Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.105208
Revised: April 22, 2025
Accepted: June 20, 2025
Published online: September 20, 2025
Processing time: 210 Days and 0.3 Hours
India has the highest tuberculosis (TB) burden in the world. Of the estimated annual 10 million TB cases, features of extra pulmonary TB are evident in up to 45%. Urogenital TB (UGTB) accounts for approximately 20% of those cases. The lack of non-sputum based diagnostic tools continue to hinder efforts to reduce the burden of UGTB. MicroRNAs (miRNAs) play a crucial role in biological pathways and can be used as a potential biomarker for TB. We evaluated urinary ex
To evaluate the potential of miRNA-155-5p, miRNA-26a-5p and miRNA-29a-3p in uEVs to diagnose UGTB in adults.
uEV characterization was done using nanoparticle tracking analysis and flow cytometry. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) for urinary uEV-miRNAs were carried out in samples from patients with suspected UGTB, or Urinary tract infections [UTI, disease controls (DC)] and healthy controls (HCs) (n = 20/group). U6 was used to normalize the qRT-PCR data. Receivers operating characteristic curves was used to calculate the diagnostic accuracy of uEV-miRNAs to differentiate UGTB from controls (DC and HCs).
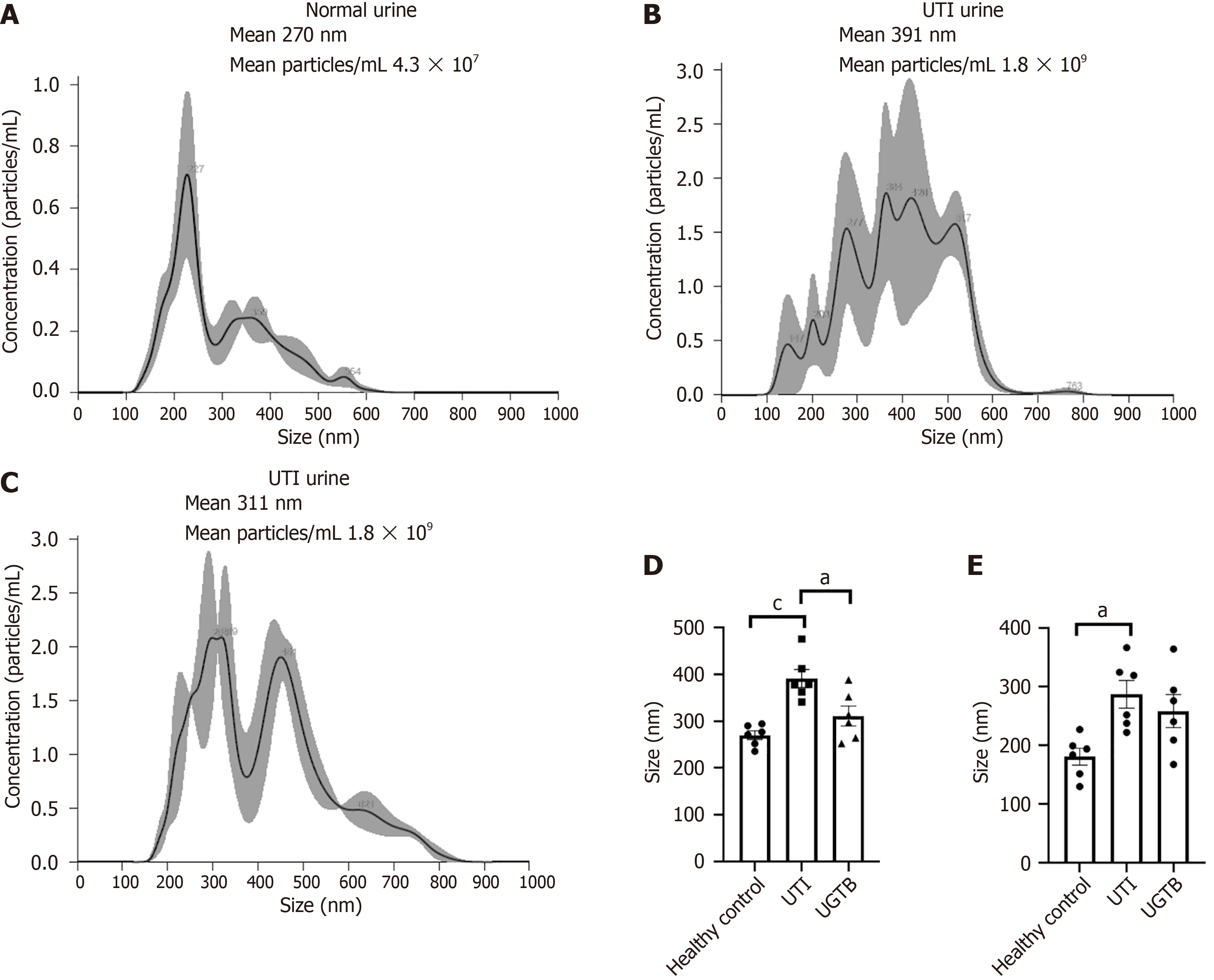
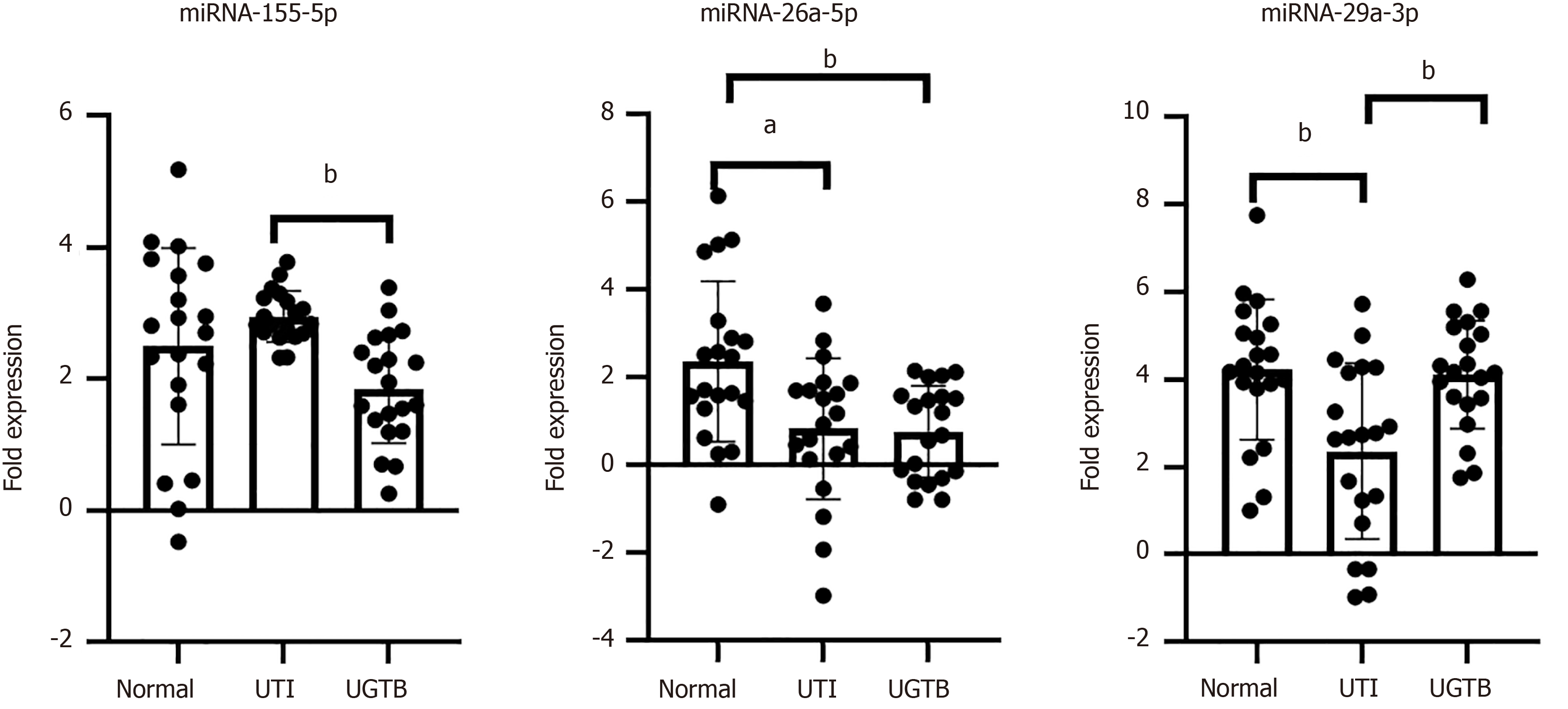
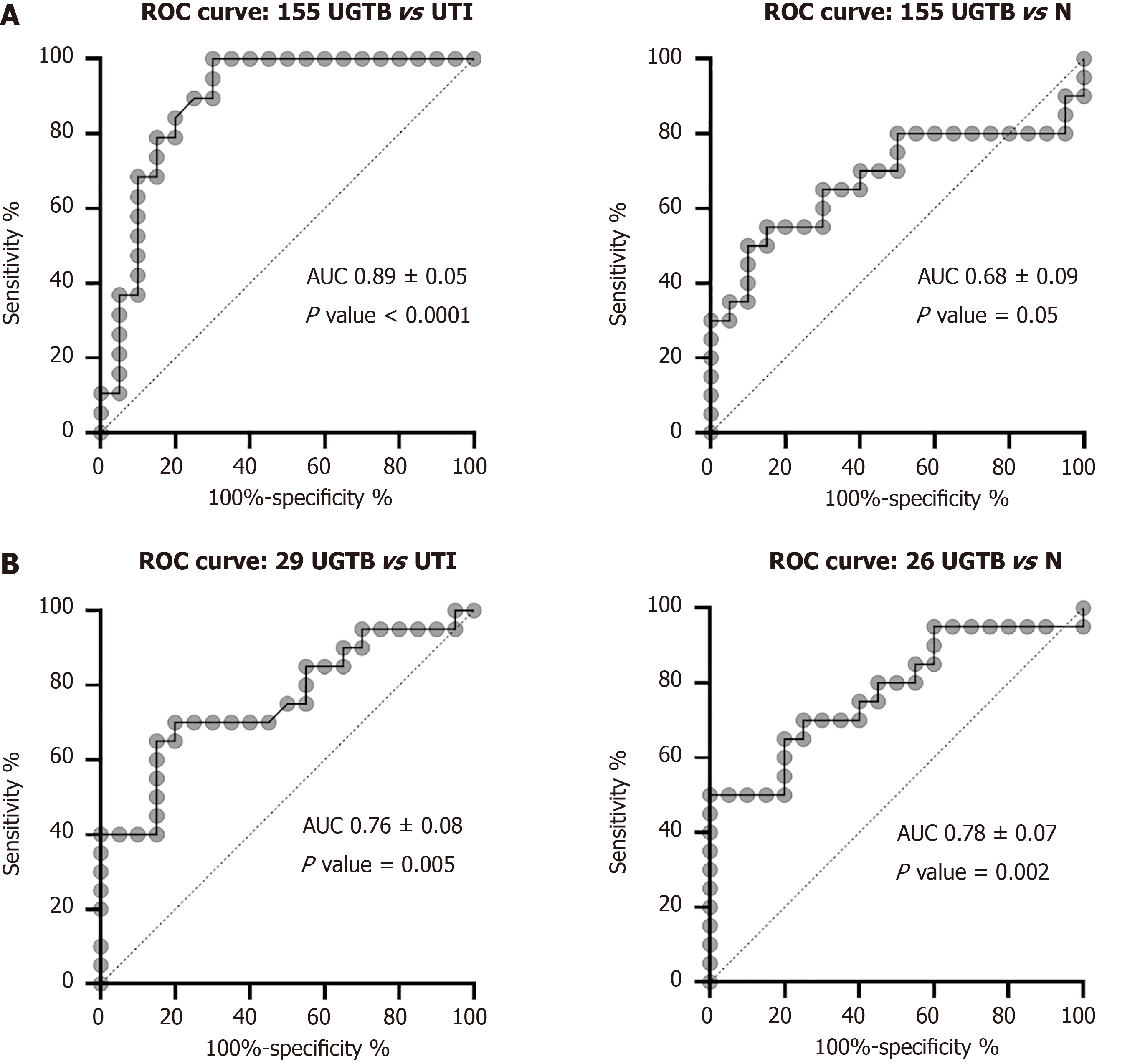
uEVs from UGTB or UTI patients had higher mean size, and also lower proportion of CD63 positive vesicles as compared to HC’s uEVs. Between UTI and UGTB, the mean size of uEVs was significantly higher in UTI cases. qRT-PCR analysis revealed a significantly lower abundance of miRNA-155-5p and miRNA-26a-5p in uEVs from UGTB relative to UTI (P value = 0.004) and HC (P value = 0.009) respectively n = 20/group). While, miRNA-29a-3p was higher in abundance in both UGTB and HCs’ uEV, relative to uEVs from UTI cases (P values = 0.004 and 0.002 respectively, n = 20/group). Moreover, miRNA-155-5p [area under curve (AUC) = 0.88, P ≤ 0.0001] and miRNA-29a-3p (AUC = 0.76, P value = 0.005) had optimal diagnostic accuracy to differentiate UGTB from DC (n = 20/groups) with a likelihood ratio of 5.2 and 4.3, respectively through receivers operating characteristic curve. While, miRNA-155-5p (AUC = 0.68, P value = 0.05) and miRNA-26a-5p (AUC = 0.78, P value = 0.002) had optimal diagnostic accuracy to differentiate UGTB from HCs with a likelihood ratio of > 2.
The differential expression of uEV-miRNAs, miRNA-155-5p and miRNA-29a-3p in UTGB and UTI cases hold promise in the specific diagnosis of UGTB. Further studies in large cohort are, however, needed to confirm the diagnostic accuracy of these uEV-miRNAs.
Core Tip: Discovery of new biomarkers from easily attainable bodily fluids is essential for controlling Urogenital tuberculosis (TB), a significant health concern. Urinary extracellular vesicle microRNAs (miRNAs) are exciting as biomarkers given their stability and non-invasive nature. miRNA-155, miRNA-26 and miRNA-29 play a crucial role in mycobacterium TB pathogenesis via regulation of posttranscriptional gene expression and subsequently apoptosis, cellular proliferation, differentiation and several other biological functions governing various facets of innate and acquired immunity. Thus, there is scope for developing miRNA studies using in vitro and in vivo models in TB which would likely lead to new technologies in diagnosis and treatment.
- Citation: Das P, Chaudhary DK, Mishra R, Tiwari S. Evaluation of urinary extracellular vesicles and microRNAs to diagnose urogenital tuberculosis. World J Exp Med 2025; 15(3): 105208
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/105208.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.105208
Tuberculosis (TB), has resulted in an estimated 1.5 million deaths globally, in 2020[1]. Although the most prevalent and contagious is the pulmonary form, mycobacterium TB (MTB) can affect any organ. Out of the estimated 10 million people with TB, features of extra pulmonary TB (EPTB) are evident in up to 45%[2]. Urogenital TB (UGTB), responsible for 15%-20% of all extra pulmonary cases, is second only to lymph-node TB[3]. Currently, Gene Xpert MTB/RIF/GeneXpert MTB/RIF ultra which are real-time quantitative PCR assay for amplifying MTB DNA and part of the rpoB gene encoding rifampicin resistance are recommended for the detection of pulmonary TB. However, so far limited studies have been carried out to evaluate its performance for the diagnosis of EPTB. Molecular tests like GeneXpert detecting MTB DNA might give false-positive test results since MTB DNA lingers in tissues even after treatment and eradication of live mycobacteria[4]. Nevertheless, lack of specific tests for UGTB and non-specific presentation causes delayed diagnosis which result in irreversible organ damage and end-stage renal failure. Biomarkers from easily attainable bodily fluids is essential if we are to reduce the burden associated with UGTB.
Outcome of TB infection is decided by the balance between the protective and pathogenic immune response. Extracellular vesicles (EVs) shed during MTB infection contain various molecules which could be screened through lipomics, proteomics and transcriptomics to identify potential biomarkers for the diagnosis of TB[5]. Amongst the EVs cargo microRNAs (miRNAs) regulate various biological functions like cell proliferation, differentiation, migration, apoptosis and autophagy which play an important role in pathogenesis of intracellular pathogens like MTB[6]. MiRNAs in EVs become even more promising candidates for TB biomarker as they are protected from RNase degradation by being enclosed within the EV lipid bilayer and remain in body fluids for prolonged periods.
Till now lipoarabinomannan, is the only approved biomarker in urine for TB diagnosis, and although commercially available they are useful in human immunodeficiency virus-positive TB cases with low CD4 cell counts only[7]. Altogether, given the ease of their assessment in urine EVs and the central role of miRNAs in TB[8], the present study was aimed at isolating and characterizing urinary EVs (uEVs) from suspected UGTB patients and identifying potential uEV-miRNAs that may be useful in the diagnosis of UGTB in adults.
The pilot study was approved by the Institutional Ethics Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences and carried out from February 2023-September 2023. Participants included in the study were between 15 years to 75 years with clinical or radiological features suggestive of UGTB, such as lower urinary tract symptoms, haematuria and/or sterile pyuria. Patients were excluded if having/found to be: (1) Previous TB history or had received anti-TB therapy (last 1 year); (2) Pregnant; (3) Human immunodeficiency virus positive; (4) One/more major complications like asthma, chronic obstructive pulmonary disease and cancers; and (5) Family history of hereditary disease.
A sample size of n = 20/group was determined based on feasibility considerations, including available resources, recruitment capacity, and disease burden in our setup. Participants were asked to provide an early-morning first-void midstream urine sample (≥ 5 mL) for examination including smear, culture and EV isolation. Midstream urine of age and gender matched healthy control (HC) and patients with urinary tract infection (UTI) enrolled as disease controls was also collected. Since routine samples were involved waiver of consent was given by Institutional Ethics Committee, however, written informed consent from healthy subjects was taken. Patients with UTI had a final diagnosis based on symptoms and signs of acute UTI and confirmed by urine culture.
Processing of urine was done within 2 hours of sample receipt. All Urine samples were divided into two parts. One part underwent urine routine and microscopic examination, culture and staining. The second part underwent a low-speed centrifugation at 2000 × g for 30 minutes at 4 °C to remove cells and debris. The supernatant was aliquoted and stored at
After centrifugation 0.1–0.2 mL of the urine sample was taken for direct smear microscopy and Acidfast Bacilli (AFB) staining with Ziehl-Neelsen stain. These slides were then microscopically examined with a light emitting diode microscope. They were examined under 10 × and 40 × for the presence of pus cells, red blood cells’s, casts, crystals and bacteria. The AFB smears were read and interpreted in accordance with the guidelines.
Urine specimen was cultured using a standard double wire loop of 4 mm diameter onto cysteine lactose electrolyte deficient agar and incubated aerobically at 37 °C for 18-24 hours. Plates were examined for bacterial growth and those showing Bacterial growth of ≥ 105 CFU/mL were taken to be significant. Identification was based on colony characteristics, Gram stain and biochemical reactions.
2 mL of the centrifuged urine sample, together with 1-2 times the volume of 2% N-acetyl-L-cysteine, NaOH and Na citrate, was vortexed for 20 seconds before it was incubated for 15 minutes at room temperature. Phosphate-buffered saline (PBS) buffer (pH = 6.8) was then added to a final volume of 45 mL and centrifuged at 3000 × g for 15 minutes at
EVs were isolated from human urine (3 mL) using commercially available isolation reagent from Invitrogen (Catalogue number: 4484452) per the manufacturer’s instructions. Simultaneously, an in-house EV isolation reagent was used to isolate EVs from an aliquot of the urine samples (3 mL) from control group using the same protocol to compare the performance of in-house developed reagent. The EVs isolated from both the reagents were suspended in 60 μL 1 × PBS and stored at -80 °C until further use.
An aliquot of EVs, isolated by the two reagents, were evaluated for their characteristic, including average concentration (particles/mL), and size of EVs, expression of CD63, an exosome enriched tetraspanin and calnexin an endoplasmic reticulum protein confirming the purity of uEVs[10], protein content in various EV preparations for isolation efficiency and Total RNA content (Supplementary Figures 1-5, Supplementary Table 1).
Measurements of protein concentration in analyzed samples were performed using the Pierce™ BCA Protein Assay (Thermo Fisher Scientific), according to the manufacturer’s instructions.
Thirty μg of uEV protein were separated on the 12% sodium dodecyl sulfate page gel and transferred to a nitrocellulose membrane. Blots were blocked with 5%non-fat milk for 1 hour and incubated with anti-CD63 (ab59479, Abcam) and calnexin (1:500, ab22595, Abcam) antibody for overnight at 4 °C. Membranes were washed with 0.1% phosphate buffered saline with tween 20 and incubated with appropriate secondary antibody for 2 hours at room temperature. Blots were developed with enhanced chemiluminescence reagent (Bio-Rad, CA, United States). Images were acquired on a Chemi Doc imaging system (Universal Hood III, Bio-Rad, CA, United States).
Nanoparticle tracking analysis (NTA) using NanoSightNS300, Malvern Instruments, United Kingdom was performed to analyze the size distribution and concentration of uEVs. Briefly, EVs were diluted in1 × PBS and loaded into a sample chamber. A green laser was used to detect the nanoparticles and five videos of 60 second duration were taken. Data was analyzed using the NTA 3.2 software (Malvern Instruments), which tracks the individual particles and calculates their size and velocity to generate a size distribution profile and determine the nanoparticle concentration.
EVs were analyzed for the presence of surface marker CD63 by flow cytometry using magnetic coated CD63-Dynabeads (Thermo Fisher Scientific, Waltham, MA, United States, Cat No. 10606D) as per the manufacturer’s instructions. Isolated EVs were resuspended in 1 × PBS and bound to magnetic-coated CD63-Dynabeads (Thermo Fisher Scientific, Waltham, MA, United States) overnight at 4 °C. The following day, the Dynabeads-bound EVs were incubated with an anti-CD63 mouse antibody (Abcam, Cat No. ab59479) over night at 4 °C along with an appropriate isotype control. After incubation, the beads were washed two times with 1 × PBS and stained with Alexa Flour 488 anti-mouse secondary antibody (detection antibody) for 1 hour at 4 °C and analyzed by flowcytometry (Beckman Coulter DxFLEX Flow Cytometer).
To retrieve suitable UGTB associated miRNAs we conducted a PubMed based search for studies comparing miRNA expression in urine of UGTB patients and healthy individuals. We could not find any studies pertaining to UGTB and majority of the studies were focused on pulmonary TB with blood/serum/plasma as the sample. The data sets were manually reviewed and only those fulfilling the following criteria were included for further analysis: (1) MiRNA expression profiling by array; and (2) Paired samples from patients with and without TB. Suitable data sets were identified and retrieved. Finally, dataset GSE34608 was selected for analysis which had miRNA profiling of whole blood RNA from 08 TB patients and 08 HCs respectively[11]. The dataset was analyzed using GEO2R tool available on the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo). Differentially expressed miRNAs were normalized and identified with specific cutoff criteria (P value = 0.05 and |log fold change| ≥ 2). Simultaneously a literature search for miRNAs playing a significant role in various biological processes integral in the pathogenesis of TB and found to be altered in multiple studies was done[12].
Total RNA was extracted using the miRNeasy Mini Kit (Qiagen, Valencia, CA, United States) according to the manufacturer’s instructions. Briefly700 μL QIAzol Lysis Reagent was used to extract RNA from EV samples through miRNeasy Mini spin columns. The RNA was eluted in 30 μL RNase-free water (Invitrogen, MA, United States) by centrifuging for 1 minute at ≥ 8000 × g (≥ 10000 rpm). RNA quality and quantity were determined by a Spectrophotometer (NanoDrop™2000/2000c) and stored at -80 °C until further use.
For expression analysis of miRNA, miRNA-specific stem-loop primers were designed using protocol as previously described[13,14] and synthesized by Eurofins Genomics India Pvt Ltd. cDNA synthesis was performed with 1 μg of total RNA template per sample by using High-Capacity cDNA Reverse Transcription Kit (applied biosystems) according to the manufacturer’s instructions. Real-time polymerase chain reaction (PCR) was performed using an ABI 7500 Sequence Detection System (Applied Biosystems, California, United States) in the presence of SYBR Green Master Mix (Takara Bio, Shiga, Japan). Standard PCR conditions were used as prescribed in the reagent protocol. Each miRNA level was normalized with U6 small nuclear RNA level. All samples were run in duplicate. Fold changes were calculated using the 2-ΔΔCT method (where Ct is the threshold cycle). The primer sequences used are shown in Table 1.
| Primer set name | Reverse transcriptase reaction primer (5’ to 3’) | Real-time quantitative PCR primer (5’ to 3’) |
| miRNA-155-5p | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACCCC | Forward: TCGCTTTAATGCTAATCGTGATA |
| Reverse: CCAGTGCAGGGTCCGAGGTA | ||
| miRNA-26a-5p | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCCTA | Forward: TCGCTTTCAAGTAATCCAGGA |
| Reverse: CCAGTGCAGGGTCCGAGGTA | ||
| miRNA-29a-3p | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAACCG | Forward: TCGCTTAGCACCATCTGAAAT |
| Reverse: CCAGTGCAGGGTCCGAGGTA | ||
| U6 | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAACGCTT | Forward: AGAAGATTAGCATGGCCCCTG |
| Reverse: CCAGTGCAGGGTCCGAGGTA |
GraphPad Prism 5 software was used for the statistical analysis. Data are presented as individual data with mean ± SD. The significance of differences between mean values was estimated using two-tailed unpaired Student’s t-test. P value < 0.05 was considered significant. Receivers operating characteristic curves was used to analyze the ability of uEV-miRNAs to differentiate UGTB from controls (disease controls and HCs), using culture as gold standard.
Out of the enrolled subjects 89 were classified as UGTB based on history, clinical presentation, AFB staining and/or Lowenstein-Jensen culture for MTB. Of 69 subjects which were negative for AFB staining and/or MTB cultures and came positive on culture for other bacteria were classified as UTI. Simultaneously 70, age and gender matched HCs were enrolled. There was no significant difference in age and sex distribution among the study groups. Table 2 provides the demographics of the subjects in each group. Amongst the 89 UGTB urine samples AFB smear was positive in 67 cases (75.3%) and negative in 22 cases (24.7%). The bacterial and MTB culture results are shown in Table 3 and Table 4 respectively.
| Characteristics | Healthy control | UTI | UGTB |
| n | 70 | 69 | 89 |
| Gender (male), n (%) | 32 (45.71) | 35 (50.72) | 43 (48.31) |
| Age (year), mean ± SD | 40 ± 15 | 42 ± 14 | 42 ± 15 |
| Age (groups), n | |||
| 18-35 years | 35 | 27 | 36 |
| 35-50 years | 15 | 18 | 19 |
| 51-65 years | 20 | 24 | 34 |
| Organism | Number |
| Escherichia coli | 40 |
| Other lactose fermenters | 5 |
| Non-lactose fermenters | 8 |
| Gram positive organisms | 17 |
| Mycobacterium tuberculosis | Culture results |
| Culture positive | 18 |
| Culture sterile | 55 |
| Contamination | 01 |
| Only smear | 15 |
NTA of uEVs revealed significant differences in the uEV size, and concentration/mL urine among the three groups Figure 1A-C shows representative NTA plot of the average concentration (particles/mL), and size of uEVs isolated from each group (n = 6/group). Significant differences in mean size of uEVs were detected in UTI urine when compared to Control and UGTB urine (P value = 0.0005 and 0.013 respectively, Figure 1D). While a higher mean size of uEVs were seen in UGTB patients’ urine compared to Control urine, the results were not statistically significant (P value = 0.24, Figure 1D). Similarly, the number of larger uEV particles (> 200 nm) were higher in the UTI and UGTB group as compared to HCs (n = 6/group, P value = 0.012 and 0.07 respectively Figure 1E).
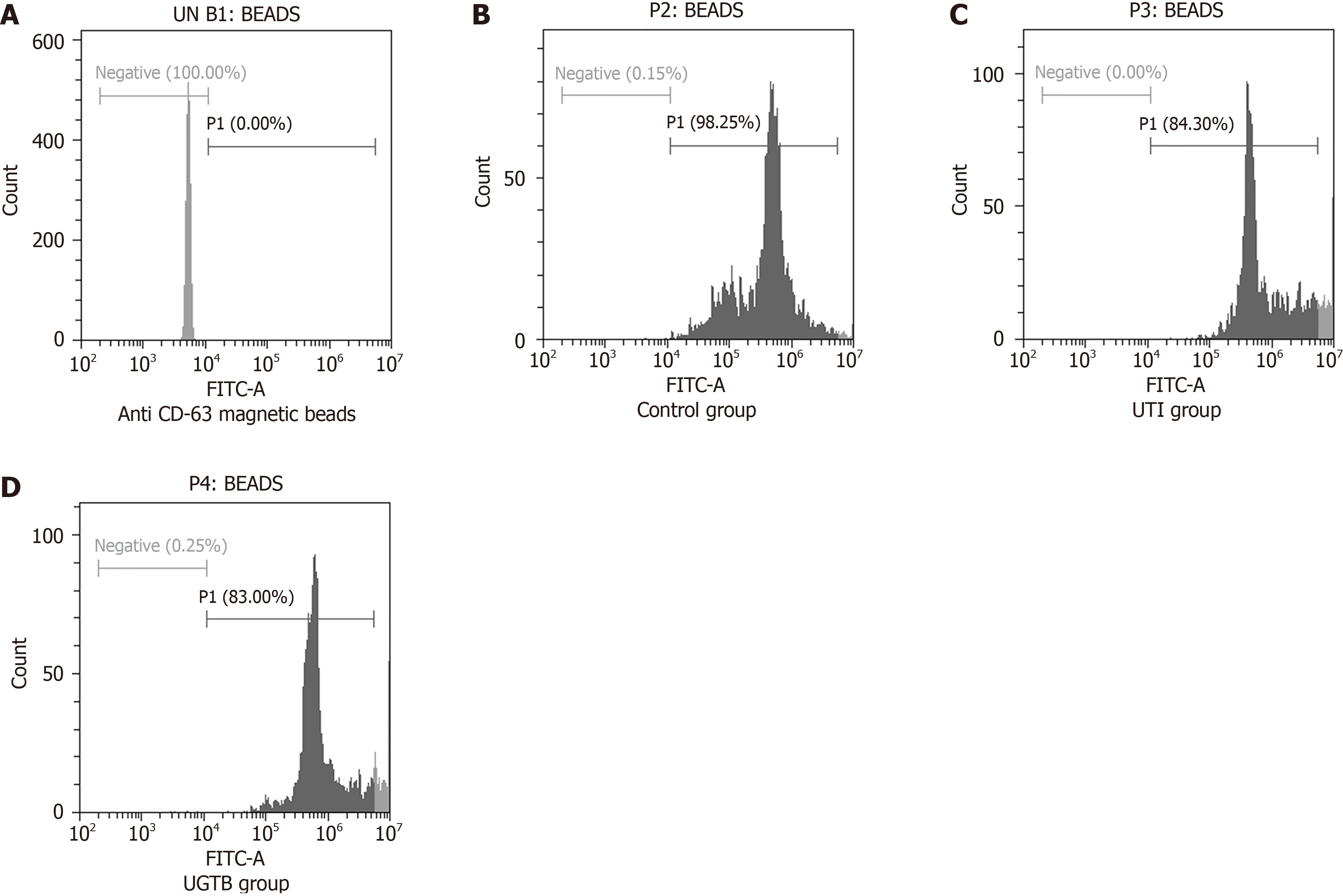
The percentage of uEV-bead complexes with positive staining for CD63, an exosome enriched tetraspanin known to be well expressed in EVs, was quantitated using flow cytometer (n = 3/group) (Figure 2). The uEVs were prepared from 3 mL urine from each group. Figure 2B-D shows the representative plot with 98.25%, 84.3% and 83% CD63 positive staining respectively in control, UTI and UGTB group.
Relative quantitation of miRNA-155-5p, miRNA-26a-5p and miRNA-29a-3p were done by RT-PCR in the uEVs in the three groups. U6 was used as an endogenous control to normalize the data[15]. These miRNAs were reported to have an association with TB pathogenesis, and were identified through miRNA profiling of serum samples from pulmonary TB infected subjects (n = 8). In our study, a lower abundance of both, miRNA-155-5p (compared to UTI, P value = 0.004) and miRNA-26a-5p (compared to HC, P value = 0.009) was recorded in uEVs of UGTB (Figure 3, n = 20/group). However, no such trend was evident in miRNA-29a-3p abundance. While a lower level of miRNA-29a-3p was seen in uEVs from UTI when compared HC and UGTB (Figure 3; n = 20/group, P value = 0.002 and 0.004 respectively).
Receivers operating characteristic curve showed an optimal diagnostic accuracy of miRNA-155-5p to differentiate UGTB from UTI as well as from HCs cases, with a likely hood ratio of 5.26 (P < 0.0001) and 2.16 (P = 0.05), respectively (Figure 4A). Moreover, miRNA-29a-3p and miRNA-26a-5p were also able to differentiate UGTB from UTI and HC cases, respectively with optimal sensitivity and specificity with a likelihood ratio of 4.33 and 2.8 respectively (Figure 4B). The cut-off values with sensitivity and specificity have been provided in Table 5.
| Classification | Cut-off | Sensitivity (%) | 95%CI | Specificity (%) | 95%CI | Likelihood ratio |
| UGTB vs UTI | ||||||
| miRNA-155-5p | > 2.680 | 78.95 | 56.67%-91.49% | 85.00 | 63.96%-94.76% | 5.263 |
| miRNA-29a-3p | < 2.945 | 65.00 | 43.29%-81.88% | 85.00 | 63.96%-94.76% | 4.333 |
| UGTB vs HC | ||||||
| miRNA-155-5p | > 2.320 | 65.00 | 43.29%-81.88% | 70.00 | 48.10%-85.45% | 2.167 |
| miRNA-26a-5p | > 1.565 | 70.00 | 48.10%-85.45% | 75.00 | 53.13%-88.81% | 2.800 |
UGTB represents up to a quarter of worldwide EPTB cases but yet remains a neglected clinical issue. More than 90% of UGTB cases occur in developing countries where it is the second most common cause of EPTB[16]. In our study UGTB was confirmed by culture in 18 out of the 89 UGTB suspects. Males were affected almost three times more as women. Similar results have been reported in various studies[3,17]. However, UGTB affecting twice as many women as men is also reported[2]. The median age of UGTB suspects in our study was 42 years. As per literature, although no age group is spared, UGTB is commonly seen in the fourth and fifth decade of life[17,18].
Given the paucibacillary nature of the disease and intermittent shedding of the bacilli collection of multiple urine samples (at least three) on consecutive days is recommended. The sensitivity of AFB smear in our study was 38% (7/18). The sensitivity of AFB smear microscopy is low as it requires the presence of 104 bacilli/mL of specimen to yield a positive result[19]. Another drawback are the false-positive results (11/18) which might be due to the presence of environmental, non-tubercular mycobacteria. Although culture is the diagnostic gold standard for UGTB its sensitivity ranges from 15%-90%[2,19]. In our study culture had a sensitivity of 24.3% (18/65). In spite of the above demerits microscopy and culture are still warranted for diagnosis, monitoring response to treatment and detecting relapse.
In this study we explored the biomarker potential of EV shed during MTB infection for diagnosing UGTB. EVs are crucial for biological processes, and their dysfunction is linked to various diseases. Their cargo (DNA, mRNA, miRNA, proteins, and lipids) has the potential to be used for diagnosing MTB infection[5]. Research on EVs in TB is still at preliminary stage. Numerous studies have shown that Mycobacteria release EVs and induce various host immune responses[20]. To our knowledge, this study is the first to analyze uEVs in UGTB suspects and identify uEV-miRNAs with the potential to diagnose UGTB. The altered uEV profile in UTI and UGTB cases compared to healthy individuals could serve as an initial clue towards the presence of a pathology and/or disease state.
In addition, we found differential levels of certain miRNAs in the uEVs of cases. miRNAs cause significant immunomodulation during TB pathogenesis and their differential expression pattern has revealed their usefulness as biomarkers. Literature search revealed several studies that have identified miRNAs (> 100 in number) to be regulated in whole blood, plasma, serum, peripheral blood mononuclear cells, sputum and pleural fluid collected from patients with active pulmonary TB. However, studies on UGTB are lacking and a miRNA signature is yet to be finalized even for TB[12]. This could be due to rapid degradation of miRNAs in the biofluid, especially in urine.
The role of miRNA-155 as a pro-inflammatory miRNA with established roles in innate and adaptive immunity, especially in macrophage activation and production of cytokines both of which are integral in the pathogenesis of TB is well characterized[12]. In our study we noticed a lower levels of miRNA-155 in uEVs from UGTB urine when compared to HC (although P value = 0.12). Its downregulation may indicate an impaired host immune response or an evasion strategy employed by MTB with selective miRNA packaging hampering intercellular communications especially during chronic infections[21]. Bidirectional trends in its level have been reported in samples of TB patients[12]. It is intricately linked to TB pathogenesis via its role in tumor necrosis factor α synthesis by targeting Src homology 2 domain-containing inositol polyphosphate 5-phosphatase 1, that promotes cell apoptosis through the modification of the phosphatidylinositol 3-kinase/protein kinase B signaling pathway[8]. It also orchestrates the interferon-gamma (IFN-γ) production in CD4+ and CD8+ T-cells by targeting suppressor of cytokine signaling-1[22]. Other notable mechanisms include contribution to Th17 cell function by suppressing the inhibitory effects of Jumonji and AT-rich interaction domain containing 2[23], fine tuning autophagy response through inhibition of the negative regulator Ras homolog enriched in brain and components of the mammalian target of rapamycin signaling pathway and polarization of macrophages towards anti-inflammatory M2 phenotype[24].
During MTB infection, miRNA-26a regulates various facets of macrophage signaling pathways which are exploited to ensure its continuum in the host. These include upregulation of Kruppel-like factor 4 which in turn favors increased arginase and decreased inducible nitric oxide synthase activity. Effects were also seen in MTB trafficking to lysosomes and favoring M2 polarization via the cyclic adenosine monophosphate response element binding and CCAAT enhancer-binding protein beta signaling axis[25]. MiRNA-26a is reported to exert negative regulatory effects on inflammatory mediators and is implicated in the modulation of the IFN-γ signaling pathway[26]. In our study lower levels of miRNA-26a were found in UGTB urine compared to HCs (P value = 0.009). Similar expression trends were cited by other studies done on MTB infected macrophages of murine and human origin[25,26]. This downregulation might also represent a protective mechanism to mitigate tissue damage caused by sustained inflammation but in the bargain potentially facilitating the persistence of MTB within genitourinary tissues.
Another key player associated with MTB infection is miRNA-29a which has been identified as a negative regulator of innate and adaptive immunity and is known to target IFN-γ mRNA, thereby modulating T-cell responses[27]. It also plays a role in regulation of WNT signaling which is an ancient and highly conserved signaling mechanism which have been associated with cellular proliferation, differentiation, apoptosis, motility, and polarization of cells[27]. Usually, an inverse correlation between the expression of miRNA-29a and IFN-γ during infection with an intracellular pathogen has been cited but we noticed no such trend in our study population[27,28]. Its elevated presence in uEVs of UGTB patients and HCs, suggests a fundamental role in immune regulation or the maintenance of immunological homeostasis. The further upregulation observed in the context of chronic infection may reflect an adaptive mechanism in response to sustained immune activation and bacterial persistence[28].
While many articles have indicated the potential of miRNAs as biomarker for TB, our study is the first to evaluate their biomarker potential in UGTB cases. Besides, we utilized an approach to analyze these uEV-miRNAs, which may be a more sensitive approach than their analysis in total urine[29]. While our study yielded promising results warranting further investigation regarding the potential diagnostic utilities of miRNA-155-5p, miRNA-26a-5p and miRNA-29a-3p, we acknowledge that there were limitations to our research. These miRNAs may offer valuable complementary insights to existing diagnostic methods by reflecting underlying immunopathological processes and indicating the presence of disease. The generalizability of our findings could be further improved by increasing the sample size. Furthermore, it may be worthwhile to explore their expression in other types of cells or tissues to better understand their role in UGTB pathogenesis. While this study successfully employed both commercially available kit and in-house developed reagent for uEV isolation, it is important to acknowledge certain methodological limitations. Specifically, a systematic comparative analysis of the isolation strategies which will dictate the choice of isolation method and can significantly influence downstream analyses, including EV yield, purity, and functional activity.
UGTB remains a significant health concern worldwide. Consequently, new diagnostic approaches are required to curtail the progression and spread of this disease. The non-coding RNAs, including, miRNA-155-5p, miRNA-26a-5p and miRNA-29a-3p play a pivotal role in governing innate and adaptive immunity, allowing the pathogen to avoid elimination by the immune system and persist in the body. We demonstrated the potential diagnostic utilities of these miRNAs in UGTB. Moreover, we provided a non-invasive approach that utilizes uEVs samples for the analysis of miRNAs in UGTB patients. EVs protect the miRNAs from the toxic environment of urine, and hence enhance the sensitivity of the miRNAs-based diagnosis. Confirmation of the diagnostic accuracy of uEV-miRNAs, miRNA-155-5p, miRNA-26a-5p and miRNA-29a-3p, in a bigger subset of patient sample can provide a basis for innovative UGTB biomarkers and expand our awareness of UGTB pathophysiology.
The authors wish to acknowledge the following persons from Department of Molecular Medicine and Biotechnology, SGPGIMS - Dr. Nishant for helping with primer designing, Ms Sukhanshi Khandpur and Ms Medha Srivastava for the technical help and Mr Chamoli and Ms Sadhna from Department of Microbiology, SGPGIMS for help with sample collection and processing.
| 1. | World Health Organization. Global tuberculosis report 2021. [cited 14 January 2025]. Available from: https://www.who.int/publications/digital/global-tuberculosis-report-2021. |
| 2. | Muneer A, Macrae B, Krishnamoorthy S, Zumla A. Urogenital tuberculosis - epidemiology, pathogenesis and clinical features. Nat Rev Urol. 2019;16:573-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Zajaczkowski T. Genitourinary tuberculosis: historical and basic science review: past and present. Cent European J Urol. 2012;65:182-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, Zumla A. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18:e199-e210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 5. | Palacios A, Gupta S, Rodriguez GM, Prados-Rosales R. Extracellular vesicles in the context of Mycobacterium tuberculosis infection. Mol Immunol. 2021;133:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Das K, Garnica O, Dhandayuthapani S. Modulation of Host miRNAs by Intracellular Bacterial Pathogens. Front Cell Infect Microbiol. 2016;6:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Paris L, Magni R, Zaidi F, Araujo R, Saini N, Harpole M, Coronel J, Kirwan DE, Steinberg H, Gilman RH, Petricoin EF 3rd, Nisini R, Luchini A, Liotta L. Urine lipoarabinomannan glycan in HIV-negative patients with pulmonary tuberculosis correlates with disease severity. Sci Transl Med. 2017;9:eaal2807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Sinigaglia A, Peta E, Riccetti S, Venkateswaran S, Manganelli R, Barzon L. Tuberculosis-Associated MicroRNAs: From Pathogenesis to Disease Biomarkers. Cells. 2020;9:2160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T, Sahoo S, Torrecilhas AC, Zheng L, Zijlstra A, Abuelreich S, Bagabas R, Bergese P, Bridges EM, Brucale M, Burger D, Carney RP, Cocucci E, Crescitelli R, Hanser E, Harris AL, Haughey NJ, Hendrix A, Ivanov AR, Jovanovic-Talisman T, Kruh-Garcia NA, Ku'ulei-Lyn Faustino V, Kyburz D, Lässer C, Lennon KM, Lötvall J, Maddox AL, Martens-Uzunova ES, Mizenko RR, Newman LA, Ridolfi A, Rohde E, Rojalin T, Rowland A, Saftics A, Sandau US, Saugstad JA, Shekari F, Swift S, Ter-Ovanesyan D, Tosar JP, Useckaite Z, Valle F, Varga Z, van der Pol E, van Herwijnen MJC, Wauben MHM, Wehman AM, Williams S, Zendrini A, Zimmerman AJ; MISEV Consortium, Théry C, Witwer KW. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1603] [Cited by in RCA: 1396] [Article Influence: 1396.0] [Reference Citation Analysis (0)] |
| 10. | Ahmad S, Deep G, Punzi HA, Su Y, Singh S, Kumar A, Mishra S, Saha AK, Wright KN, VonCannon JL, Dell'Italia LJ, Meredith WJ, Ferrario CM. Chymase Activity in Plasma and Urine Extracellular Vesicles in Primary Hypertension. Kidney360. 2024;5:1613-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 11. | Maertzdorf J, Weiner J 3rd, Mollenkopf HJ; TBornotTB Network, Bauer T, Prasse A, Müller-Quernheim J, Kaufmann SH. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci U S A. 2012;109:7853-7858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 12. | Pattnaik B, Patnaik N, Mittal S, Mohan A, Agrawal A, Guleria R, Madan K. Micro RNAs as potential biomarkers in tuberculosis: A systematic review. Noncoding RNA Res. 2022;7:16-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Kramer MF. Stem-loop RT-qPCR for miRNAs. Curr Protoc Mol Biol. 2011;Chapter 15:Unit 15.10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3676] [Cited by in RCA: 3907] [Article Influence: 195.4] [Reference Citation Analysis (0)] |
| 15. | Duan ZY, Cai GY, Li JJ, Bu R, Wang N, Yin P, Chen XM. U6 can be used as a housekeeping gene for urinary sediment miRNA studies of IgA nephropathy. Sci Rep. 2018;8:10875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Grange JM, Yates MD, Ormerod LP. Factors determining ethnic differences in the incidence of bacteriologically confirmed genitourinary tuberculosis in south east England. J Infect. 1995;30:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Mantica G, Ambrosini F, Riccardi N, Vecchio E, Rigatti L, De Rose AF, Van der Merwe A, Terrone C, Bartoletti R, Bonkat G. Genitourinary Tuberculosis: A Comprehensive Review of a Neglected Manifestation in Low-Endemic Countries. Antibiotics (Basel). 2021;10:1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Berta M, Sturm G, Juri L, Cosiansi MC, Barzón S, Barnes AI, Rojo SC. [Bacteriological diagnosis of renal tuberculosis: an experience at the regional tuberculosis laboratory in Córdoba Province, Argentina]. Rev Argent Microbiol. 2011;43:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Schorey JS, Cheng Y, McManus WR. Bacteria- and host-derived extracellular vesicles - two sides of the same coin? J Cell Sci. 2021;134:jcs256628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | O'Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 22. | Hirschberger S, Hinske LC, Kreth S. MiRNAs: dynamic regulators of immune cell functions in inflammation and cancer. Cancer Lett. 2018;431:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 23. | Escobar TM, Kanellopoulou C, Kugler DG, Kilaru G, Nguyen CK, Nagarajan V, Bhairavabhotla RK, Northrup D, Zahr R, Burr P, Liu X, Zhao K, Sher A, Jankovic D, Zhu J, Muljo SA. miR-155 activates cytokine gene expression in Th17 cells by regulating the DNA-binding protein Jarid2 to relieve polycomb-mediated repression. Immunity. 2014;40:865-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Wang J, Yang K, Zhou L, Minhaowu, Wu Y, Zhu M, Lai X, Chen T, Feng L, Li M, Huang C, Zhong Q, Huang X. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013;9:e1003697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 25. | Sahu SK, Kumar M, Chakraborty S, Banerjee SK, Kumar R, Gupta P, Jana K, Gupta UD, Ghosh Z, Kundu M, Basu J. MicroRNA 26a (miR-26a)/KLF4 and CREB-C/EBPβ regulate innate immune signaling, the polarization of macrophages and the trafficking of Mycobacterium tuberculosis to lysosomes during infection. PLoS Pathog. 2017;13:e1006410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Ni B, Rajaram MV, Lafuse WP, Landes MB, Schlesinger LS. Mycobacterium tuberculosis decreases human macrophage IFN-γ responsiveness through miR-132 and miR-26a. J Immunol. 2014;193:4537-4547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | He J, Xiong J, Huang Y. miR-29 as diagnostic biomarkers for tuberculosis: a systematic review and meta-analysis. Front Public Health. 2024;12:1384510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, Hua M, Li N, Yao H, Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol. 2011;12:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 530] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 29. | Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, Algehainy N, Alanazi MA, Abou-Samra AB, Kumar R, Al-Shabeeb Akil AS, Macha MA, Mir R, Bhat AA. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 359] [Article Influence: 359.0] [Reference Citation Analysis (0)] |