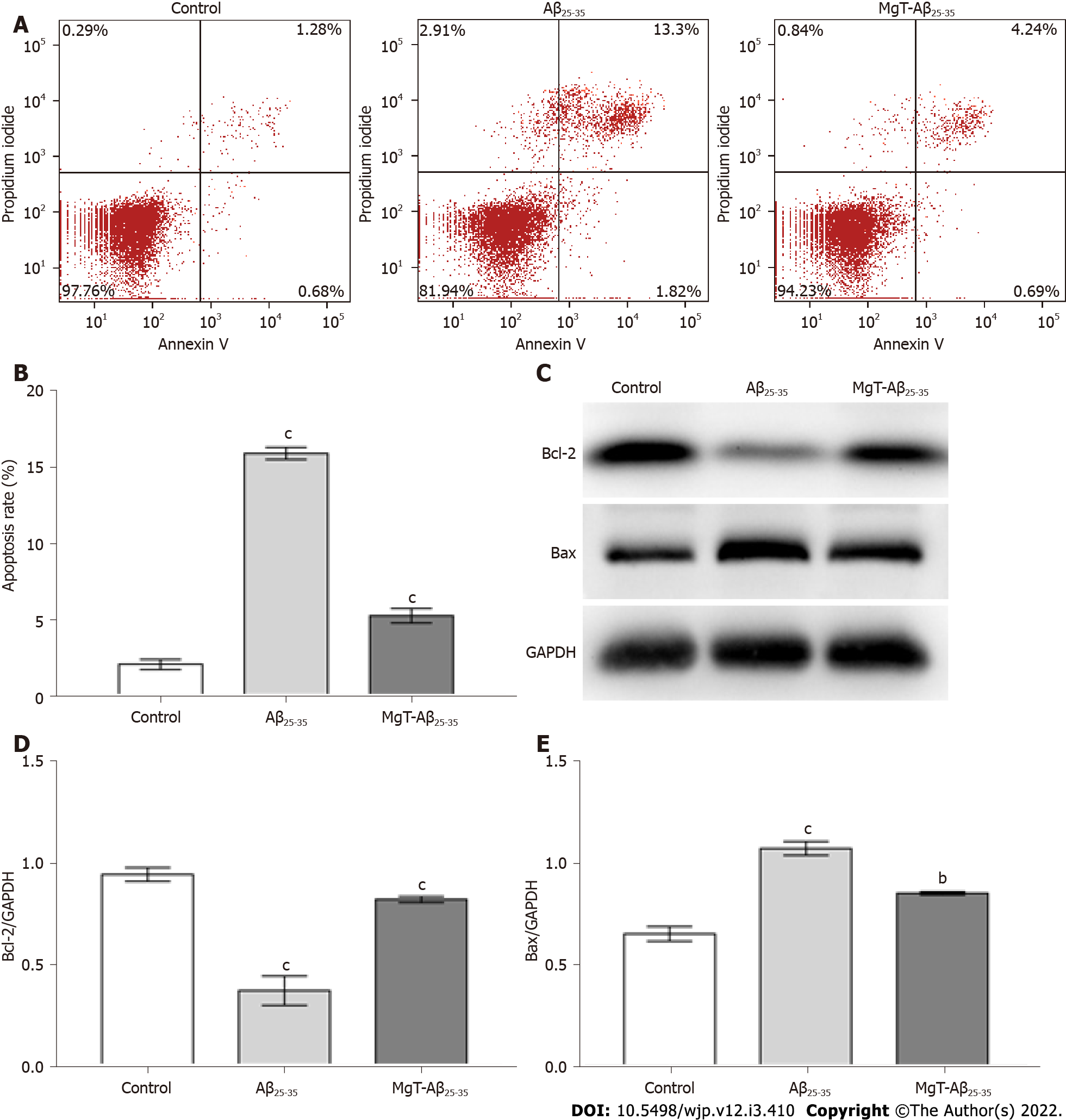
The effects of MgT treatment in regulating apoptosis and apoptotic-associated proteins expression were also measured, aiming to further assess the neuroprotective effect of MgT against neuronal damage in the Aβ25-35-treated HT22 cell. As displayed in Figure 3A and B, Aβ25-35-administrated group owned a higher apoptosis rate of HT22 cells than control group (P < 0.001), and the apoptosis rate was obviously reduced after MgT intervention (P < 0.001). What’s more, the Aβ25-35-administrated group had a lower B-cell lymphoma 2 (Bcl-2) protein (an anti-apoptotic molecule[22]) expression level and a higher Bcl-2-associated X (Bax) protein (a pro-apoptotic molecule[23]) expression level than control group (both P < 0.001), while MgT treatment effectively promoted Bcl-2 expression (P < 0.001) and blocked Bax expression (P < 0.01) (Figure 3C-E).
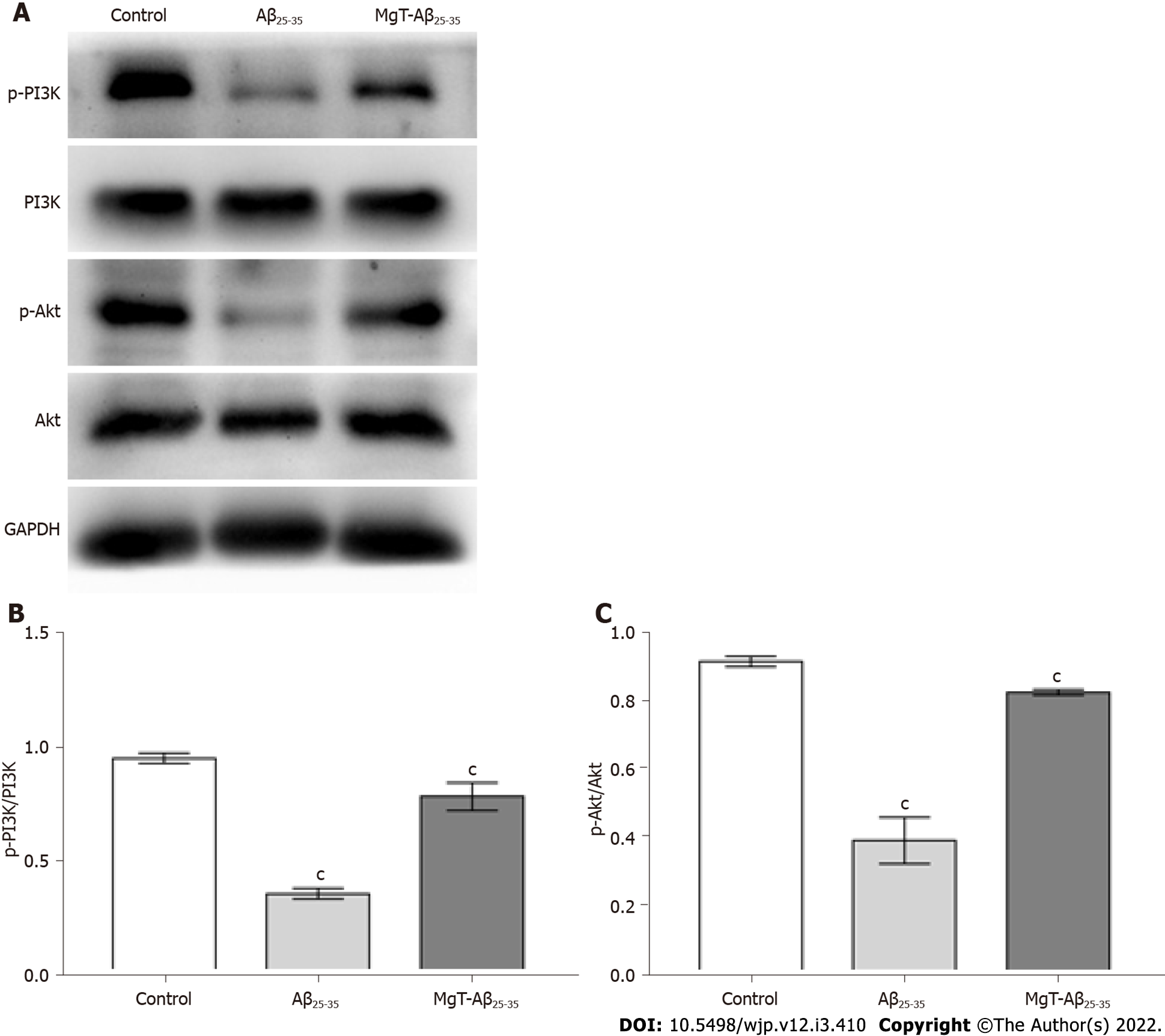
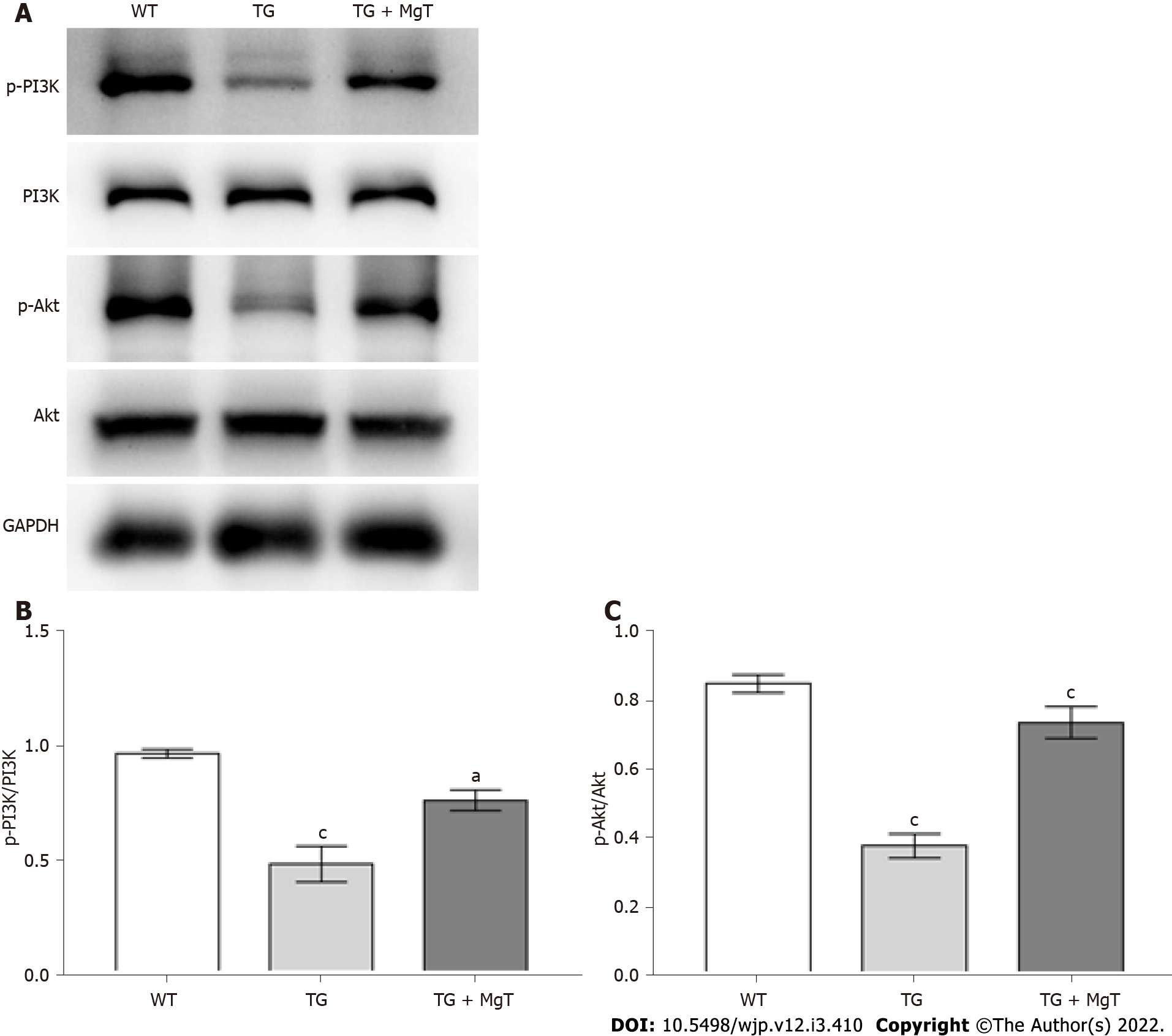
The effects of MgT administration on regulating PI3K/Akt pathway, which was a classical pathway related to cell apoptosis[24], were also detected. As shown in Figure 4, Aβ25-35-exposed cells showed lower ratios of phosphorylated (p)-PI3K/PI3K and p-Akt/Akt than control cells (both P < 0.001). After MgT administration, these two ratios were significantly upregulated (both P < 0.001).
The behavioral performance was recorded with the Morris water maze method to assess the effect of MgT intervention against memory deficit in AD mouse. Compared with WT group, TG group exhibited prolonged escape latency, while the escape latency was shortened in the TG + MgT group vs TG group (Figure 5A). The number of platform crossings and the percentage of target quadrant exploration time were significantly decreased in the TG group vs WT group (both P < 0.01), while these two cognitive scores were increased after MgT administration (crossings, P < 0.01; target quadrant exploration time; P < 0.05) (Figure 5B–D). The TG group had a longer latency to locate the removed platform than WT group (P < 0.001), and the latency was shorter in the TG + MgT group vs TG group (P < 0.01) (Figure 5E). Nevertheless, no obvious differences regarding the swimming speed and body weight were discovered among all groups (Figure 5F and G).
Compared with WT group, elevated expression of HIF-1α, NOX4 (a reliable marker of oxidative stress[25,26]) and Aβ1–42 proteins was seen in the TG group (HIF-1α and Aβ1–42, P < 0.001; NOX4, P < 0.01), while these indexes were all decreased in the TG + MgT group vs TG group (all P < 0.01) (Figure 6).
The effects of MgT administration in ameliorating neuronal apoptosis and regulating the expression of apoptotic-associated proteins were also examined to further demonstrate the neuroprotective effect of MgT on APP/PS1 mouse hippocampus. As listed in Figure 7A and B, the apoptosis rate of hippocampal neuron was elevated in the TG group vs WT group (P < 0.01), while TG + MgT group had a significant lower apoptosis rate than TG group (P < 0.01). Moreover, the downregulation of Bcl-2 expression and the upregulation of Bax expression were noticed in TG group vs WT group (both P < 0.001), while MgT treatment promoted Bcl-2 expression (P < 0.01) and suppressed Bax expression (P < 0.001) (Figure 7C-E).
The effect of MgT administration on the PI3K/Akt pathway was also detected in the in vivo experiment of this study. As shown in Figure 8, p-PI3K/PI3K and p-Akt/Akt ratios were reduced in TG group vs WT group (both P < 0.001), while these two ratios were obviously elevated after MgT administration (p-PI3K/PI3K ratio, P < 0.05; p-Akt/Akt ratio, P < 0.001).
It was demonstrated that oxidative stress could trigger neuronal damage in the hippocampus tissues of the brain, which is the vital pathological mechanism of neurodegenerative diseases, including AD[27]. Recently, the findings of the in vitro study certified that extracellular magnesium concentration could act as a regulator that effectively influenced the level of MDA, a pathological marker closely associated with oxidative stress damage[13,14]. Several researches indicated that MgT could elevate the level of brain magnesium via oral administration[10,12]. Therefore, this research attempted to validate the effects of MgT against oxidative stress and neuronal damage in the Aβ25-35-treated HT22 cell and the hippocampus of APP/PS1 mouse, and investigated the involved mechanism.
Growing evidences have proved that during the pathological progression of neurogenerative disease, such as AD, abnormal oxidative stress resulted in the generation of ROS and hippocampal neuronal apoptosis thus leading to the deterioration of brain function[27,28]. The in vitro experiment part of this study, oxidative stress, was detected by assessing the ROS level and cell apoptosis was detected by measuring the apoptosis rate and quantifying the expression of apoptosis-associated proteins. The in vitro data revealed that MgT remarkably blocked the oxidative stressors Aβ25-35-induced[28] oxidative damage and apoptosis in the HT22 cells as proved by the elevation of cell viability, the reduction of ROS generation, the decrease of apoptosis rate and Bax expression, and the upregulation of Bcl-2 expression after MgT administration. In line with these in vitro results, the in vivo data confirmed the suppressive effect of MgT treatment against oxidative stress-triggered hippocampal neuronal damage via downregulating the expression level of the oxidative stress marker NOX4 protein and inhibiting the apoptosis of the hippocampal neuron in the AD mouse model. Additionally, it has been confirmed that the increased ROS induced by oxidative stress can lead to abnormal production of Aβ which can worsen the pathological process of AD[29]. In our in vivo study, the measurement of Aβ1–42 expression by western blotting confirmed the inhibitory effect of MgT against Aβ production in the AD mouse model.
Numerous researches verified the key role of HIF-1α in the mediation of oxygen homeostasis within the cellular environment. A close relationship was discovered between HIF-1α level and oxygen balance: HIF-1α level remained low under the physiological situation while it was significantly elevated under the hypoxia condition[30,31]. Moreover, recent study revealed that the high glucose-triggered oxidative stress accelerated Aβ aggregation via the regulation of the ROS/HIF-1α mechanism in vitro, which supported a strong relationship between ROS and HIF-1α, and that the crosstalk between the two could deteriorate the Aβ production under abnormal oxidative stress condition[32]. Another research also indicated the crosstalk between HIF-1α and ROS in RAW 264.7 cell model[33]. Therefore, the effect of MgT administration on HIF-1α expression was also investigated. The observations from in vivo and in vitro investigations indicated that MgT significantly suppressed the HIF-1α overexpression in Aβ25-35-treated HT22 cells and APP/PS1 mice.
PI3K/Akt pathway is an important cellular pathway occupying a pivotal role in the mediation of cell apoptosis[34]. A recent study demonstrated that Rotundifuran-induced ROS production could lead to cell apoptosis via suppressing the PI3K/Akt pathway in the cervical cancer cell model[35]. Another study also showed that inhibition of apoptosis was correlated with the ROS-mediated PI3K/Akt pathway in a streptozotocin-treated INS-1 cell model[24]. Based on the above findings, dysregulation of the PI3K/Akt signaling pathway supports the relationship between oxidative stress and apoptosis. The present experimental procedure also detected the effect of MgT administration on the PI3K/Akt pathway. According to the results from Western blotting, the PI3K/Akt pathways were downregulated in Aβ25-35-administrated HT22 cells and APP/PS1 mice, which were restored by MgT administration.
In light of the findings that MgT administration exhibited neuroprotective effects against oxidative stress and hippocampal neuronal apoptosis in this AD mouse model, which were the vital pathological mechanisms underlying the cognitive deficit of AD[3,36], the cognitive ability of MgT-treated APP/PS1 mouse was measured. In this experiment, the results acquired from the Morris water maze test confirmed that MgT treatment ameliorated the cognitive deficit in this AD animal model, but the further mechanism underlying the memory protective effect of MgT needs to be further investigated.
There are several limitations in this experiment. In this study, APP/PS1 mice were applied as the animal model of AD. Although this animal model was a typical and common model of AD and it could be employed to mimic the cognitive impairment and pathological changes of AD[16], it might not reflect all types of this disease. Therefore, it is necessary to conduct further explorations to validate the above-mentioned effects of MgT on other types of Alzheimer’s disease, animal models of other neurodegenerative diseases and clinical trials.
It can be demonstrated in this study that MgT intervention has neuroprotective effects against oxidative stress and hippocampal neuronal damage in Aβ25–35-treated HT22 cells and AD mouse model. Our study suggests a promising therapeutic agent for the amelioration of oxidative stress and hippocampal neuronal damage-associated neurodegenerative disorders.
The increasing prevalence of Alzheimer’s disease (AD) in the elderly population has posed a huge financial and medical burden on the society. Effective methods to block the progression of the cognitive deterioration in AD patients are urgently required. As oxidative stress accounts for a pivotal role in the pathological mechanism of neurodegenerative diseases, including AD, anti-oxidative stress treatments may provide a promising therapeutic direction. Recent study had explored the anti-malondialdehyde effect of magnesium in vitro, however the potential anti-oxidative stress damage effect of Magnesium-L-threonate (MgT) still remains to be verified.
This research investigated the suppressive effect of MgT against oxidative stress injury, thus developing a therapeutic reference basis for the future explorations.
This research aimed to determine the neuroprotective effect of MgT against oxidative stress damage and explore the related mechanism which may bring a research foundation for the feasibility of MgT.
As the cell and animal models, amyloid β (Aβ)25-35-treated HT22 cells and APPswe/PS1dE9 (APP/PS1) mice were treated with MgT administration. After the MgT administration, cell counting kit-8 detection was applied to analysis the viability of HT22 cells and the Morris Water Maze test was used to record the cognition of APP/PS1 mice. Reactive oxygen species (ROS) production of HT22 cells and cell apoptosis of both models were all quantified by using the flow cytometry assay. The expression of hypoxia-inducible factor (HIF)-1α, NADPH oxidase (NOX) 4, Aβ1-42, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X (Bax) and phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) pathway proteins was quantified by Western blotting.
MgT effectively suppressed the HT22 cellular injury triggered by Aβ25-35-induced oxidative stress by elevating the viability, blocking the ROS formation and downregulating HIF-1α. MgT significantly ameliorated the impaired cognitive performance of APP/PS1 mouse and inhibited the upregulation of Aβ1-42, NOX4 and HIF-1α protein expression. In addition, MgT obviously suppressed the cell apoptosis, regulated apoptotic-related proteins and upregulated the PI3K/Akt pathway in both models. In future research, further explorations are required to confirm the above-mentioned effects of MgT in more disease models.
This study demonstrates the protective effect of MgT against oxidative stress injury in Aβ25-35-treated HT22 cells and APP/PS1 mice.
This study provides a promising therapeutic agent to ameliorate the oxidative stress damage-associated neurodegenerative diseases. More investigations to demonstrate this effect of MgT on other types of Alzheimer’s disease, in vivo models of other neurodegenerative diseases and clinical experiments are required in further research.
The authors would like to acknowledge Xi-Yan Wang for editing assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aguzzi A, Switzerland; Al-Shahi Salman R, United Kingdom S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Fan S, Zheng Y, Liu X, Fang W, Chen X, Liao W, Jing X, Lei M, Tao E, Ma Q, Zhang X, Guo R, Liu J. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer's disease. Drug Deliv. 2018;25:1091-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 2. | Kola A, Dudek D, Valensin D. Metal Complexation Mechanisms of Polyphenols Associated to Alzheimer's Disease. Curr Med Chem. 2021;28:7278-7294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Sieteiglesias V, González-Burgos E, Bermejo-Bescós P, Divakar PK, Gómez-Serranillos MP. Lichens of Parmelioid Clade as Promising Multitarget Neuroprotective Agents. Chem Res Toxicol. 2019;32:1165-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Tang KS. The potential role of nanoyttria in alleviating oxidative stress biomarkers: Implications for Alzheimer's disease therapy. Life Sci. 2020;259:118287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Montine TJ, Montine KS, McMahan W, Markesbery WR, Quinn JF, Morrow JD. F2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid Redox Signal. 2005;7:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer's disease. J Neurochem. 2006;96:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Mecocci P, Boccardi V, Cecchetti R, Bastiani P, Scamosci M, Ruggiero C, Baroni M. A Long Journey into Aging, Brain Aging, and Alzheimer's Disease Following the Oxidative Stress Tracks. J Alzheimers Dis. 2018;62:1319-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 8. | de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 1018] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 9. | Cilliler AE, Ozturk S, Ozbakir S. Serum magnesium level and clinical deterioration in Alzheimer's disease. Gerontology. 2007;53:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Slutsky I, Abumaria N, Wu LJ, Huang C, Zhang L, Li B, Zhao X, Govindarajan A, Zhao MG, Zhuo M, Tonegawa S, Liu G. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Li W, Yu J, Liu Y, Huang X, Abumaria N, Zhu Y, Huang X, Xiong W, Ren C, Liu XG, Chui D, Liu G. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer's disease mouse model. Mol Brain. 2014;7:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 12. | Abumaria N, Yin B, Zhang L, Li XY, Chen T, Descalzi G, Zhao L, Ahn M, Luo L, Ran C, Zhuo M, Liu G. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J Neurosci. 2011;31:14871-14881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Altura BM, Gebrewold A, Zhang A, Altura BT. Low extracellular magnesium ions induce lipid peroxidation and activation of nuclear factor-kappa B in canine cerebral vascular smooth muscle: possible relation to traumatic brain injury and strokes. Neurosci Lett. 2003;341:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Zhao H, Zhang X, Zhang B, Qu X. Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities. Open Life Sci. 2021;16:719-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Fang WL, Zhao DQ, Wang F, Li M, Fan SN, Liao W, Zheng YQ, Liao SW, Xiao SH, Luan P, Liu J. Neurotropin® alleviates hippocampal neuron damage through a HIF-1α/MAPK pathway. CNS Neurosci Ther. 2017;23:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 16. | Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Liu J, Li L, Suo WZ. HT22 hippocampal neuronal cell line possesses functional cholinergic properties. Life Sci. 2009;84:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Zhao ZY, Luan P, Huang SX, Xiao SH, Zhao J, Zhang B, Gu BB, Pi RB, Liu J. Edaravone protects HT22 neurons from H2O2-induced apoptosis by inhibiting the MAPK signaling pathway. CNS Neurosci Ther. 2013;19:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Fan S, Zhang B, Luan P, Gu B, Wan Q, Huang X, Liao W, Liu J. PI3K/AKT/mTOR/p70S6K Pathway Is Involved in Aβ25-35-Induced Autophagy. Biomed Res Int. 2015;2015:161020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Huang C, Gan D, Luo F, Wan S, Chen J, Wang A, Li B, Zhu X. Interaction Mechanisms Between the NOX4/ROS and RhoA/ROCK1 Signaling Pathways as New Anti- fibrosis Targets of Ursolic Acid in Hepatic Stellate Cells. Front Pharmacol. 2019;10:431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Zheng Y, Fang W, Fan S, Liao W, Xiong Y, Liao S, Li Y, Xiao S, Liu J. Neurotropin inhibits neuroinflammation via suppressing NF-κB and MAPKs signaling pathways in lipopolysaccharide-stimulated BV2 cells. J Pharmacol Sci. 2018;136:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Ji KY, Kim KM, Kim YH, Shim KS, Lee JY, Kim T, Chae S. Serum Starvation Sensitizes Anticancer Effect of Anemarrhena asphodeloides via p38/JNK-Induced Cell Cycle Arrest and Apoptosis in Colorectal Cancer Cells. Am J Chin Med. 2021;49:1001-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Alzain AA, Brisson L, Delaye PO, Pénichon M, Chadet S, Besson P, Chevalier S, Allouchi H, Mohamed MA, Roger S, Enguehard-Gueiffier C. Bioinspired imidazo[1,2-a:4,5-c']dipyridines with dual antiproliferative and anti-migrative properties in human cancer cells: The SAR investigation. Eur J Med Chem. 2021;218:113258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Wang J, Dong Z, Lou L, Yang L, Qiu J. MiR-122 Participates in Oxidative Stress and Apoptosis in STZ-Induced Pancreatic β Cells by Regulating PI3K/AKT Signaling Pathway. Int J Endocrinol. 2021;2021:5525112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Fakih D, Zhao Z, Nicolle P, Reboussin E, Joubert F, Luzu J, Labbé A, Rostène W, Baudouin C, Mélik Parsadaniantz S, Réaux-Le Goazigo A. Chronic dry eye induced corneal hypersensitivity, neuroinflammatory responses, and synaptic plasticity in the mouse trigeminal brainstem. J Neuroinflammation. 2019;16:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Balkrishna A, Rustagi Y, Bhattacharya K, Varshney A. Application of Zebrafish Model in the Suppression of Drug-Induced Cardiac Hypertrophy by Traditional Indian Medicine Yogendra Ras. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1335] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 28. | Tan MA, Zakharova E, An SSA. Diaportheone A Analogues Instigate a Neuroprotective Effect by Protecting Neuroblastoma SH-SY5Y Cells from Oxidative Stress. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 469] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 30. | Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, Xu H, Zhang YW. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem. 2007;282:10873-10880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 31. | Li RL, He LY, Zhang Q, Liu J, Lu F, Duan HX, Fan LH, Peng W, Huang YL, Wu CJ. HIF-1α is a Potential Molecular Target for Herbal Medicine to Treat Diseases. Drug Des Devel Ther. 2020;14:4915-4949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Lee HJ, Ryu JM, Jung YH, Lee SJ, Kim JY, Lee SH, Hwang IK, Seong JK, Han HJ. High glucose upregulates BACE1-mediated Aβ production through ROS-dependent HIF-1α and LXRα/ABCA1-regulated lipid raft reorganization in SK-N-MC cells. Sci Rep. 2016;6:36746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Lu Y, Rong J, Lai Y, Tao L, Yuan X, Shu X. The Degree of Helicobacter pylori Infection Affects the State of Macrophage Polarization through Crosstalk between ROS and HIF-1α. Oxid Med Cell Longev. 2020;2020:5281795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Yan W, Ma X, Zhao X, Zhang S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther. 2018;12:3961-3972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 35. | Gong G, Shen YL, Lan HY, Jin JM, An P, Zhang LJ, Chen LL, Peng W, Luan X, Zhang H. The Cyr61 Is a Potential Target for Rotundifuran, a Natural Labdane-Type Diterpene from Vitex trifolia L., to Trigger Apoptosis of Cervical Cancer Cells. Oxid Med Cell Longev. 2021;2021:6677687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Shao L, Dong C, Geng D, He Q, Shi Y. Ginkgolide B protects against cognitive impairment in senescence-accelerated P8 mice by mitigating oxidative stress, inflammation and ferroptosis. Biochem Biophys Res Commun. 2021;572:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |