Published online Sep 9, 2025. doi: 10.5409/wjcp.v14.i3.103873
Revised: March 22, 2025
Accepted: April 21, 2025
Published online: September 9, 2025
Processing time: 187 Days and 19.2 Hours
For over half a century, the administration of maternal corticosteroids before anticipated preterm birth has been regarded as a cornerstone intervention for enhancing neonatal outcomes, particularly in preventing respiratory distress syndrome. Ongoing research on antenatal corticosteroids (ACS) is continuously refining the evidence regarding their efficacy and potential side effects, which may alter the application of this treatment. Recent findings indicate that in resource-limited settings, the effectiveness of ACS is contingent upon meeting specific conditions, including providing adequate medical support for preterm newborns. Future studies are expected to concentrate on developing evidence-based strategies to safely enhance ACS utilization in low- and middle-income countries.
To analyze the clinical effectiveness of antenatal corticosteroids in improving outcomes for preterm newborns in a tertiary care hospital setting in Kazakhstan, following current World Health Organization guidelines.
This study employs a comparative retrospective cohort design to analyze single-center clinical data collected from January 2022 to February 2024. A total of 152 medical records of preterm newborns with gestational ages between 24 and 34 weeks were reviewed, focusing on the completeness of the ACS received. Quantitative variables are presented as means with standard deviations, while frequency analysis of qualitative indicators was performed using Pearson's χ2 test (χ²) and Fisher's exact test. If statistical significance was identified, pairwise comparisons between the three observation groups were conducted using the Bonferroni correction.
The obtained data indicate that the complete implementation of antenatal steroid prophylaxis (ASP) improves neonatal outcomes, particularly by reducing the frequency of birth asphyxia (P = 0.002), the need for primary resuscitation (P = 0.002), the use of nasal continuous positive airway pressure (P = 0.022), and the need for surfactant replacement therapy (P = 0.038) compared to groups with incomplete or no ASP. Furthermore, complete ASP contributed to a decrease in morbidity among preterm newborns (e.g., respiratory distress syndrome, intrauterine pneumonia, cerebral ischemia, bronchopulmonary dysplasia, etc.), improved Apgar scores, and reduced the need for re-intubation and the frequency of mechanical ventilation. However, it was associated with an increased incidence of uterine atony in postpartum women (P = 0.0095).
In a tertiary hospital setting, the implementation of ACS therapy for pregnancies between 24 and 34 weeks of gestation at high risk for preterm birth significantly reduces the incidence of neonatal complications and related interventions. This, in turn, contributes to better outcomes for this cohort of children. However, the impact of ACS on maternal outcomes requires further thorough investigation.
Core Tip: This retrospective cohort study was aimed to evaluate the effect of antenatal steroid prophylaxis (ASP) on the general condition of preterm newborns. It demonstrates collected clinical data from January 2022 to February 2024 in Kazakhstan, Almaty region. 152 premature newborns with gestational ages between 24 and 34 weeks were analyzed depending on the completeness of ASP among them. Results show effect of ASP in prevention of respiratory distress syndrome and incidences of comorbidities related to respiratory, cardiovascular and digestive systems. In conclusion, findings suggest that ASP significantly improves neonatal outcomes, particularly in reducing birth asphyxia, primary resuscitation measures, need for lung ventilation including mechanical ventilation and nasal continuous positive airway pressure, and re-intubation cases.
- Citation: Sairankyzy S, Kinayatova I, Amangeldi D, Zhumatova A, Bozhbanbayeva N, Ismailova A, Akhtayeva N, An O. Comparative analysis on the efficacy of antenatal corticosteroids in preterm newborns in a Kazakhstani Tertiary Care Hospital setting. World J Clin Pediatr 2025; 14(3): 103873
- URL: https://www.wjgnet.com/2219-2808/full/v14/i3/103873.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i3.103873
Preterm birth remains a major global health issue, contributing significantly to perinatal and neonatal morbidity and mortality. According to the World Health Organization (WHO), 13.4 million preterm newborns were born worldwide in 2020, with complications of prematurity causing approximately 900000 neonatal deaths in 2019 - accounting for nearly 35% of all deaths within the first 28 days of life[1-4].
According to a 2023 report by the Ministry of Healthcare of the Republic of Kazakhstan, preterm births account for approximately 5% of all live births, with 20000 to 22000 premature newborns delivered annually in Kazakhstan. Since 2008, national guidelines have defined preterm newborns as those born at ≥ 24 weeks of gestation with a birth weight of at least 500 g or up to 37 weeks of gestation with a weight below 2500 g.
Neonatal respiratory distress syndrome (RDS) is a leading cause of respiratory failure in preterm infants, resulting from surfactant deficiency and lung immaturity. The incidence of RDS rises with decreasing gestational age and birth weight and is particularly high in neonates born before 34 weeks of gestation. A 2014 UNICEF report identified RDS as the most common complication among preterm newborns, affecting 44.15% of cases, followed by transient tachypnea of the newborn, birth asphyxia, sepsis, apnea, and pneumonia[5].
Antenatal prophylaxis with corticosteroids in pregnant women at risk of preterm birth promotes the development of the fetal respiratory system. Glucocorticoid receptors in the fetal lungs respond to antenatal corticosteroids (ACS) administration, stimulating lung cell differentiation and surfactant production[6-8]. For more than fifty years, ACS have been regarded as a foundational intervention for enhancing neonatal outcomes, particularly in preventing RDS. Ongoing research on ACS is continuously refining the evidence regarding their efficacy and potential side effects, which may alter the application of this treatment. Recent findings indicate that in resource-limited settings, the effectiveness of ACS is contingent upon meeting specific conditions, including the provision of adequate medical support for preterm newborns[9].
A 2015 cluster-randomized trial conducted across some low- and middle-income regions, published in Lancet (London, England), raised critical concerns regarding the benefits and risks of ACS in resource-limited settings. The study observed that, despite a fourfold increase in ACS coverage among pregnant women at risk for preterm birth, there was no reduction in neonatal mortality. Instead, the intervention group experienced higher rates of neonatal mortality, stillbirths, and maternal infections, as well as an increase in infectious complications among mothers[9].
In response to these concerns, the WHO conducted a follow-up, placebo-controlled, randomized trial across multiple Low- and Middle-Income Countries (LMICs) to further assess the efficacy and safety of ACS in improving outcomes for preterm newborns. This study, known as ACTION-I, specifically examined the administration of antenatal dexamethasone to women at risk of preterm birth between 26 and 34 weeks of gestation. The findings confirmed that, under specialized medical conditions, antenatal dexamethasone effectively reduced neonatal mortality and severe respiratory distress within the first 24 hours after birth, with no adverse effects reported for either mothers or newborns. Based on these results, the WHO updated its recommendations in 2022, supporting the use of ACS in cases of preterm birth risk.
The updated guidelines recommend ACS for women at high risk of preterm birth between 24 and 34 weeks of gestation, provided certain conditions are met: Reliable gestational age assessment, a strong likelihood of preterm delivery within 7 days of starting treatment, absence of maternal infection signs, availability of specialized healthcare providers, and access to high-quality medical care at birth. Essential care includes resuscitation, thermal support, feeding assistance, infection management, and respiratory support, including continuous positive airway pressure[10,11].
All of the above underscores the importance of further evaluating the impact of antenatal corticosteroid therapy for preventing respiratory distress syndrome in preterm newborns, with an emphasis on its clinical application and feasibility in LMICs.
In Kazakhstan, this topic remains insufficiently studied, despite advancements in neonatal care and the imple
We believe that the complete administration of antenatal corticosteroids to pregnant women at risk of preterm birth between 24 and 34 weeks of gestation reduces the incidence of neonatal complications.
Aim: To analyze the clinical effectiveness of ACS in improving outcomes for preterm neonates in a tertiary care hospital setting in Kazakhstan, in accordance with current WHO guidelines.
A retrospective cohort analysis was conducted using clinical data collected from January 2022 to February 2024 at the Enbekshikazakh Multidisciplinary Central District Hospital in Issyk (EMCDH Issyk) in the Almaty region of Kazakhstan. The study encompassed 152 preterm newborns, representing a sample size calculated with a 95% confidence level. During the study period, 250 preterm infants were born in the neonatal care unit of EMCDH Issyk. Assuming a population proportion of 50% (P = 0.5), the required sample size for a reliable analysis at a 95% confidence level is 152 individuals. The sample size was calculated using the formula:
Sample Size n = [Z1−α/22 d2 × (N−1)] + p × (1−p) DEFF × N × p × (1−p).
n = required sample size.
DEFF = design effect (accounts for the impact of the sampling design on the precision of estimates).
N = total population size.
p = estimated proportion of the population possessing the characteristic of interest.
d = desired margin of error.
Z = Z-value corresponding to the chosen confidence level (for a 95% confidence level, the Z-value is 1.96).
The studied newborns were divided into groups based on whether their mothers received a full, partial, or no dose of antenatal steroid prophylaxis (ASP). According to the Clinical Protocol of the Ministry of Health of the Republic of Kazakhstan from 2023 and the WHO recommendations on antenatal corticosteroids for improving preterm birth outcomes from 2022, ASP is administered to women at risk of preterm birth between 24 and 34 weeks of gestation. ASP was considered complete when all women at risk of preterm birth received 6 mg of dexamethasone intramuscularly twice a day for two days. If at least one dose was missed, ASP was considered incomplete. The study included newborns with a gestational age of 24 weeks + 0 day to 33 weeks + 6 days. Participants included both singleton and multiple pregnancies between 24 and 34 weeks of gestation. Exclusion criteria were based on the Ministry of Health's 2023 guidelines and WHO recommendations, omitting pregnancies below 24 weeks and above 34 weeks. Regarding other factors influencing preterm newborn outcomes, particularly maternal comorbidities and gestational age, no significant differences were found (P > 0.05). Additionally, all newborns in the study received the same neonatal care and medical support. Among the 152 newborns, 75 (49.3%) were male, and 77 (50.7%) were female, with a mean birth weight of 1725.03 ± 622.11 g. The majority of newborns (n = 105; 69.1%) were born between 28 and 33 weeks of gestation.
Data on the health status of both mothers and newborns were obtained from the integrated medical system "Damumed" of the Ministry of Health of the Republic of Kazakhstan. The study involved a review of medical records for women during pregnancy and the postpartum period, which included various factors such as age, presence of harmful habits, obstetric complications (e.g., polyhydramnios, oligohydramnios, preeclampsia, and gestational diabetes), use of in vitro fertilization, and intrauterine infections. Additionally, the medical records of the newborns were reviewed, including information on congenital pathologies, complications, and medical procedures received by the preterm newborns.
Descriptive statistical analyses were performed using Microsoft Excel 2016 and IBM SPSS Statistics (Version 13). Variables such as maternal age, Apgar scores, newborn weight, and gestational age were assessed for normal distribution and are presented as means ± SD. The Kolmogorov-Smirnov test with Lilliefors correction was used to verify distribution normality. Frequency analyses of qualitative indicators were conducted using Pearson’s χ2 test. For the analysis of categorical data, the χ² test was employed. Upon identifying statistical significance, pairwise comparisons among the three observation groups were conducted using the Bonferroni correction. When contingency tables contained values of less than 5 Fisher's exact test was applied
This study was reviewed and approved by the Ethics Committee of the Kazakhstan Medical University “School of Public Health” (Approval No: IRB - A171, dated September 18, 2024).
The analysis focused on the obstetric and gynecological histories of mothers who delivered preterm newborns, with comparisons based on their receipt of antenatal steroid prophylaxis (Table 1). Table 1 further details the association between ASP administration and various maternal and neonatal outcomes, highlighting statistically significant P values for several parameters. Notably, mothers who did not receive antenatal steroids exhibited a higher incidence of emergency deliveries and adverse neonatal outcomes, particularly in cases where labor had already commenced upon hospital admission. Conditions such as in vitro fertilization (IVF) (P = 0.0012), preterm premature rupture of membranes
| Parameters | Obstetric and gynecological history of mothers of preterm newborns based on the extent of antenatal steroid prophylaxis | P value | |||
| Complete, n = 71 | Absent, n = 49 | Incomplete, n = 32 | Total, n = 152 | ||
| Multiple pregnancy | Р = 0.056, α = 0.0167 | ||||
| Yes | 12 (57.1) | 2 (9.5) | 7 (33.3) | 21 (100) | |
| No | 59 (45.0) | 47 (35.9) | 25 (19.1) | 131 (100) | |
| Abortion | χ² = 3.73, P = 0.155 | ||||
| Yes | 30 (52.6) | 13 (22.8) | 14 (24.6) | 57 (100) | |
| No | 41 (43.2) | 36 (37.9) | 18 (18.9) | 95 (100) | |
| Intrauterine infections | Р = 0.623, α = 0.0167 | ||||
| Yes | 1 (25.0) | 3 (75.0) | 0 (0.0) | 4 (100) | |
| No | 70 (47.3) | 46 (31.1) | 32 (21.6) | 148 (100) | |
| IVF | Р = 0.0012, α = 0.0167; Group 1 – Group 2: P = 1.000; Group 1 – Group 3: Р = 1.000; Group 2 – Group 3: Р = 1.000 | ||||
| Yes | 2 (66.7) | 0 (0.0) | 1 (33.3) | 3 (100) | |
| No | 69 (46.3) | 49 (32.9) | 31 (20.8) | 149 (100) | |
| Gestational hypertension | χ² = 0.906, P = 0.636 | ||||
| Yes | 15 (53.6) | 7 (25.0) | 6 (21.4) | 28 (100) | |
| No | 56 (45.2) | 42 (33.9) | 26 (21.0) | 124 (100) | |
| Gestational diabetes | Р = 1.0, α = 0.0167 | ||||
| Yes | 2 (25.0) | 1 (12.5) | 5 (62.5) | 8 (100) | |
| No | 69 (47.9) | 48 (33.3) | 27 (18.8) | 144 (100) | |
| Gestational thrombocytopenia | Р = 0.056, α = 0.0167 | ||||
| Yes | 11 (55.0) | 2 (10.0) | 7 (35.0) | 20 (100) | |
| No | 60 (45.5) | 47 (35.6) | 25 (18.9) | 132 (100) | |
| Fetal growth restriction | Р = 0.203, α = 0.0167 | ||||
| Yes | 3 (27.3) | 6 (54.5) | 2 (18.2) | 11 (100) | |
| No | 68 (48.2) | 43 (30.5) | 30 (21.3) | 141 (100) | |
| Preeclampsia | χ² = 4.317, P = 0.115 | ||||
| Yes | 17 (51.5) | 6 (18.2) | 10 (30.3) | 33 (100) | |
| No | 54 (45.4) | 43 (36.1) | 22 (18.5) | 119 (100) | |
| Eclampsia | Р = 0.408, α = 0.0167 | ||||
| Yes | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100) | |
| No | 71 (47.0) | 48 (31.8) | 32 (21.2) | 151 (100) | |
| HELLP syndrome | Р = 1.000, α = 0.0167 | ||||
| Yes | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) | |
| No | 71 (47.0) | 49 (32.5) | 31 (20.5) | 151 (100.0) | |
| Bleeding | Р = 0.484, α = 0.0167 | ||||
| Yes | 4 (36.4) | 5 (45.5) | 2 (18.2) | 11 (100) | |
| No | 67 (47.5) | 44 (31.2) | 30 (21.3) | 141 (100) | |
| Threatening fetal condition | χ² = 3.014, P = 0.222 | ||||
| Yes | 10 (34.5) | 10 (34.5) | 9 (31.0) | 29 (100) | |
| No | 61 (49.6) | 39 (31.7) | 23 (18.7) | 123 (100) | |
| Polyhydramnios | Р = 0.647, α = 0.0167 | ||||
| Yes | 4 (50.0) | 1 (12.5) | 3 (37.5) | 8 (100) | |
| No | 67 (46.5) | 48 (33.3) | 29 (20.1) | 144 (100) | |
| Oligohydramnios | χ² = 0.339, P = 0.844 | ||||
| Yes | 19 (47.5) | 14 (35.0) | 7 (17.5) | 40 (100) | |
| No | 52 (46.4) | 35 (31.3) | 25 (22.3) | 112 (100) | |
| PPROM | Р = 0.023, α = 0.0167 | ||||
| Yes | 4 (28.6) | 9 (64.3) | 1 (7.1) | 14 (100) | |
| No | 67 (48.6) | 40 (29.0) | 31 (22.5) | 138 (100) | |
| Atonic period | χ² = 9.304, Р = 0.0095; Group 1 – Group 2: P = 0.006 (α < 0.0167); Group 1 – Group 3: P = 0.426; Group 2 – Group 3: P = 0.380 | ||||
| Yes | 39 (59.1) | 15 (22.7) | 12 (18.2) | 66 (100) | |
| No | 32 (37.2) | 34 (39.5) | 20 (23.3) | 86 (100) | |
| Uterine scar | Р = 0.019, α = 0.0167 | ||||
| Yes | 13 (41.9) | 15 (48.4) | 3 (9.7) | 31 (100) | |
| No | 58 (47.9) | 34 (28.1) | 29 (24.0) | 121 (100) | |
| Cesarean section | χ² = 0.658, P = 0.720 | ||||
| Yes | 49 (45.0) | 36 (33.0) | 24 (22.0) | 109 (100) | |
| No | 22 (51.2) | 13 (30.2) | 8 (18.6) | 43 (100) | |
| Adhesive process | Р = 0.154, α = 0.0167 | ||||
| Yes | 3 (100) | 0 (0.0) | 0 (0.0) | 3 (100) | |
| No | 68 (45.6) | 49 (32.9) | 32 (21.5) | 149 (100) | |
| Chorioamnionitis | Р = 0.794, α = 0.0167 | ||||
| Yes | 5 (55.6) | 2 (22.2) | 2 (22.2) | 9 (100) | |
| No | 66 (46.2) | 47 (32.9) | 30 (21.0) | 143 (100) | |
| Bleeding | Р = 0.642, α = 0.0167 | ||||
| Yes | 4 (36.4) | 5 (45.5) | 2 (18.2) | 11 (100) | |
| No | 67 (47.5) | 44 (31.2) | 30 (21.3) | 141 (100) | |
| Induction with misoprostol | Р = 0.624, α = 0.0167 | ||||
| Yes | 7 (58.3) | 3 (25.0) | 2 (16.7) | 12 (100) | |
| No | 64 (45.7) | 46 (32.9) | 30 (21.4) | 140 (100) | |
| Recurrent pregnancy loss | 0.743 | ||||
| Yes | 7 (53.8) | 3 (23.1) | 3 (23.1) | 13 (100) | |
| No | 64 (46.0) | 46 (33.1) | 29 (20.9) | 139 (100) | |
In addition to obstetric and gynecological histories, chronic medical conditions among pregnant women were evaluated, as detailed in Table 2. Chronic maternal conditions did not differ statistically between the groups (P > 0.05). Notably, among women with preterm births, anemia was diagnosed in 117 cases, and chronic pyelonephritis in 77 cases. Other diseases were identified less frequently and varied in occurrence.
| Parameters | Chronic medical conditions of mothers of preterm newborns based on the extent of antenatal steroid prophylaxis | P value | |||
| Complete, n = 71 | Absent, n = 49 | Incomplete, n = 32 | Total, n = 152 | ||
| Anemia | 0.146 | ||||
| Yes | 59 (50.4) | 34 (29.1) | 24 (20.5) | 117 (100) | |
| No | 12 (34.3) | 15 (42.9) | 8 (22.9) | 35 (100) | |
| Chronic pyelonephritis | 0.615 | ||||
| Yes | 39 (50.6) | 23 (29.9) | 15 (19.5) | 77 (100) | |
| No | 32 (42.7) | 26 (34.7) | 17 (22.7) | 75 (100) | |
| Chronic cholecystitis | 0.65 | ||||
| Yes | 3 (60.0) | 0 (0.0) | 2 (40.0) | 5 (100) | |
| No | 68 (46.3) | 49 (33.3) | 30 (20.4) | 147 (100) | |
| Chronic gastritis | 0.763 | ||||
| Yes | 2 (50,.0) | 2 (50.0) | 0 (0.0) | 4 (100) | |
| No | 69 (46.6) | 47 (31.8) | 32 (21.6) | 148 (100) | |
| Chronic glomerulonephritis | 0.605 | ||||
| Yes | 0 (0.0) | 1 (100) | 0 (0.0) | 1 (100) | |
| No | 71 (47.0) | 48 (31.8) | 32 (21.2) | 151 (100) | |
| Chronic pancreatitis | 0.467 | ||||
| Yes | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100) | |
| No | 70 (46.4) | 49 (32.5) | 32 (21.2) | 151 (100) | |
| Thyrotoxic goiter | 0.522 | ||||
| Yes | 3 (75.0) | 0 (0.0) | 1 (25.0) | 4 (100) | |
| No | 68 (45.9) | 49 (33.1) | 31 (20.9) | 148 (100) | |
| Hypothyroidism | 1 | ||||
| Yes | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (100) | |
| No | 70 (46.7) | 48 (32.0) | 32 (21.3) | 150 (100) | |
| Viral hepatitis B | 1 | ||||
| Yes | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100) | |
| No | 71 (47.0) | 48 (31.8) | 32 (21.2) | 151 (100) | |
| Viral hepatitis C | 1 | ||||
| Yes | 1 (50.0) | 0 (0.0) | 1 (50.0) | 2 (100) | |
| No | 70 (46.7) | 49 (32.7) | 31 (20.7) | 150 (100) | |
The analysis also considered additional health conditions beyond the obstetric history, as outlined in Table 3. The results between the groups were statistically insignificant (P > 0.05), except for varicose veins of the lower extremities, which were significantly more frequent in women who received complete antenatal steroid prophylaxis (P = 0.049).
| Parameters | Additional health history of mothers of preterm newborns based on the extent of antenatal steroid prophylaxis | P value | |||
| Complete, n = 71 | Absent, n = 49 | Incomplete, n = 32 | Total, n = 152 | ||
| ARVI | 0.282 | ||||
| Yes | 5 (71.4) | 0 (0.0) | 2 (28.6) | 7 (100) | |
| No | 66 (45.5) | 49 (33.8) | 30 (20.7) | 145 (100) | |
| Medical allergy | 0.714 | ||||
| Yes | 5 (50.0) | 4 (40.0) | 1 (10.0) | 10 (100) | |
| No | 66 (46.5) | 45 (31.7) | 31 (21.8) | 142 (100) | |
| Contact dermatitis | 0.642 | ||||
| Yes | 1 (50.0) | 0 (0.0) | 1 (50.0) | 2 (100) | |
| No | 70 (46.7) | 49 (32.7) | 31 (20.7) | 150 (100) | |
| Obesity | 0.903 | ||||
| Yes | 11 (45.8) | 7 (29.2) | 6 (25.0) | 24 (100) | |
| No | 60 (46.9) | 42 (32.8) | 26 (20.3) | 128 (100) | |
| Varicose veins | 0.028; α = 0.0167 | ||||
| Yes | 15 (71.4) | 4 (19.0) | 2 (9.5) | 21 (100) | |
| No | 56 (42.7) | 45 (34.4) | 30 (22.9) | 131 (100) | |
| Myopia | 0.759 | ||||
| Yes | 3 (42.9) | 3 (42.9) | 1 (14.3) | 7 (100) | |
| No | 68 (46.9) | 46 (31.7) | 31 (21.4) | 145 (100) | |
| Retinal angiopathy | 0.723 | ||||
| Yes | 4 (44.4) | 4 (44.4) | 1 (11.1) | 9 (100) | |
| No | 67 (46.9) | 45 (31.5) | 31 (21.7) | 143 (100) | |
Given that the primary therapeutic benefit of ACS is the prevention of RDS during the early neonatal period, we chose to highlight this pathology and present the findings in detail.
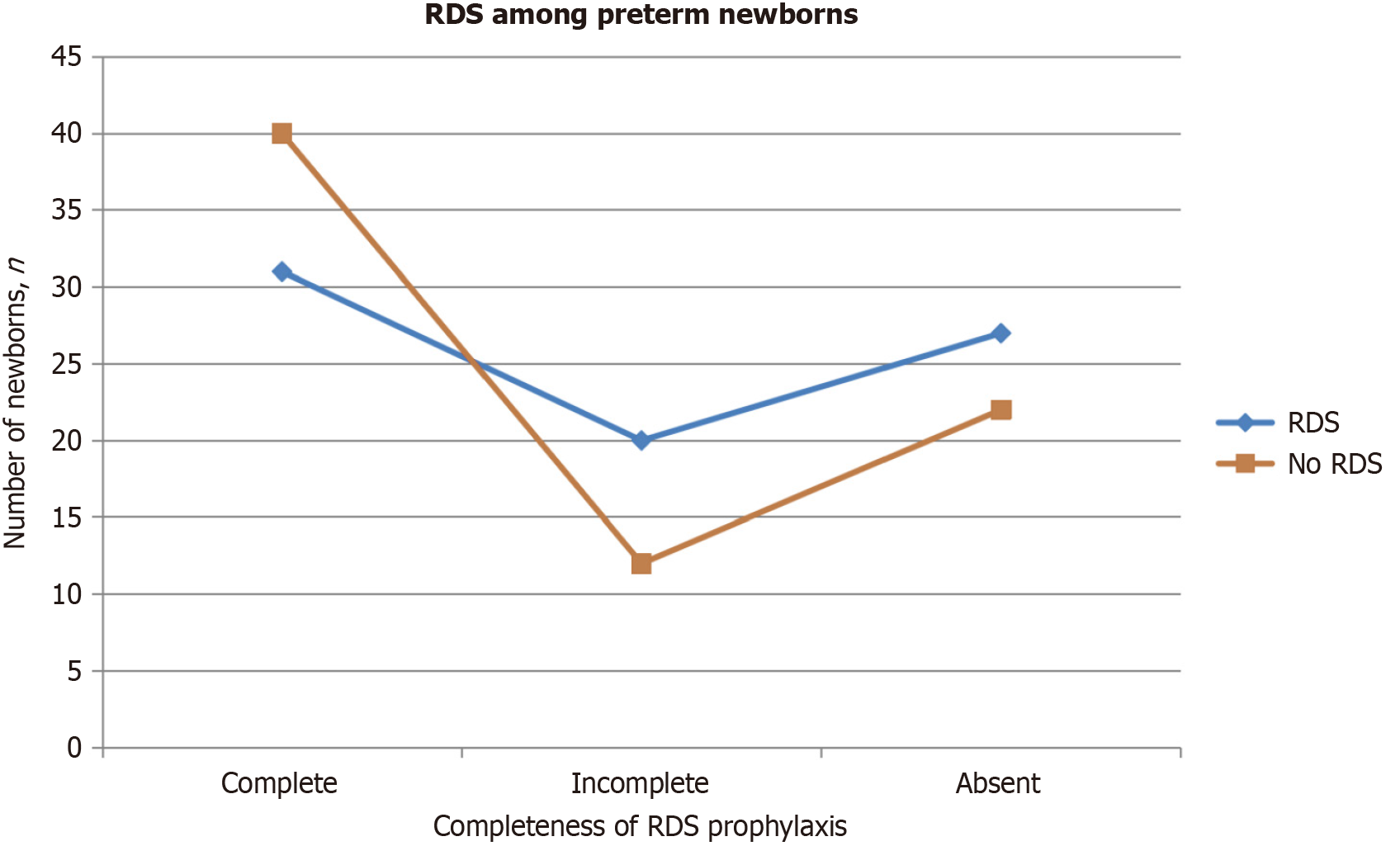
RDS was diagnosed in 78 out of the total number of newborns (51.3%). Among these, 31 premature newborns (41.0%) received a full dose of ACS, while 20 newborns (25.6%) received a partial dose and 27 newborns (34.6%) did not receive any.
Conversely, RDS was absent in 74 newborns (48.7%). Of these, 40 newborns (54.1%) received a full dose of ACS, 12 (16.2%) had a partial dose, and 22 (29.7%) did not receive any.
Although the P value (P = 0.170) indicates no statistically significant correlation between the occurrence of RDS in newborns and the completeness of ACS, the quantitative data suggests a higher prevalence of RDS among premature newborns who did not receive ACS. Furthermore, the data illustrates that the majority of newborns who did not develop RDS had received full prenatal dexamethasone treatment, as depicted in Figure 1.
The analysis revealed a statistically significant relationship between the incidence of birth asphyxia and the completeness of ASP prophylaxis (P = 0.002; see Table 4). In cases of moderate asphyxia, which affected 85 newborns, 34 (40.0%) had received full ASP, 30 (35.3%) had not received any prophylaxis, and 21 (24.7%) had received partial prophylaxis. Among the 34 newborns (100.0%) who did not experience asphyxia, 25 (73.5%) had received complete prophylaxis, 6 (17.6%) had no prophylaxis, and 3 (8.8%) had received partial prophylaxis. Regarding severe asphyxia, observed in 33 newborns, 12 (36.4%) had received full ASP, 13 (39.4%) had not received prophylaxis, and 8 (24.2%) had received partial prophylaxis. The presence of severe asphyxia in preterm newborns who did not receive ASP therapy led to a higher frequency of primary resuscitation measures in these groups compared to infants who received full or partial doses of ASP (P = 0.002). Bonferroni pairwise comparison showed that a complete course of antenatal steroids significantly improves neonatal resuscitation outcomes compared to both the absence of treatment (P = 0.00853) and an incomplete course (P = 0.00338). However, there was no significant difference between no steroid prophylaxis and an incomplete course (P = 1.000), suggesting that an incomplete course may not offer significant benefits.
| Asphyxia level | Full dose of ASP, n = 71 | No ASP, n = 49 | Partial dose of ASP, n = 32 | Total, n = 152 | P value (α = 0.0167) |
| None | 25 (73.5) | 6 (17.6) | 3 (8.8) | 34 (100) | 0.002; Group 1 – Group 2: P = 0.003; Group 1 – Group 3: P = 0.001; Group 2 – Group 3: P = 0.42 |
| Moderate | 34 (40.0) | 30 (35.3) | 21 (24.7) | 85 (100) | |
| Severe | 12 (36.4) | 13 (39.4) | 8 (24.2) | 33 (100) |
Pairwise comparisons using Bonferroni correction (α = 0.0167) revealed a significant difference between the Full-dose ASP and No ASP groups (P = 0.003), with a higher proportion of neonates without asphyxia in the Full-dose ASP group. A significant difference was also observed between the Full-dose ASP and Partial-dose ASP groups (P = 0.001), again demonstrating a higher proportion of neonates without asphyxia in the Full-dose ASP group. No significant difference was observed between the No ASP and Partial-dose ASP groups (P = 0.42). These findings suggest that Full-dose ASP is associated with a higher likelihood of neonates being born without asphyxia compared to those receiving partial or no ASP.
In evaluating neonatal outcomes, the Apgar scores of preterm newborns were assessed at both the first and fifth minutes after birth, as shown in Table 5. The average Apgar score in the first minute was higher in the group that received full antenatal steroid prophylaxis (5.38 ± 1.84) compared to the group with incomplete prophylaxis (4.81 ± 1.64) and those with no prophylaxis (4.92 ± 1.77). By the fifth minute, the scores improved across all groups, with the fully treated group scoring 6.55 ± 1.73, compared to 5.97 ± 1.62 in the incomplete group and 5.94 ± 1.74 in those without prophylaxis. However, statistical analysis indicated no significant relationship between Apgar scores and the completeness of ASP, as reflected in the P values of 0.214 (first minute) and 0.101 (fifth minute).
| Parameter | ACS | P value | |||
| Complete, n = 71 | Absent, n = 49 | Incomplete, n = 32 | Total, n = 152 | ||
| Apgar score at the 1st minute | 5.38 ± 1.84 | 4.92 ± 1.77 | 4.81 ± 1.64 | 5.11 ± 1.78 | 0.214 |
| Apgar score at the 5th minute | 6.55 ± 1.73 | 5.94 ± 1.74 | 5.97 ± 1.62 | 6.23 ± 1.72 | 0.101 |
Table 6 presents the prevalence of major and associated comorbidities among preterm newborns. The data indicate that respiratory system complications are the most commonly observed issues.
| Parameters | Prevention of fetal RDS | P value | |||
| Complete, n = 71 | Absent, n = 49 | Incomplete, n = 32 | Total, n = 152 | ||
| Comorbidities | |||||
| Cerebral ischemia | 0.121 | ||||
| Yes | 41 (41.0) | 37 (37.0) | 22 (22.0) | 100 (100.0) | |
| No | 30 (57.7) | 12 (23.1) | 10 (19.2) | 52 (100.0) | |
| Congenital pneumonia | 0.051 | ||||
| Yes | 16 (32.7) | 19 (38.8) | 14 (28.6) | 49 (100.0) | |
| No | 55 (53.4) | 30 (29.1) | 18 (17.5) | 103 (100.0) | |
| Respiratory failure | 0.588 | ||||
| Yes | 16 (47.1) | 9 (26.5) | 9 (26.5) | 34 (100.0) | |
| No | 55 (46.6) | 40 (33.9) | 23 (19.5) | 118 (100.0) | |
| Cerebral depression | 0.829 | ||||
| Yes | 16 (51.6) | 9 (29.0) | 6 (19.4) | 31 (100.0) | |
| No | 55 (45.5) | 40 (33.1) | 26 (21.5) | 121 (100.0) | |
| Hypoxic-ischemic encephalopathy | 0.592 | ||||
| Yes | 13 (56.5) | 6 (26.1) | 4 (17.4) | 23 (100.0) | |
| No | 58 (45.0) | 43 (33.3) | 28 (21.7) | 129 (100.0) | |
| Bronchopulmonary dysplasia | 0.575 | ||||
| Yes | 6 (37.5) | 7 (43.8) | 3 (18.8) | 16 (100.0) | |
| No | 65 (47.8) | 42 (30.9) | 29 (21.3) | 136 (100.0) | |
| Congenital hydrocephalus | 0.347 | ||||
| Yes | 0 (0.0) | 1 (100) | 0 (0.0) | 1 (100.0) | |
| No | 71 (47.0) | 48 (31.8) | 32 (21.2) | 151 (100.0) | |
| Heart failure | 0.951 | ||||
| Yes | 7 (50.0) | 4 (28.6) | 3 (21.4) | 14 (100.0) | |
| No | 64 (46.4) | 45 (32.6) | 29 (21.0) | 138 (100.0) | |
| Atrial septal defect | 0.882 | ||||
| Yes | 3 (42.9) | 2 (28.6) | 2 (28.6) | 7 (100.0) | |
| No | 68 (46.9) | 47 (32.4) | 30 (20.7) | 145 (100.0) | |
| Patent ductus arteriosus | 0.397 | ||||
| Yes | 4 (66.7) | 2 (33.3) | 0 (0.0) | 6 (100.0) | |
| No | 67 (45.9) | 47 (32.2) | 32 (21.9) | 146 (100.0) | |
| Ventricular septal defect | 0.389 | ||||
| Yes | 1 (33.3) | 2 (66.7) | 0 (0.0) | 3 (100.0) | |
| No | 70 (47.0) | 47 (31.5) | 32 (21.5) | 149 (100.0) | |
| Transient tachypnea in a newborn | 0.730 | ||||
| Yes | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (100.0) | |
| No | 70 (46.7) | 48 (32.0) | 32 (21.3) | 150 (100.0) | |
| Neonatal jaundice | 0.531 | ||||
| Yes | 18 (40.0) | 17 (37.8) | 10 (22.2) | 45 (100.0) | |
| No | 53 (49.5) | 32 (29.9) | 22 (20.6) | 107 (100.0) | |
| Necrotizing enterocolitis | 0.825 | ||||
| Yes | 4 (50.0) | 3 (37.5) | 1 (12.5) | 8 (100.0) | |
| No | 67 (46.5) | 46 (31.9) | 31 (21.5) | 144 (100.0) | |
| Сongenital malformations of the gastrointestinal tract | 0.481 | ||||
| Yes | 1 (50.0) | 0 (0.0) | 1 (50.0) | 2 (100.0) | |
| No | 70 (46.7) | 49 (32.7) | 31 (20.7) | 150 (100.0) | |
| Extreme immaturity | 0.886 | ||||
| Yes | 9 (50.0) | 6 (33.3) | 3 (16.7) | 18 (100.0) | |
| No | 62 (46.3) | 43 (32.1) | 29 (21.6) | 134 (100.0) | |
| Massive pulmonary hemorrhage occurred in the perinatal period | 0.636 | ||||
| Yes | 2 (66.7) | 1 (33.3) | 0 (0.0) | 3 (100.0) | |
| No | 69 (46.3) | 48 (32.2) | 32 (21.5) | 149 (100.0) | |
| Gastrointestinal hemorrhage | 0.845 | ||||
| Yes | 1 (33.3) | 1 (33.3) | 1 (33.3) | 3 (100.0) | |
| No | 70 (47.0) | 48 (32.2) | 31 (20.8) | 149 (100.0) | |
| Intraventricular hemorrhage | 0.151 | ||||
| Yes | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) | |
| No | 71 (47.0) | 49 (32.5) | 31 (20.5) | 151 (100.0) | |
| DIC of blood | 0.322 | ||||
| Yes | 1 (16.7) | 3 (50.0) | 2 (33.3) | 6 (100.0) | |
| No | 70 (47.9) | 46 (31.5) | 30 (20.5) | 146 (100.0) | |
| Anemia in premature newborns | 0.501 | ||||
| Yes | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (100.0) | |
| No | 70 (46.7) | 48 (32.0) | 32 (21.3) | 150 (100.0) | |
| Periventricular leukomalacia | 0.119 | ||||
| Yes | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | |
| No | 71 (47.3) | 47 (31.3) | 32 (21.3) | 150 (100.0) | |
| Proliferative retinopathy | 0.730 | ||||
| Yes | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (100.0) | |
| No | 70 (46.7) | 48 (32.0) | 32 (21.3) | 150 (100.0) | |
Cerebral Ischemia (CI) was present in 100 newborns (65.8%). Among these, 41 newborns (41.0%) received complete ASP, 22 (22.0%) received partial prophylaxis, and 37 (37.0%) did not receive any ASP. In contrast, among the 52 newborns (34.2%) without CI, 30 (57.7%) received full prophylaxis, 10 (19.2%) had partial prophylaxis, and 12 (23.1%) had no prophylaxis.
The prevalence of congenital pneumonia (CP) was noted in 49 newborns (32.2%), while 103 newborns (67.8%) were free from CP. Among those with CP, 16 (32.7%) had complete ASP, 14 (28.6%) had partial prophylaxis, and 19 (38.8%) had no prophylaxis. For the 103 newborns without CP, 55 (53.4%) received complete prophylaxis, 18 (17.5%) had partial prophylaxis, and 30 (29.1%) had no prophylaxis. The P value (P = 0.051) indicates a statistically significant association between congenital pneumonia and the completeness of ASP.
Bronchopulmonary dysplasia (BPD) was diagnosed in 16 newborns (10.5%). Of these, 6 (37.5%) received full prophylaxis, 3 (18.8%) had partial prophylaxis, and 7 (43.8%) had no prophylaxis. Among the 136 newborns (89.5%) without BPD, 65 (47.8%) received full prophylaxis, 29 (21.3%) had partial prophylaxis, and 42 (30.9%) received no prophylaxis. The P value (P = 0.575) shows no statistically significant correlation between the presence of BPD and the completeness of ASP.
Several newborns were diagnosed with congenital heart defects, primarily heart failure, patent ductus arteriosus (PDA), and atrial septal defect (ASD): Heart failure (HF) was observed in 14 newborns (9.2%). Of these, 4 (28.6%) received full prophylaxis, 3 (21.4%) had partial prophylaxis, and 7 (50.0%) had no prophylaxis. Among the 138 newborns (90.8%) without HF, 64 (46.4%) received complete prophylaxis, 29 (21.0%) had partial prophylaxis, and 45 (32.6%) had none. PDA was present in 6 newborns (3.9%); of these, 3 (50.0%) had full prophylaxis, 2 (33.3%) had partial prophylaxis, and 1 (16.7%) had none. For the 146 infants without PDA, 67 (45.9%) received complete prophylaxis, 32 (21.9%) had partial, and 47 (32.2%) had none. ASD was diagnosed in 7 newborns (4.6%). Among them, 3 (42.9%) received full prophylaxis, 2 (28.6%) had partial prophylaxis, and 2 (28.6%) received none. For the 145 infants without ASD, 68 (46.9%) received complete prophylaxis, 30 (20.7%) had partial prophylaxis, and 47 (32.4%) had none.
The prevalence of conditions such as congenital hydrocephalus, ventricular septal defect, neonatal jaundice, necrotizing enterocolitis, congenital gastrointestinal malformations, proliferative retinopathy, periventricular leukomalacia, disseminated intravascular coagulation, and anemia did not differ significantly between preterm newborns with and without antenatal steroid prophylaxis. Detailed information on these conditions is presented in Table 6.
The study also examined mortality rates among preterm newborns. Out of 152 newborns, 13 (8.6%) died. Of these, 4 (30.8%) had received complete ASP, 3 (23.1%) had partial prophylaxis, and 6 (46.2%) had no prophylaxis. Among the 139 survivors (91.4%), 67 (48.2%) received full prophylaxis, 29 (20.9%) had partial prophylaxis, and 43 (30.9%) had none. Although the P value (P = 0.437) did not indicate a statistically significant association between mortality and the completeness of ASP, the data suggest that the absence of prophylaxis may contribute to higher mortality rates among preterm newborns.
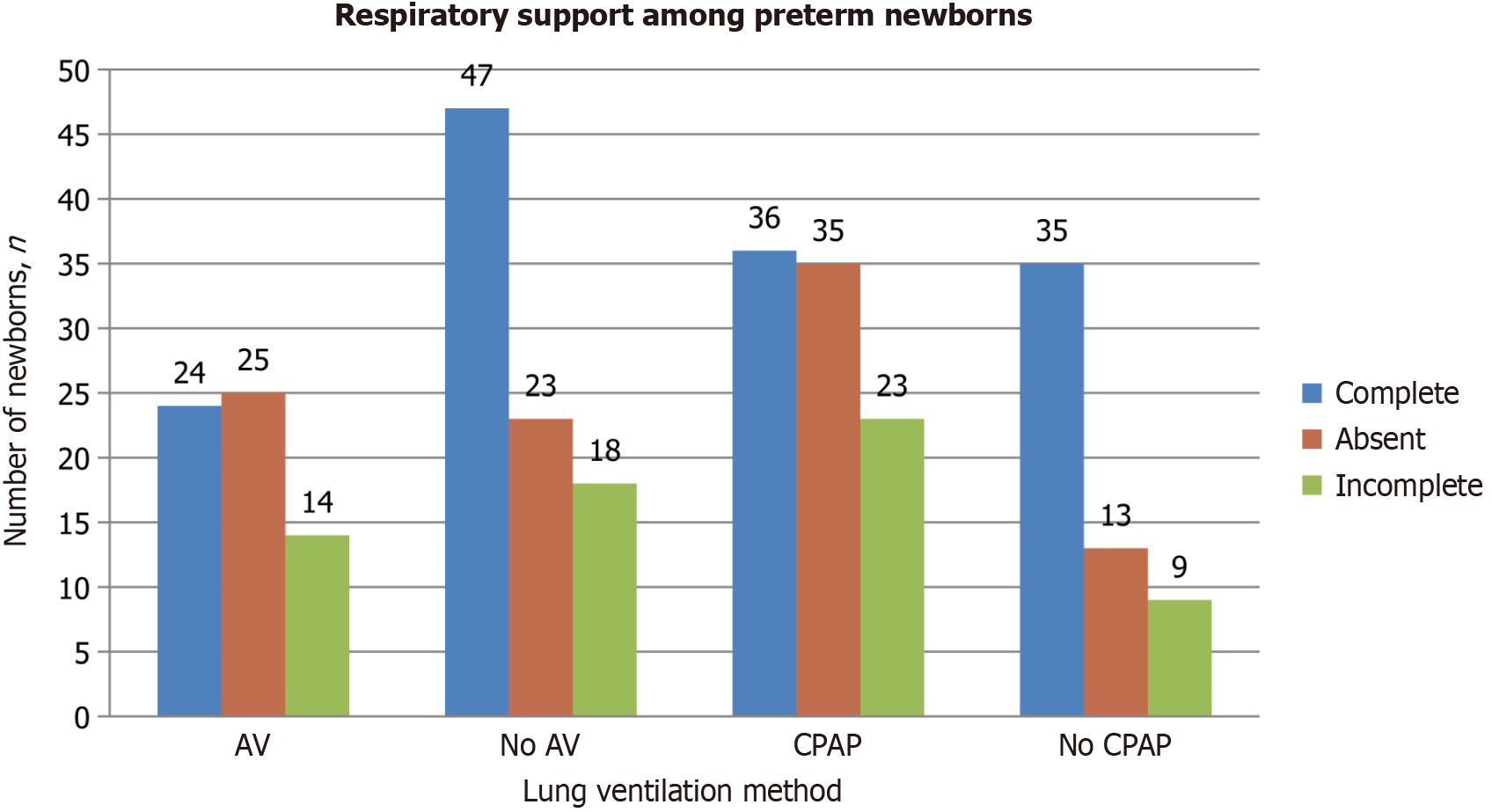
The bar chart in Figure 2 illustrates the differences in the need for continuous positive airway pressure (CPAP) therapy and mechanical ventilation among preterm newborns with varying levels of ASP. Mechanical ventilation was required for 63 newborns (41.4%). Of these, 24 (38.1%) had received complete ASP, 14 (22.2%) had partial prophylaxis, and 25 (39.7%) had not received any prophylaxis. The data suggest that incomplete or absent prophylaxis is associated with an increased need for ventilator support. In contrast, among the 88 newborns (57.9%) who did not require ventilation, 47 (53.4%) had received full prophylaxis, 18 (20.5%) had partial prophylaxis, and 23 (26.1%) had no prophylaxis. These findings indicate that newborns who did not require artificial ventilation were more likely to have received complete or partial ASP.
Non-invasive CPAP (NCPAP) support was needed for 94 newborns (61.8%), while 58 newborns (38.2%) did not require it. Among those needing NCPAP, 36 (38.3%) had received full prophylaxis, 23 (24.5%) had partial prophylaxis, and 35 (37.2%) had no prophylaxis. This suggests that a lack of complete antenatal steroid treatment, or incomplete dosing, may increase the need for NCPAP therapy in preterm newborns. Conversely, among the 58 newborns who did not require NCPAP, 35 (61.4%) had received complete ASP, 9 (15.8%) had partial prophylaxis, and 13 (22.8%) had no prophylaxis. The data imply that full ASP is effective in reducing the need for NCPAP in preterm infants.
The P value (P = 0.022) indicates a statistically significant association between the need for NCPAP and the completeness of ASP, emphasizing the importance of thorough antenatal steroid administration in reducing the requirement for respiratory support in preterm newborns. However, pairwise comparisons did not show statistically significant results between the study groups.
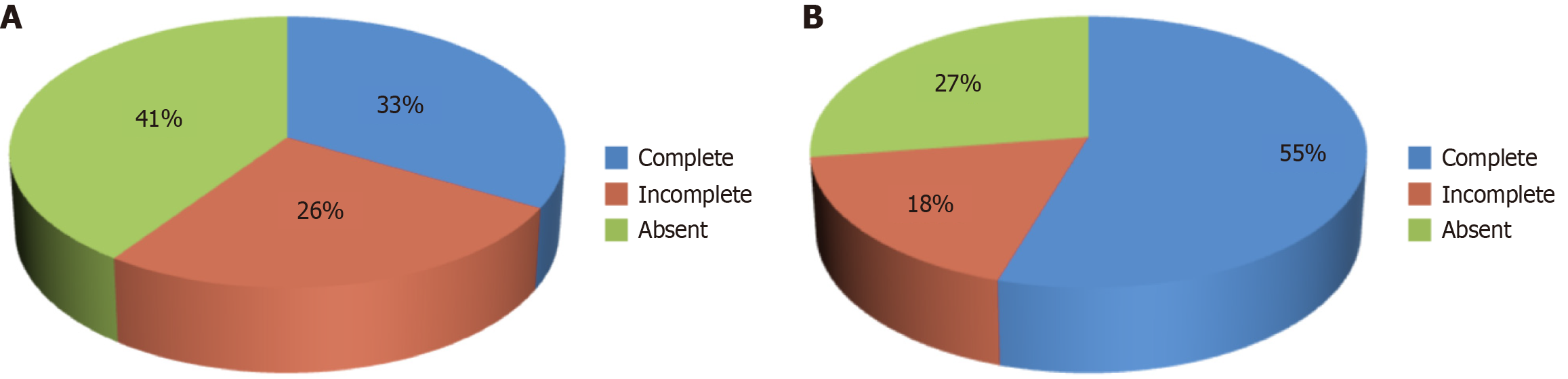
The data indicate that 37.5% (57 newborns) required surfactant therapy, while 62.5% (95 newborns) did not (Figure 3). The analysis suggests that preterm newborns who received incomplete or no ASP were more likely to require surfactant administration. Among those who required surfactant therapy, 23 newborns (40.4%) had no prior dexamethasone prophylaxis, and 15 (26.3%) had received incomplete prophylaxis. Only 19 newborns (33.3%) who needed surfactant therapy had received complete ASP. Conversely, of the 95 newborns who did not require surfactant therapy, 52 (54.7%) had complete prophylaxis, 17 (17.9%) had incomplete prophylaxis, and 26 (27.4%) had no prophylaxis at all. The statistical analysis shows a significant association between the administration of surfactant therapy and the completeness of ASP, as indicated by a P value of 0.038. This finding underscores the importance of complete antenatal steroid administration in reducing the need for surfactant therapy in preterm newborns. However, Bonferroni pairwise comparisons were also statistically non-significant.
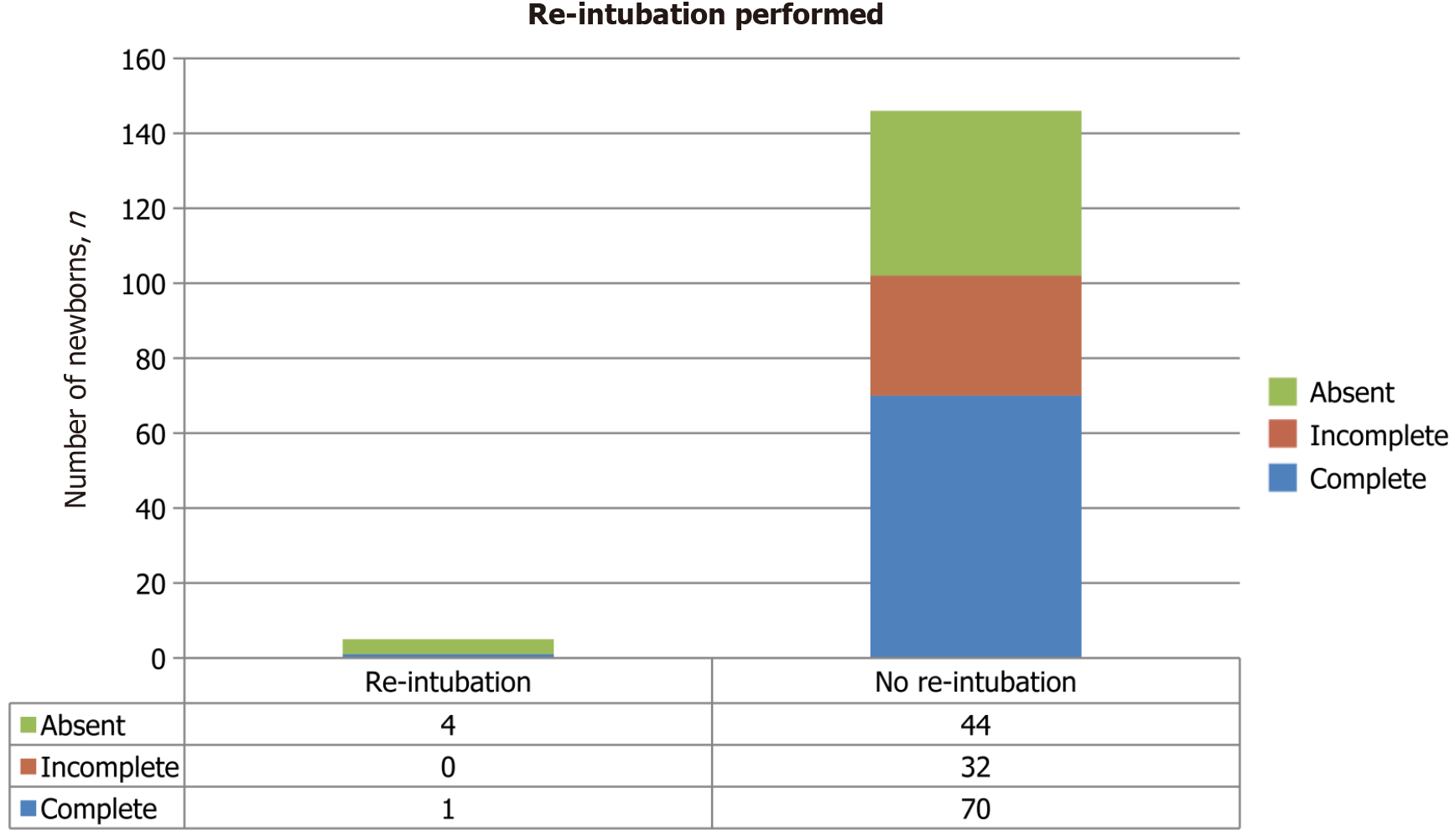
In rare cases, newborns were re-intubated (Figure 4). Newborns who did not receive ASP underwent re-intubation more frequently than those who received incomplete and complete doses. The result is approaching statistical significance (P = 0.058).
Our study's findings align with previous research while also revealing some deviations from the existing literature. For instance, studies by Bonanno et al[12] and Blankenship et al[13] demonstrated that the administration of antenatal corticosteroids is effective in reducing neonatal mortality compared to the absence of corticosteroid administration[12,13]. Our study is consistent with the results of American research (2016), published in Reproductive Health, in which the absence of ASP prevention may lead to a greater percentage of deaths among premature newborns[14].
A study by Battarbee[15] demonstrated a reduction in the risks of respiratory distress syndrome (P = 0.003), intraventricular hemorrhage, necrotizing enterocolitis, and neonatal mortality (P = 0.01) with ASP. Additionally, it was found that there was no increased risk of neonatal infection after administration of antenatal corticosteroids even after preterm pre-labor rupture of membranes. In contrast, our study did not find statistically significant associations between the completeness of ASP and the incidence of RDS, intraventricular hemorrhage, or necrotizing enterocolitis. Additionally, no correlation was found between ASP and the occurrence of neonatal sepsis. Nevertheless, we observed that the absence of ASP may contribute to an increased mortality rate among preterm newborns.
Furthermore, we found that the absence of ASP prevention may lead to a greater percentage of deaths among premature newborns. The Taiwanian cohort study (2023) and Korean (2019) found that children exposed to one course of antenatal corticosteroids were significantly more likely to have an increased risk of serious infection during the first 12 months of life, with adjusted hazard ratios for overall serious infection (P < 0.001), sepsis (P = 0.02), pneumonia (P < 0.001)[16,17]. According to the results of this study, antenatal steroid prevention has shown effectiveness in intrauterine pneumonia (P = 0.05). Further investigation is required to determine if specific factors predispose certain newborns to life-threatening infections following ASP.
The studies of Battarbee[15] and Roberts et al[18] are consistent with our research, which suggests that antenatal corticosteroid use is effective in women with premature rupture of membranes and pregnancy-related hypertension syndromes. In this study, we found that many women with premature rupture of membranes (P = 0.023) did not receive ASP, likely due to active management protocols favoring immediate delivery over expectant management. Thus, the full effectiveness of ASP in such cases remains uncertain.
Evidence from one randomized controlled trial from the Cochrane Database (2021) suggests that prophylactic corticosteroids before elective cesarean section at term probably reduce admission to the neonatal intensive care unit for respiratory morbidity. The respiratory morbidity after treatment with antenatal corticosteroids was 2.3% compared with 5.1% in the control group[18]. Our study also supports the beneficial effects of ASP in reducing the need for re-intubation and CPAP in preterm newborns. However, our findings did not establish a statistically significant correlation between the need for mechanical ventilation and the completeness of ASP (P = 0.135). It is possible that combining the complete and incomplete ASP groups in future analyses could yield more robust correlations.
According to the Serbian study (Belgrade, 2022), they suggested that the level of maternal hyperglycemia after the administration of ASP may be reduced by altering the timing and dosing intervals. Betamethasone may offer advantages over dexamethasone in pregnancies complicated by gestational diabetes mellitus. Consequently, in the future, the administration of ASP must be individualized, and population-based approaches should be discontinued. Nevertheless, there is a need for more studies here[19].
Research work by Zhu et al[20], published in Frontiers in Pharmacology (2023), highlighted the challenges in twin pregnancies, where the risk of adverse outcomes is higher due to increased prematurity rates and complexities related to corticosteroid administration. There are problems with timing, dosage, repeat courses, and types of corticosteroids that affect twins’ outcomes. So, there is no optimal usage for them in comparison with singletons[18,20]. On the other hand, our data suggest that there is a trend toward statistical significance (P = 0.056) between multiple pregnancies and the effectiveness of antenatal corticosteroid prophylaxis. We conclude that multiple pregnancies may impact the effectiveness of ASP. Perhaps we are mistaken because in this study, with multiple births, each child was counted separately and this could affect the results.
The Indian study (2024) observed that antenatal betamethasone exacerbated hyperglycemia in pregnant women, irrespective of their pre-existing glycemic status[17,20-23]. In this study, we did not find an association between antenatal corticosteroid prophylaxis (ACP) and the development of hyperglycemia in women. However, the results showed that uterine atony occurred more frequently in women who received a full dose of ACP. This may be related to the hormonal therapy administered before delivery, which might have affected the timely contraction of the uterus. This interesting finding warrants further investigation, possibly in a larger cohort of women, to more thoroughly explore the effects of ACP not only on neonatal outcomes but also on maternal health.
Our findings align with recommendations from American (2024) and United Kingdom (2017) studies, advocating for a single course of antenatal corticosteroids to accelerate fetal lung maturation in women at risk of preterm delivery[24-26]. Notably, our study showed that incomplete or absent ASP increased the likelihood of needing surfactant therapy, indicating a significant correlation between surfactant administration and the completeness of ASP.
We would also like to highlight the strengths and limitations of our study. One of the strengths of this study is its comprehensive analysis, encompassing multiple parameters across obstetric, antenatal, and neonatal factors. Unlike studies focusing on isolated outcomes, our approach allows for a broader evaluation of how various factors interact to affect neonatal health.
However, a limitation of our study is the absence of logistic regression analysis. There are several reasons why we did not apply this particular statistical method. First, reliable results from logistic regression require a sufficiently large sample size. With a cohort of 152 newborns, we believe there may be insufficient statistical power to detect meaningful correlations between the completeness of ACP and outcomes using this method. Moreover, the primary objective of our study was to compare outcomes between groups, rather than to predict complex relationships. Therefore, logistic regression could be considered excessive in this context. All of this underscores that the statistical methods we employed - the χ2 test and Fisher’s exact test with Bonferroni correction - are appropriate and justified, considering the study’s aims, sample size, and its relatively simple design.
Moreover, the retrospective design of this study may have led to an underestimation of maternal infections or sepsis due to potential gaps or inconsistencies in medical records.
Additionally, the effectiveness of partial antenatal corticosteroid courses remains uncertain, as some studies indicate reduced efficacy compared to complete courses, potentially influencing neonatal outcomes in our study. Variability in the timing, dosage, and frequency of ACS administration may have affected neonatal respiratory and systemic outcomes, contributing to inconsistencies in our findings. Despite adjusting for multiple factors, variations in maternal health conditions, gestational age at birth, and neonatal characteristics could have also influenced the results, limiting the generalizability of the conclusions.
The scientific work lacks information on long-term outcomes in newborns, such as survival beyond the neonatal period and neurodevelopment, which is also a limitation of the study. Future research should focus on the follow-up of preterm newborns.
The obtained data indicate a significant association between the complete implementation of ASP and the occurrence of birth asphyxia (P = 0.012), as well as the need for primary resuscitation measures (P = 0.001). Although pairwise comparisons did not yield statistically significant results, the use of NCPAP (P = 0.022) and the need for surfactant replacement therapy (P = 0.038) remain important parameters indicating the effectiveness of ASP in newborns. Complete ASP was also associated with a reduced need for invasive mechanical ventilation and re-intubation. Furthermore, the results of this study demonstrated that improvement in these indicators contributed to reduced morbidity and increased survival rates among preterm newborns.
It can also be concluded that full administration of ASP may provoke postpartum bleeding in women, as a significant association was found between a complete ASP course and the development of uterine atony (P = 0.0095). This highlights the need for improved postpartum management in women who have received a full ASP course and calls for further in-depth investigation.
For us, this study lays the foundation for further research (catamnesis) to evaluate the long-term impact of ASP on the growth, cognitive development, and overall health of preterm newborns. In clinical practice, we can actively take measures to improve the care of preterm newborns and the management of postpartum women, which undoubtedly creates conditions for enhancing the quality of life for this cohort of children in the long term.
Gratitude to S.D. Asfendiyarov Kazakh National Medical University for providing the resources and facilities necessary to carry out this research. Additionally, gratitude to the Neonatal Care Department of the Enbekshikazakh Multidisciplinary Central District Hospital for their assistance in data collection.
| 1. | Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, Thorp JM, Caritis SN, Prasad M, Tita AT, Saade GR, Sorokin Y, Rouse DJ, Blackwell SC, Tolosa JE; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215:103.e1-103.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 359] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 2. | Aslamzai M, Froogh, BA, Mukhlis AH, Faizi OA, Sajid SA, Hakimi Z. Factors associated with respiratory distress syndrome in preterm neonates admitted to a tertiary hospital in Kabul city: A retrospective cross-sectional study. Glob Pediatr. 2023;3:100035. [DOI] [Full Text] |
| 3. | Ohuma EO, Moller AB, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, Okwaraji YB, Mahanani WR, Johansson EW, Lavin T, Fernandez DE, Domínguez GG, de Costa A, Cresswell JA, Krasevec J, Lawn JE, Blencowe H, Requejo J, Moran AC. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023;402:1261-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 505] [Article Influence: 252.5] [Reference Citation Analysis (0)] |
| 4. | Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, Prieto-Merino D, Cousens S, Black RE, Liu L. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022;6:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 675] [Article Influence: 225.0] [Reference Citation Analysis (0)] |
| 5. | Wardlaw T, You D, Hug L, Amouzou A, Newby H. UNICEF Report: enormous progress in child survival but greater focus on newborns urgently needed. Reprod Health. 2014;11:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001;32:76-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Bird AD, McDougall AR, Seow B, Hooper SB, Cole TJ. Glucocorticoid regulation of lung development: lessons learned from conditional GR knockout mice. Mol Endocrinol. 2015;29:158-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | McDougall ARA, Aboud L, Lavin T, Cao J, Dore G, Ramson J, Oladapo OT, Vogel JP. Effect of antenatal corticosteroid administration-to-birth interval on maternal and newborn outcomes: a systematic review. EClinicalMedicine. 2023;58:101916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Althabe F, Belizán JM, McClure EM, Hemingway-Foday J, Berrueta M, Mazzoni A, Ciganda A, Goudar SS, Kodkany BS, Mahantshetti NS, Dhaded SM, Katageri GM, Metgud MC, Joshi AM, Bellad MB, Honnungar NV, Derman RJ, Saleem S, Pasha O, Ali S, Hasnain F, Goldenberg RL, Esamai F, Nyongesa P, Ayunga S, Liechty EA, Garces AL, Figueroa L, Hambidge KM, Krebs NF, Patel A, Bhandarkar A, Waikar M, Hibberd PL, Chomba E, Carlo WA, Mwiche A, Chiwila M, Manasyan A, Pineda S, Meleth S, Thorsten V, Stolka K, Wallace DD, Koso-Thomas M, Jobe AH, Buekens PM. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Vogel JP, Ramson J, Darmstadt GL, Qureshi ZP, Chou D, Bahl R, Oladapo OT. Updated WHO recommendations on antenatal corticosteroids and tocolytic therapy for improving preterm birth outcomes. Lancet Glob Health. 2022;10:e1707-e1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Jobe AH, Kemp MW, Kamath-Rayne B, Schmidt AF. Antenatal corticosteroids for low and middle income countries. Semin Perinatol. 2019;43:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Bonanno C, Wapner RJ. Antenatal corticosteroids in the management of preterm birth: are we back where we started? Obstet Gynecol Clin North Am. 2012;39:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Blankenship SA, Brown KE, Simon LE, Stout MJ, Tuuli MG. Antenatal corticosteroids in preterm small-for-gestational age infants: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Goldenberg RL, Thorsten VR, Althabe F, Saleem S, Garces A, Carlo WA, Pasha O, Chomba E, Goudar S, Esamai F, Krebs NF, Derman RJ, Liechty EA, Patel A, Hibberd PL, Buekens PM, Koso-Thomas M, Miodovnik M, Jobe AH, Wallace DD, Belizán JM, McClure EM. The global network antenatal corticosteroids trial: impact on stillbirth. Reprod Health. 2016;13:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Battarbee AN. Use of Antenatal Corticosteroids in Preterm Prelabor Rupture of Membranes. Obstet Gynecol Clin North Am. 2020;47:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Yao TC, Chang SM, Wu CS, Tsai YF, Sheen KH, Hong X, Chen HY, Wu AC, Tsai HJ. Association between antenatal corticosteroids and risk of serious infection in children: nationwide cohort study. BMJ. 2023;382:e075835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 17. | Ogawa M, Matsuda Y, Kobayashi A, Shimada E, Akizawa Y, Mitani M, Makino Y, Matsui H. Ritodrine Should Be Carefully Administered during Antenatal Glucocorticoid Therapy Even in Nondiabetic Pregnancies. ISRN Obstet Gynecol. 2013;2013:120735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 471] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 19. | Babović IR, Dotlić J, Sparić R, Jovandaric MZ, Andjić M, Marjanović Cvjetićanin M, Akšam S, Bila J, Tulić L, Kocijančić Belović D, Plešinac V, Plesinac J. Gestational Diabetes Mellitus and Antenatal Corticosteroid Therapy-A Narrative Review of Fetal and Neonatal Outcomes. J Clin Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Zhu J, Li S, Zhao Y, Xiong Y. The role of antenatal corticosteroids in twin pregnancy. Front Pharmacol. 2023;14:1072578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Satyaraddi A, Sooragonda BG, Satyaraddi AA, Khadilkar K, Ks S, Kiran L, Kannan S. Antenatal Corticosteroids and Their Effects on Maternal Glycemic Status: A Prospective Observational Study From an Indian Tertiary Referral Center. Cureus. 2024;16:e60043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Jolley JA, Rajan PV, Petersen R, Fong A, Wing DA. Effect of antenatal betamethasone on blood glucose levels in women with and without diabetes. Diabetes Res Clin Pract. 2016;118:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Langen ES, Kuperstock JL, Sung JF, Taslimi M, Byrne J, El-Sayed YY. Maternal glucose response to betamethasone administration. Am J Perinatol. 2015;30:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Sotiriadis A, McGoldrick E, Makrydimas G, Papatheodorou S, Ioannidis JP, Stewart F, Parker R. Antenatal corticosteroids prior to planned caesarean at term for improving neonatal outcomes. Cochrane Database Syst Rev. 2021;12:CD006614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine. Good clinical practice advice: Antenatal corticosteroids for fetal lung maturation. Int J Gynaecol Obstet. 2019;144:352-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Ryu YH, Oh S, Sohn J, Lee J. The Associations between Antenatal Corticosteroids and In-Hospital Outcomes of Preterm Singleton Appropriate for Gestational Age Neonates according to the Presence of Maternal Histologic Chorioamnionitis. Neonatology. 2019;116:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |