Published online Aug 12, 2025. doi: 10.5410/wjcu.v14.i2.109933
Revised: June 21, 2025
Accepted: July 29, 2025
Published online: August 12, 2025
Processing time: 77 Days and 13.7 Hours
Β-elemene is widely used to treat a variety of cancers, including bladder cancer (BLCA). However, the anti-cancer target, effective constituents and mechanism was unclear.
To investigate the therapeutic effect and underlying mechanism of β-elemene in BLCA.
We first mined the GEPIA2 database to explore the association between the GM3
Our results revealed a significantly reduced expression of GM3 in BLCA tissues. Notably, BLCA patients with higher GM3 expression exhibited prolonged overall survival and disease-free survival. In vitro studies demonstrated that β-elemene significantly affected BLCA cell viability, leading to a marked upregulation of GM3 expression, increased apoptotic cell populations, and a notable reduction in cell migration and invasion. WB analysis showed that β-elemene enhanced GM3 protein expression while simultaneously decreasing phosphorylated epidermal growth factor receptor (p-EGFR) levels. Additionally, overexpression or RNAi of GM3 in BLCA cells resulted in corresponding changes in epidermal growth factor receptor and p-EGFR expression levels.
This study provides preliminary evidence for further investigation into the molecular mechanisms of β-elemene in the treatment of BLCA.
Core Tip: We believe that our study makes a significant contribution to the literature because GEPIA2 database deep mining revealed that GM3 was poorly expressed in bladder cancer (BLCA), and this study showed that β-elemene inhibited BLCA cells by increasing GM3 expression. Moreover, cluster analysis showed that GM3 increased cell adhesion and inhibited the invasion and migration of BLCA cells by expressing membrane-related signaling proteins. Further, we believe that this paper will be of interest to the readership of your journal because the use of β-elemene in anti-cancer medication is advantageous owing to its significant anti-tumor activity against various types of cancer and its reduced toxicity, which ensures that its impact on the quality of life of patients with cancer is minimal and the drug is well tolerated. Thus, this study provides a preliminary basis and strong evidence for further research on the underlying molecular mechanisms of β-elemene in the treatment of BLCA.
- Citation: Li FY, Wang Y, Zhang YJ. β-elemene inhibits bladder cancer cell through the expression of cell membrane protein GM3. World J Clin Urol 2025; 14(2): 109933
- URL: https://www.wjgnet.com/2219-2816/full/v14/i2/109933.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v14.i2.109933
Bladder cancer (BLCA) is the most common malignancy of the urinary tract[1]. In 2020, there were 573278 new cases of BLCA globally, with a mortality rate of 212536[2]. Routine treatments for BLCA include transurethral resection of bladder tumor (TURBT) followed by intravesical chemotherapy or Bacille Calmette–Guerin biological therapy[3,4]. While TURBT provides some therapeutic benefit, 50%-80% of patients experience recurrence, and nearly 30% develop malignant tumors. The 5-year survival rate after treatment remains low, at 50%-60%. Thus, surgical treatment alone has limited impact on the biological behavior of BLCA[5,6].
Progress in elucidating BLCA’s molecular biology and genetics has enhanced treatment strategies for localized and advanced disease. Current treatment options for muscle-invasive and advanced BLCA now include targeted therapies, immune checkpoint inhibitors, and antibody–drug conjugates[7]. Hypersialylation of the tumor glycocalyx represents a critical immune evasion strategy in cancer, where sialic acid-rich glycans actively impair immune detection and suppress antitumor responses[8]. Similar to programmed death ligand-1, which induces T cell exhaustion, sialoglycans enable tumors to evade immune surveillance through interactions with recently identified sialic acid-binding receptors.
Siglecs are transmembrane receptors that bind sialic acid and are expressed on immune, hematopoietic, and neural cells[9]. ST3 Beta-Galactoside Alpha-2,3-Sialyltransferase 5, GM3 (ST3GAL5) is a key ganglioside component containing sphingosine, glucose, galactose, and sialic acid. Through its extracellular domain, GM3 interacts with diverse membrane proteins (integrins, growth factor receptors, tetraspanins) to initiate signaling cascades that coordinate critical cellular processes: Adhesion, growth, and migration[10]. Downregulation of GM3 is significantly associated with adverse clinicopathological features-including muscle invasion, high tumor grade, and poor prognosis-in patients with BLCA[11]. Integrated bioinformatics analysis and experimental validation, GM3 was identified as a key sialyltransferase implicated in BLCA pathogenesis[12,13]. This finding is mechanistically supported by previous studies demonstrating that GM3, the specific ganglioside synthesized by GM3, plays a critical role in BLCA development. In addition, GM3 has been reported to inhibit transforming growth factor-beta (TGF-β) signaling by facilitating the localization of the TGF-β type I receptor (TβRI) into lipid rafts and enhancing its ubiquitination and subsequent degradation[14]. Moreover, TGFβ-driven tumorigenesis is critically contingent upon epidermal growth factor receptor (EGFR) activation and expression of the p63 ΔN isoform[15].
Β-elemene is a natural lipid-soluble plant compound used in traditional Chinese medicine (TCM), extracted from the dried rhizome of Rhizoma zedoariae (Zingiberaceae family)[16]. It exhibits wide-ranging antitumor effects against multiple cancer types[17-20], due to its low toxicity and effective anticancer properties[21,22].
Network pharmacological analysis revealed a significant association between β-elemene and GM3 expression. Our experimental validation demonstrates that β-elemene upregulates GM3 while downregulating both EGFR and phosphorylated-EGFR (p-EGFR), ultimately inhibiting the migration and invasion capabilities of BLCA cells.
Β-elemene injections were produced by Dalian Huali Jingang Pharmaceutical Co. Ltd. (Dalian, China) with the National Medical Product Administration approval number Chinese medicine H10960114. GM3 negative control (NC) & inter
To investigate the relationship between GM3 gene expression and the development of BLCA, we used the GEPIA 2 database (http://gepia2.cancer-pku.cn) to analyze GM3 expression profiles from the Genotypic Tissue Expression project and The Cancer Genome Atlas dataset. A dot plot was generated to compare GM3 expression levels between BLCA and normal tissues. The log2FC cutoff was set to 1, and the P value threshold was set to 0.01. A survival curve was used to assess the correlation between GM3 expression and BLCA stage, as well as patient prognosis, including disease-free survival (DFS) and overall survival (OS).
TCCSUP and HT-1376 BLCA cells were obtained from the National Collection of Authenticated Cell Cultures (Shanghai, China) and maintained in minimum essential medium (MEM; iCELL, Shanghai, China), supplemented with 10% fetal bovine serum (FBS; Hyclone, United States) and 1% penicillin-streptomycin. Cells were cultured at 37 °C under 5% CO2 and passaged every 2-3 days.
The CCK-8 assay (Abbkine, Wuhan, China) was used to assess the effect of β-elemene on BLCA cell viability. Cells were plated in 96-well plates (5000 cells/well) and treated with five concentrations of β-elemene (0, 25, 50, 100, and 200 µg/mL) for 24 hours, in triplicate. Cell survival was determined by adding 10 µl of CCK-8 solution per well, and absorbance was measured at 450 nm using a microplate reader (PerkinElmer, PE, United States).
Total RNA was extracted from cells using the Trizol reagent (ThermoFisher, MA, United States) according to the manufacturer's instructions. RNA was reverse transcribed using the Hifair® II 1st Strand cDNA Synthesis SuperMix Kit (Yisheng, Shanghai, China) following standard protocols. cDNA samples were amplified using the Applied Biosystems 12K real-time polymerase chain reaction (PCR) Detection System (ABI, Waltham, MA, United States) with Hieff® quantitative PCR (qPCR) SYBR Green Master Mix (YiSheng, Shanghai, China). The PCR conditions were as follows: Initial denaturation at 95 °C for 5 minutes, followed by 40 cycles of denaturation at 95 °C for 10 seconds, annealing at
TCCSUP and HT-1376 BLCA cells were cultured on glass coverslips in six-well plates and treated with or without β-elemene. Following fixation with 4% paraformaldehyde, cells were permeabilized with 0.5% Triton X-100 and blocked with goat serum for 30 minutes at room temperature. Cells were then incubated with primary antibodies overnight at
Cells were seeded into 6-well plates at a density of 2 × 105 cells/well and cultured until 80%-90% confluence was reached. Once the cells formed a monolayer, a wound was created using a sterile 200-μL micropipette tip. The cells were treated with or without the indicated concentrations of β-elemene in serum-free medium. Photographs of the cell monolayer were taken before and 24 hours after treatment using an Olympus IX71 inverted microscope. The areas of cancer cell migration were measured using ImageJ software.
To evaluate the effect of β-elemene on BLCA cell invasion, a transwell invasion assay was performed using a BioCoat Matrigel Invasion Chamber (Corning) according to the manufacturer’s instructions. Different groups of cells (4 × 104 cells/well) were seeded in triplicate into the upper chamber, which was coated with Matrigel (354234, BD). The lower chambers were filled with medium containing 10% fetal bovine serum (FBS). After a 48-hour incubation period, cells that had invaded to the lower surface of the membranes were fixed, stained with crystal violet (21155722, Biosharp), and photographed. The number of invading cells was counted in five random fields on each membrane.
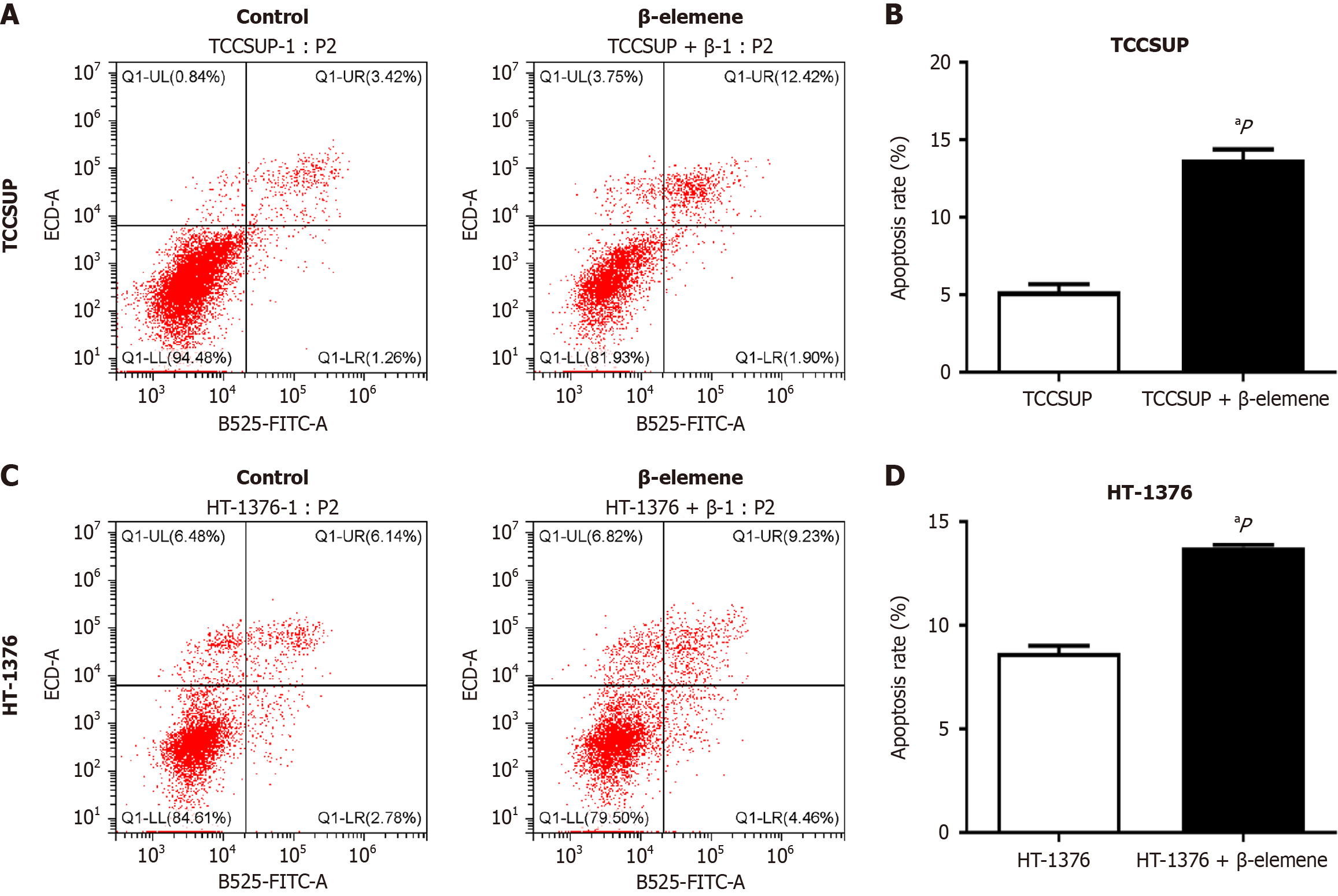
Cells were treated with β-elemene or saline control for 24 hours, followed by harvesting with 0.25% trypsin-EDTA solution and washing with phosphate-buffered saline. Each well was then supplemented with 5 µL of Annexin V-AbFluor™ 488 and 2 µL of propidium iodide (PI), following the manufacturer’s instructions. Cells were incubated at room temperature for 15 minutes, and the concentration was adjusted to 1 × 106 cells/mL. Apoptotic cell death was quantitatively determined using a CytExpert Flow Cytometer and analyzed with FlowJo software (Beckman Coulter, Pasadena, California, United States).
When TCCSUP and HT-1376 BLCA cells reached 60%-80% confluency, GM3 siRNA (NC & interference groups) was diluted in Opti-MEM Medium and mixed with Lipofectamine 3000 (1:1 ratio). The mixture was incubated at room temperature for 10 minutes to form transfection complexes, then added to cells for 6 hours. GM3 knockdown efficiency was assessed via qPCR.
For GM3 overexpression, cells were transfected with the GM3 plasmid or empty vector using Lipofectamine 3000. After selection, stable GM3-overexpressing cell lines were established, and overexpression efficiency was confirmed by qPCR.
Harvested cells were lysed in a high-salt extraction buffer composed of 10 mmol/L Hepes, 400 mmol/L NaCl, 0.1 mmol/L, ethylene glycol tetraacetic acid, 0.5 mmol/L DL-Dithiothreitol, 5% glycerol, and 0.5 mmol/L Phenylmethylsulfonyl Fluorid, at pH 7.8, with a protease inhibitor cocktail (25% of the total available inhibitors). Protein concentrations in the cell lysates were determined using the bicinchoninic acid assay method. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (10%) and 25 µg of protein was loaded per lane. After a 1.5-hour transfer, proteins were transferred to polyvinylidene fluoride membranes, which were pre-activated with methanol (100%) for 1 minute at 22 °C and blocked with 5% non-fat milk. Membranes were incubated overnight at 4 °C with primary antibodies, followed by a 1-hour incubation with secondary antibodies at room temperature. After each incu
Data are expressed as mean ± SD. Student's t-test was used to compare differences between two groups, while one-way analysis of variance was used for comparisons between multiple groups, using GraphPad Prism software (version 5.0). Statistical significance was set at P ≤ 0.05.
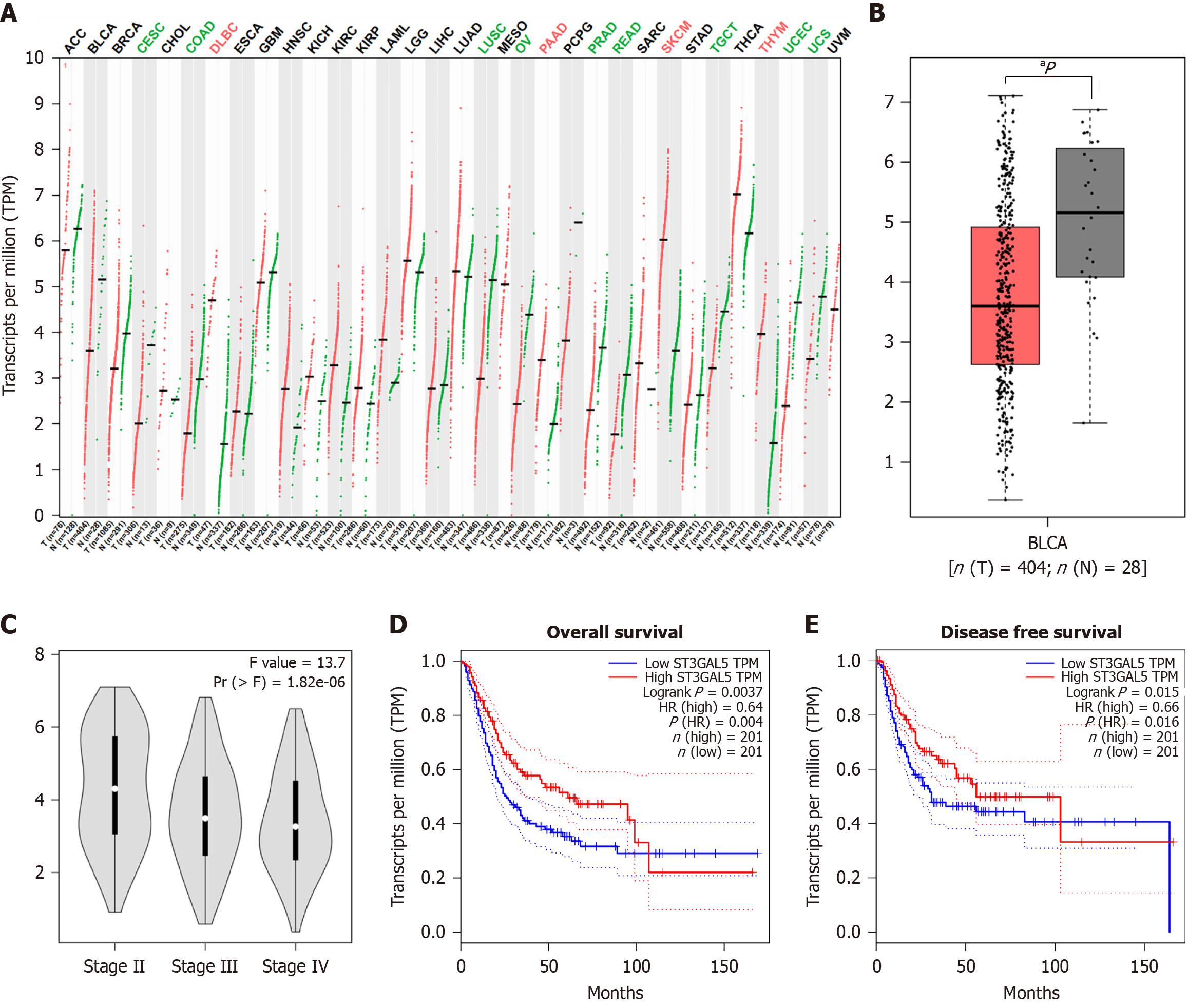
We first used the GEPIA 2 database to analyze GM3 mRNA expression levels in various human cancers and their paired normal samples. Compared to normal samples, GM3 was downregulated in 15 types of tumors, such as ovarian cancer, breast cancer, endometrial carcinoma, and lung squamous cell carcinoma, and upregulated in 13 types of tumors (all P < 0.05) (Figure 1A). We then examined GM3 expression in BLCA and found that GM3 Levels were lower in BLCA tissues than in normal tissues (Figure 1B). Furthermore, GM3 expression decreased with increasing pathological grade (Figure 1C). To assess the prognostic value of GM3 in BLCA, we explored the relationship between GM3 expression levels and clinical characteristics of BLCA patients using the GEPIA 2 database. High GM3 expression was associated with better DFS and OS in patients with BLCA (HR = 0.64, log-rank P = 0.004; HR = 0.66, log-rank P = 0.016) (Figure 1D and E).
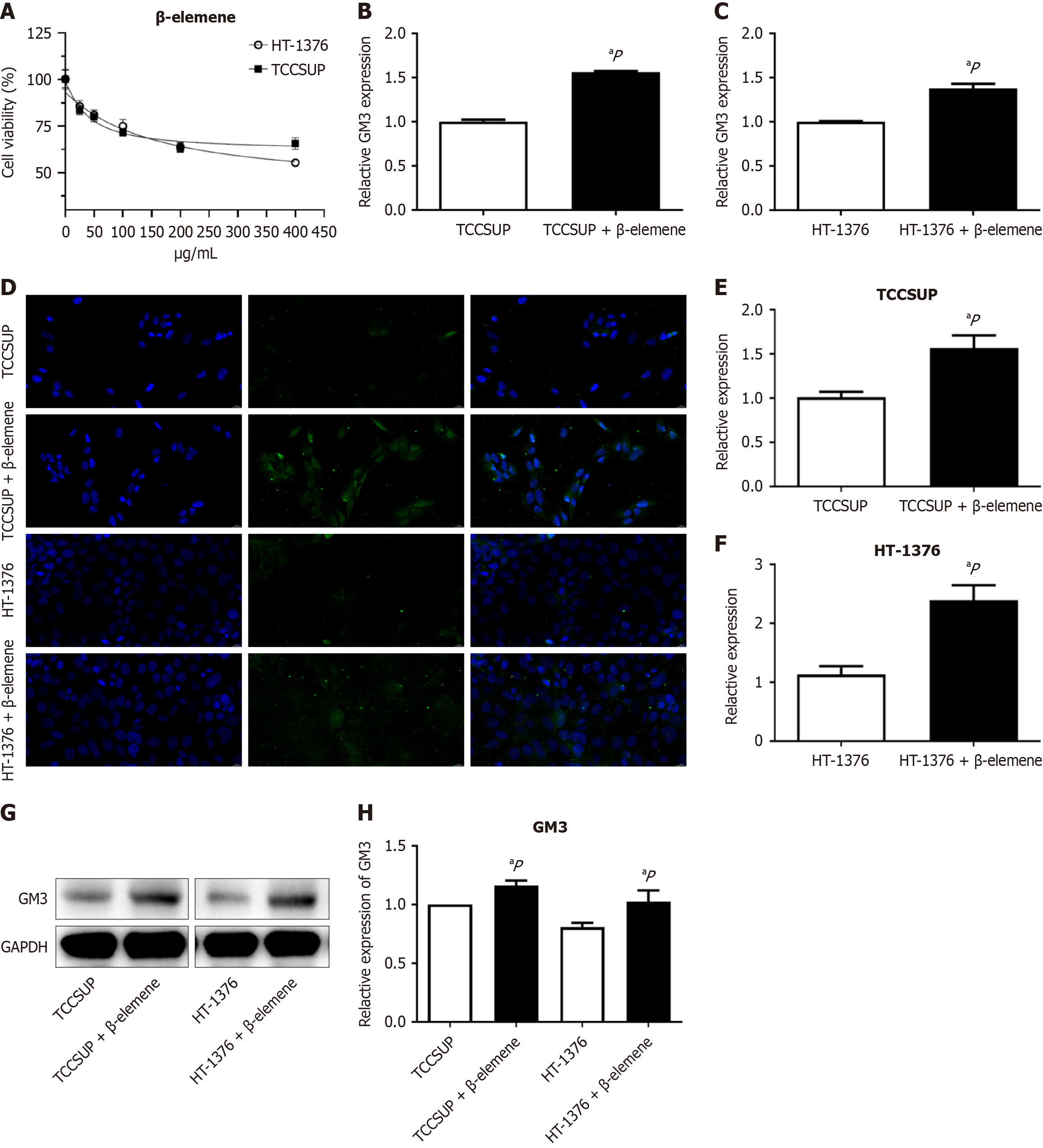
The GEPIA 2 database indicated that GM3 had tumor-suppressive effects in various malignancies. To investigate the antitumor effect of β-elemene, we examined whether it modulated GM3 expression in two BLCA cell lines. CCK8 assays showed that cell viability decreased with increasing concentrations of β-elemene, with a plateau effect observed at concentrations above 200 μg/mL (Figure 2A). Therefore, 200 μg/mL was used in subsequent experiments. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) demonstrated that β-elemene increased GM3 expression (Figure 2B and C), and immunofluorescence confocal imaging confirmed this trend (Figure 2D) in TCCSUP and HT-1376 BLCA cell lines. These results show a statistically significant difference (P < 0.05) (Figure 2E and F). Similar results were obtained by Western blot. Β-elemene increased the overall expression levels of GM3 protein in TCCSUP and HT-1376 cells (Figure 2G), and the differences were statistically significant (P < 0.05) (Figure 2H).
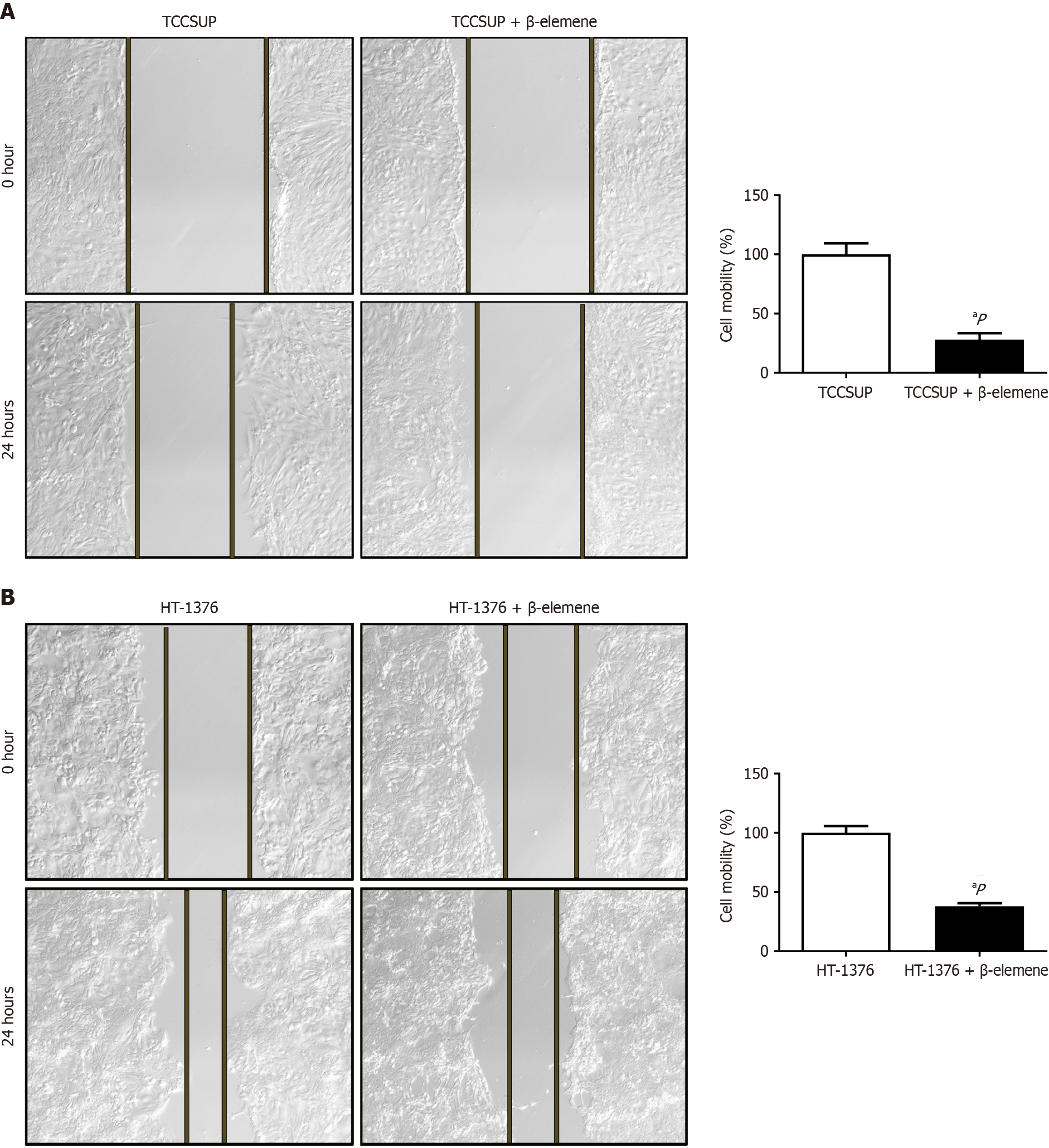
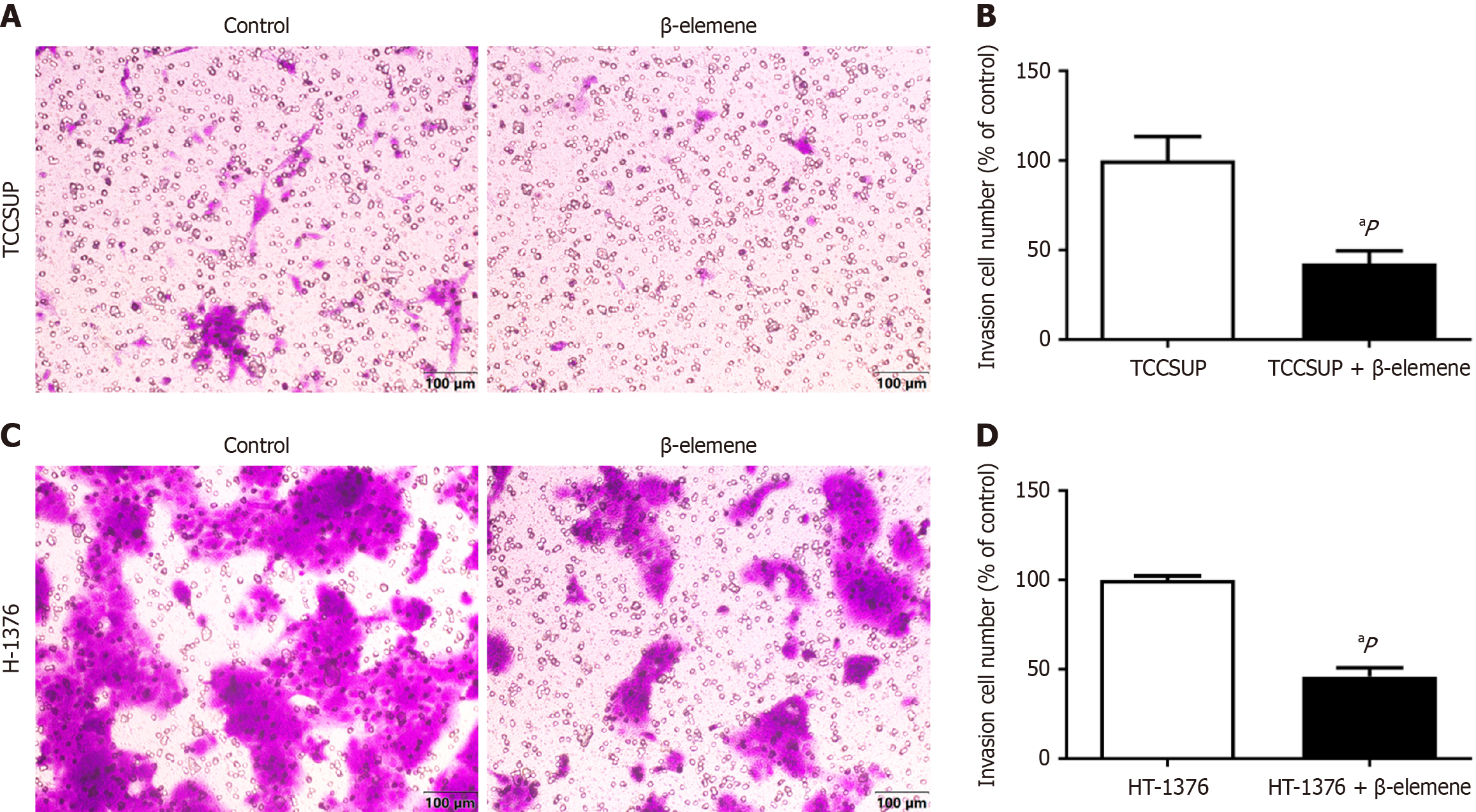
We used wound healing and transwell assays to explore the effects of β-elemene on BLCA cell malignancy. Treatment with β-elemene for 24 hours significantly reduced the migratory distance of TCCSUP and HT-1376 cells (Figure 3A and B). Crystal violet staining revealed that the number of BLCA cells penetrating the positron emission tomography (PET) membrane was significantly reduced after 24 hours of β-elemene treatment (Figure 4). Thus, β-elemene markedly inhibited cell migration and invasion in BLCA cells (all P < 0.05).
To assess the effects of β-elemene on apoptosis in BLCA cells, we employed Annexin V–AbFluor™ 488 and PI staining. CytExpert analysis showed a significant increase in apoptosis in BLCA cells treated with β-elemene (Figure 5), with statistical significance at P < 0.05.
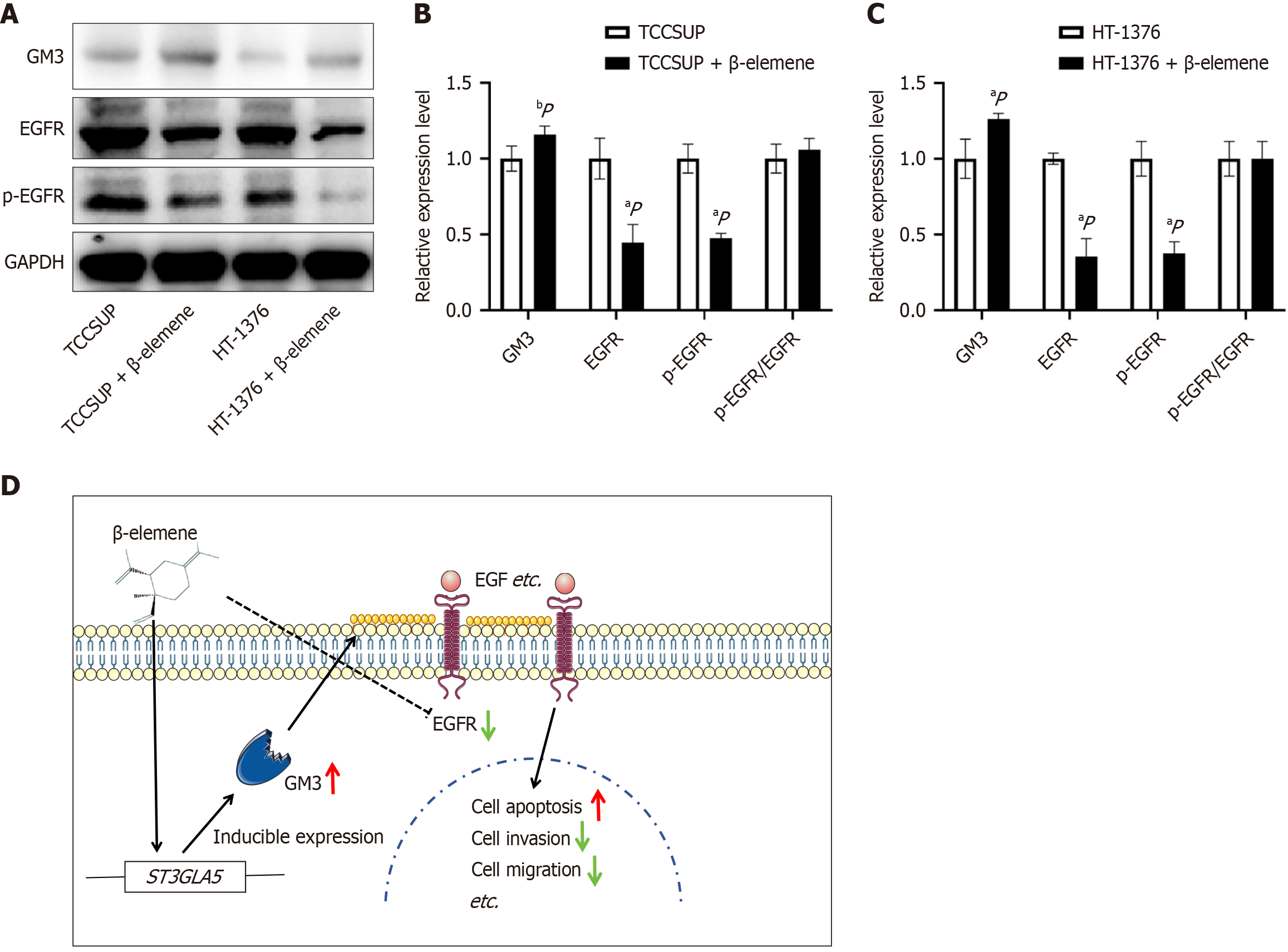
Β-elemene compound enhances GM3 expression and suppresses EGFR expression and phosphorylation, thereby exerting an anti-cancer effect.
GM3 is an integral component of the cell membrane, while EGFR is a critical receptor for cellular signaling. Western blot analysis confirmed that β-elemene significantly elevated GM3 expression (P < 0.05), while concurrently reducing EGFR and p-EGFR levels (P < 0.05) (Figure 6A). These results were consistent across both BLCA cell lines. However, β-elemene did not alter the p-EGFR/EGFR ratio, and no significant difference was observed between the two cell lines (Figure 6B and C). The mechanistic diagram of β-elemene clarifies its potential therapeutic role in BLCA. Both GM3 and EGFR proteins are expressed on the membrane surface, where their expression levels exhibit a competitive and complementary relationship. Β-elemene upregulated GM3 gene and protein expression while downregulating EGFR protein and its phosphorylated form. This modulation promoted apoptosis and inhibited the migration and invasion of BLCA cells (Figure 6D).
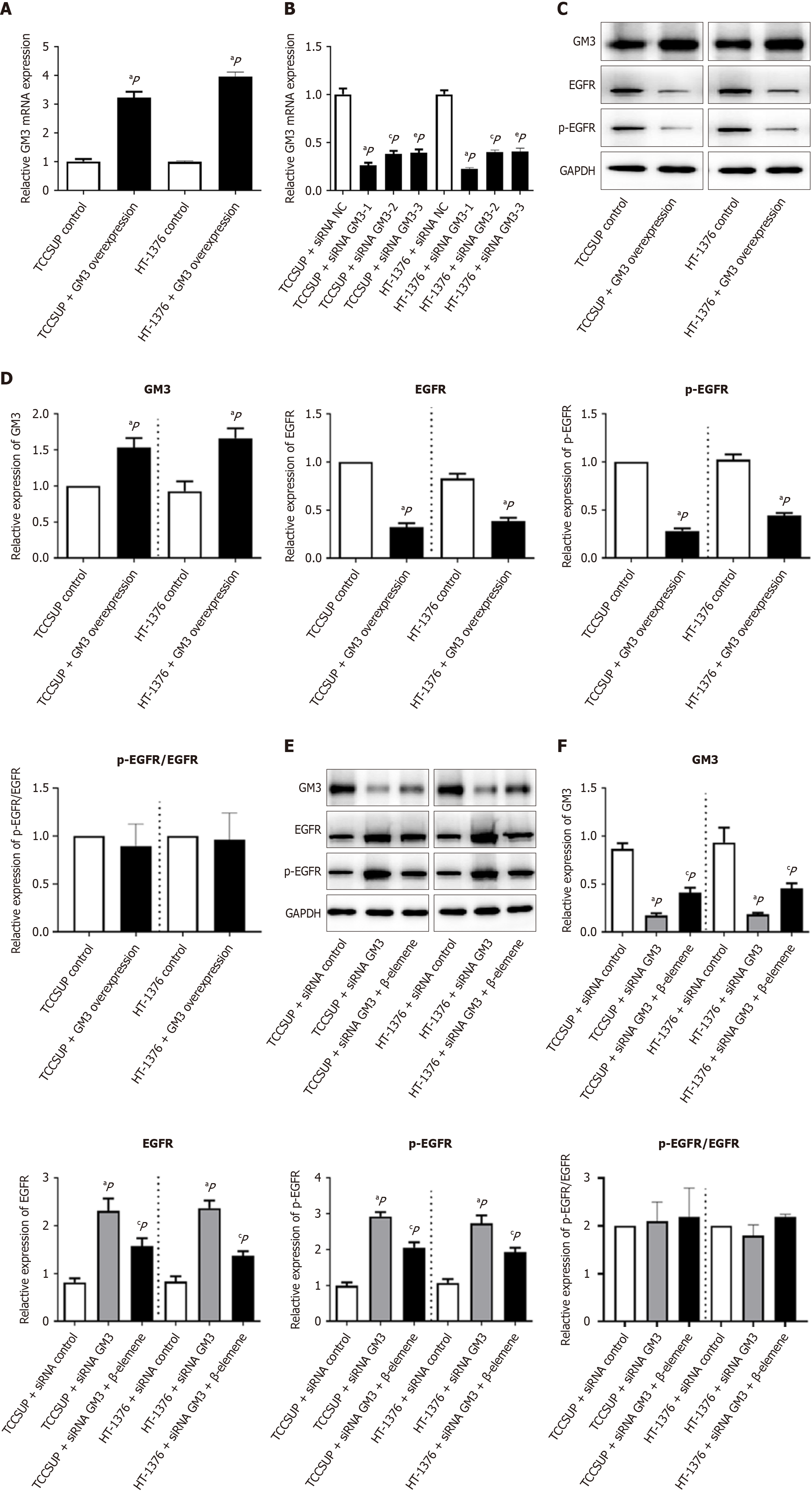
GM3 overexpression/interference can affect GM3-EGFR/pEGFR expression, and GM3-EGFR/pEGFR expression is repaired by β-elemene after GM3 interference.
GM3 overexpression constructs were transfected into cells to establish stable GM3 expression. mRNA analysis showed that GM3 expression in the transfected cells was 3-4 times higher than in control cells (P < 0.05) (Figure 7A). siRNA-mediated interference targeting the GM3 sequence reduced GM3 expression by approximately 50% (P < 0.05) (Figure 7B). In BLCA cells, GM3 protein expression increased after GM3 overexpression, while EGFR and p-EGFR protein levels decreased; however, the p-EGFR/EGFR ratio remained unchanged (Figure 7C and D). In contrast, siRNA interference significantly reduced GM3 protein expression in BLCA cells, and treatment with β-elemene restored GM3 protein expression, along with inverse changes in EGFR and p-EGFR levels. However, the p-EGFR/EGFR ratio did not change significantly (Figure 7E and F).
BLCA is a leading cause of tumor-related mortality and is 3–4 times more common in males than females[23,24]. The development of BLCA is complex, involving multiple genes, processes, and stages. BLCA can be broadly categorized into two subgroups: Non-muscle-invasive BLCA, which has a more favorable prognosis, and muscle-invasive BLCA, which has a five-year survival rate of less than 60%. TURBT does not prevent BLCA progression, and the efficacy of chemotherapy is limited by side effects and the development of resistance[25]. TCM herbs, known for their anticancer properties, low toxicity, and minimal side effects, may serve as adjuncts to chemotherapy, improving clinical outcomes. Β-elemene, a naturally occurring plant compound extracted from turmeric, has demonstrated various beneficial effects, including antitumor activity. In China, a β-elemene emulsion is used as a Class II non-cytotoxic antitumor agent[26]. With documented activity against solid tumors (including lung cancer, brain tumors and gastrointestinal cancers), β-elemene represents a promising candidate for repurposing in oncology[16]. Β-elemene demonstrates tumor-specific cell cycle modulation, arresting progression at distinct checkpoints (G0/G1, S, or G2/M) through cell multiplication pathways. For example, in human hepatocellular carcinoma HepG2 cells, β-elemene reduced alpha-tubulin expression and inhibited microtubule polymerization, causing G2/M- and S-phase arrest. Additionally, β-elemene inhibits tumor growth by regulating cyclin-dependent kinase (CDK) activity and cell cycle checkpoints[27]. In human nasopharyngeal carcinoma,
GM3 is closely related to the biological behavior and prognosis of BLCA and plays a crucial role in regulating cell adhesion and signal transduction[31]. In this study, GEPIA 2 database analysis revealed that GM3 expression was low in BLCA tissue, and lower GM3 expression was associated with reduced OS and DFS. Cell experiments demonstrated that
EGFR resides in cell membrane and consists of four distinct regions: Extracellular ligand binding sites (exon domains serving as receptors), the basic near-membrane region, the transmembrane helix region, and the intracellular protein kinase region[32]. In glioblastoma studies, β-elemene is also used to reduce resistance to gefitinib (an EGFR inhibitor) and improve gefitinib efficacy[33]. Additionally, β-elemene can enhance erlotinib sensitivity in NSCLC with mutated EGFR[34] while inducing ferroptosis in NSCLC cells with wild-type EGFR[35]. Similarly, β-elemene combined with cetuximab inhibits the occurrence of EMT in KRAS-mutated colorectal cancer cells[36].
GM3 has been identified as a potential predictive marker and therapeutic target for BLCA, but effective or specific drugs targeting GM3 are lacking. Network pharmacological analysis indicated that β-elemene is associated with GM3. Experimental results confirmed that β-elemene modulates GM3 expression at both the gene and protein levels in BLCA cells. Moreover, β-elemene can enhance GM3 protein expression after siRNA-mediated GM3 gene silencing. Therefore, β-elemene could serve as a promising therapeutic agent for GM3-targeted therapy. However, this study has some short
In conclusion, the findings of this study suggest that β-elemene enhance GM3 and inhibit EGFR expression, thereby modulating membrane-associated EGFR/p-EGFR signaling pathways. This modulation could suppress BLCA metastasis and invasion while promoting BLCA apoptosis.
| 1. | Ruiz de Porras V, Font A. Molecular Mechanisms of Tumor Progression and New Therapeutic Strategies for Urological Cancers. Int J Mol Sci. 2023;24:15795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8001] [Article Influence: 8001.0] [Reference Citation Analysis (2)] |
| 3. | Aragon-Ching JB, Werntz RP, Zietman AL, Steinberg GD. Multidisciplinary Management of Muscle-Invasive Bladder Cancer: Current Challenges and Future Directions. Am Soc Clin Oncol Educ Book. 2018;38:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 1547] [Article Influence: 515.7] [Reference Citation Analysis (0)] |
| 5. | Teoh JY, Kamat AM, Black PC, Grivas P, Shariat SF, Babjuk M. Recurrence mechanisms of non-muscle-invasive bladder cancer - a clinical perspective. Nat Rev Urol. 2022;19:280-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 6. | Audisio A, Buttigliero C, Delcuratolo MD, Parlagreco E, Audisio M, Ungaro A, Di Stefano RF, Di Prima L, Turco F, Tucci M. New Perspectives in the Medical Treatment of Non-Muscle-Invasive Bladder Cancer: Immune Checkpoint Inhibitors and Beyond. Cells. 2022;11:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: A Review. JAMA. 2020;324:1980-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 1060] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 8. | Lübbers J, Rodríguez E, van Kooyk Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front Immunol. 2018;9:2807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Xu Z, Wu KL, Yu L, Wang C, Ding H, Gao Y, Sun H, Wu YH, Xia M, Chen Y, Xiao H. Siglec-15/sialic acid axis as a central glyco-immune checkpoint in breast cancer bone metastasis. Proc Natl Acad Sci U S A. 2024;121:e2312929121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Inokuchi JI, Inamori KI, Kabayama K, Nagafuku M, Uemura S, Go S, Suzuki A, Ohno I, Kanoh H, Shishido F. Biology of GM3 Ganglioside. Prog Mol Biol Transl Sci. 2018;156:151-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Ouyang S, Liu JH, Ni Z, Ding GF, Wang QZ. Downregulation of ST3GAL5 is associated with muscle invasion, high grade and a poor prognosis in patients with bladder cancer. Oncol Lett. 2020;20:828-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Tan Z, Fu S, Feng R, Huang Y, Li N, Wang H, Wang J. Identification of Potential Biomarkers for Progression and Prognosis of Bladder Cancer by Comprehensive Bioinformatics Analysis. J Oncol. 2022;2022:1802706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Jian Y, Chen Q, Al-Danakh A, Xu Z, Xu C, Sun X, Yu X, Yang D, Wang S. Identification and validation of sialyltransferase ST3Gal5 in bladder cancer through bioinformatics and experimental analysis. Int Immunopharmacol. 2024;138:112569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Zhang J, van der Zon G, Ma J, Mei H, Cabukusta B, Agaser CC, Madunić K, Wuhrer M, Zhang T, Ten Dijke P. ST3GAL5-catalyzed gangliosides inhibit TGF-β-induced epithelial-mesenchymal transition via TβRI degradation. EMBO J. 2023;42:e110553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Sundqvist A, Vasilaki E, Voytyuk O, Bai Y, Morikawa M, Moustakas A, Miyazono K, Heldin CH, Ten Dijke P, van Dam H. TGFβ and EGF signaling orchestrates the AP-1- and p63 transcriptional regulation of breast cancer invasiveness. Oncogene. 2020;39:4436-4449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Tan T, Li J, Luo R, Wang R, Yin L, Liu M, Zeng Y, Zeng Z, Xie T. Recent Advances in Understanding the Mechanisms of Elemene in Reversing Drug Resistance in Tumor Cells: A Review. Molecules. 2021;26:5792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Chen Y, Zhu Z, Chen J, Zheng Y, Limsila B, Lu M, Gao T, Yang Q, Fu C, Liao W. Terpenoids from Curcumae Rhizoma: Their anticancer effects and clinical uses on combination and versus drug therapies. Biomed Pharmacother. 2021;138:111350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Tan Q, Lu J, Liang J, Zhou Y, Yang C, Zhang Z, Li C. A review of traditional Chinese medicine Curcumae Rhizoma for treatment of glioma. Int Rev Neurobiol. 2023;172:303-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Zhu J, Jiang X, Luo X, Zhao R, Li J, Cai H, Ye XY, Bai R, Xie T. Combination of chemotherapy and gaseous signaling molecular therapy: Novel β-elemene nitric oxide donor derivatives against leukemia. Drug Dev Res. 2023;84:718-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 20. | Liang Y, Li S. β-elemene Suppresses Migration of Esophageal Squamous Cell Carcinoma by Modulating Expression of MMP9 through the PI3K/Akt/NF-κB Pathway. Comb Chem High Throughput Screen. 2023;26:2304-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Yu X, Xu M, Li N, Li Z, Li H, Shao S, Zou K, Zou L. β-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer. Biochem Biophys Res Commun. 2017;490:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, Guo J, Chen X, Wang Y. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 23. | Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38:1895-1904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 568] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 24. | Yang Y, Zhang H, Liu Z, Zhao F, Liang G. EPDR1 is related to stages and metastasize in bladder cancer and can be used as a prognostic biomarker. BMC Urol. 2021;21:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Minoli M, Kiener M, Thalmann GN, Kruithof-de Julio M, Seiler R. Evolution of Urothelial Bladder Cancer in the Context of Molecular Classifications. Int J Mol Sci. 2020;21:5670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Zhai B, Zeng Y, Zeng Z, Zhang N, Li C, Zeng Y, You Y, Wang S, Chen X, Sui X, Xie T. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. Int J Nanomedicine. 2018;13:6279-6296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 27. | Dai ZJ, Tang W, Lu WF, Gao J, Kang HF, Ma XB, Min WL, Wang XJ, Wu WY. Antiproliferative and apoptotic effects of β-elemene on human hepatoma HepG2 cells. Cancer Cell Int. 2013;13:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Lu X, Wang Y, Luo H, Qiu W, Han H, Chen X, Yang L. β-elemene inhibits the proliferation of T24 bladder carcinoma cells through upregulation of the expression of Smad4. Mol Med Rep. 2013;7:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Su P, Ahmad B, Zou K, Zou L. β-Elemene Enhances the Chemotherapeutic Effect of 5-Fluorouracil in Triple-Negative Breast Cancer via PI3K/AKT, RAF-MEK-ErK, and NF-κB Signaling Pathways. Onco Targets Ther. 2020;13:5207-5222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Do MH, Thanh HD, To PK, Kim MS, Moon C, Jung C. CD46 protects the bladder cancer cells from cetuximab-mediated cytotoxicity. Sci Rep. 2022;12:22420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 31. | Cao S, Hu X, Ren S, Wang Y, Shao Y, Wu K, Yang Z, Yang W, He G, Li X. The biological role and immunotherapy of gangliosides and GD3 synthase in cancers. Front Cell Dev Biol. 2023;11:1076862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 32. | Mitchell RA, Luwor RB, Burgess AW. Epidermal growth factor receptor: Structure-function informing the design of anticancer therapeutics. Exp Cell Res. 2018;371:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 33. | Mu L, Wang T, Chen Y, Tang X, Yuan Y, Zhao Y. β-Elemene enhances the efficacy of gefitinib on glioblastoma multiforme cells through the inhibition of the EGFR signaling pathway. Int J Oncol. 2016;49:1427-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Xu C, Jiang ZB, Shao L, Zhao ZM, Fan XX, Sui X, Yu LL, Wang XR, Zhang RN, Wang WJ, Xie YJ, Zhang YZ, Nie XW, Xie C, Huang JM, Wang J, Wang J, Leung EL, Wu QB. β-Elemene enhances erlotinib sensitivity through induction of ferroptosis by upregulating lncRNA H19 in EGFR-mutant non-small cell lung cancer. Pharmacol Res. 2023;191:106739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 35. | Zhao LP, Wang HJ, Hu D, Hu JH, Guan ZR, Yu LH, Jiang YP, Tang XQ, Zhou ZH, Xie T, Lou JS. β-Elemene induced ferroptosis via TFEB-mediated GPX4 degradation in EGFR wide-type non-small cell lung cancer. J Adv Res. 2024;62:257-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 36. | Chen P, Li X, Zhang R, Liu S, Xiang Y, Zhang M, Chen X, Pan T, Yan L, Feng J, Duan T, Wang D, Chen B, Jin T, Wang W, Chen L, Huang X, Zhang W, Sun Y, Li G, Kong L, Chen X, Li Y, Yang Z, Zhang Q, Zhuo L, Sui X, Xie T. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10:5107-5119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |