Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.103154
Revised: February 28, 2025
Accepted: June 3, 2025
Published online: September 20, 2025
Processing time: 275 Days and 3.3 Hours
Hypertension is a significant global health concern and serves as a critical risk factor for cardiovascular diseases, stroke, and renal failure. Traditional Chinese Medicine (TCM) has been utilized for an extended period to address hyperten
To explore the differential plasma metabolites and associated pathways in hypertensive patients with YDYHS using gas chromatography-mass spectrometry (GC-MS) to elucidate the distinctive blood metabolite pattern in this patient population.
GC-MS was used to analyze plasma samples from 51 hypertensive patients with YDYHS and 20 healthy controls. Chemometric methods, including principal component analysis and partial least squares discriminant analysis, were employed to identify potential biochemical patterns. Simultaneously, the high-quality Kyoto Encyclopedia of Genes and Genomes metabolic pathways database was used to identify associated metabolic pathways. Using variable importance in projection and receiver operating characteristic curve analyses, potential biomarkers were extracted to assess their clinical utility.
Metabolomic profiling of hypertensive patients with YDYHS identified 20 potential biomarkers (4-hydroxybenzoic acid, pectin, 1,2,3-trihydroxybenzene, D-ribose, 3-hydroxybutyric acid, quinic acid, L-lysine, oleic acid, 2-hydroxybutyric acid, linoleic acid, citric acid, alpha-tocopherol, D-glucuronic acid, glycerol, N-acetyl-L-aspartic acid, beta-mannosylglycerate, indolelactic acid, L-glutamic acid, D-maltose, L-aspartic acid) and four metabolic pathways (linoleic acid metabolism; alanine, aspartate and glutamate metabolism; D-glutamine and D-glutamate metabolism; arginine biosynthesis). The identified differential metabolites may serve as potential biomarkers for distinguishing hypertensive patients with YDYHS from healthy controls. The area under the curve values ranged from 0.750 to 0.866. Receiver operating characteristic curve analysis showed that these differential metabolites can effectively classify hypertensive patients with YDYHS and healthy individuals.
The metabolomic analysis revealed a distinct blood metabolite pattern in hypertensive patients with YDYHS compared to the healthy control group, highlighting the potential role of the identified 20 biomarkers and four metabolic pathways in these patients. These findings may serve as an important material basis for understanding the occurrence and development of the disease, providing a scientific foundation for future clinical diagnosis.
Core Tip: Metabolomic profiling of hypertensive patients with Yin deficiency and Yang hyperactivity syndrome identified 20 potential biomarkers and four metabolic pathways. These biomarkers and pathways distinguished hypertensive patients with Yin deficiency and Yang hyperactivity syndrome from healthy controls. These potential biomarkers and pathways suggest the underlying metabolic basis of Yin deficiency and Yang hyperactivity syndrome in hypertensive patients. The metabolomic profiles provide novel insights into the mechanisms of Yin deficiency and Yang hyperactivity syndrome in hypertensive patients.
- Citation: Jialiken D, Dai J, Fan YD, Zhang HT, Shan JJ, Xu WC, Zou C. Gas chromatography-mass spectrometry-based plasma metabolomics analysis in hypertensive patients with Yin deficiency and Yang hyperactivity syndrome. World J Exp Med 2025; 15(3): 103154
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/103154.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.103154
Hypertension is a global public health issue, posing a significant burden on society. Long-term and persistent hypertension, which can result in target organ damage or failure, accounts for approximately 10.4 million deaths worldwide annually and contributes to around 10% of the total healthcare expenditure globally[1]. In comparison to solely relying on Western medicine, the integration of Chinese and Western medicine offers a safe and effective approach to reducing blood pressure in Chinese patients with essential hypertension, improving clinical symptoms without increasing the incidence of adverse events[2].
In Traditional Chinese Medicine (TCM), hypertension is classified under the categories of “vertigo” and “headache”. The target organ damage caused by hypertension has garnered increasing attention in clinical practice, aligning with the TCM pathogenesis theory that emphasizes liver and kidney disorders. The development of hypertension is linked to an imbalance between vasodilation (Yin) and vasoconstriction (Yang). Through a review of 2910 studies on TCM treatment for hypertension, 47 common syndromes have been identified; among them, Yin deficiency and Yang hyperactivity syndrome (YDYHS) (25.25%) is the most prevalent, followed by liver-Yang hyperactivity syndrome (15.7%) and phlegm-dampness congestion syndrome (PDCS) (8.59%)[3]. As a major pathogenetic factor in hypertension, YDYHS has also been included in Chinese guidelines for hypertension management. However, syndrome differentiation often depends on the subjective experience of the physician and lacks standardized rules.
In 2020, several key technology seminars on the development of new drugs in TCM were organized in China, focusing on how to achieve objectivity in TCM evidence. Chinese medicine summarizes the “syndrome” of diseases through observation, olfaction, inquiry, and palpation, indicating the complexity and integrity of the “syndrome”[4]. Metabolomics enables the acquisition of individualized disease management insights by measuring biological samples like blood, urine, and saliva to obtain each patient's metabolic profile[5], which, to some extent, aligns with the TCM principle of "treatment based on syndrome differentiation." Previous studies have shown that 33 potential biomarkers, such as androstenedione and lyso-phosphatidylcholine, and 16 related metabolic pathways, such as steroid hormone and lipid metabolism, are present in prehypertensive patients with excessive liver fire syndrome[6]. Compared with the control group, the contents of acetone, very-low-density lipoprotein, and low-density lipoprotein in hypertension patients with PDCS increased, while the contents of lactate, serine, glucose, methionine, and alanine decreased. Abnormalities were found in lipid metabolism, amino acid metabolism, and glucose metabolism[7]. Although there were studies on YDYHS[8,9] the sample sizes were less than 20 cases.
Herein, we aimed to further expand the sample size to investigate changes in metabolites and metabolic pathways in the plasma of hypertensive patients with YDYHS. Additionally, this study sought to explore the mechanisms of YDYHS in patients with high blood pressure. Plasma metabolomics methods based on gas chromatography-mass spectrometry (GC-MS) and multiple bioinformatics tools were applied to analyze plasma samples from hypertensive patients with YDYHS and healthy controls (HCs). The results obtained will contribute to revealing the metabolic basis of YDYHS in hypertensive patients (Figure 1).
All participants provided written informed consent. All samples were collected from the Institute of Hypertension or Department of Cardiology, Affiliated Hospital of Nanjing University of Chinese Medicine. Patients who met the inclusion criteria were enrolled in the study. Diagnosis was provided by the same physician. This study was approved by the Ethics Committee at the Affiliated Hospital of Nanjing University of Chinese Medicine (2019NL-190-02).
The inclusion criteria for the experimental group were as follows: (1) Met the diagnostic criteria for primary hypertension according to the Chinese Guidelines for the Prevention and Treatment of Hypertension 2018 Revision[10], and hypertensive patients with blood pressure < 140/90 mmHg after treatment with antihypertensive drugs; (2) Met the YDYHS diagnostic criteria from the Clinical Research Guidelines for New Drugs in Chinese Medicine (2002). Primary diagnostic criteria for YDYHS were the presence of at least two primary (vertigo; headache; soreness of the waist; weakness of the knees; and dysphoria with feverish sensation in chest, palms, and soles) and two secondary manifestations (palpitation; tinnitus; insomnia; and forgetfulness); (3) Aged 35–70 years; and (4) Voluntarily signed an informed consent form.
The inclusion criteria for the control group were as follows: Good previous physical fitness, no history of hypertension, coronary heart disease, diabetes, etc., and history taking, physical examination, and laboratory tests at the time of consultation were within the normal range or showed abnormalities without clinical significance. Cases were selected from the physical examination center or Phase I ward, Affiliated Hospital of Nanjing University of Chinese Medicine.
The exclusion criteria for the experimental group were as follows: (1) Secondary hypertension; (2) Serious life-threatening diseases such as acute myocardial infarction, stroke, heart failure (NYHA Class IV), and malignant arrhythmia; (3) Liver function indexes [aspartate aminotransferase (AST) or alanine aminotransferase (ALT)] greater than two times the upper limit of normal; (4) Patients with a history of mental illness or legal disability; and (5) Participation in other clinical trials.
Instrument: Trace 1310-TSQ8000 Evo Triple quadrupole gas chromatography-mass spectrometry (Thermo Fisher Scientific, United States); Allegra 64R high-speed refrigerated centrifuge (Beckman Coulter, United States); Vortex-Genie 2 vortex mixer (Scientific Industries, United States); BSA124S-CW analytical balance (Sartorius, Germany); Savant SPD1010 vacuum centrifuge concentrator (Thermo Fisher Scientific, United States).
Reagents: Methoxylamine hydrochloride (Sigma-Aldrich, Batch No. WXBD0638V); pyridine (Sigma-Aldrich, Batch No. STBJ2924); 1,2-¹³C myristic acid (Sigma-Aldrich, Batch No. MBC6775); N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) (Sigma-Aldrich, Batch No. BCCD2584); methanol (Merck, Batch No. I1150307122).
Samples were thawed on ice. A 100 μL aliquot was transferred to a 1.5 mL centrifuge tube, and 400 μL of methanol solution (containing 12.5 μg/mL of 1,2-¹³C myristic acid) was added, vortexed for 3 min, and then centrifuged at 4 °C for 10 min at 13000 rpm. Then, 100 μL of supernatant was evaporated in a centrifuge concentrator (45 °C, 15 kPa). To the dried residue, 30 μL of methoxylamine hydrochloride in pyridine solution (10 mg/mL) was added, vortexed for 5 min, and incubated at 30 °C for 1.5 h. Subsequently, 30 μL of BSTFA (containing 1% TMCS) was added, vortexed for 1 min, and incubated at 37 °C for 0.5 h. After derivatization, the mixture was centrifuged at 13000 rpm for 10 min, and 40 μL of the supernatant was transferred to a sample vial for GC-MS analysis.
Aliquots from all study samples were pooled. A 100 μL volume of this pooled mixture was used as a quality control (QC) sample and processed according to the steps described above for individual samples.
A TG-5MS capillary column (0.25 mm × 30 m × 0.25 μm) was used. The temperature program was: 0–1 min, 60 °C; 1–14 min, ramp from 60 °C to 320 °C (specify rate if available, e.g., at 20 °C/min); 14–19 min, 320 °C. The split ratio was 20:1, and the injection volume was 1 μL. Mass spectrometry was performed in full scan mode with a scan range of m/z 50–500. The carrier gas was high-purity helium (purity > 99.999%) at a flow rate of 1.2 mL/min.
The spectral data obtained by GC-MS were processed using MS-DIAL software, and metabolites were identified against the National Institute of Standards and Technology (NIST) database. The data matrices were analyzed by principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) using the MetaboAnalyst website (https://www.metaboanalyst.ca) to identify differential metabolites and associated metabolic pathways. Receiver operating characteristic curves of the differential metabolites were plotted using MedCalc software to assess their discriminatory power.
For quantitative data, those following a normal distribution are expressed as the mean ± SD, and an independent samples t-test was used for comparisons. Data not following a normal distribution are expressed as median and interquartile range [median (IQR: P25–P75)], and the Wilcoxon rank sum test was used for comparisons. Qualitative data, presented as counts (n) and percentages (%), were compared between groups using the χ² test (or Fisher's exact test, as appropriate). Data were processed and analyzed using SPSS 25.0 software, and P < 0.05 was considered statistically significant.
A total of 71 participants were recruited and divided into the hypertension (YDYHS) group (n = 51) and the HC group (n = 20). The baseline comparisons are summarized in Table 1. The two groups showed statistically significant differences in white blood cell count (WBC), ALT, AST, gamma-glutamyl transferase (GGT), blood urea nitrogen (BUN), and blood glucose (Glu) (P < 0.05).
| Project | Healthy subjects | Hypertension (YDYHS) | Normal reference | t/Z | P value |
| WBC (× 109/L) | 5.4860 ± 1.04219 | 6.7019 ± 1.52323 | 4.00-10.00 | 3.254 | 0.002a |
| RBC (× 1012/L) | 4.7685 ± 0.41509 | 4.8728 ± 0.50817 | 4.00-5.50 | 0.809 | 0.422 |
| Hb (g/L) | 141.85 ± 14.147 | 145.79 ± 13.779 | 120-160 | 1.062 | 0.292 |
| PLT (× 109/L) | 236.45 ± 51.953 | 235.53 ± 47.571 | 100-300 | -0.070 | 0.944 |
| ALT (U/L) | 14 (12, 17) | 22 (15, 27) | < 42 | -3.677 | 0.000a |
| AST (U/L) | 17 (15, 19) | 18 (16, 24) | < 38 | -2.315 | 0.021a |
| TBil (μmol/L) | 11.41 (8.84, 16.28) | 11.10 (9.19, 15.28) | 5.10-28.00 | -0.319 | 0.750 |
| ALP (U/L) | 77.45 ± 18.349 | 83.12 ± 22.374 | 45-125 | 1.005 | 0.319 |
| GGT (U/L) | 14 (12, 19) | 22 (16, 30) | 11-50 | -3.232 | 0.001a |
| CR (μmol/L) | 72.4 (59.9, 84.4) | 67.1 (59.8, 76.6) | 44.0-110.0 | -0.917 | 0.359 |
| BUN (mmol/L) | 4.12 (3.51, 4.87) | 4.96 (4.13, 5.90) | 2.86-8.20 | -2.899 | 0.004a |
| Glu (mmol/L) | 4.54 (4.31, 4.86) | 5.54 (5.21, 5.95) | 3.89-6.11 | -5.474 | 0.000a |
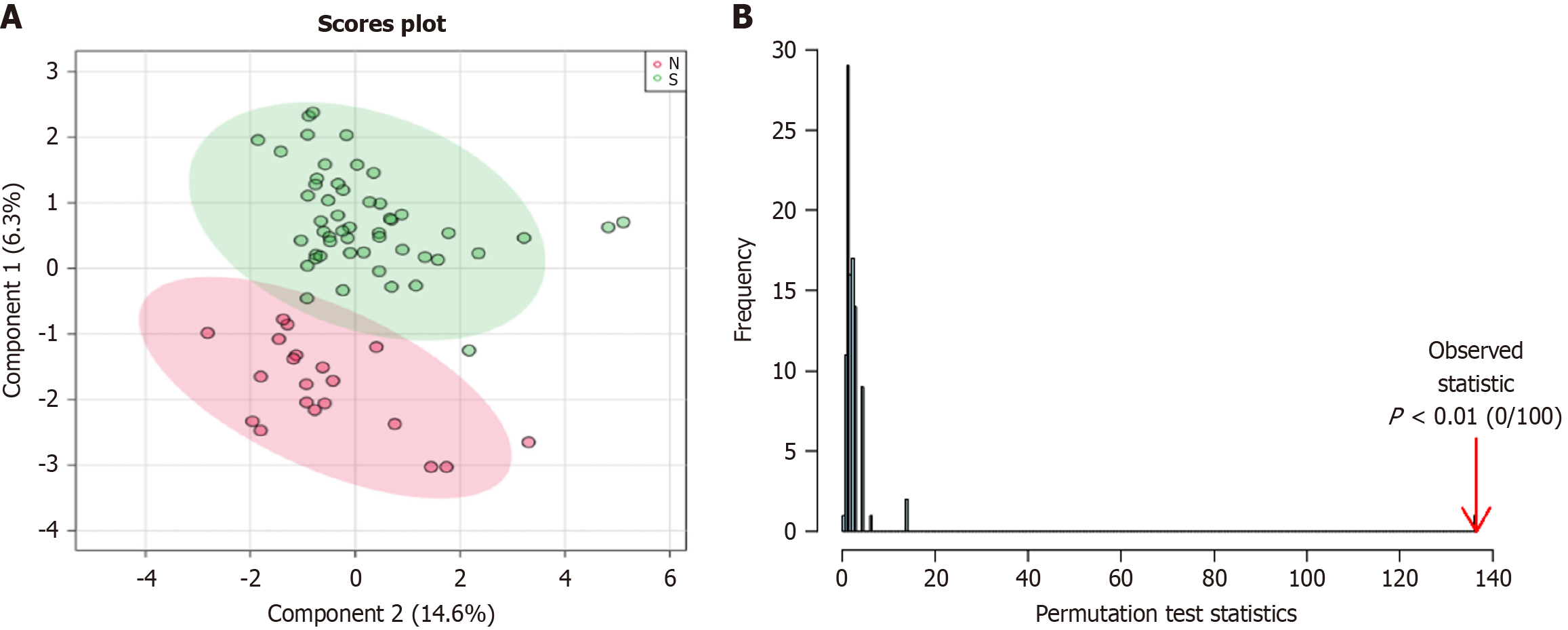
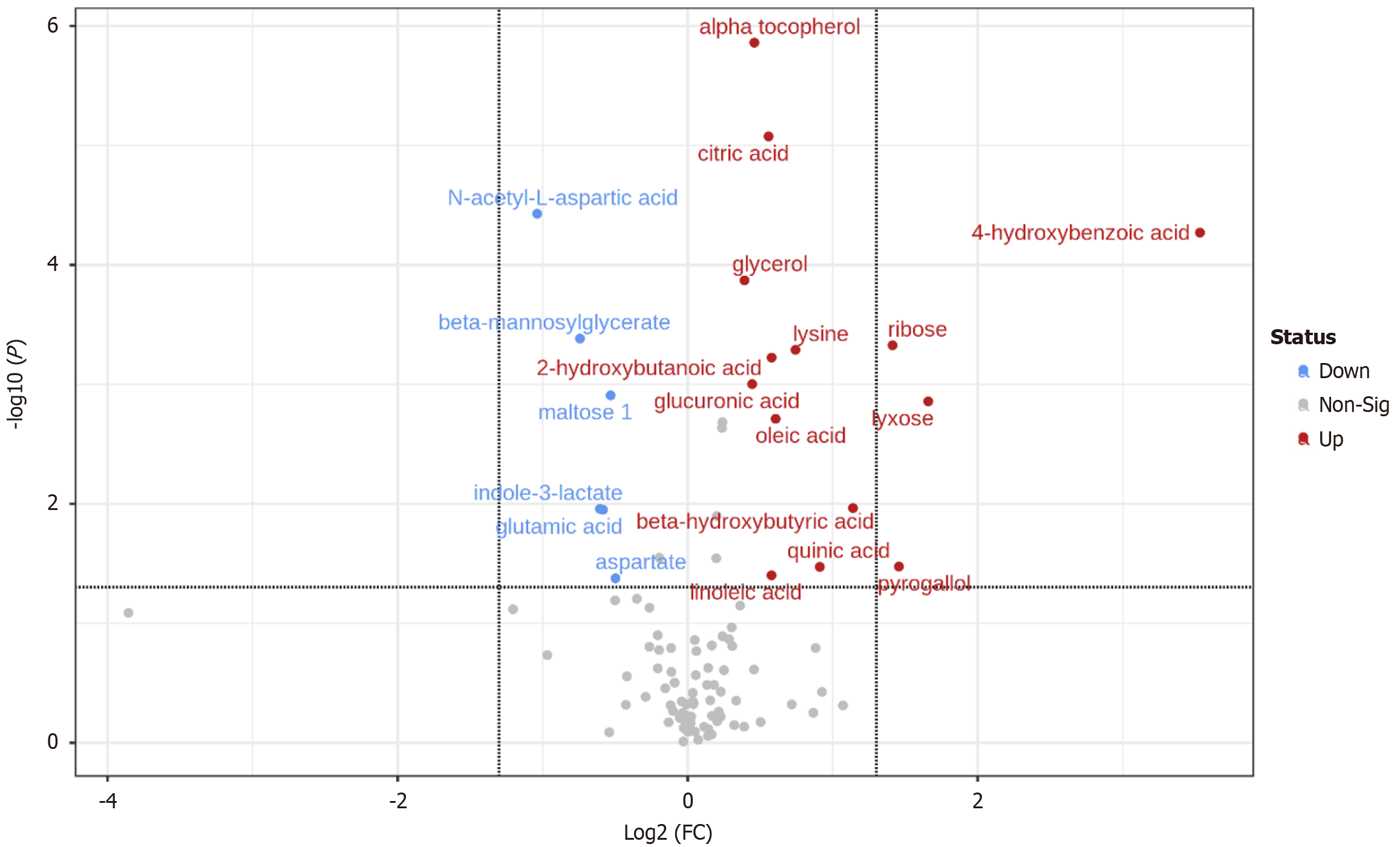
QC samples were closely distributed and concentrated (Supplementary Figure 1), and the analytical system was stable during the process. QC total ion flow diagram is shown in Supplementary Figure 2. With the help of Metaboanalyst website to plot PCA and PLS-DA, there was a clear separation between the two groups of metabolites (Figure 2), suggesting the existence of metabolic differences between the two groups. The volcano plot and heat map of the different metabolites were plotted by setting “P < 0.05, fold change = 1.2” (Figure 3), and the peak ratio of metabolites in the disease group/healthy group is expressed by fold change.
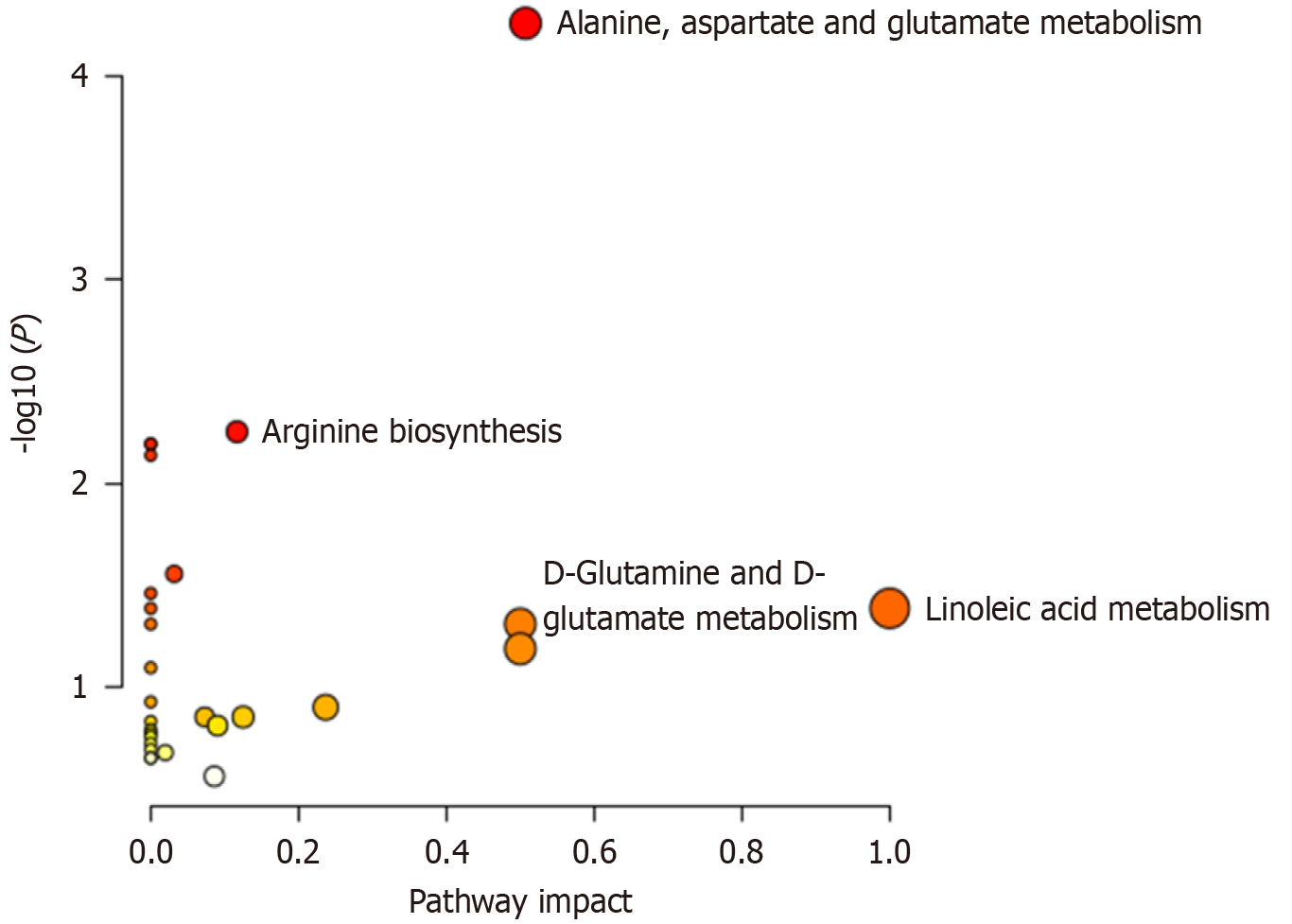
After analysis, 20 differential metabolites existed in the two groups, including 14 up-regulated and 6 down-regulated metabolites (Table 2), mainly involving five metabolic pathways, including lipid metabolism and amino acid metabolism (Table 3 and Figure 4). The ROC curves of differential metabolites were further plotted using MedCalc software (Supplementary Figure 3), and the AUC values were distributed in the range of 0.684-0.866.
| No. | Var ID | HMDB | KEGG | FC | P value | PubChem | Trend |
| 1 | 4-Hydroxybenzoic acid | HMDB0000500 | C00156 | 11.566 | < 0.01 | 135 | ↑ |
| 2 | Pectin | HMDB0003402 | C08348 | 3.1576 | < 0.01 | 65550 | ↑ |
| 3 | 1,2,3-Trihydroxybenzene | HMDB0013674 | C01108 | 2.7443 | 0.033 | 3446 | ↑ |
| 4 | D-Ribose | HMDB0000283 | C00121 | 2.6621 | < 0.01 | 5779 | ↑ |
| 5 | 3-Hydroxybutyric acid | HMDB0000357 | C01089 | 2.2031 | 0.011 | 441 | ↑ |
| 6 | Quinic acid | HMDB0003072 | C00296 | 1.8801 | 0.034 | 6508 | ↑ |
| 7 | L-Lysine | HMDB0000182 | C00047 | 1.6739 | < 0.01 | 6508 | ↑ |
| 8 | Oleic acid | HMDB0000207 | C00712 | 1.5228 | ≤ 0.01 | 445639 | ↑ |
| 9 | 2-Hydroxybutyric acid | HMDB0000008 | C05984 | 1.4935 | 0.01 | 11266 | ↑ |
| 10 | Linoleic acid | HMDB0000673 | 159 | 14928 | 0.040 | 5280450 | ↑ |
| 11 | Citric acid | HMDB0000094 | C00158 | 1.4718 | 0.01 | 311 | ↑ |
| 12 | Alpha-tocopherol | NA | NA | 1.375 | 0.01 | NA | ↑ |
| 13 | D-Glucuronic acid | HMDB0000127 | C00191 | 1.3608 | < 0.01 | 94715 | ↑ |
| 14 | Glycerol | HMDB0000131 | C00116 | 1.3121 | < 0.01 | 753 | ↑ |
| 15 | N-Acetyl-L-aspartic acid | HMDB0000812 | C01042 | 0.48705 | 0.01 | 65065 | ↓ |
| 16 | Beta-mannosylglycerate | NA | NA | 0.59765 | < 0.01 | NA | ↓ |
| 17 | Indolelactic acid | HMDB0000671 | C02043 | 0.65739 | 0.011 | 92904 | ↓ |
| 18 | L-Glutamic acid | HMDB0000148 | C00025 | 0.66721 | 0.011 | 33032 | ↓ |
| 19 | D-Maltose | HMDB0000163 | C00208 | 0.69193 | 0.01 | 10991489 | ↓ |
| 20 | L-Aspartic acid | HMDB0000191 | C00049 | 0.70828 | 0.042 | 5960 | ↓ |
| Pathway | Hits/Total | Expected | Impact | P value |
| Linoleic acid metabolism | 1/5 | 0.041935 | 1 | 0.041 |
| Alanine, aspartate, and glutamate metabolism | 4/28 | 0.23484 | 0.50722 | < 0.01 |
| D-Glutamine and D-glutamate metabolism | 1/6 | 0.050323 | 0.5 | 0.049 |
| Arginine biosynthesis | 2/14 | 0.11742 | 0.11675 | < 0.01 |
In the baseline comparison, values of all safety indicators were within normal ranges. The YDYHS group showed statistically significant increases in WBC, ALT, AST, GGT, BUN, and Glu when compared with the HC group. In TCM, YDYHS-type hypertension is characterized by insufficient Yin fluid, resulting in a relative Yang excess, causing hypertension symptoms. Changes in WBC in these patients may be associated with their immune status and inflammatory responses. An elevated WBC count may correlate with chronic inflammation, metabolic syndrome, and an increased risk of cardiovascular diseases. This suggests that a certain level of inflammation may contribute to alterations in WBC levels. The increases in ALT, AST, GGT, BUN, and Glu observed in YDYHS-type hypertensive patients indicate that these patients are more likely to experience damage to organ function and target organs compared to healthy individuals. The results suggest that hypertensive patients with YDYHS may have overactivated inflammatory reactions, liver metabolic disorders, potential for hyperhomocysteinemia (inferred from BUN), and blood glucose abnormalities that can distinguish them from healthy participants.
A study involving 2935 healthy subjects divided them into three groups based on WBC distribution: Group 1 (< 4.7 × 109/L), group 2 (4.7-5.999 × 109/L), and group 3 (≥ 6.0 × 109/L). After an average follow-up of 4.5 years, 908 subjects had systolic blood pressure ≥ 140 mmHg. Compared to group 1, the risk ratio of hypertension in group 2 was 1.07 (95% confidence interval: 0.90-1.26), with a higher risk ratio in group 3. It suggests that the incidence rate of hypertension increases with the leukocyte count, which may promote the process of atherosclerosis through leukocyte adherence to endothelial cells, leading to an increase in systemic arterial stiffness and causing hypertension[11]. A study investigating the potential correlation between WBC count and blood pressure levels revealed that an elevated WBC count may serve as an independent risk factor for hypertension, implying a significant role for inflammation in the pathophysiological mechanisms underlying hypertension. Consequently, incorporating WBC count as an indicator in clinical practice can facilitate early intervention and management strategies for assessing the risk of hypertension more effectively[12].
One cross-sectional study evaluated the association between elevated liver enzymes and hypertension. The study found that compared with the normal blood pressure group, the average concentrations of serum ALT, AST, and GGT in the hypertension group were significantly increased, indicating a relatively higher incidence of elevated liver enzymes in patients with hypertension[13]. Another large sample study of 21293 healthy participants, followed up for 3.9 years (average) with hypertension as the observation endpoint, reached similar conclusions. In addition, in the subgroup analysis of participants with ALT ≤ 2 times the normal limit (80 U/L) (n = 20983), this study showed that high ALT and GGT were significantly associated with the incidence of hypertension[14]. Hyperhomocysteinemia and dysglycemia are recognized risk factors for hypertension. BUN is associated with homocysteine levels. A study has shown that BUN serves as an independent risk factor in male hypertensive patients[15]. Another study found that compared to a lower baseline of Glu (< 5.6 mmol/L), women with a higher baseline of Glu (≥ 5.6 mmol/L and < 7.0 mmol/L) had a significantly increased risk of developing new-onset hypertension[16]. Additionally, a 2019 study indicated that fasting blood glucose serves as a predictive factor for future hypertension in middle-aged Japanese men and women, which supports fasting blood glucose as an effective biomarker for identifying the risk of hypertension in middle-aged populations and emphasizes the importance of early management and intervention in preventing cardiovascular diseases. The results of our study align with these previous findings[17].
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid, has anti-inflammatory, antioxidant, cardioprotective, and vasodilatory effects. As an important target of Lei-gong-gen formula granule in the intervention of hypertension, 4-hydroxybenzoic acid exhibits good binding ability with NOS3, SRC, PIK3CA, and AKT, which may act on vascular smooth muscle by stimulating endothelial cells to release nitric oxide (NO), further promoting vasodilation and lowering blood pressure[18]. There are various known forms of hydroxybenzoic acid compounds, such as 2,3-dihydroxybenzoic acid (pyrocatechuic acid), 2,5-dihydroxybenzoic acid (gentisic acid), and 3,4-dihydroxyphenylacetic acid (protocatechuic acid), which can activate the Nrf2 signaling pathway, increase the expression of antioxidant enzymes, and reduce hypertensive endothelial dysfunction caused by oxidative stress[19]. This suggests that 4-hydroxybenzoic acid may also protect the vascular endothelium and alleviate hypertension similarly. 4-Hydroxybenzoic acid may be in a relatively balanced state in normal individuals, with a compensatory increase in the disease state for self-regulation.
Compared with HCs, the levels of linoleic acid (LA) were increased in hypertensive patients with YDYHS. LA can increase the risk of cardiovascular disease by inducing arachidonic acid to promote inflammation[20], leading to increased oxidized LA metabolites, inducing the formation of foam cells and causing endothelial cell dysfunction[21]. In addition, LA induces the expression of vascular cell adhesion molecule-1 in endothelial cells through nuclear factor-kappaB signal transduction, activating the proinflammatory response of vascular endothelial cells and leading to atherosclerosis[22]. Vascular endothelial dysfunction leads to the interaction between atherosclerosis and hypertension, which is the central link in the event chain where hypertension further leads to damage to important target organs such as the heart, brain, and kidney. It is speculated that patients with YDYHS may have more prominent vascular endothelial damage problems than healthy individuals, which affects the occurrence and development of hypertension. LA is also the most prevalent fatty acid found in arterial plaques, and its stable oxidation product—hydroxyoctadecadienoic acid—serves as a marker of oxidative stress and a component of oxidized lipids in atherosclerosis (AS). This compound promotes the progression of AS and increases the risk of cardiovascular events[21]. Based on these findings, this study suggests that LA may exert a bidirectional regulatory effect. Furthermore, it is possible that LA has upstream or downstream activators that play a dual role in the cardiovascular system, warranting further investigation.
The alanine, aspartate, and glutamate metabolism pathway is a key pathway in regulating oxidative stress in cardiovascular diseases[23]. Oxidative stress is a pathological process that occurs when there is an imbalance between oxidation and antioxidant activity in the body. Moderate levels of reactive oxygen species (ROS) play an important role in cell signaling, but excessive levels of ROS can lead to damage to proteins, lipids, and even cell death. Oxidative stress is one of the mechanisms of hypertension, which leads to dysfunction of the vascular endothelium, destruction of vascular structure, and acceleration of the atherosclerosis process, and finally causes hypertension. Elevated levels of aspartic acid and its metabolites, including quinolinic acid, in patients with hypertension are linked to a heightened risk of cardiovascular disease. Quinolinic acid, the final product of tryptophan metabolism, is closely associated with inflammation, oxidative stress, and endothelial dysfunction[24]. Abnormal glutamate metabolism impacts vascular function by affecting the proliferation and migration of vascular smooth muscle cells. This mechanism is particularly evident in pulmonary hypertension and other cardiovascular diseases. Future research should further investigate the specific pathways of glutamate metabolism and its role in various cardiovascular conditions to develop more effective therapeutic strategies[25]. The decrease in alanine, aspartic acid, and glutamate metabolites in patients with YDYHS suggests the activation of oxidative stress, which may lead to excessive consumption of these metabolites or inhibition of antioxidant production in pathological conditions.
Glutamine is a precursor substance of glutamate. Glutamine first enters the cell through transporters and then is converted into glutamate in mitochondria through the catalytic action of glutaminase. Glutamate is further converted into α-ketoglutaric acid through glutamate dehydrogenase, alanine transaminase, or aspartate transaminase. α-Ketoglutaric acid is an important intermediate product in the tricarboxylic acid cycle, and metabolic disorders of this substance can affect energy metabolism in the body[26]. A study has shown that direct injection of glutamic acid into the cerebral cistern of experimental dogs resulted in an increase in blood pressure. Further analysis suggested that this might be related to the involvement of glutamic acid in central blood pressure regulation and an increase in sympathetic nervous tension[27]. D-Glutamine plays a significant role in angiogenesis through a nitric oxide synthase-dependent mechanism, which promotes the growth and migration of endothelial cells. The metabolism of glutamine is essential for the biosynthesis of L-arginine, thereby influencing the production of endothelium-derived relaxing factor by endothelial cells[28]. Hypertensive patients with YDYHS can also exhibit altered glutamate metabolism, possibly manifested as energy utilization disorders and high sympathetic activity.
Nitric oxide synthase exists in the endothelial cells of blood vessels, and under certain conditions, L-arginine is decomposed into NO and L-citrulline[29]. The arginine metabolic pathway plays a crucial role in the pathogenesis and management of hypertension by regulating NO production and the urea cycle. Endothelial dysfunction, oxidative stress, and vascular remodeling, which arise from metabolic abnormalities, are fundamental mechanisms underlying hypertension. Arginine supplementation and the regulation of enzyme activity offer promising new treatment options. Future research should integrate genetic background and metabolic phenotype to create more targeted intervention strategies[30]. NO is an endothelial relaxing factor that soothes vascular smooth muscle, dilates blood vessels, and regulates blood pressure. Research has shown[31] that oral L-citrulline combined with L-arginine can rapidly enhance the bioavailability of NO, which has certain clinical significance in regulating blood pressure. Conversely, when the arginine biosynthesis pathway is affected, NO generation also decreases, leading to a decrease in vasodilation function and a rise in blood pressure. In addition, L-arginine is an important dietary amino acid. Supplementing L-arginine plays a role in reducing inflammation and oxidative stress pathways, lowering blood pressure, decreasing left ventricular damage, and reducing abdominal fat[25]. The imbalance of arginine biosynthesis may also be one of the characteristic pathways in hypertensive patients with YDYHS.
In summary, metabolomic profiling of hypertensive patients with YDYHS identified 20 potential biomarkers and four metabolic pathways. These distinguished hypertensive patients with YDYHS from healthy controls. These potential biomarkers and pathways suggest the substantial metabolic basis of YDYHS in hypertensive patients. The metabolomic profiles highlight modernized insights into the mechanisms of YDYHS in these patients.
| 1. | Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1966] [Article Influence: 393.2] [Reference Citation Analysis (3)] |
| 2. | Mohammed SAD, Hanxing L, Fang L, Algradi AM, Alradhi M, Safi M, Shumin L. Integrated Chinese herbal medicine with Western Medicine versus Western Medicine in the effectiveness of primary hypertension treatment: A systematic review and meta-analysis of randomized controlled trials. J Ethnopharmacol. 2023;300:115703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Tan Y, L Huang. Literature research on TCM syndrome types and syndrome elements distribution laws of hypertension. Zhonghua Zhongyiyao Xuekan. 2015;33. [DOI] [Full Text] |
| 4. | Yuan X, Wang YQ. Metabonomic in research of basic materiel of syndromes of traditional Chinese medicine. Zhonghua Zhongyiyao Xuekan. 2013;31. [DOI] [Full Text] |
| 5. | Müller J, Bertsch T, Volke J, Schmid A, Klingbeil R, Metodiev Y, Karaca B, Kim SH, Lindner S, Schupp T, Kittel M, Poschet G, Akin I, Behnes M. Narrative review of metabolomics in cardiovascular disease. J Thorac Dis. 2021;13:2532-2550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Li SM, Li C, Yang WQ, Li YL. [Non-targeted metabolomics study and biomarker screening of prehypertensive liver-fire hyperactivity syndrome based on UPLC-Q-Exactive MS technology]. Zhongguo Zhong Yao Za Zhi. 2021;46:2881-2888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wu TM, Chen JS, Xue WJ, Gao XL. NMR metabonomics study on serum samples of young and middle-age hypertension patients with phlegm dampness retention syndrome. Zhongguo Zhongyiyao Xinxi Zazhi. 2016;23:21-25. [DOI] [Full Text] |
| 8. | Fan QL, Wang GJ, Akiye, Hao HP, Zhu J, Ning C, Chen XH. A metabonomic study on hypertension due to yin deficiency and yang excess. Nanjing Zhongyiyao Daxue Xuebao. 2010;26:409-411. [DOI] [Full Text] |
| 9. | Zhu J, Dong HQ, Fang ZY. Study on the metabolomics of hypertension patient with yin deficiency and yang excess syndrome. Shenzhen Zhongxiyi Jiehe Zazhi. 2013;23:142-147. [DOI] [Full Text] |
| 10. | Writing Group of 2018 Chinese Guidelines for the Management of Hypertension CHL, Chinese Society of Cardiology, Chinese Medical Doctor Association Hypertension Committee, Hypertension Branch of China International Exchange and Promotive Association for Medical and Health Care, Hypertension Branch of Chinese Geriatric Medical Association. 2018 Chinese guidelines for the management of hypertension. Zhongguo Xinxueguan Zazhi. 2019;24:24-56. [DOI] [Full Text] |
| 11. | Ishida S, Kondo S, Funakoshi S, Satoh A, Maeda T, Kawazoe M, Yoshimura C, Tada K, Takahashi K, Ito K, Yasuno T, Masutani K, Nakashima H, Arima H. White blood cell count and incidence of hypertension in the general Japanese population: ISSA-CKD study. PLoS One. 2021;16:e0246304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Tsuda K. A link between white blood cell count and blood pressure levels. Hypertens Res. 2024;47:537-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Rahman S, Islam S, Haque T, Kathak RR, Ali N. Association between serum liver enzymes and hypertension: a cross-sectional study in Bangladeshi adults. BMC Cardiovasc Disord. 2020;20:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Liu YH, Chen SC, Lee WH, Chen YC, Huang JC, Wu PY, Hung CH, Kuo CH, Su HM. Liver-function parameters are associated with incident hypertension in a large Taiwanese population follow-up study. J Hum Hypertens. 2023;37:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Yang Q, Lu Y, Deng Y, Xu J, Zhang X. Homocysteine level is positively and independently associated with serum creatinine and urea nitrogen levels in old male patients with hypertension. Sci Rep. 2020;10:18050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Liu J, Cheng NN, Zhou ZY, Zhang Y, Yang J, Liu LS, Song Y, Huang X, Tang GF, Wang BY, Qin XH, Xu XP, Kong XQ. Effect of fasting blood glucose on risk of new-onset hypertension in rural Chinese population: a 15-year follow-up cohort. BMC Cardiovasc Disord. 2021;21:531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Li Q, Lan T, He S, Chen W, Li X, Zhang W, Liu Y, Zhang Q, Chen X, Han Y, Su Z, Zhu D, Guo H. A network pharmacology-based approach to explore the active ingredients and molecular mechanism of Lei-gong-gen formula granule on a spontaneously hypertensive rat model. Chin Med. 2021;16:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Tatsumi Y, Morimoto A, Asayama K, Sonoda N, Miyamatsu N, Ohno Y, Miyamoto Y, Izawa S, Ohkubo T. Fasting Blood Glucose Predicts Incidence of Hypertension Independent of HbA1c Levels and Insulin Resistance in Middle-Aged Japanese: The Saku Study. Am J Hypertens. 2019;32:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Juurlink BH, Azouz HJ, Aldalati AM, AlTinawi BM, Ganguly P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr J. 2014;13:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Chandra A, Røsjø H, Svensson M, Vigen T, Ihle-Hansen H, Orstad EB, Rønning OM, Lyngbakken MN, Nygård S, Berge T, Schmidt EB, Omland T, Tveit A, Eide IA. Plasma linoleic acid levels and cardiovascular risk factors: results from the Norwegian ACE 1950 Study. Eur J Clin Nutr. 2020;74:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Vangaveti V, Baune BT, Kennedy RL. Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Ther Adv Endocrinol Metab. 2010;1:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Dichtl W, Ares MP, Jönson AN, Jovinge S, Pachinger O, Giachelli CM, Hamsten A, Eriksson P, Nilsson J. Linoleic acid-stimulated vascular adhesion molecule-1 expression in endothelial cells depends on nuclear factor-kappaB activation. Metabolism. 2002;51:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Alam MA, Kauter K, Withers K, Sernia C, Brown L. Chronic l-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats. Food Funct. 2013;4:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Salminen A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J Mol Med (Berl). 2021;99:1-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Chen S, Lin S, Liu W, Lin Q, Yang Y, Qiu Q, Zong Y, Xiao T, Hou C, Xie L. Serum Metabolomic Profile in Hypoxia-Induced Pulmonary Hypertension Mice after C75 Treatment. Front Biosci (Landmark Ed). 2023;28:251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Zhong SJ, Li J, Li L, Huang SM, Yang M, Qiu H, Cheng B, Hu ZX. Exploring the intervention mechanism of Shenmai injection on hypertensive heart failure based on bioinformatics. Zhongyi Xuebao. 2021;36:160-164. [DOI] [Full Text] |
| 27. | Chelly J, Kouyoumdjian JC, Mouillé P, Huchet AM, Schmitt H. Effects of L-glutamic acid and kainic acid on central cardiovascular control. Eur J Pharmacol. 1979;60:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Peyton KJ, Liu XM, Yu Y, Yates B, Behnammanesh G, Durante W. Glutaminase-1 stimulates the proliferation, migration, and survival of human endothelial cells. Biochem Pharmacol. 2018;156:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Whitworth JA. Relationship between white blood cell count and incident hypertension. Am J Hypertens. 2004;17:861; author reply 861-861; author reply 862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 31. | Morita M, Hayashi T, Ochiai M, Maeda M, Yamaguchi T, Ina K, Kuzuya M. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem Biophys Res Commun. 2014;454:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |