Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.104431
Revised: April 21, 2025
Accepted: July 18, 2025
Published online: September 20, 2025
Processing time: 235 Days and 16.1 Hours
Autoimmune diseases frequently present with ophthalmological manifestations, posing significant diagnostic and therapeutic challenges. This review delved into the complex interplay between autoimmunity and ocular health, highlighting common manifestations such as uveitis, keratitis, and optic neuritis. We explored advanced diagnostic tools and techniques to improve early detection and accurate diagnosis. Additionally, the review addressed current therapeutic strategies, emphasizing the need for tailored treatments to manage ocular symptoms effectively while minimizing systemic side effects. By overcoming these cha
Core Tip: Early detection of symptoms related to ocular manifestations in autoimmune diseases, leading to vision-threatening conditions such as uveitis and optical neuritis, is critical for preventing irreversible damage. Advanced diagnostic tools and useful serological biomarkers offer improved accuracy but remain underutilized. Effective management requires balancing symptom control with minimizing immunosuppression risks and emphasizes a multidisciplinary approach involving ophthalmologists and rheumatologists. Continued research is vital to address unmet clinical needs and optimize outcomes.
- Citation: El Kaouri I, Deligiannis I, Bakopoulou K, Sdralis PP, Shoumnalieva-Ivanova V, Shumnalieva R, Velikova T. Ophthalmological manifestations in autoimmune diseases: Overcoming diagnostic and therapeutic challenges. World J Exp Med 2025; 15(3): 104431
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/104431.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.104431
Autoimmune diseases are a group of disorders in which the immune system mistakenly targets the body’s own cells, tissues, or organs, resulting in chronic inflammation and tissue damage[1]. These diseases are mostly systemic and involve multiple organ systems with various presentations. Simply, they are not homogenous when it comes to variables like their pathogenesis, symptoms, and prevalence.
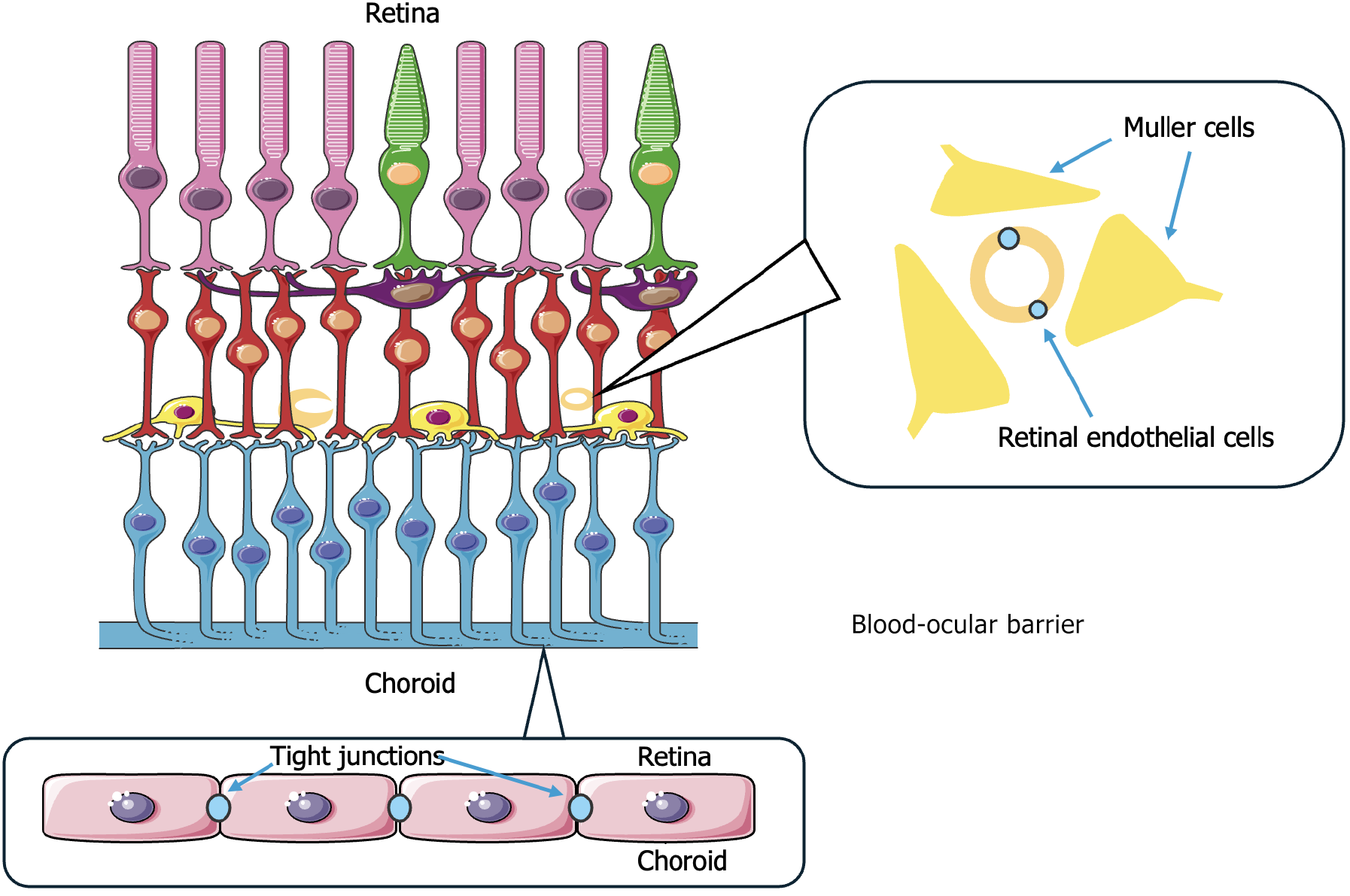
The global burden of autoimmune diseases is high with studies showing that it affects approximately 5% of the United States population and around 5%-10% of the industrialized world population[2,3]. One of the organ systems frequently affected by autoimmune diseases is the eye, which is particularly vulnerable due to its immune-privileged status (Figure 1). Ophthalmological manifestations often reflect underlying systemic activity and at times precede the systemic symptoms of the condition. Common complications involving the eye include dry eye disease in Sjörgen’s syndrome (SS), uveitis in ankylosing spondylitis (AS), scleritis, episcleritis, and keratitis in rheumatoid arthritis (RA), and retinal vasculitis in systemic lupus erythematosus (SLE) and granulomatosis with polyangiitis (GPA). The conditions mentioned above and many others can range from mild ocular symptoms to severe complications threatening the patient’s vision. Early detection and treatment of ocular symptoms are important for preventing irreversible damage and improving outcomes[3].
Due to the nature of ophthalmological conditions and the vast differential diagnosis, diagnosing ocular manifestations as part of a systemic condition remains challenging. Advances in diagnostic techniques, both in imaging and laboratory, including optical coherence tomography (OCT), OCT angiography (OCTA), functional imaging, and serological biomarkers like aquaporin 4 (AQP4), have improved diagnostic accuracy, but their use is not widespread yet. When treating these conditions, clinicians should aim to strike a balance between controlling the symptoms and minimizing the adverse effects of immunosuppression, highlighting the need for ongoing research[4-6].
The aim of this review was threefold. Our objective was to delve deeper into the available advanced diagnostic tools and discuss the challenges clinicians face when treating patients with autoimmune diseases. We aimed to discuss current therapeutic and emerging approaches focusing on unmet clinical needs and to highlight the importance of a multidisciplinary approach involving rheumatologists, ophthalmologists, and other clinical and research specialists to optimize patient outcomes. This review provided a comprehensive understanding of the topics mentioned earlier.
The research strategy for this paper focused on a comprehensive review of the literature to explore ocular manifestations in autoimmune diseases. PubMed, MEDLINE, Scopus, and Web of Science databases were utilized to gather evidence-based data. Key topics included diagnostic challenges, advances in imaging technologies, and therapeutic approaches like corticosteroids (CS) and biologics. Emphasis was placed on multidisciplinary management and the integration of biomarkers in diagnostics.
The search terms included combinations of the following keywords with Boolean operators: (“ophthalmological manifestations” OR “ocular manifestations” OR “eye involvement”) AND (“autoimmune diseases” OR “autoimmune disorders” OR “systemic autoimmune diseases”) AND (“diagnosis” OR “diagnostic challenges” OR “diagnostic tools”) AND (“therapy” OR “treatment” OR “therapeutic challenges” OR “immunotherapy”) AND (“uveitis” OR “keratitis” OR “optic neuritis” OR “dry eye syndrome”) AND (“biomarkers” OR “imaging techniques” OR “optical coherence tomo
Articles were critically reviewed for relevance, methodology, and outcomes. This approach ensured a robust synthesis of current knowledge while identifying gaps for future research managing ocular involvement in autoimmune disease.
Autoimmune inflammatory diseases are able to affect nearly every part of the eye and orbit, sometimes presenting as the first or single manifestation of an underlying systemic disease[7]. Ophthalmic involvement may appear either preceding or during active disease or years after the diagnosis[8]. Common manifestations include orbital or intraocular symptoms (e.g., uveitis, keratitis) or adnexal inflammation [e.g., optic neuritis (ON)] and often render emergencies requiring urgent treatment[9]. An observational cross-sectional study performed by Cifuentes-González et al[10] concluded that the most common ocular manifestation of autoimmune disease, reported in approximately 1 out of 3 patients (30.86%), was dry eye sensation[10]. The eye is historically recognized as an immune-privileged tissue. Ocular immune privilege is mediated by physical barriers, such as the blood-ocular barrier or the absence of lymphatic drainage from the anterior chamber, and immunoregulatory molecules, which protect the eye from local inflammatory processes[11]. Disruption of this protective environment by autoimmune conditions, often in the background of vasculitis or multisystemic disease, can result in a range of minor to vision-threatening symptoms that frequently are underestimated in general clinical practice[8].
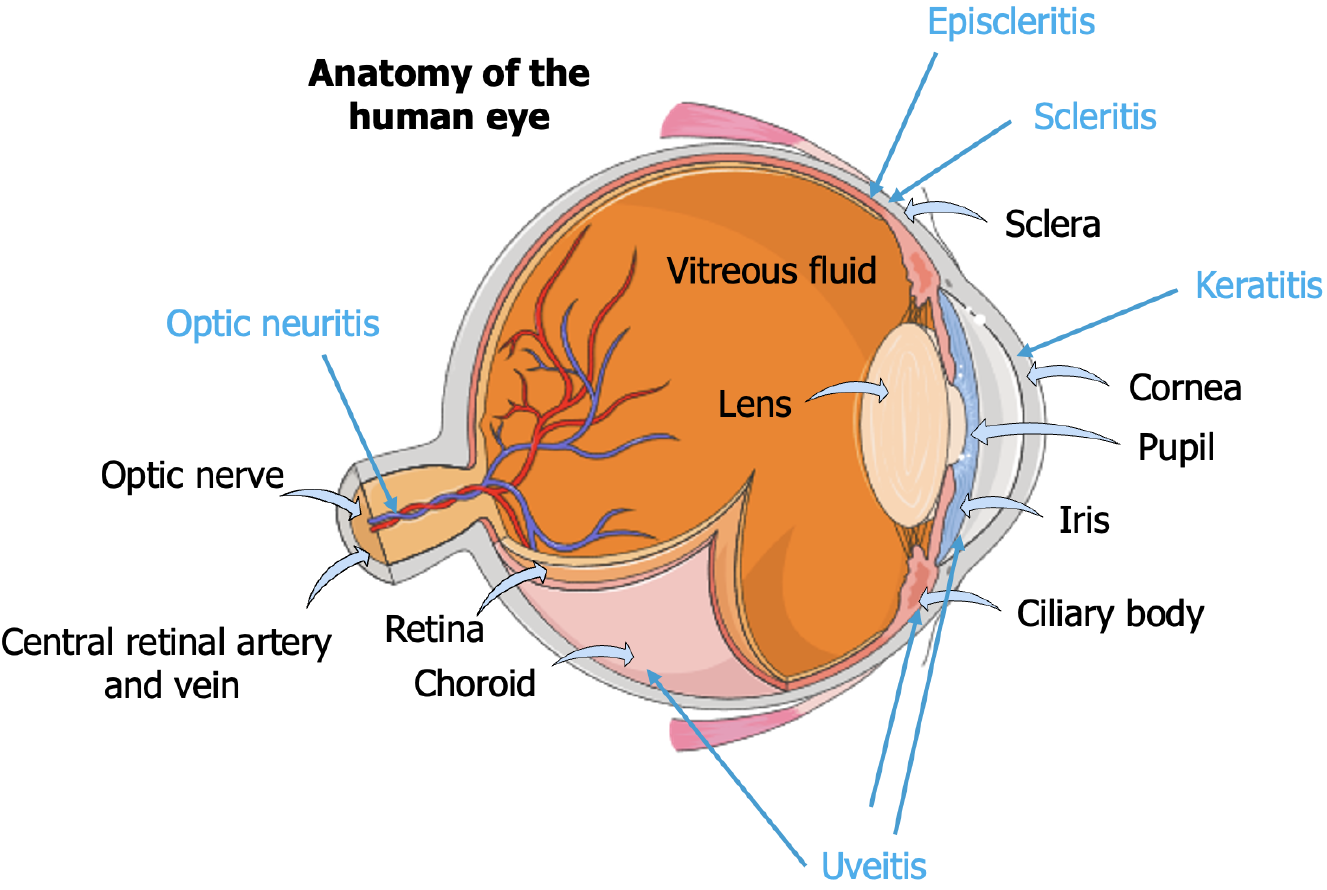
In Figure 2, we present the basic anatomy of the human eye, highlighting its many structures while labeling different conditions that can affect the eye and their locations.
Intraocular inflammation of the uvea (uveitis) is an important manifestation of various autoimmune diseases[9]. Uveitis can affect the iris, ciliary body, or choroid and is classified according to anatomical involvement into anterior, inter
| Condition/manifestation | Primary location | Clinical features | Common autoimmune diseases | Non-autoimmune/differential diagnoses | Complications | Ref. |
| Uveitis | Uvea (iris, ciliary body, choroid) | Redness, pain, photophobia, blurred vision, floaters | RA, SS, BD, CD, UC, MS, celiac, sarcoidosis | HSV, VZV, CMV, toxoplasmosis, TB, trauma, leukemia, Lyme disease | Macular edema, glaucoma, cataracts, Horner’s syndrome | [7,9,12-27,60-63] |
| Keratitis | Cornea | Redness, photophobia, blurred vision, pain, FB sensation | RA, SS, SLE, celiac, Hashimoto’s, vasculitis | HSV, HHV-6, fungi, bacteria, trichiasis, trauma | Ulceration, perforation, corneal thickening | [9,17,21,30-38,61,62,64] |
| Optic neuritis | Optic nerve | Visual impairment (unilateral or bilateral), pain with eye movement | MS, NMO, MOG-AD, sarcoidosis, SLE, SS | Syphilis, TB, Lyme disease, compressive lesions, toxins | Blindness, demyelination, optic atrophy | [40-42,46-53,65] |
| Episcleritis | Episcleral tissue | Mild pain, redness, irritation | RA, SS, CD, UC, dermatomyositis | Syphilis, Lyme disease, TB, HZV, hypersensitivity reactions | Generally self-limiting | [16,54,56,66-70] |
| Scleritis | Sclera | Severe pain, photophobia, possible vision loss | RA, SS, CD, UC, GPA, PAN, BD | Monkeypox, TB, HZV, syphilis, post-op, bisphosphonates | Necrotizing inflammation, retinal vasculitis, vision loss | [10,16,54-59,62,66,67,71] |
| Keratoconjunctivitis sicca | Cornea/Conjunctiva | Dryness, photophobia, gritty sensation | RA, SS, CD, celiac, GBS, MG, GD, Hashimoto’s, psoriasis, SSc | Medications, meibomian gland dysfunction, environment, HIV/AIDS | Corneal damage, infection risk | [72,73] |
| Conjunctivitis | Conjunctiva | Redness, edema, lacrimation, discomfort | RA, CD, psoriasis, celiac | Allergens, irritants, viruses (e.g., SARS-CoV-2, flavivirus) | Persistent inflammation, scarring | [61,62,74,75] |
| Blepharitis | Eyelids | Burning, photophobia, irritation, redness | RA, CD, MG, psoriasis | Staph, VZV, meibomian dysfunction, rosacea | Chronic discomfort, eyelid scarring | [76,77] |
| Retinal vasculitis | Retina | Floaters, scotoma, color vision changes | RA, SS, BD, CD | CMV, TB, syphilis, Lyme disease, flavivirus | Vision loss, retinal ischemia | [60,61,78] |
| Macular edema | Macula | Blurry vision, micro/macropsia, color impairment | RA, BD, GBS, DM1 | RVO, tumors, trauma, radiation | Vision loss, distortion | [79,80] |
| Choroiditis | Choroid | Floaters, metamorphopsia, central vision loss | RA | TB, vector-borne infections | Vision distortion, scarring | [60,81] |
| Photophobia | Nonspecific | Light intolerance | SS, GBS, Hashimoto’s | Migraine, TBI, optic/chiasmal pathology | Underlying diagnosis-dependent | [82,83] |
| Retinal detachment | Retina | Flashes, floaters, shadow over vision | RA, BD, UC, MS, SSc | Trauma, tumors, medications | Vision loss | [84] |
| Vitreal/retinal hemorrhage | Vitreous/retina | Floaters, cobwebs, haziness | BD, MS, GBS, dermatomyositis | Endocarditis, trauma, PCV, flavivirus | Vision loss | [62,72,77,85] |
| Retinal vein occlusion | Retina | Vision loss, RAPD | BD, UC, celiac, dermatomyositis | Polycythemia vera, thrombophilia, drugs | Edema, hemorrhage, ischemia | [86] |
| Glaucoma | Optic nerve | Progressive vision loss, dark adaptation delay | BD, MS, GD, DM1 | Ocular HTN, steroids, surgery, trauma | Irreversible blindness | [87] |
| Corneal ulcers | Cornea | Pain, redness, photophobia, discharge | SS, CD, celiac | Bacteria, HHV-6, trauma | Vision loss, perforation | [71,72] |
| Diplopia | EOMs/CNS | Double vision | GBS, MG, GD, Hashimoto’s, SSc, dermatomyositis | Orbital mass, refractive error, neuromuscular issues | Falls, impaired depth perception | [88,89] |
| Optic atrophy | Optic nerve | Vision loss, field defects | BD | Ischemic, infectious, compressive, toxic | Permanent vision loss | [65] |
| Cataracts | Lens | Blurry vision, glare, color desaturation | CD, Celiac, MS, DM1, psoriasis, SSc, dermatomyositis | Corticosteroids, trauma, radiation | Progressive vision loss | [90] |
RA is another autoimmune disease frequently associated with uveitis, particularly anterior uveitis[20]. Ocular symptoms usually precede joint involvement, including pain, photophobia, redness without discharge, and decreased vision[21]. Although the exact mechanism linking RA and uveitis is not fully understood, one transcription factor, signaling transducer and activator of transcription 3 (STAT3), is shown to play an important role in the pathogenesis of the symptoms[22]. According to Escobar et al[22], STAT3 is needed for the differentiation of Th17 CD4+ cells, which in turn produce interleukin (IL) 17 but also for the expression of microRNA155 (miR-155) in the same type of cells. In their study miR-155 and STAT3-deficient mice failed to develop experimental autoimmune uveitis. Thus, it became evident that STAT3 and miR-155 form an axis that sets the ground for the pathogenesis of uveitis through IL-17.
Moreover, Behçet’s disease frequently causes recurrent uveitis, characterized by non-granulomatous necrotizing obliterative retinal vasculitis that can affect any part of the uvea[23]. Posterior and panuveitis are the most common forms while typical complaints include blurred vision, photophobia, pain, and conjunctival hyperemia[24]. Ocular symptoms usually do not precede systemic manifestations, but their significance should not be overlooked as severe complications such as retinal lesions, cataract, and glaucoma can arise[8,25,26]. Intense inflammation (hypopyon) can be seen in around 20% of patients with ocular involvement and is associated with a poor prognosis of blindness.
Finally, other autoimmune diseases, such as AS and multiple sclerosis (MS), have also been associated with uveitis; in AS anterior uveitis is present in 20%-40% of patients in developed countries[12] while intermediate uveitis is common in MS[27]. More specifically, uveitis in MS is thought to be secondary to inflammation of the central nervous system (CNS), but the exact mechanism is still under investigation.
In general proinflammatory cytokines, most importantly IL-6, play an important role in developing immune-related uveitis. Murray et al[28] revealed that in some autoimmune uveitis, the levels of IL-6 in the aqueous humor are drastically elevated without a similar increase in serum levels[28]. Moreover, Ooi et al[29] showed that IL-6 anti-TGF-β activity could counteract the fine balance of the anterior chamber-associated immune deviation, which is crucial for maintaining ocular immune privilege.
Keratitis, or inflammation of the cornea, can also be manifested in various autoimmune diseases. In SS lacrimal gland dysfunction can result in keratoconjunctivitis sicca (KCS) [or dry eye syndrome (DES)] due to abnormal tear film function[30]. Patients experience photosensitivity, itching, and foreign body sensations, which worsen during low blinking rates[31]. Complications of untreated KCS are severe and may include corneal ulceration and in some cases perforation[21]. Early diagnosis is therefore important to prevent such consequences.
KCS is also common in SLE, affecting more than one-third of patients[9]. KCS in SLE often coincides with the development of secondary SS. According to Chen et al[32], symptom severity correlates with anti-dsDNA antibody titers and low C3 Levels. Corneal involvement in SLE may also present as erosions, keratoendothelitis, or peripheral ulcerative keratitis (PUK)[33] with typical manifestations including painful eyes, hyperlacrimation, and blurred vision. However, in a great percentage of patients, the disease remains silent until complications arise, thus underscoring the importance of regular eye examinations for SLE patients. Interestingly, Monov et al[34] recently described a rare case of retinal vasculitis as the first manifestation of SLE, implying that the inclusion of ocular manifestations among the classification criteria for SLE would enable earlier establishment of the diagnosis and therapeutic interventions[34].
In RA DES results from immune-mediated damage to the lacrimal glands by B and T lymphocytes[35]. Symptoms include roughness, redness, and impaired vision[17], and studies have shown that KCS is present in 29% of patients with RA while bilateral PUK is found in 10% of cases[21,36,37].
PUK is associated with systemic vasculitis syndromes such as ANCA-associated vasculitis (AAV) and polyarteritis nodosa[38]. It is associated with the deposition of immune complexes in the periphery of the cornea, conserving the central cornea due to the lack of blood supply[39]. Typical symptoms include pain, redness, frequent lacerations, and blurred vision, but the diagnosis is made by slit lamp examination of crescent-shaped ulcers[21].
ON is an acute inflammatory condition that causes optic nerve demyelination, often causing permanent visual im
The mechanism of the development of optic nerve damage involves demyelination and axonal injury with anti-AQP4 antibodies or anti-myelin oligodendrocyte glycoprotein playing a central role while at the same time serving as biomarkers. AQP4 is a water channel expressed on the surface of astrocytes in the CNS[43]. Axonal injury is mediated by AQP4 antibodies with the involvement of the complement system, eventually leading to astrocytic lysis[44]. This damage was also shown to be independent of complement participation[45].
Moreover, ON in MOG-AD and NMO is more extensive than in MS, involving more optic nerve segments and often involving the anterior optic nerve in MOG-AD and the posterior optic chiasm or tract in NMO[46,47]. The authors of three separate studies[47-49] reported that optic chiasm involvement occurred in the majority (60%-75%) of patients with NMO but is less common in MOG-AD and rare in MS. Consecutively, radiological differences can help differentiate these diseases as patients with NMO and MOG-AD have more extensive optic nerve involvement. Magnetic resonance imaging is important for diagnosing ON and distinguishing it from noninflammatory optic neuropathy while contrast-enhanced orbital imaging can help differentiate demyelinating disease[50].
Additionally, longitudinally extensive ON lesions are a key feature of NMO and MOG-AD, whereas MS typically shows shorter lesion involvement[51]. A study by Dunker et al[52] showed that lesions greater than 17.5 mm or involving the intracanalicular portion of the optic nerve are associated with poorer visual outcomes[52]. On the other hand a more recent study by Kupersmith et al[53] suggested that the shorter the lesion, less than 17.5 mm, the better the recovery[53].
Similarly, although less frequently, episcleritis and scleritis can occur in autoimmune disease[8]. Scleritis (inflammation of the sclera) presents with severe eye pain, photophobia, and possible vision loss. In contrast episcleritis (inflammation of the tissue between the sclera and conjunctiva) is usually less uncomfortable and has a slower course[54].
Scleritis, especially in RA and GPA, can result in serious complications such as necrotizing inflammation[55] and retinal vasculitis leading to visual loss[56]. It usually presents with discharge, photophobia, and decreased visual acuity as opposed to episcleritis, which is characterized by mild pain, irritation, and redness limited to a single area of the eye[57]. GPA-associated scleritis presents with severe bilateral pain and diffuse inflammation with anterior scleritis being the most common[58]. Systemic vasculitides such as polyarteritis nodosa and Behçet’s disease are also associated with scleritis[54]. Prompt diagnosis and treatment are important because of the sight-threatening nature of scleritis and its association with systemic diseases[59].
A summary of ocular manifestations associated with autoimmune diseases is presented in Table 1, emphasizing the most common clinical symptoms and complications of conditions like uveitis, keratitis, and ON. The main autoimmune diseases and non-autoimmune diseases that may cause uveitis (i.e., uveitis associated with autoimmune diseases[7,9,12], in sarcoidosis[13-17], tuberculosis[18-19], rheumatoid arthritis[20,21], experimental autoimmune uveitis[22], uveitis in Behçet disease[23-26], multiple sclerosis[27]; viral infections[60-63]), keratitis (autoimmune diseases[9], sarcoidosis[17], rheumatoid arthritis[21], Sjögren Syndrome[30-32], SLE[33,34], rheumatoid arthritis[35-38], viral infections[61,62,64], ON (management[40-42] multiple sclerosis[46-49], visual performance[53], optic atrophy[65], episcleritis (associated with sarcoidosis[16], noninfectious[54], systemic disease[56], infections[66-70], scleritis (rheumatological diseases[10], sarcoidosis[16], noninfectious and systemic[54-59], viral infections[62,66,67,71]), KCS cornea[72,73], conjunctivitis[61,62,74,75], blepharitis[76,77], retinal vasculitis[60,61,78], macular edema[79,80], choroiditis[60,81], photophobia[82,83], retinal detachment[84], vitreal/retinal hemorrhage[62,72,77,85], retinal vein occlusion[86], glaucoma[87], corneal ulcers[71,72], diplopia[88,89], optic atrophy[65], and cataracts[90] and their complications are presented in Table 1.
Eye involvement in the context of systemic autoimmune disease can prove to be a severe sight-threatening complication. Therefore, early diagnosis and timely management are required even in asymptomatic patients. Early screening should ideally include an ophthalmologic examination as an integral part of routine multisystemic assessment of the patient with the autoimmune disease. However, the commonly offered methods of traditional eye examination have several potential limitations.
A significant rate-limiting issue arises from the lack of specificity in the traditionally encountered tests. Thus, the lack of standardized diagnostic criteria makes it essential to approach each patient with a history of (poly) autoimmunity with a high degree of suspicion. Diagnosis is often made on the basis of clinical examination combined with the patient’s history and commonly relies on the exclusion of numerous disease mimickers. A careful anamnesis and clinical examination can often be followed by testing visual fields, whereas more specific imaging modalities can also be offered in conjunction with autoantibody serologic testing. As dry eye sensation is the most commonly encountered mani
As we have already pinpointed, the complex and usually nonspecific symptomatology of autoimmune ophthalmic involvement further hinders and delays the diagnosis. Additionally, early ophthalmic involvement may not yield significant findings, and eye examination may remain unremarkable without pathognomonic features[92]. Similarly, the variable nature of autoimmune disease progress makes it difficult to predict the timeline of ophthalmic involvement. For example ocular involvement can occur early on, coinciding with initial disease diagnosis or even years after disease establishment[8].
As evidenced by Table 1, many autoimmune diseases can exhibit similar ocular manifestations. For instance KCS can occur in primary SS or secondary SS in the context of RA. ON, retinal vasculitis, and uveitis are also some frequently encountered autoimmune manifestations of various systemic diseases. This overlap can prove to be quite a conundrum, especially in the case of polyautoimmunity in which a causal relationship will be difficult to establish[93]. Additionally, the impact of various medications employed in treating autoimmune diseases further complicates the effect-causality question. For example CS and hydroxychloroquine can mediate glaucoma and retinopathy evolution, respectively[94]. Consequently, the rarity and the extremely variable nature of ophthalmic involvement make it necessary that the patient is routinely evaluated by a multidisciplinary team, including ophthalmologists, rheumatologists, and other specialists. The emphasis should be on a personalized approach for monitoring and effectively managing each patient[8,93].
The utilization of various imaging modalities, including OCT, FA, color fundus photography, and OCTA, constitutes an integral part of the diagnosis and management of autoimmune disorders targeting the eye. These methods aim to improve diagnostic accuracy, add prognostic value, and improve treatment planning.
OCT provides a high-resolution image of the retina and the choroid plexus, and subsequently it can effectively highlight structural abnormalities and change secondary to autoimmune processes. Currently, OCT is also employed to document disease progression as in the cases of ON and autoimmune retinopathy according to retinal thickness changes[95]. As an OCT evolution OCTA can additionally map changes involving both superficial and deep retinal microvascular networks, optic disc perfusion, and choroidal vasculature flow, which may not be apparent in traditional OCT. For instance OCTA in patients with MS reveals reduced retinal plexus densities, preferentially involving the superficial vascular networks[96].
OCTA can additionally quantify changes in the retinal and choroidal microvasculature by measuring the foveal avascular zone extent and describing the density, caliber, complexity, and perimeter indices of microcirculation. OCTA findings in patients with SLE ocular involvement were consistent with enlargement of the foveal avascular zone and reduced density of the parafoveal vessels of the deep retinal capillary plexus. An inverse relationship between the SLE index and parafoveal vessel density in deep retinal capillary plexus has also been established. OCT can also detect choroidal involvement based on choroidal thickening and subretinal fluid, particularly in the presence of antiphospholipid antibodies[97,98].
Both OCT and OCTA provide insightful although different details for assessing ocular manifestations. While OCT has an advantage in assessing structural components, OCTA also has an advantage in capturing the minute details of vascular networks without being able to detect vascular leakage. Using both modalities together improves the precision of diagnosis and the effectiveness of disease progression monitoring.
The inability of OCTA to assess active inflammation through blood vessel leakage detection necessitates complementary imaging with FA. FA allows for the visualization of blood flow in the retina and choriocapillaris following the injection of a fluorescent dye into the systemic circulation. This examination can pinpoint areas of blockage and dye leakage in the retinal vasculature that can prompt the diagnosis of retinal vasculitis, which is commonly encountered in autoimmune processes. Another benefit of FA lies in its ability to diagnose complications of autoimmune diseases associated with neovascularization and macular edema.
FA in patients with SLE demonstrated macular edema and the formation of epiretinal membranes. FA was able to measure disease activity based on the early subclinical changes in the retinas of patients with SLE. In RA FA can detect signs of vasculitis and retinal inflammation, including lack of capillary perfusion and capillary leakage while it can also indicate macular changes, including macular edema. Similar findings can be replicated by OCTA, which can detect vascular changes, including retinal capillary loss and caliber changes[97,99].
The presence of specific autoantibodies in the sera of patients can narrow down the differential diagnoses, optimize treatment, and monitor disease activity. However, scientific knowledge surrounding the correlation of systemic biomarkers with ocular involvement in autoimmune diseases is limited. Nevertheless, among symptomatic patients with autoimmune eye manifestations, some inflammatory biomarkers and antibodies are present. A specific category of biomarkers can be found in tear fluid. It was therefore suggested that these tear film biomarkers, including lactoferrin and lysozyme, could indicate ocular surface inflammation encountered in SS. Inflammatory biomarkers, such as matrix metalloproteinase 9, can diagnose inflammation like KCS. Another useful application of biomarkers found in ocular fluids (vitreous, aqueous humor, tears) could be in the elucidation of the pathogenetic mechanisms and serve as a measure to monitor the response to therapy[100].
The prognostic value of biomarkers can be supported by the results of the study published by Cifuentes-González et al[10] in which they concluded that the presence of rheumatoid factor as well as anti-cyclic citrullinated peptide antibodies correlated closely with the incidence of severe sight-threatening complications, including PUK, sclerosing keratitis, and scleritis. Similarly, in AAV positive proteinase-3 ANCA antibodies were associated with anterior segment involvement, whereas in patients with myeloperoxidase-ANCA, the optic nerve was more commonly affected. Moreover, polyautoimmunity was shown to have a positive correlation with the presence of antinuclear antibodies (ANAs), IgG, and IgM anti-cardiolipin antibodies, anti-Smith antibodies, anti-ribonucleoproteins antibodies, and anti-Sjögren syndrome-related antigen A. Reduced concentrations of C3 additionally had predictive value for the occurrence of photophobia (P = 0.05)[10]. The presence of TRab (thyrotropin receptor autoantibodies) is frequently encountered in the serum of patients with eye pain, correlating with Grave's ophthalmopathy[101]. Red eye and diplopia were accompanied by positivity for both PR3 antibodies and myeloperoxidase-ANCA antibodies in patients with AAV[10,102].
Similarly, antiphospholipid antibodies can be encountered in some patients complaining of decreased visual acuity. Dry eyes have the most diversity regarding the presence of positive antibodies, especially in patients with SS, including positive anti-Sjögren syndrome-related antigen A, Anti-La, rheumatoid factor, ANAs, salivary protein 1, autoantibodies against muscarinic acetylcholine receptors M3, and autoantibodies against kallikrein 13. In the same context dry eye sensation is associated with the positivity of anti-cyclic citrullinated peptide in the sera of patients with RA while in patients with SLE ANAs and anti-dsDNA were encountered. Ocular involvement with diplopia correlated with the presence of lupus anticoagulants, anti-cardiolipin antibodies, and B2GP in patients with antiphospholipid syndrome while muscarinic acetylcholine receptor positivity was typical for myasthenia gravis[10]. Finally, AQP4-IgG demonstrates 98% specificity for neuromyelitis optica spectrum disorders, aiding differentiation from MS-related ON.
With the evolution of artificial intelligence (AI) technologies, the potential for in-depth ophthalmologic analyses is great. Integrating machine-learning and deep-learning technologies with ophthalmic imaging can improve diagnosis, management, and prediction protocols and aid in the decision-making process in clinics. Deep-learning models based on deep neural networks can help classify and predict the evolution of disease. For instance fundus images can be analyzed to screen and quantify the likelihood of ocular involvement in the setting of systemic autoimmune disease. Traditional machine-learning protocols can aid in establishing a correlation between retinal findings or ocular structure and systemic disease. Ocular biomarkers can also be potentially extracted by identifying specific imaging features that indicate the presence of a specific autoimmune disease. AI-assisted image analysis can be used to extract data from OCT and OCTA to analyze changes in retinal vasculature, including vascular tortuosity, length density, area density, bifurcation count, and the arteriole-to-venule caliber ratio[95].
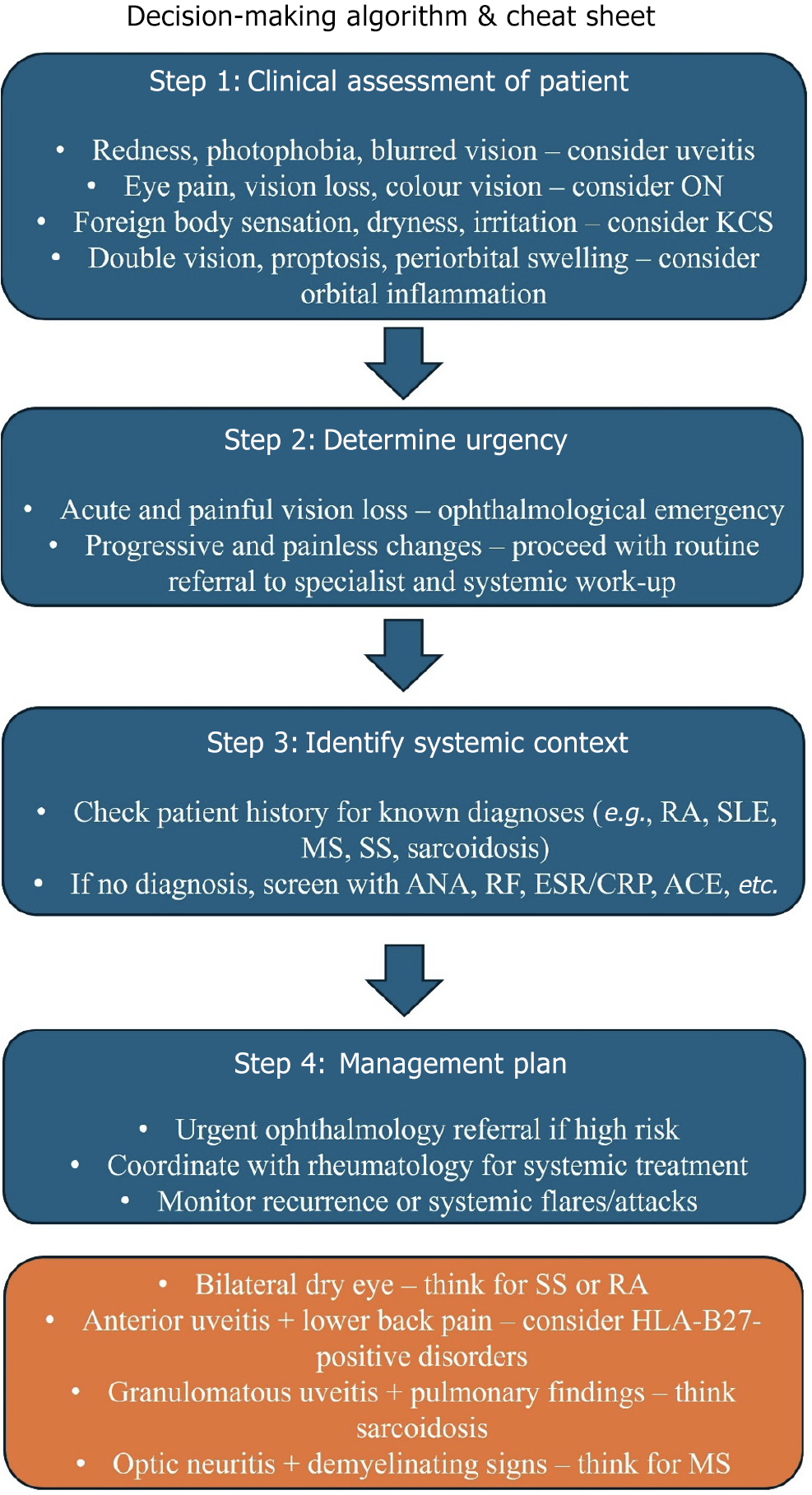
In Figure 3, we present a simple clinical decision-making algorithm to help physicians identify autoimmune disease in patients presenting with isolated ocular symptoms and vice versa.
Reducing overall inflammation and attaining total symptom remission are the two main objectives of managing AI presentations in the eyes. This is essential to avoid worsening symptoms, further deterioration[103-105], and potential permanent blindness[106-110].
Depending on the severity and clinical manifestations of the symptoms, treatment for autoimmune-related uveitis is gradual. Initial management typically starts with topical CS, particularly for anterior disease in which symptoms like eye redness and photophobia are common[111-113]. Additionally, sympathomimetics or parasympatholytics can be used to prevent posterior synechiae by promoting ciliary muscle relaxation through pupil dilation[114]. Concerning more severe manifestations or in the event of inadequacy of topical treatment, systemic CS becomes mandatory. To rapidly reduce inflammation the first-line approach frequently consists of high-dose systemic CS, including prednisone, administered at doses greater than 60 mg daily[113]. An intravenous (IV) burst of high-dose CS with dosages up to 1000 mg/day over 3 days may be used in acute cases[115]. Doctors may opt for aggressive management with higher CS doses to control complications rapidly. Particularly for patients with recurrent or chronic uveitis, the goal of the treatment is to maintain disease control with the lowest possible effective drug dosage to minimize side effects[116]. Following a favorable reaction, the dosage of CS is gradually reduced (tampering) to a maintenance dose of 7.5 mg/day to lower the chance of long-term complications, such as cataract or glaucoma development[113].
Ocular dryness in cases of keratitis can be managed by avoiding dry or windy environments and minimizing activities associated with a low blinking rate[117-119]. Topical CS and lubricating eye drops are used to treat more complicated cases, reduce DES symptoms, and encourage healing. Additionally, systemic immunosuppressants may also be introduced into regimens to manage the underlying inflammation more effectively[117-120]. In cases where patients do not adequately respond to medical therapy and maximal lubrication agents, punctal occlusion-occlusion of the tear drainage system at the level of the puncta lacrimalia proves beneficial.
ON, especially when associated with MS, can be controlled with high-dose IV CS, particularly for acute cases, to reduce inflammation and assist the healing process. Typical regimens include IV methylprednisolone 1 g/day for 3-7 days, followed by oral steroids[121]. Timely intervention is necessary to minimize permanent CNS and visual damage. Therefore, treatment should be started early and in an aggressive manner[122].
Lastly, caution is also necessary for scleritis and episcleritis. Low-dose CS or topical nonsteroidal anti-inflammatory drugs may be effective treatments for episcleritis, which typically resolves independently[105]. Scleritis has a more severe course and can lead to serious problems. Therefore, systemic CS or even immunosuppressive medication may be required[123]. Patients with systemic autoimmune disease are often treated with medications, including mycophenolate mofetil, methotrexate, and azathioprine as we will discuss later.
Although CS is still the mainstay of treatment for ocular symptoms, prolonged use of these medications carries a number of substantial concerns, such as an increased risk of infection and other severe adverse effects (e.g., hyperglycemia, Cushing’s syndrome development)[124]. Consequently, patients with autoimmune ocular involvement benefit greatly from adjunctive therapy. In clinical practice immunosuppressive medications are commonly combined with CS to enhance illness control[103,105,108,110] and reduce dependence[125-128]. The most widely used immunosuppressive agents include methotrexate and mycophenolate mofetil[129-131]. They are chosen due to their effectiveness in controlling inflammation and relatively manageable side effects.
Methotrexate is typically preferred as a first-line agent for non-infectious inflammatory eye diseases while mycophenolate mofetil is typically favored in chronic cases where prolonged immunosuppression is necessary. Other commonly employed drugs include azathioprine and cyclosporine. Azathioprine is valued for its relative safety in long-term use and is often considered particularly suitable for younger patients[129,132] while cyclosporine (a T cell inhibitor) is widely used worldwide for managing chronic uveitis, even though a study by Kaçmaz et al[133] found that it has significant adverse effects, particularly in older adults. The same study concluded that cyclophosphamide and other alkylating agents, though less commonly used due to their significant toxicity[134,135], can be reserved for severe cases that fail to respond to first-line agents.
Furthermore, the treatment of many illnesses, including autoimmune disease, has been completely transformed by the advent of biologic medicines in recent years[103]. Because these biologics help achieve remission while reducing the need for CS, they are especially beneficial for patients who do not respond well to traditional therapy regimens. Tumor necrosis factor-alpha (TNF-α) inhibitors such as adalimumab have demonstrated significant improvements in visual acuity and inflammation reduction (the usual dose is 40 mg at 2-week intervals)[136]. The possible effectiveness of other biological medicines is also being studied. For instance recent clinical studies have studied IL-6 receptor inhibitors like tocilizumab and sarilumab as possible therapies (the usual dose is 8 mg/kg IV every 4 weeks)[137-140]. Without any unanticipated safety issues, the phase 1/2 STOP-uveitis study found that patients taking tocilizumab had better visual acuity and less vitreous haze[141]. Additionally, non-TNF biologics have been developed mainly for the treatment of RA. However, they are now tested in small clinical trials in ocular inflammatory disease, including inhibition of T cell activation via suppression of growth factor by inhibiting IL-2 receptor signaling with daclizumab, co-stimulation of T cells via a fusion protein of CTLA-4 with abatacept, or inhibiting B cell responses via the anti-CD20mAb rituximab[103].
Concerning NMO-associated disorders, inebilizumab, an anti-AQP4 monoclonal IgG antibody, has shown great promise as shown during a 28-week-long randomized control trial (N-MOmentum trial)[142]. More than 80% of the patients treated reported no attacks after 4 years while most of the attacks occurred during the first year of treatment, implying that long-term use of inebilizumab results in enhanced efficacy. Moreover, no safety concerns were raised during the use of this agent. Another anti-AQP4 antibody, satralizumab, was also proven beneficial in treating NMO spectrum ocular autoimmune disease with more than 70% of patients remaining attack-free after 4 years of treatment[143]. While satralizumab reduces neuromyelitis optica spectrum disorder relapse rates by 70%, its long-term safety profile remains under investigation with upper respiratory infections reported in 15% of trial participants (N-MOmentum trial).
Targeted therapy can be achieved using intravitreal injections of CS or biologic drugs, especially in situations of uveitis and other inflammatory disorders that are severe or resistant to treatment[110]. These techniques can lower the possibility of systemic side effects by directly delivering therapeutic medicines to the impacted ocular tissues.
Successful interdisciplinary therapeutic management for patients presenting with ocular symptoms linked to systemic autoimmune diseases has been documented in a number of examples in the literature. For instance a patient with ocular symptoms associated with an autoimmune disease was given a thorough treatment regimen that included systemic immunosuppressives, high-dose glucocorticoids, rituximab, and intraocular medications such as intravitreal beva
The management of ocular manifestations in autoimmune diseases must balance the effectiveness of treatment against the potential for adverse effects, particularly with long-term corticosteroid use as evident in Table 2. Revolutionary treatment options are being developed into novel biological drugs targeting immune pathways. Targeted therapy deve
| Ocular symptoms | Sever characteristics | Treatment options | Details | Ref. |
| Uveitis | Mild/moderate | Topical CS | Initial management for anterior uveitis; reduces redness and photophobia | [111-114] |
| Severe | Systemic CS, systemic immunosuppressants | High-dose prednisone (> 60 mg daily); IV CS for acute cases (1000 mg/day for 3 days) | [113,115,125-127] | |
| Recurrent | Gradual CS taper | Reduce to 7.5 mg/day maintenance to prevent complications like cataracts | [113,116] | |
| Keratitis | Mild | Environmental modifications | Avoid dry/windy conditions; avoid activities with reduced blinking | [117-119] |
| Severe | Topical CS, lubricating drops, systemic immunosuppressants | Controls dryness, reduces inflammation | [117-119,125-128] | |
| ON | Acute | High-dose IV CS | IV methylprednisolone 1 g/day for 3-7 days; followed by oral CS | [121,122] |
| Episcleritis | Mild | Low-dose CS or NSAIDs | Often self-resolving | [105] |
| Scleritis | Severe | Systemic CS, immunosuppressants | Controls inflammation | [105,123,125-128] |
| Chronic ocular inflammation | Chronic cases | Immunosuppressive agents | Methotrexate (first line for non-infectious cases), mycophenolate mofetil (chronic use), azathioprine, and cyclosporine | [129-133] |
| Refractory cases | Resistant to initial treatments | Alkylating agents (e.g., cyclophosphamide) | Reserved for severe, non-responsive cases due to toxicity | [134,135] |
As we described extensively the ophthalmological manifestations of autoimmune diseases are complex and often challenging to diagnose and manage. Also, there are many challenges regarding the variability in disease presentation from mild inflammation to severe vision-threatening complications, complicated diagnosis, and rarely timely treatment.
One major gap in the current knowledge is understanding the precise immunological mechanisms that compel ocular inflammation and how they influence systemic disease. In line with this future perspectives circle around advancing molecular understanding, refining and broadening diagnostic tools, and exploring novel targeted therapies. Novel fields for research for ocular manifestations of autoimmune diseases may include investigation of genetic factors, fine immune and molecular mechanisms, and novel molecules promising to change the current treatment effectiveness[145].
Advancements in diagnostic innovations are also transforming the field. High-resolution imaging modalities, such as OCT and confocal microscopy, enable earlier detection of subtle changes in ocular tissues, allowing for more precise monitoring[146]. Additionally, the identification of novel biomarkers has the potential to predict disease activity and therapeutic responses. However, these tools need to be proven before being applied widely in clinical settings. Therapeutic innovations have also made significant strides. Monoclonal antibodies targeting specific cytokines have shown promise in controlling ocular manifestations in patients with systemic autoimmune diseases[147].
Despite these advances challenges remain, including the risk of adverse effects, treatment resistance, and the need for long-term immunosuppression. Looking ahead, personalized medicine offers significant potential for improving patient care. We can develop more targeted, effective, and safe therapies by combining genetic and molecular profiling with clinical data. Collaboration among specialists, such as rheumatologists, ophthalmologists, and immunologists, is essential in effective management strategies. Furthermore, ongoing research into immune modulation and regenerative therapies provides hope for preventing vision loss and improving the quality of life for patients with autoimmune-related ocular diseases.
Ocular manifestations of autoimmune diseases can substantially impact daily life, including vision, comfort, and quality of life. Certain symptoms, such as dryness, photophobia, or ocular pain, can interfere with daily activities, such as reading, studying, or working, but more serious disorders, such as uveitis or ON, can result in vision loss if not add
Early detection and treatment are critical for protecting vision and avoiding additional issues. CS, immunosuppressive drugs, and adjunctive therapy are examples of management options designed to decrease inflammation and reduce long-term harm. As previously stated high-dose CS are critical in acute cases such as ON, whereas maintenance dosages are carefully managed to balance efficacy and side effects[113,121]. Delayed therapy raises the likelihood of irreversible ocular and systemic consequences, emphasizing the importance of early detection. A multidisciplinary approach is required for the best care, which includes coordination between ophthalmologists, rheumatologists, and other experts. This integrated method guarantees that the disease is managed comprehensively, both on an ocular and a systemic level. It also enables personalized treatment strategies, which meet individual patient needs and improve outcomes[109].
We present Table 3, which includes biomarkers that can link ocular symptoms with specific autoimmune disease[148] and their respective specificity and sensitivity[149-153].
| Condition | Key biomarkers | Sensitivity (%) | Specificity (%) | Comments | Ref. |
| NMOSD | AQP4-IgG (live cell-based assay) | 80% | 100% | Highly specific; distinguishes NMOSD from MS-related optic neuritis | [148] |
| MS-related optic neuritis | No specific serological marker | Diagnosis is clinical and radiological; CSF oligoclonal bands may assist | [149] | ||
| MOG-AD | MOG-IgG (cell-based assay) | 89.1% | 93.3% | Important for recurrent optic neuritis with optic disc edema; often in children/young adults | [150] |
| Sarcoidosis-associated uveitis | ACE, lysozyme | ACE: 38.2%-84.0%; lysozyme: 60.0%-78.0% | ACE: 83.0%-97.8%; lysozyme: 76.0%-95.0% | Elevated levels support diagnosis; imaging often essential | [151] |
| TB-associated uveitis | QuantiFERON-TB gold | 62%-95% | 92%-100% | Latent TB testing critical in endemic areas or atypical uveitis | [152] |
| Behçet’s disease | HLA-B51 | HLA-B51 is the strongest genetic association; supportive rather than diagnostic | [153] |
Autoimmune diseases are a heterogeneous group of disorders that significantly impact global health. These diseases often involve multiple organ systems, including the eyes, which are particularly sensitive due to the immune-privileged environment. Ocular manifestations include uveitis, keratitis, and ON, ranging from mild to severe and potentially threatening vision. Early detection and treatment are critical for preventing irreversible damage. Advances in diagnostic tools, such as OCT and serological biomarkers, have improved diagnostic accuracy but are not yet widely implemented in clinical practice. More research is needed in this direction to perfect diagnostic possibilities and improve patient care.
Managing ocular symptoms in autoimmune illnesses requires a delicate balance between symptom control and reducing the negative effects of immunosuppression. New medicines, such as TNF-α inhibitors and IL-6 receptor blockers, provide targeted solutions for refractory cases. More importantly, a multidisciplinary strategy combining ophthalmologists, rheumatologists, and other experts is required to improve patient outcomes. Continued research and innovation are critical for meeting unmet clinical requirements and increasing the quality of treatment for afflicted patients.
| 1. | Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 766] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 2. | Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34:J168-J177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Generali E, Cantarini L, Selmi C. Ocular Involvement in Systemic Autoimmune Diseases. Clin Rev Allergy Immunol. 2015;49:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Mecê P, Gocho K, Harmening W, Rossi E, Young L. Editorial: Advances in optical imaging for ophthalmology: new developments, clinical applications and perspectives. Front Ophthalmol (Lausanne). 2024;4:1496015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Jonnal RS. Toward a clinical optoretinogram: a review of noninvasive, optical tests of retinal neural function. Ann Transl Med. 2021;9:1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Cohen DA, Gise R, Gaier ED. Serum Biomarkers in Neuro-Ophthalmology: When to Test. Semin Ophthalmol. 2021;36:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Vodopivec I, Lobo AM, Prasad S. Ocular inflammation in neurorheumatic disease. Semin Neurol. 2014;34:444-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Glover K, Mishra D, Singh TRR. Epidemiology of Ocular Manifestations in Autoimmune Disease. Front Immunol. 2021;12:744396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Agarwal A, Sepah YJ, Nguyen QD. Ocular Manifestations of Systemic Autoimmune Diseases. In: Ikezu T, Gendelman H, editors. Neuroimmune Pharmacology. Cham: Springer, 2017: 553–573. [DOI] [Full Text] |
| 10. | Cifuentes-González C, Uribe-Reina P, Reyes-Guanes J, Muñoz-Ortiz J, Muñoz-Vargas PT, Rojas-Carabali W, Nova-Florián DV, De-Los-Ríos AS, Mantilla-Hernández RD, de-la-Torre A. Ocular Manifestations Related to Antibodies Positivity and Inflammatory Biomarkers in a Rheumatological Cohort. Clin Ophthalmol. 2022;16:2477-2490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073-3083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, Androudi S. A Focus on the Epidemiology of Uveitis. Ocul Immunol Inflamm. 2018;26:2-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 387] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 13. | Rothova A. Ocular involvement in sarcoidosis. Br J Ophthalmol. 2000;84:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 199] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Jamilloux Y, Kodjikian L, Broussolle C, Sève P. Sarcoidosis and uveitis. Autoimmun Rev. 2014;13:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Pasadhika S, Rosenbaum JT. Ocular Sarcoidosis. Clin Chest Med. 2015;36:669-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Zur Bonsen LS, Pohlmann D, Rübsam A, Pleyer U. Findings and Graduation of Sarcoidosis-Related Uveitis: A Single-Center Study. Cells. 2021;11:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Umur KA, Tayfun B, Oguzhan O. Different ophthalmologic manifestations of sarcoidosis. Curr Opin Ophthalmol. 2012;23:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Ang M, Wong WL, Li X, Chee SP. Interferon γ release assay for the diagnosis of uveitis associated with tuberculosis: a Bayesian evaluation in the absence of a gold standard. Br J Ophthalmol. 2013;97:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Gineys R, Bodaghi B, Carcelain G, Cassoux N, Boutin LTH, Amoura Z, Lehoang P, Trad S. QuantiFERON-TB gold cut-off value: implications for the management of tuberculosis-related ocular inflammation. Am J Ophthalmol. 2011;152:433-440.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 403] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Bhamra M, Gondal I, Amarnani A, Betesh S, Zhyvotovska A, Scott W, Alvarez M, Lazzaro D, Mcfarlane I. Ocular Manifestations of Rheumatoid Arthritis: Implications of Recent Clinical Trials. Int J Clin Res Trials. 2019;4:139. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Escobar T, Yu CR, Muljo SA, Egwuagu CE. STAT3 activates miR-155 in Th17 cells and acts in concert to promote experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2013;54:4017-4025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behçet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 426] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 24. | Saip S, Akman-Demir G, Siva A. Neuro-Behçet syndrome. Handb Clin Neurol. 2014;121:1703-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Plekhanov AN, Fomina AS, Sverkunova OP, Ivanova JV. Autoimmune Uveitis. Review. Oftalʹmologiâ. 2019;16:5-11. [DOI] [Full Text] |
| 26. | Paovic J, Paovic P, Sredovic V. Behcet's disease: systemic and ocular manifestations. Biomed Res Int. 2013;2013:247345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Messenger W, Hildebrandt L, Mackensen F, Suhler E, Becker M, Rosenbaum JT. Characterisation of uveitis in association with multiple sclerosis. Br J Ophthalmol. 2015;99:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Murray PI, Hoekzema R, van Haren MA, de Hon FD, Kijlstra A. Aqueous humor interleukin-6 levels in uveitis. Invest Ophthalmol Vis Sci. 1990;31:917-920. [PubMed] |
| 29. | Ooi KG, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis: is there a correlation with clinical phenotype? Clin Med Res. 2006;4:294-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Virdee S, Greenan-Barrett J, Ciurtin C. A systematic review of primary Sjögren's syndrome in male and paediatric populations. Clin Rheumatol. 2017;36:2225-2236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Baer AN, Walitt B. Update on Sjögren Syndrome and Other Causes of Sicca in Older Adults. Rheum Dis Clin North Am. 2018;44:419-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Balint G, Watson Buchanan W, Kean CA, Kean W, Rainsford KD. Sjögren's syndrome. Inflammopharmacology. 2024;32:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Dammacco R. Systemic lupus erythematosus and ocular involvement: an overview. Clin Exp Med. 2018;18:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 34. | Monov S, Hristova R, Dacheva R, Toncheva R, Shumnalieva R, Shoumnalieva-Ivanova V, Monova D. Acute necrotizing retinal vasculitis as onset of systemic lupus erythematosus: A case report. Medicine (Baltimore). 2017;96:e5754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Abd-Allah NM, Hassan AA, Omar G, Hamdy M, Abdelaziz STA, Abd El Hamid WM, Moussa RA. Dry eye in rheumatoid arthritis: relation to disease activity. Immunol Med. 2020;43:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Kemeny-Beke A, Szodoray P. Ocular manifestations of rheumatic diseases. Int Ophthalmol. 2020;40:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Aboud S, Abd Elkhalek M, Aly N, Abd Elaleem E. Ocular involvement and its manifestations in rheumatoid arthritis patients. Delta J Ophthalmol. 2017;18:57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, Doctor PP, Tauber J, Foster CS. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology. 2012;119:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 39. | Yagci A. Update on peripheral ulcerative keratitis. Clin Ophthalmol. 2012;6:747-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Winter A, Chwalisz B. MRI Characteristics of NMO, MOG and MS Related Optic Neuritis. Semin Ophthalmol. 2020;35:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J. 2012;6:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Turk MA, Hayworth JL, Nevskaya T, Pope JE. Ocular Manifestations in Rheumatoid Arthritis, Connective Tissue Disease, and Vasculitis: A Systematic Review and Metaanalysis. J Rheumatol. 2021;48:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 547] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 44. | Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, Adzemovic M, Bauer J, Berger T, Fujihara K, Itoyama Y, Lassmann H. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 438] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 45. | Nishiyama S, Misu T, Nuriya M, Takano R, Takahashi T, Nakashima I, Yasui M, Itoyama Y, Aoki M, Fujihara K. Complement-dependent and -independent aquaporin 4-antibody-mediated cytotoxicity in human astrocytes: Pathogenetic implications in neuromyelitis optica. Biochem Biophys Rep. 2016;7:45-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Mealy MA, Whetstone A, Orman G, Izbudak I, Calabresi PA, Levy M. Longitudinally extensive optic neuritis as an MRI biomarker distinguishes neuromyelitis optica from multiple sclerosis. J Neurol Sci. 2015;355:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Storoni M, Davagnanam I, Radon M, Siddiqui A, Plant GT. Distinguishing optic neuritis in neuromyelitis optica spectrum disease from multiple sclerosis: a novel magnetic resonance imaging scoring system. J Neuroophthalmol. 2013;33:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Khanna S, Sharma A, Huecker J, Gordon M, Naismith RT, Van Stavern GP. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol. 2012;32:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, Vucic S, Gorman MP, Benson LA, Alper G, Riney CJ, Barnett M, Parratt JD, Hardy TA, Leventer RJ, Merheb V, Nosadini M, Fung VS, Brilot F, Dale RC. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22:470-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 50. | Youl BD, Turano G, Miller DH, Towell AD, MacManus DG, Moore SG, Jones SJ, Barrett G, Kendall BE, Moseley IF. The pathophysiology of acute optic neuritis. An association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991;114 (Pt 6):2437-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 163] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Akaishi T, Nakashima I, Takeshita T, Kaneko K, Mugikura S, Sato DK, Takahashi T, Nakazawa T, Aoki M, Fujihara K. Different etiologies and prognoses of optic neuritis in demyelinating diseases. J Neuroimmunol. 2016;299:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Dunker S, Wiegand W. Prognostic value of magnetic resonance imaging in monosymptomatic optic neuritis. Ophthalmology. 1996;103:1768-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Kupersmith MJ, Alban T, Zeiffer B, Lefton D. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain. 2002;125:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Nevares A, Raut R, Libman B, Hajj-Ali R. Noninfectious Autoimmune Scleritis: Recognition, Systemic Associations, and Therapy. Curr Rheumatol Rep. 2020;22:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Sainz de la Maza M, Foster CS, Jabbur NS. Scleritis associated with rheumatoid arthritis and with other systemic immune-mediated diseases. Ophthalmology. 1994;101:1281-1286; discussion 1287. [PubMed] |
| 56. | Akpek EK, Thorne JE, Qazi FA, Do DV, Jabs DA. Evaluation of patients with scleritis for systemic disease. Ophthalmology. 2004;111:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Giordano N, D'Ettorre M, Biasi G, Fioravanti A, Moretti L, Marcolongo R. Retinal vasculitis in rheumatoid arthritis: an angiographic study. Clin Exp Rheumatol. 1990;8:121-125. [PubMed] |
| 58. | Akpek EK, Uy HS, Christen W, Gurdal C, Foster CS. Severity of episcleritis and systemic disease association. Ophthalmology. 1999;106:729-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Cocho L, Gonzalez-Gonzalez LA, Molina-Prat N, Doctor P, Sainz-de-la-Maza M, Foster CS. Scleritis in patients with granulomatosis with polyangiitis (Wegener). Br J Ophthalmol. 2016;100:1062-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Abroug N, Khairallah M, Zina S, Ksiaa I, Amor HB, Attia S, Jelliti B, Khochtali S, Khairallah M. Ocular Manifestations of Emerging Arthropod-Borne Infectious Diseases. J Curr Ophthalmol. 2021;33:227-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Zina SM, Hoarau G, Labetoulle M, Khairallah M, Rousseau A. Ocular Manifestations of Flavivirus Infections. Pathogens. 2023;12:1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 62. | Venkatesh A, Patel R, Goyal S, Rajaratnam T, Sharma A, Hossain P. Ocular manifestations of emerging viral diseases. Eye (Lond). 2021;35:1117-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Duplechain A, Conrady CD, Patel BC, Baker S. Uveitis. 2023 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 64. | Singh P, Gupta A, Tripathy K. Keratitis. 2023 Aug 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 65. | Ahmad SS, Blair K, Kanukollu VM. Optic Atrophy. 2024 Mar 1. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 66. | Shields MK, Furtado JM, Lake SR, Smith JR. Syphilitic scleritis and episcleritis: A review. Asia Pac J Ophthalmol (Phila). 2024;13:100073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Héron E, Bourcier T. [Scleritis and episcleritis]. J Fr Ophtalmol. 2017;40:681-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Aldebasi TM, Alasiri AA, Alnahdi MA, Alfarhan A. Tubercular Episcleritis: A Review of Literature. Middle East Afr J Ophthalmol. 2022;29:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Schonberg S, Stokkermans TJ. Episcleritis. 2023 Aug 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 71. | Lagina A, Ramphul K. Scleritis. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 72. | Nowinska AK, Machalińska A, Módis L, Koprowski R, Rechichi M. Ocular Manifestations of Systemic Diseases. J Ophthalmol. 2018;2018:7851691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Golden MI, Meyer JJ, Zeppieri M, Patel BC. Dry Eye Syndrome. 2024 Feb 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 74. | Li R, Zhang J, Zhang Y, Wang L, Qi X, Chen Y. Does SARS-CoV-2 infection cause persistent ocular symptoms?: A cross-sectional study after the lifting of lockdown in Chongqing, China. Medicine (Baltimore). 2023;102:e36798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Hashmi MF, Gurnani B, Benson S. Conjunctivitis. 2024 Jan 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 76. | Bernardes TF, Bonfioli AA. Blepharitis. Semin Ophthalmol. 2010;25:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Eberhardt M, Zeppieri M, Rammohan G. Blepharitis. 2025 Feb 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 78. | O'Keefe GD, Kim LA, Agarwal A, Tripathy K, Palestine A, Lim JI, Hossain HA. Retinal Vasculitis. [cited 14 July 2025]. Available from: https://eyewiki.org/Retinal_Vasculitis. |
| 79. | Porter D. What Is Macular Edema? [cited 14 July 2025]. Available from: https://www.aao.org/eye-health/diseases/what-is-macular-edema. |
| 80. | Kohli P, Tripathy K, Patel BC. Macular Edema. 2024 Apr 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 81. | Salvador GLO, Basso ACN, Barbieri PP, Leitao CA, Teixeira BCA, Neto AC. Central nervous system and spinal cord tuberculosis: Revisiting an important disease. Clin Imaging. 2021;69:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 82. | Katz BJ, Digre KB. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol. 2016;61:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 83. | Wu Y, Hallett M. Photophobia in neurologic disorders. Transl Neurodegener. 2017;6:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 84. | Feldman BH, Phelps P, Miller AM, Barash A, Murchison A, Justin GA, Tsui JC, Bhagat N, Lim JI, Lai KE, Patel SJ, Karth PA, Gullapalli V, Starr M. Retinal Detachment. [cited June 23 2025]. Available from: https://eyewiki.org/Retinal_Detachment. |
| 85. | Kanukollu VM, Ahmad SS. Retinal Hemorrhage. 2023 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 86. | Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010;8:1886-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 87. | Dietze J, Blair K, Zeppieri M, Havens SJ. Glaucoma. 2024 Mar 16. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 88. | Tan A, Faridah H. The two-minute approach to monocular diplopia. Malays Fam Physician. 2010;5:115-118. [PubMed] |
| 89. | Najem K, Asuncion RMD, Margolin E. Diplopia. 2024 Feb 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 90. | Hilliard GC, Feldman BH, Heersink S, Patel AS, DelMonte DW, Hossain K, Baartman B, Anderson D, Stelzner K, Ryburn C, Hopkins N. Cataract. [cited June 24 2025]. Available from: https://eyewiki.org/Cataract. |
| 91. | Schwartz TM, Keenan RT, Daluvoy MB. Ocular Involvement in Rheumatoid Arthritis. [cited June 24 2025]. Available from: https://www.aao.org/eyenet/article/ocular-involvement-in-rheumatoid-arthritis. |
| 92. | Canamary AM Jr, Takahashi WY, Sallum JMF. Autoimmune retinopathy: A Review. Int J Retina Vitreous. 2018;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Baig IF, Lee AG, Babu J, Al-Zubidi N, Vickers A. Ophthalmologic Manifestations of Autoimmune Diseases. [cited June 24 2025] Available from: https://eyewiki.org/Ophthalmologic_Manifestations_of_Autoimmune_Diseases. |
| 94. | Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 403] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 95. | Miao H, Zou Z, Xu J, Gao Y. Advancing systemic disease diagnosis through ophthalmic image‐based artificial intelligence. MedComm: Future Med. 2024;3. [DOI] [Full Text] |
| 96. | Murphy OC, Kalaitzidis G, Vasileiou E, Filippatou AG, Lambe J, Ehrhardt H, Pellegrini N, Sotirchos ES, Luciano NJ, Liu Y, Fitzgerald KC, Prince JL, Calabresi PA, Saidha S. Optical Coherence Tomography and Optical Coherence Tomography Angiography Findings After Optic Neuritis in Multiple Sclerosis. Front Neurol. 2020;11:618879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 97. | Fouad SA, Esmat Mahmoud Ali SM, Rezk Alnaggar ARL, Mahfouz S, Essam M, El-Gendy H. Structural Retinal Assessment Using Optical Coherence Tomography and Fundus Fluorescein Angiography in Systemic Lupus Erythematosus Patients. J Clin Rheumatol. 2021;27:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | An Q, Gao J, Liu L, Liao R, Shuai Z. Analysis of Foveal Microvascular Abnormalities in Patients with Systemic Lupus Erythematosus Using Optical Coherence Tomography Angiography. Ocul Immunol Inflamm. 2021;29:1392-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Ventura Valenzuela ME, González Díaz V, Martinez-Bonilla G, Bernard Medina AG, Uribe Martinez JF, Rosal Arteaga CA, Perez-Topete S, Salazar García J, Sánchez Sánchez S, Cerpa-Cruz S. AB0679 ophthalmic findings by structural spectral domain optical coherence tomography in patients with systemic lupus erythematosus. Ann Rheum Dis. 2023;82:1542-1543. |
| 100. | Peng J, Feinstein D, DeSimone S, Gentile P. A Review of the Tear Film Biomarkers Used to Diagnose Sjogren's Syndrome. Int J Mol Sci. 2024;25:10380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 101. | Nicolì F, Lanzolla G, Mantuano M, Ionni I, Mazzi B, Leo M, Sframeli A, Posarelli C, Maglionico MN, Figus M, Nardi M, Marcocci C, Marinò M. Correlation between serum anti-TSH receptor autoantibodies (TRAbs) and the clinical feature of Graves' orbitopathy. J Endocrinol Invest. 2021;44:581-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 102. | Ungprasert P, Crowson CS, Cartin-Ceba R, Garrity JA, Smith WM, Specks U, Matteson EL, Makol A. Clinical characteristics of inflammatory ocular disease in anti-neutrophil cytoplasmic antibody associated vasculitis: a retrospective cohort study. Rheumatology (Oxford). 2017;56:1763-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 103. | Lee RW, Dick AD. Current concepts and future directions in the pathogenesis and treatment of non-infectious intraocular inflammation. Eye (Lond). 2012;26:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 104. | Kümpfel T, Giglhuber K, Aktas O, Ayzenberg I, Bellmann-Strobl J, Häußler V, Havla J, Hellwig K, Hümmert MW, Jarius S, Kleiter I, Klotz L, Krumbholz M, Paul F, Ringelstein M, Ruprecht K, Senel M, Stellmann JP, Bergh FT, Trebst C, Tumani H, Warnke C, Wildemann B, Berthele A; Neuromyelitis Optica Study Group (NEMOS). Update on the diagnosis and treatment of neuromyelitis optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part II: Attack therapy and long-term management. J Neurol. 2024;271:141-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 78] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 105. | Abdel-Aty A, Gupta A, Del Priore L, Kombo N. Management of noninfectious scleritis. Ther Adv Ophthalmol. 2022;14:25158414211070879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 106. | Zlatanović G, Veselinović D, Cekić S, Zivković M, Dorđević-Jocić J, Zlatanović M. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosn J Basic Med Sci. 2010;10:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 107. | Palejwala NV, Walia HS, Yeh S. Ocular manifestations of systemic lupus erythematosus: a review of the literature. Autoimmune Dis. 2012;2012:290898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 108. | Negrini S, Emmi G, Greco M, Borro M, Sardanelli F, Murdaca G, Indiveri F, Puppo F. Sjögren's syndrome: a systemic autoimmune disease. Clin Exp Med. 2022;22:9-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 233] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 109. | Van Bentum RE, Van den Berg JM, Wolf SE, Van der Bijl J, Tan HS, Verbraak FD, van der Horst-Bruinsma IE. Multidisciplinary management of auto-immune ocular diseases in adult patients by ophthalmologists and rheumatologists. Acta Ophthalmol. 2021;99:e164-e170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford). 2019;58:i43-i54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 236] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 111. | van Laar JAM, Rothova A, Missotten T, Kuijpers RWAM, van Hagen PM, van Velthoven MEJ. Diagnosis and treatment of uveitis; not restricted to the ophthalmologist. J Clin Transl Res. 2015;1:94-99. [PubMed] |
| 112. | Charkoudian LD, Ying GS, Pujari SS, Gangaputra S, Thorne JE, Foster CS, Jabs DA, Levy-Clarke GA, Nussenblatt RB, Rosenbaum JT, Suhler EB, Kempen JH. High-dose intravenous corticosteroids for ocular inflammatory diseases. Ocul Immunol Inflamm. 2012;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, Nussenblatt RB, Stiehm ER, Tessler H, Van Gelder RN, Whitcup SM, Yocum D. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 664] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 114. | Selmi C. Diagnosis and classification of autoimmune uveitis. Autoimmun Rev. 2014;13:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 115. | Reed JB, Morse LS, Schwab IR. High-dose intravenous pulse methylprednisolone hemisuccinate in acute Behçet retinitis. Am J Ophthalmol. 1998;125:409-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 116. | Gallego-Pinazo R, Dolz-Marco R, Martínez-Castillo S, Arévalo JF, Díaz-Llopis M. Update on the principles and novel local and systemic therapies for the treatment of non-infectious uveitis. Inflamm Allergy Drug Targets. 2013;12:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Price EJ, Rauz S, Tappuni AR, Sutcliffe N, Hackett KL, Barone F, Granata G, Ng WF, Fisher BA, Bombardieri M, Astorri E, Empson B, Larkin G, Crampton B, Bowman SJ; British Society for Rheumatology Standards, Guideline and Audit Working Group. The British Society for Rheumatology guideline for the management of adults with primary Sjögren's Syndrome. Rheumatology (Oxford). 2017;56:e24-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 118. | Foulks GN, Forstot SL, Donshik PC, Forstot JZ, Goldstein MH, Lemp MA, Nelson JD, Nichols KK, Pflugfelder SC, Tanzer JM, Asbell P, Hammitt K, Jacobs DS. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul Surf. 2015;13:118-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 119. | Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T, Fisher BA, Gottenberg JE, Hernandez-Molina G, Kocher A, Kostov B, Kruize AA, Mandl T, Ng WF, Retamozo S, Seror R, Shoenfeld Y, Sisó-Almirall A, Tzioufas AG, Vitali C, Bowman S, Mariette X; EULAR-Sjögren Syndrome Task Force Group. EULAR recommendations for the management of Sjögren's syndrome with topical and systemic therapies. Ann Rheum Dis. 2020;79:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 343] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 120. | Kuklinski E, Asbell PA. Sjogren's syndrome from the perspective of ophthalmology. Clin Immunol. 2017;182:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 121. | Songthammawat T, Srisupa-Olan T, Siritho S, Kittisares K, Jitprapaikulsan J, Sathukitchai C, Prayoonwiwat N. A pilot study comparing treatments for severe attacks of neuromyelitis optica spectrum disorders: Intravenous methylprednisolone (IVMP) with add-on plasma exchange (PLEX) versus simultaneous ivmp and PLEX. Mult Scler Relat Disord. 2020;38:101506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 122. | Carnero Contentti E, Rojas JI, Cristiano E, Marques VD, Flores-Rivera J, Lana-Peixoto M, Navas C, Papais-Alvarenga R, Sato DK, Soto de Castillo I, Correale J. Latin American consensus recommendations for management and treatment of neuromyelitis optica spectrum disorders in clinical practice. Mult Scler Relat Disord. 2020;45:102428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 123. | Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000;130:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 236] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 124. | Kapugi M, Cunningham K. Corticosteroids. Orthop Nurs. 2019;38:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 125. | Wang MM, Huang T, Li JX, Yao Y, Chen Y, Fu KK, Miao WR, Han Y. Optic Neuritis Leading to Vision Loss: A Case of MOG-Associated Disease with Successful Immunotherapy. Am J Case Rep. 2024;25:e943112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 126. | Carnero Contentti E, Correale J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflammation. 2021;18:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 127. | Rosenbaum JT, Sibley CH, Lin P. Retinal vasculitis. Curr Opin Rheumatol. 2016;28:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 128. | Shen J, Su Z, Feng L. Sarcoid uveitis: A case report and systematic review of literature. Adv Ophthalmol Pract Res. 2022;2:100047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 129. | Nederlands Oogheelkundig Genootschap. Richtlijnen en standpunten. [cited June 24 2025]. Available from: https://www.oogheelkunde.org/sites/www.oogheelkunde.org/files/richtlijnen/Richtlijn-Uveitis-def-geautoriseerde-versie.pdf. |
| 130. | Schwartzman S. Advancements in the management of uveitis. Best Pract Res Clin Rheumatol. 2016;30:304-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 131. | Rosenbaum JT, Bodaghi B, Couto C, Zierhut M, Acharya N, Pavesio C, Tay-Kearney ML, Neri P, Douglas K, Pathai S, Song AP, Kron M, Foster CS. New observations and emerging ideas in diagnosis and management of non-infectious uveitis: A review. Semin Arthritis Rheum. 2019;49:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 132. | Wakefield D, Abu El-Asrar A, McCluskey P. Treatment of severe inflammatory eye disease in patients of reproductive age and during pregnancy. Ocul Immunol Inflamm. 2012;20:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 133. | Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Jabs DA, Levy-Clarke GA, Foster CS. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 134. | Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol. 2014;8:1891-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 135. | Dick AD, Rosenbaum JT, Al-Dhibi HA, Belfort R Jr, Brézin AP, Chee SP, Davis JL, Ramanan AV, Sonoda KH, Carreño E, Nascimento H, Salah S, Salek S, Siak J, Steeples L; Fundamentals of Care for Uveitis International Consensus Group. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis: Fundamentals Of Care for UveitiS (FOCUS) Initiative. Ophthalmology. 2018;125:757-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 136. | Posarelli C, Arapi I, Figus M, Neri P. Biologic agents in inflammatory eye disease. J Ophthalmic Vis Res. 2011;6:309-316. [PubMed] |
| 137. | Zhang C, Zhang M, Qiu W, Ma H, Zhang X, Zhu Z, Yang CS, Jia D, Zhang TX, Yuan M, Feng Y, Yang L, Lu W, Yu C, Bennett JL, Shi FD; TANGO Study Investigators. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): an open-label, multicentre, randomised, phase 2 trial. Lancet Neurol. 2020;19:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 138. | Ringelstein M, Ayzenberg I, Harmel J, Lauenstein AS, Lensch E, Stögbauer F, Hellwig K, Ellrichmann G, Stettner M, Chan A, Hartung HP, Kieseier B, Gold R, Aktas O, Kleiter I. Long-term Therapy With Interleukin 6 Receptor Blockade in Highly Active Neuromyelitis Optica Spectrum Disorder. JAMA Neurol. 2015;72:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 139. | Araki M, Matsuoka T, Miyamoto K, Kusunoki S, Okamoto T, Murata M, Miyake S, Aranami T, Yamamura T. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology. 2014;82:1302-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 140. | Lotan I, Charlson RW, Ryerson LZ, Levy M, Kister I. Effectiveness of subcutaneous tocilizumab in neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2020;39:101920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 141. | Sepah YJ, Sadiq MA, Chu DS, Dacey M, Gallemore R, Dayani P, Hanout M, Hassan M, Afridi R, Agarwal A, Halim MS, Do DV, Nguyen QD. Primary (Month-6) Outcomes of the STOP-Uveitis Study: Evaluating the Safety, Tolerability, and Efficacy of Tocilizumab in Patients With Noninfectious Uveitis. Am J Ophthalmol. 2017;183:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 142. | Rensel M, Zabeti A, Mealy MA, Cimbora D, She D, Drappa J, Katz E. Long-term efficacy and safety of inebilizumab in neuromyelitis optica spectrum disorder: Analysis of aquaporin-4-immunoglobulin G-seropositive participants taking inebilizumab for ⩾4 years in the N-MOmentum trial. Mult Scler. 2022;28:925-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 143. | Kleiter I, Traboulsee A, Palace J, Yamamura T, Fujihara K, Saiz A, Javed A, Mayes D, Büdingen HV, Klingelschmitt G, Stokmaier D, Bennett JL. Long-term Efficacy of Satralizumab in AQP4-IgG-Seropositive Neuromyelitis Optica Spectrum Disorder From SAkuraSky and SAkuraStar. Neurol Neuroimmunol Neuroinflamm. 2023;10:e200071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 144. | Ikić Matijašević M, Kilić P, Ikić L, Galić I, Brzović Šarić V, Galić E. Rituximab, Intravitreal Bevacizumab and Laser Photocoagulation for Treatment of Macrophage Activation Syndrome and Retinal Vasculitis in Lupus: A Case Report. Int J Mol Sci. 2023;24:2594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 145. | Musa M, Chukwuyem E, Ojo OM, Topah EK, Spadea L, Salati C, Gagliano C, Zeppieri M. Unveiling Ocular Manifestations in Systemic Lupus Erythematosus. J Clin Med. 2024;13:1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 146. | Hari N, Patel P, Ross J, Hicks K, Vanholsbeeck F. Optical coherence tomography complements confocal microscopy for investigation of multicellular tumour spheroids. Sci Rep. 2019;9:10601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 147. | Zong Y, Miyagaki M, Yang M, Zhang J, Zou Y, Ohno-Matsui K, Kamoi K. Ophthalmic Use of Targeted Biologics in the Management of Intraocular Diseases: Current and Emerging Therapies. Antibodies (Basel). 2024;13:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 148. | Redenbaugh V, Montalvo M, Sechi E, Buciuc M, Fryer JP, McKeon A, Lennon VA, Mills JR, Weinshenker BG, Wingerchuk DM, Chen JJ, Tariq Bhatti M, Lopez Chiriboga AS, Pittock SJ, Flanagan EP. Diagnostic value of aquaporin-4-IgG live cell based assay in neuromyelitis optica spectrum disorders. Mult Scler J Exp Transl Clin. 2021;7:20552173211052656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 149. | Jendretzky KF, Bajor A, Lezius LM, Hümmert MW, Konen FF, Grosse GM, Schwenkenbecher P, Sühs KW, Trebst C, Framme C, Wattjes MP, Meuth SG, Gingele S, Skripuletz T. Clinical and paraclinical characteristics of optic neuritis in the context of the McDonald criteria 2017. Sci Rep. 2024;14:7293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 150. | Gastaldi M, Scaranzin S, Jarius S, Wildeman B, Zardini E, Mallucci G, Rigoni E, Vegezzi E, Foiadelli T, Savasta S, Banfi P, Versino M, Benedetti L, Novi G, Mancardi MM, Giacomini T, Annovazzi P, Baroncini D, Ferraro D, Lampasona V, Reindl M, Waters P, Franciotta D. Cell-based assays for the detection of MOG antibodies: a comparative study. J Neurol. 2020;267:3555-3564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 151. | Giorgiutti S, Jacquot R, El Jammal T, Bert A, Jamilloux Y, Kodjikian L, Sève P. Sarcoidosis-Related Uveitis: A Review. J Clin Med. 2023;12:3194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 152. | Yakin M, Kesav N, Cheng SK, Caplash S, Gangaputra S, Sen HN. The Association between QuantiFERON-TB Gold Test and Clinical Manifestations of Uveitis in the United States. Am J Ophthalmol. 2021;230:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 153. | Takeno M. The association of Behçet's syndrome with HLA-B51 as understood in 2021. Curr Opin Rheumatol. 2022;34:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |