Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.107711
Revised: May 1, 2025
Accepted: June 24, 2025
Published online: September 20, 2025
Processing time: 135 Days and 20.8 Hours
Ovarian cancer (OC) is the most lethal gynecological cancer among females, and its early diagnosis could help for better outcomes of the patients.
To investigate the utility of serum insulin-like growth factors-binding proteins 2 (IGFBP2), secreted phosphoprotein 1 (SPP1), thrombospondin 1 protein (TSP1) and D-dimer levels in addition to currently used biomarkers [cancer antigen 125 (CA125) and human epididymis protein 4 (HE4)] in the diagnosis of epithelial OC (EOC).
This is a case-control study that included fifty females diagnosed with EOC, 10 females with benign ovarian masses recruited from the Egyptian National Cancer Institute, and 30 healthy females as a control group. All subjects were assessed for serum HE4, CA125, IGFBP2, TSP1 and SPP1 measurement by enzyme-linkedimmunosorbent assay.
There was a statistically significant difference in serum levels between EOC, benign ovarian masses, and healthy control groups regarding CA125 and SPP1 (P < 0.001 for both markers), while HE4 and IGFBP2 increased significantly in EOC compared to healthy control groups (P < 0.001 for all markers) with no significant difference between EOC and benign ovarian masses groups. However, there was no statistically significant difference among EOC, benign ovarian masses, and healthy control groups regarding the TSP1 serum levels (P = 0.051). Receiver operating characteristic analysis revealed that combined assessment of SPP1 with CA125 or TSP1 increased the diagnosis of EOC patients to a sensitivity, specificity, and area under curve of (93.3%, 100%, 0.968; respectively, P < 0.001).
SPP1 may be a potential marker for the differentiation between benign and malignant ovarian masses, while IGFBP2 can differentiate between healthy females and females with ovarian masses. Combining SPP1 with CA125 or TSP1 provides high sensitivity and specificity for the detection of EOC patients.
Core Tip: Serum secreted phosphoprotein 1 (SPP1) can be a potential marker for the differentiation of patients with benign from those with malignant ovarian masses. insulin-like growth factors-binding proteins 2 (IGFBP2) and D-dimer could be potential markers for the diagnosis of patients with ovarian masses in relation to healthy controls. IGFBP2 could be a useful, sensitive diagnostic marker for epithelial ovarian cancer (EOC) patients. Also, combining SPP1 with cancer antigen 125 or thrombospondin 1 protein provides a high sensitivity (93.3%) and specificity (100%) for the detection of EOC patients.
- Citation: Ibrahim NH, Abdellateif MS, Serag DS, Laymouna A, Elaguizy MS, Halim RMA. Clinical significance of differential plasma proteins levels in the diagnosis of epithelial ovarian cancer. World J Exp Med 2025; 15(3): 107711
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/107711.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.107711
Ovarian cancer (OC) is the most lethal gynecological cancer all over the world[1]. About one hundred and twenty-five thousand people die from OC each year, with a five-year survival rate of approximately 46%[2,3]. Epithelial OC (EOC) accounts for 90 % of OC[1]. Early diagnosis of OC has been a challenge due to lacking of signs and symptoms at early stage[4].
Plasma proteins differentially expressed in EOC patients have been evaluated and found to be useful markers in early diagnosis of cancer[5]. The screening procedures routinely used for the diagnosis of OC include ultrasound, transvaginal sonography, and computed tomography scans. Currently, cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) are well-established biomarkers in OC diagnosis[6].
CA125 is a large transmembrane glycoprotein, which is encoded by mucin 16 gene[7]. CA125 is overexpressed in EOC and could be detected in the serum with a sensitivity of only 50%-62% in early-stage OC[6,8,9]. Additionally, CA125 is reported to be elevated in other physiological or pathological conditions, such as pregnancy, endometriosis, and inflammatory diseases of the peritoneum, making its specificity low[6,9].
Another important serum biomarker that has a higher specificity than CA125 for the diagnosis of EOC is the HE4[10,11]. It was also found to be elevated in multiple abdominal tumors, endometrial cancer, and pancreatic cancer[12-15]. Therefore, the assessment of HE4 alone could not be useful for the diagnosis of EOC[16].
Insulin-like growth factors-binding proteins 2 (IGFBP2) is a protein-binding hormone that is implicated in glucose and lipid metabolism[17,18]. Additionally, it controls cell survival, growth, proliferation, migration, and differentiation[19]. Furthermore, it has been suggested that IGFBP2 contributes to the pathophysiology of several cancers, such as breast cancer[20], OC[21], pancreatic cancer[22], thyroid cancer[23], and lung cancer[24].
Thrombospondin 1 protein (TSP1) is a glycoprotein with a potent antiangiogenic effect[25]. It has a pivotal function in cell proliferation, cell adhesion, apoptosis, angiogenesis, metastasis[26-28]. It was also proposed to be regulated by p53, a gene which is mutated in advanced OC[25].
Secreted phosphoprotein 1 (SPP1) is a secreted arginine, glycine, and aspartic acid containing phosphorylated glycoprotein[29]. It is synthesized by various cells including osteoclasts, macrophages, epithelial cells, and endothelial cells[30]. Previous studies reported that the expression of SPP1 is high in numerous tumors such as breast, colon, prostate, and lung cancers[31,32]. It promotes the progression of cancer through modulation of vascular endothelial growth factor expression and regulation of extracellular matrix protein[33]. However, the localization and function of SPP1 in OC are still unknown[34].
Therefore, evaluating the expression levels of IGFBP2, TSP1, and SPP1 in EOC patients could provide clinical value in the diagnosis of those patients. Additionally, the diagnostic significance of these proteins was correlated to the conventionally available markers including CA125 and HE4. Hence, it will help for the proper detection of EOC patients, especially those with early-stage disease. Moreover, these proteins could open the way for finding a targeted therapy that could improve the EOC patients’ outcomes.
Subjects included in the study were grouped into 3 groups: (1) Malignant disease group that consisted of 50 female patients diagnosed with EOC; (2) Benign disease group that consisted of 10 female patients diagnosed with benign ovarian masses; and (3) A control group that consisted of 30 healthy females. Patients recruited in the study were those attended the out patient clinic at the Egyptian National Cancer Institute, Cairo University during the period from November 2023 to December 2024. Patients were excluded if they have other hematological malignancies, solid tumors, previously received chemotherapy or radiotherapy. All recruited individuals signed an informed consent for participation in the study. The study protocol was approved by the Institutional Review Board of the National Cancer Institute, Cairo University, which is in accordance with the 2013 declaration of Helsinki.
All patients were subjected to detailed history taking, full clinical examination, complete laboratory and radiological assessment. Venous blood samples (3 mL) were collected from all study groups for the measurement ofCA125, HE4, IGFBP2, TSP1, SPP1 and D-dimer. The collected blood samples were centrifuged, separated into serum samples and stored at -20 °C until time of measurement.
Serum IGFBP2, TSP1, and SPP1 were measured by enzyme-linkedimmunosorbent assay using a commercial kit according to the manufacturer’s instructions as follow: (1) ELK Biotechnology, ELK1138 for IGFBP2; (2) ELK Biotechnology, ELK1047 for SPP1; and (3) ELK Biotechnology, ELK1685 for TSP1.
Data analyses were analyzed using IBM Statistical Package for the Social Sciences Statistics Version 22. Quantitative data were expressed as median and range according to the performed normality test. Qualitative data were presented as frequency and percentage. Comparison between categorical variables were done using χ2 test or Fisher exact as appropriate, while comparisons between numerical variables were performed using the one-way analysis of variance test or Kruskal-Walli’s test. The area under the receiver operating characteristic (ROC) curve was done to assess the diagnostic value of the tested markers in the vaginal cancer patients. Correlations between the assessed markers were performed using the Spearman correlation coefficient. All tests were two-tailed, and the significant level was considered at P value < 0.05.
The mean ages of the involved patients were 48.4 years ± 8 years for the control females, 50.7 years ± 8.3 years for patients with benign ovarian mass, and 52.3 years ± 14.5 years for EOC patients. A positive family history of OC was found in 6/50 (12.0%) EOC patients and only 1/10 (10.0%) in the benign group. Distant metastasis was reported in 34/50 (68.0%) of the EOC group. Other clinical features were illustrated in Table 1.
| Variables | Control (n = 30) | Benign (n = 10) | Malignant (n = 50) | |
| Age (years) | mean ± SD | 48.4 ± 8 | 50.7 ± 8.3 | 52.3 ± 14.5 |
| Median (range) | 49 (23-54) | 48.5 (41-66) | 50.5 (25-86) | |
| Family history | Positive | 1(10.0) | 6 (12.0) | |
| Negative | 9 (90.0) | 44 (88.0) | ||
| Metastasis | Positive | 34 (68.0) | ||
| Negative | 16 (32.0) | |||
| Site of metastasis | Omentum/peritoneum | 29 (58.0) | ||
| Ileum | 1 (2.0) | |||
| Ileum and rectum | 1 (2.0) | |||
| Pancreas | 1 (2.0) | |||
| Liver | 1 (2.0) | |||
| Lymph nodes | 1 (2.0) | |||
| Tumor markers | Carcinoembryonic antigen | 65.6 (3.34-116.2) | ||
| CA19-9 | 475.95 (41.3-7174) | |||
| CA15-3 | 47.25 (27.39-353.3) | |||
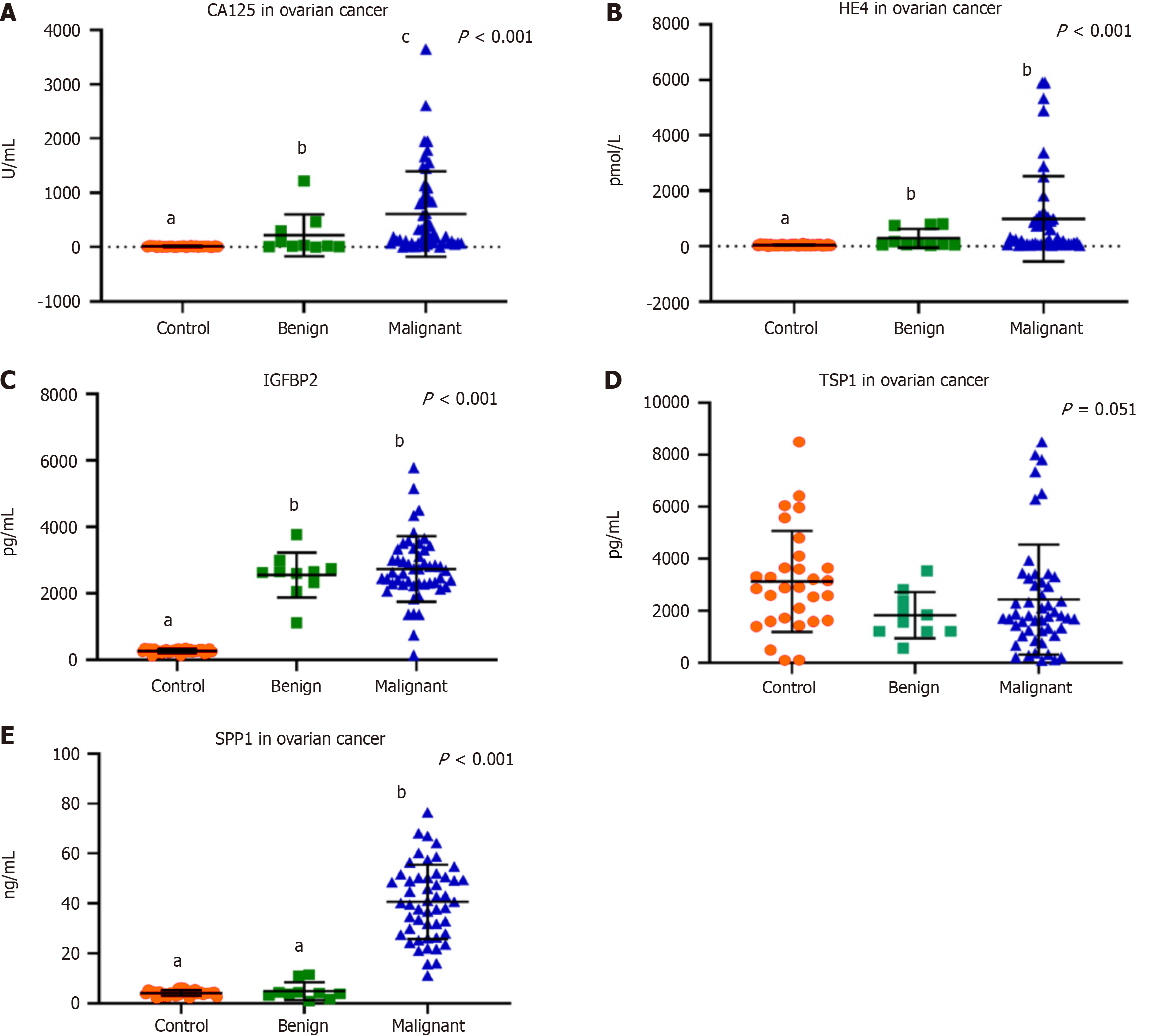
There was a significant increase in CA125 levels in EOC patients compared to the benign group and control females [232 (5.5-3649) U/mL, 28.6 (3.5-1217) U/mL, and 12.2 (3.1-25.9) U/mL, respectively, P < 0.001] (Figure 1A). HE4 was substantially elevated in the benign and malignant disease patients compared to the control group [93.4 (37-800) pmol/L, 283 (37-5908) pmol/L, and 41.6 (3.8-83.8) pmol/L, respectively, P < 0.001] (Figure 1B). IGFBP2 was also significantly increased in benign and malignant disease patients compared to the control group [2641 (1114-3773) pg/mL, 2669 (146-5785) pg/mL, and 273 (117-353) pg/mL, respectively, P < 0.001] (Figure 1C). On the other hand, there was no significant difference regarding the TSP1 serum levels in patients with benign and malignant ovarian masses in relation to the control group [1702 (560-3540) pg/mL, 1803 (92-8500) pg/mL, and 2899 (95-8500) pg/mL, respectively, P = 0.051] (Figure 1D). While SPP1 was significantly elevated in EOC patients in comparison to the benign ovarian disease and control groups [40.4 (11-76.5) ng/mL, 3.98 (0.85-11.5) ng/mL, and 4.2 (1.9-6.3) ng/mL, respectively, P < 0.001] (Figure 1E). Moreover, there was a significant increase in the D-dimer levels in EOC patients and the benign group compared to the control females [832 (262-3429) ng/mL and 744 (50-1000) ng/mL compared to 0.1 (0-0.5) ng/mL, respectively, P < 0.001] (Table 2).
| Control (n = 30) | Benign (n = 10) | Malignant (n = 50) | P value | |
| Cancer antigen 125 (U/mL) | 12.2 (3.1-25.9)a | 28.6 (3.5-1217)b | 232 (5.5-3649)c | < 0.001 |
| Human epididymis protein 4 (pmol/L) | 41.6 (3.8-83.8)a | 93.4 (37-800)b | 283 (37-5908)b | < 0.001 |
| Insulin-like growth factors-binding proteins 2 (pg/mL) | 273 (117-353)a | 2641 (1114-3773)b | 2669 (146-5785)b | < 0.001 |
| Thrombospondin 1 protein (pg/mL) | 2899 (95-8500) | 1702 (560-3540) | 1803 (92-8500) | 0.051 |
| Secreted phosphoprotein 1 (ng/mL) | 4.2 (1.9-6.3)a | 3.98 (0.85-11.5)a | 40.4 (11-76.5)b | < 0.001 |
| D-dimer (ng/mL) | 0.1 (0-0.5)a | 832 (262-3429)b | 744 (50-1000)b | < 0.001 |
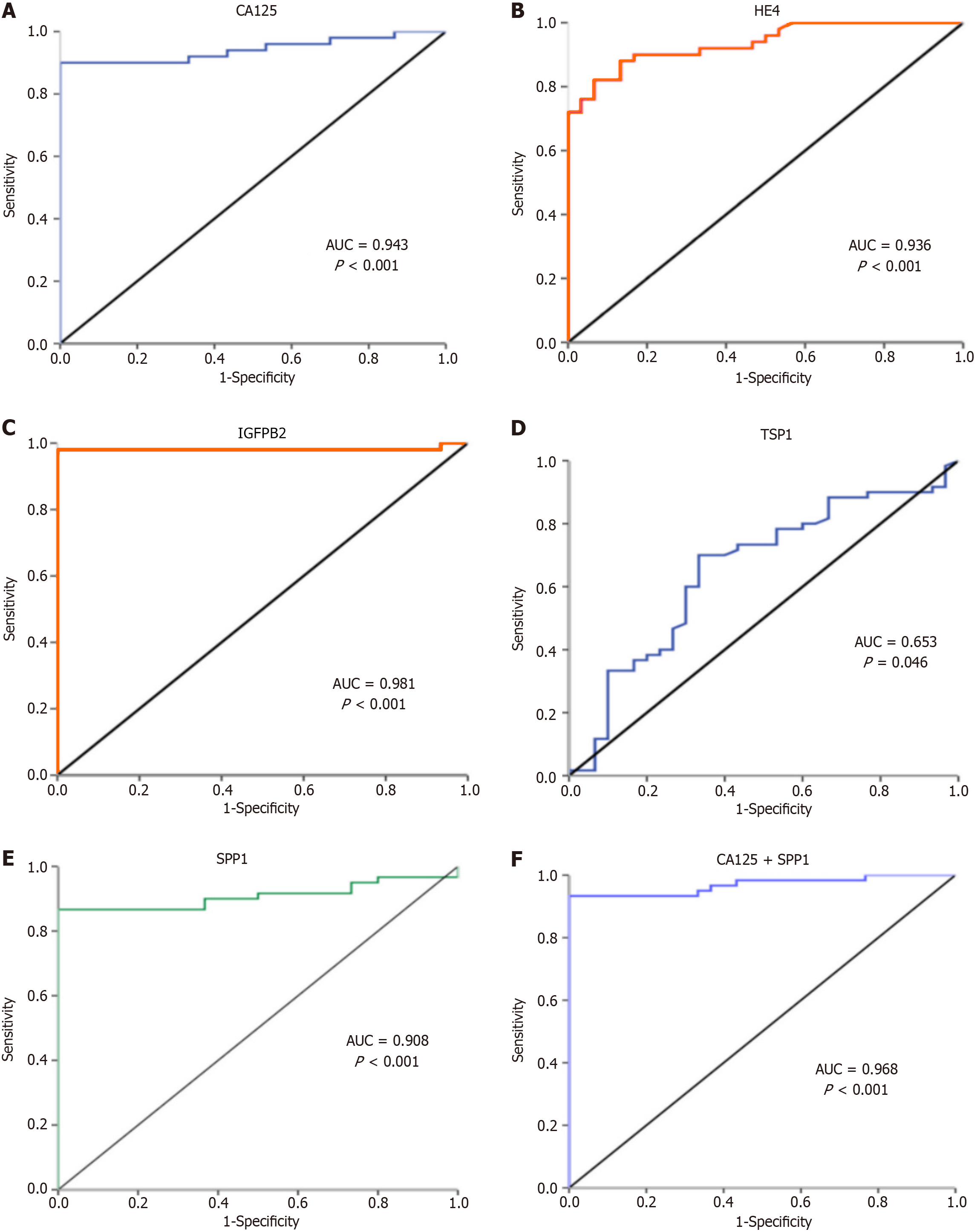
The ROC curve analysis revealed that the sensitivity, specificity and area under the ROC curve (AUC) of CA125 were 90%, 100%, 0.943; respectively P < 0.001 (Figure 2A) at a cutoff of 41.1 U/mL, and those of HE4 were 82%, 93.3%, and 0.936, respectively P < 0.001 at a cutoff of 66.4 pmol/L (Figure 2B). IGFBP2 achieved a sensitivity of 98% and specificity of 100% with AUC = 0.981 at a cutoff of pg/mL (P < 0.001) for the diagnosis of EOC patients (Figure 2C). TSP1 achieved a 70% sensitivity and 66.7% specificity at a cutoff of 2460 pg/mL with AUC = 0.653 (P = 0.046) (Figure 2D). While SPP1 showed a sensitivity, specificity, and AUC of 86.7%, 100%, and 0.908, respectively, P < 0.001 at a cutoff of 8.64 ng/mL (Figure 2E).
Furthermore, combined expressions of SPP1 and CA125 or TSP1 provide 93.3% sensitivity and 100% specificity for the detection of EOC patients with AUC = 0.968 (P < 0.001) (Figure 2F). Similarly, combining CA125 and HE4 increased the sensitivity and specificity to 91.7% and 93.3%, respectively, with AUC = 0.962. While combining TSP1 with CA125 did not add a notable value for their diagnostic accuracy (Table 3).
| Test variables | Area under the receiver operating characteristic curve | Cutoff value | Sensitivity | Specificity | P value |
| CA125 | 0.943 | 41.1 | 90% | 100% | < 0.001 |
| HE4 | 0.936 | 66.4 | 82% | 93.3% | < 0.001 |
| Insulin-like growth factors-binding proteins 2 | 0.981 | 553.1 | 98% | 100% | < 0.001 |
| TSP1 | 0.653 | 2460 | 70% | 66.7% | 0.046 |
| SPP1 | 0.908 | 8.64 | 86.7% | 100% | < 0.001 |
| TSP1 + SPP1 | 0.968 | - | 93.3% | 100% | < 0.001 |
| CA125 + SPP1 | 0.968 | - | 93.3% | 100% | < 0.001 |
| CA125 + HE4 | 0.962 | - | 91.7% | 93.3% | < 0.001 |
| CA125 + TSP1 | 0.949 | - | 95% | 83.3% | < 0.001 |
Regression analysis showed that the age of the patients [odds ratio (OR) = 1.103, P < 0.001], CA125 (OR = 1.072, P = 0.002), HE4 (OR = 1.069, P = 0.001), IGFBP2 (OR = 1.009, P = 0.044), and SPP1 (OR = 1.375, P = 0.007) were significantly associated with OC incidence (Table 4).
| Odds ratio | 95%CI for Exp(B) | P value | ||
| Lower | Upper | |||
| Age | 1.103 | 1.049 | 1.159 | 0.001 |
| Family history | 0 | 0.00 | 0.00 | 0.999 |
| Menopausal status | 0 | 0.00 | 0.00 | 0.999 |
| Cancer antigen 125 | 1.072 | 1.025 | 1.122 | 0.002 |
| Human epididymis protein 4 | 1.069 | 1.030 | 1.111 | 0.001 |
| Insulin-like growth factors-binding proteins 2 | 1.009 | 1.000 | 1.017 | 0.044 |
| Thrombospondin 1 protein | 1.000 | 1.000 | 1.000 | 0.080 |
| Secreted phosphoprotein 1 | 1.375 | 1.092 | 1.732 | 0.007 |
| D-dimer | 1.674 | 0.000 | 3611171.830 | 0.945 |
There was a significant positive correlation between IGFBP2 and TSP1 in EOC patients (r = 0.306, P = 0.018). Also, SPP1 correlated significantly with HE4 (r = 0.255, P = 0.050). Other correlations were illustrated in Table 5.
| HE4 | IGFBP2 | TSP1 | SPP1 | CA19-9 | CA15-3 | D-dimer | ||
| CA125 | r value | 0.365 | 0.131 | 0.001 | 0.183 | -0.071 | -0.446 | 0.046 |
| P value | 0.004 | 0.318 | 0.995 | 0.161 | 0.879 | 0.268 | 0.791 | |
| HE4 | r value | -0.018 | 0.101 | 0.255 | -0.536 | 0.759 | 0.220 | |
| P value | 0.891 | 0.444 | 0.050 | 0.215 | 0.029 | 0.204 | ||
| IGFBP2 | r value | 0.306 | 0.031 | -0.643 | 0.470 | 0.376 | ||
| P value | 0.018 | 0.815 | 0.119 | 0.240 | 0.026 | |||
| TSP1 | r value | -0.058 | -0.643 | 0.349 | -0.298 | |||
| P value | 0.707 | 0.119 | 0.396 | 0.082 | ||||
| SPP1 | r value | 0.107 | 0.325 | -0.009 | ||||
| P value | 0.819 | 0.432 | 0.959 | |||||
Although CA125 is the most widely used tumor marker for OC diagnosis[35], it has high false positive and false negative rates, where most of the patients having elevated CA125 levels were found to be in advanced stages of cancer. However, it may have a more important role in OC prognosis[36].
HE4 is a protease inhibitor present in the epithelium of the distal epididymis and found to be overexpressed in the tissue of ovarian carcinoma and secreted in the blood stream[37,38]. In 2009, HE4 was approved by the Food and Drug Administration as a marker for the diagnosis and monitoring of OC[39]. Since then, many studies have evaluated its diagnostic performance in OC patients and revealed that it had an average sensitivity of 79.4% for differentiating patients with benign from malignant ovarian masses with an average specificity of 84.1% at cutoff values ranging from 67-72 pmol/L[40].
Although HE4 differentiated controls from patients with ovarian masses in the present study, which was in agreement with that done by Chang et al[41], Moore et al[42], Hamed et al[43], Wang et al[44], Zheng et al[45], it didn’t differentiate between benign and malignant ovarian masses, which was in contradiction with the same studies, which all found that HE4 differentiated between benign and malignant ovarian masses with reasonable sensitivities and specificities.
The results of the present study revealed that serum IGFBP2 levels were significantly increased in EOC patients in comparison to controls. These data are in agreement with Russell et al[46], who found in their study a significant up-regulation of IGFBP2 in OC patients compared to controls. Similarly, Lancaster et al[47], reported that serum IGFBP2 levels were increased significantly (4.1-fold) in patients with OC compared to healthy controls. However, they found that IGFBP2 was able to differentiate patients with benign from those with malignant ovarian masses as it was increased 2.7-fold in patients with malignant ovarian masses[47]. The same results were concluded by Gershtein et al[48], who found that patients with malignant ovarian tumors had significantly higher levels of IGFBP2 compared to controls and patients with benign ovarian masses.
Regarding TSP1, there were contradictory reports about the role of TSP1 in the process of carcinogenesis. While multiple reports have considered TSP1 to have an inhibitory role in angiogenesis and tumor progression[49-51], some other reports have identified it as an oncogenic protein[52-54]. The results of the current study revealed that TSP1 serum levels did not significantly differ in patients with benign and malignant ovarian tumors compared to the control group. These data are inconsistent with the results of the study by Karavasilis et al[55], who found that most of the cases included in their study showed weak or absent tissue expression of TSP1. Correspondingly, Periyasamy et al[5], also studied serum levels of TSP1 and found that they significantly decreased in OC patients compared to controls and to patients with benign ovarian masses. While Alvarez et al[25] reported a TSP1 overexpression in OC tissue specimens in most of their studied patients.
SPP1 was found to be overexpressed in many cancer types, and in 2002, Kim et al[56] reported the association of OC with significantly increased SPP1 levels compared to healthy individuals and patients with benign ovarian disease. In accordance with this, the results of the present study showed that serum SPP1 levels were significantly increased in EOC patients compared to healthy controls as well as those with benign ovarian masses. These data are also in agreement with many reports in literature[5,57-59].
The present results also revealed that D-dimer was able to differentiate healthy female subjects from patients with ovarian masses, while its levels were not significantly different in patients with malignant ovarian masses when compared to those with benign diseases. This was in contradiction to the results of the studies by McKendry et al[59] and Farzaneh et al[60], who demonstrated that D-dimer was able to differentiate between patients with OC from those with benign ovarian disease.
The discrepancy in results of the present study with the literature regarding HE4, IGFBP2, and TSP1 levels in OC patients compared to their levels in patients with benign ovarian disease may be attributed to the small number of patients with benign ovarian disease who were included in the current study.
To better evaluate the expected study outcomes, further analysis was performed to assess the diagnostic potential of IGFBP2, SPP1, and TSP1 in relation to CA125 and HE4. The ROC curve analysis revealed that IGFBP2 achieved a sensitivity of 98% and a specificity of 100% at a cutoff of pg/mL for the diagnosis of EOC patients. TSP1 achieved a 70% sensitivity and 66.7% specificity at a cutoff of 2460 pg/mL. While SPP1 showed a sensitivity and specificity of 86.7% and 100%, respectively, at a cutoff of 8.64 ng/mL. Moreover, combined expressions of SPP1 with CA125 or TSP1 provide 93.3% sensitivity and 100% specificity for the detection of EOC patients. Similarly, combining CA125 and HE4 increased the sensitivity and specificity to 91.7% and 93.3%, respectively. While combining TSP1 with CA125 did not add a notable value for their diagnostic accuracy. These data are in consistency with that reported by Periyasamy et al[5], who tried to predict a combination model for the diagnosis of OC using a panel of (adepsin, CA125, IGFBP2, SPP1, and TSP1). They proposed that combining TSP1 with CA125 showed a 90.24% sensitivity and 94.87% specificity.
In conclusion, in addition to the well-established CA125 and HE4 biomarkers used for the diagnosis of OC, serum SPP1 can be a potential marker for the differentiation of patients with benign from those with malignant ovarian masses. While IGFBP2 and D-dimer could be potential markers for the diagnosis of patients with ovarian masses in relation to healthy controls. IGFBP2 could be a useful, sensitive diagnostic marker for EOC patients. Also, combining SPP1 with CA125 or TSP1 provides a high sensitivity (93.3%) and specificity (100%) for the detection of EOC patients. The limitations of the current work are the small number of recruited patients, especially those with benign ovarian disease, that could make a spectrum bias. Therefore, these data should be validated on a larger scale of patients with different pathological types.
We express our gratitude to all participating patients in the study.
| 1. | Ren X, Wu X, Hillier SG, Fegan KS, Critchley HO, Mason JI, Sarvi S, Harlow CR. Local estrogen metabolism in epithelial ovarian cancer suggests novel targets for therapy. J Steroid Biochem Mol Biol. 2015;150:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 3. | Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1446] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 4. | Li Y, Wang ZC, Luo L, Mu CY, Xu J, Feng Q, Li SB, Gu B, Ma P, Lan T. The clinical value of the combined detection of sEGFR, CA125 and HE4 for epithelial ovarian cancer diagnosis. Eur Rev Med Pharmacol Sci. 2020;24:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Periyasamy A, Gopisetty G, Subramanium MJ, Velusamy S, Rajkumar T. Identification and validation of differential plasma proteins levels in epithelial ovarian cancer. J Proteomics. 2020;226:103893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Montagnana M, Benati M, Danese E. Circulating biomarkers in epithelial ovarian cancer diagnosis: from present to future perspective. Ann Transl Med. 2017;5:276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Williams KA, Terry KL, Tworoger SS, Vitonis AF, Titus LJ, Cramer DW. Polymorphisms of MUC16 (CA125) and MUC1 (CA15.3) in relation to ovarian cancer risk and survival. PLoS One. 2014;9:e88334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Liu Q, Cheng Z, Luo L, Yang Y, Zhang Z, Ma H, Chen T, Huang X, Lin SY, Jin M, Li Q, Li X. C-terminus of MUC16 activates Wnt signaling pathway through its interaction with β-catenin to promote tumorigenesis and metastasis. Oncotarget. 2016;7:36800-36813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Sölétormos G, Duffy MJ, Othman Abu Hassan S, Verheijen RH, Tholander B, Bast RC Jr, Gaarenstroom KN, Sturgeon CM, Bonfrer JM, Petersen PH, Troonen H, CarloTorre G, Kanty Kulpa J, Tuxen MK, Molina R. Clinical Use of Cancer Biomarkers in Epithelial Ovarian Cancer: Updated Guidelines From the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 10. | Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 406] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 11. | Yanaranop M, Anakrat V, Siricharoenthai S, Nakrangsee S, Thinkhamrop B. Is the Risk of Ovarian Malignancy Algorithm Better Than Other Tests for Predicting Ovarian Malignancy in Women with Pelvic Masses? Gynecol Obstet Invest. 2017;82:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Gion M, Peloso L, Trevisiol C, Squarcina E, Zappa M, Fabricio AS. An epidemiology-based model as a tool to monitor the outbreak of inappropriateness in tumor marker requests: a national scale study. Clin Chem Lab Med. 2016;54:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Mi D, Zhang YX, Wang CJ, Feng Q, Qi P, Chen SQ. Diagnostic and prognostic value of serum human epididymis protein 4 in patients with primary fallopian tube carcinoma. J Obstet Gynaecol Res. 2016;42:1326-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Bignotti E, Ragnoli M, Zanotti L, Calza S, Falchetti M, Lonardi S, Bergamelli S, Bandiera E, Tassi RA, Romani C, Todeschini P, Odicino FE, Facchetti F, Pecorelli S, Ravaggi A. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br J Cancer. 2011;104:1418-1425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Huang T, Jiang SW, Qin L, Senkowski C, Lyle C, Terry K, Brower S, Chen H, Glasgow W, Wei Y, Li J. Expression and diagnostic value of HE4 in pancreatic adenocarcinoma. Int J Mol Sci. 2015;16:2956-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, Timmerman D, De Moor B, Vergote I. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011;104:863-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | Hjortebjerg R, Flyvbjerg A, Frystyk J. Insulin growth factor binding proteins as therapeutic targets in type 2 diabetes. Expert Opin Ther Targets. 2014;18:209-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol Metab. 2019;19:86-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Shen J, Ding Y. Multifaceted roles of insulinlike growth factor 2 mRNA binding protein 2 in human cancer (Review). Mol Med Rep. 2025;31:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Almawi WY, Zidi S, Sghaier I, El-Ghali RM, Daldoul A, Midlenko A. Novel Association of IGF2BP2 Gene Variants With Altered Risk of Breast Cancer and as Potential Molecular Biomarker of Triple Negative Breast Cancer. Clin Breast Cancer. 2023;23:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 21. | Yuan J, Li X, Wang F, Liu H, Guan W, Xu G. Insulin-like growth factor 2 mRNA-binding protein 2 is a therapeutic target in ovarian cancer. Exp Biol Med (Maywood). 2023;248:2198-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Deng H, Yao H, Zhou S, He C, Huang Y, Li Y, Chen H, Shu J. Pancancer analysis uncovers an immunological role and prognostic value of the m6A reader IGF2BP2 in pancreatic cancer. Mol Cell Probes. 2024;73:101948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Abdellateif MS, Shaarawy S, Elesawy YF, Mansour M, Tharwat E, Ibrahim NH, Eissa MS. The Role of Vitamin D, Platelet-Derived Growth Factor and Insulin-Like Growth Factor 1 in the Progression of Thyroid Diseases. Asian Pac J Cancer Prev. 2020;21:2083-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Gong L, Liu Q, Jia M, Sun X. Systematic analysis of IGF2BP family members in non-small-cell lung cancer. Hum Genomics. 2024;18:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Alvarez AA, Axelrod JR, Whitaker RS, Isner PD, Bentley RC, Dodge RK, Rodriguez GC. Thrombospondin-1 expression in epithelial ovarian carcinoma: association with p53 status, tumor angiogenesis, and survival in platinum-treated patients. Gynecol Oncol. 2001;82:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65:700-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Lawler J. Thrombospondins. Curr Drug Targets. 2008;9:820-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Jeanne A, Schneider C, Martiny L, Dedieu S. Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front Pharmacol. 2015;6:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Oldberg A, Franzén A, Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83:8819-8823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 791] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 30. | Ahmed M, Behera R, Chakraborty G, Jain S, Kumar V, Sharma P, Bulbule A, Kale S, Kumar S, Mishra R, Raja R, Saraswati S, Kaur R, Soundararajan G, Kumar D, Thorat D, Sanyal M, Ramdasi A, Ghosh P, Kundu GC. Osteopontin: a potentially important therapeutic target in cancer. Expert Opin Ther Targets. 2011;15:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7:4060-4066. [PubMed] |
| 32. | Kumari A, Kashyap D, Garg VK. Osteopontin in cancer. Adv Clin Chem. 2024;118:87-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 33. | Cui R, Takahashi F, Ohashi R, Yoshioka M, Gu T, Tajima K, Unnoura T, Iwakami S, Hirama M, Ishiwata T, Iwase A, Takahashi K. Osteopontin is involved in the formation of malignant pleural effusion in lung cancer. Lung Cancer. 2009;63:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Zeng B, Zhou M, Wu H, Xiong Z. SPP1 promotes ovarian cancer progression via Integrin β1/FAK/AKT signaling pathway. Onco Targets Ther. 2018;11:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Zhang R, Siu MKY, Ngan HYS, Chan KKL. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int J Mol Sci. 2022;23:12041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 114] [Reference Citation Analysis (0)] |
| 36. | Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. 2021;1875:188503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 37. | James NE, Chichester C, Ribeiro JR. Beyond the Biomarker: Understanding the Diverse Roles of Human Epididymis Protein 4 in the Pathogenesis of Epithelial Ovarian Cancer. Front Oncol. 2018;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Rowswell-Turner RB, Singh RK, Urh A, Yano N, Kim KK, Khazan N, Pandita R, Sivagnanalingam U, Hovanesian V, James NE, Ribeiro JR, Kadambi S, Linehan DC, Moore RG. HE4 Overexpression by Ovarian Cancer Promotes a Suppressive Tumor Immune Microenvironment and Enhanced Tumor and Macrophage PD-L1 Expression. J Immunol. 2021;206:2478-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Chudecka-Głaz A, Strojna A, Michalczyk K, Wieder-Huszla S, Safranow K, Skwirczyńska E, Jurczak A. Evaluation of He4 Use in the Diagnosis of Ovarian Cancer: First and Second Recurrence, and an Analysis of HE4 Concentration during Second- and Third-Line Chemotherapy. Diagnostics (Basel). 2023;13:452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 40. | Olsen M, Lof P, Stiekema A, van den Broek D, Wilthagen EA, Bossuyt PM, Lok CAR. The diagnostic accuracy of human epididymis protein 4 (HE4) for discriminating between benign and malignant pelvic masses: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2021;100:1788-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Chang X, Ye X, Dong L, Cheng H, Cheng Y, Zhu L, Liao Q, Zhao Y, Tian L, Fu T, Chen J, Cui H. Human epididymis protein 4 (HE4) as a serum tumor biomarker in patients with ovarian carcinoma. Int J Gynecol Cancer. 2011;21:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, Skates SJ. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol. 2011;118:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | Hamed EO, Ahmed H, Sedeek OB, Mohammed AM, Abd-Alla AA, Abdel Ghaffar HM. Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn Pathol. 2013;8:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Wang J, Gao J, Yao H, Wu Z, Wang M, Qi J. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta-analysis. Tumour Biol. 2014;35:6127-6138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Zheng LE, Qu JY, He F. The diagnosis and pathological value of combined detection of HE4 and CA125 for patients with ovarian cancer. Open Med (Wars). 2016;11:125-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Russell MR, Graham C, D'Amato A, Gentry-Maharaj A, Ryan A, Kalsi JK, Ainley C, Whetton AD, Menon U, Jacobs I, Graham RLJ. A combined biomarker panel shows improved sensitivity for the early detection of ovarian cancer allowing the identification of the most aggressive type II tumours. Br J Cancer. 2017;117:666-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Lancaster JM, Sayer RA, Blanchette C, Calingaert B, Konidari I, Gray J, Schildkraut J, Schomberg DW, Marks JR, Berchuck A. High expression of insulin-like growth factor binding protein-2 messenger RNA in epithelial ovarian cancers produces elevated preoperative serum levels. Int J Gynecol Cancer. 2006;16:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Gershtein ES, Isaeva ER, Kushlinsky DN, Korotkova EA, Ermilova VD, Laktionov KP, Adamyan LV. Insulin-Like Growth Factors (IGF) and IGF-Binding Proteins (IGFBP) in the Serum of Patients with Ovarian Tumors. Bull Exp Biol Med. 2016;160:814-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Fontanini G, Boldrini L, Calcinai A, Chinè S, Lucchi M, Mussi A, Angeletti CA, Basolo F, Bevilacqua G. Thrombospondins I and II messenger RNA expression in lung carcinoma: relationship with p53 alterations, angiogenic growth factors, and vascular density. Clin Cancer Res. 1999;5:155-161. [PubMed] |
| 50. | Grossfeld GD, Ginsberg DA, Stein JP, Bochner BH, Esrig D, Groshen S, Dunn M, Nichols PW, Taylor CR, Skinner DG, Cote RJ. Thrombospondin-1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst. 1997;89:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 217] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155:441-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Yamashita Y, Kurohiji T, Tuszynski GP, Sakai T, Shirakusa T. Plasma thrombospondin levels in patients with colorectal carcinoma. Cancer. 1998;82:632-638. [PubMed] [DOI] [Full Text] |
| 53. | Kasper HU, Ebert M, Malfertheiner P, Roessner A, Kirkpatrick CJ, Wolf HK. Expression of thrombospondin-1 in pancreatic carcinoma: correlation with microvessel density. Virchows Arch. 2001;438:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Straume O, Akslen LA. Expresson of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Karavasilis V, Malamou-Mitsi V, Briasoulis E, Tsanou E, Kitsou E, Pavlidis N. Clinicopathologic study of vascular endothelial growth factor, thrombospondin-1, and microvessel density assessed by CD34 in patients with stage III ovarian carcinoma. Int J Gynecol Cancer. 2006;16 Suppl 1:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Kim JH, Skates SJ, Uede T, Wong KK, Schorge JO, Feltmate CM, Berkowitz RS, Cramer DW, Mok SC. Osteopontin as a potential diagnostic biomarker for ovarian cancer. JAMA. 2002;287:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 57. | Milivojevic M, Boskovic V, Atanackovic J, Milicevic S, Razic S, Kotlica BK. Evaluation of osteopontin and CA125 in detection of epithelial ovarian carcinoma. Eur J Gynaecol Oncol. 2013;34:83-85. [PubMed] |
| 58. | Rani S, Sehgal A, Kaur J, Pandher DK, Punia RS. Osteopontin as a Tumor Marker in Ovarian Cancer. J Midlife Health. 2022;13:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 59. | McKendry K, Duff S, Huang Y, Redha M, Scanlon Á, Abu Saadeh F, Gleeson N, O'Leary J, Norris L, O'Toole S. The value of human epididymis 4, D-dimer, and fibrinogen compared with CA 125 alone in triaging women presenting with pelvic masses: a retrospective cohort study. Acta Obstet Gynecol Scand. 2021;100:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Farzaneh F, Salimnezhad M, Hosseini MS, Ashraf Ganjouee T, Arab M, Talayeh M. D-dimer, Fibrinogen and Tumor Marker Levels in Patients with benign and Malignant Ovarian Tumors. Asian Pac J Cancer Prev. 2023;24:4263-4268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |