Copyright
©The Author(s) 2016.
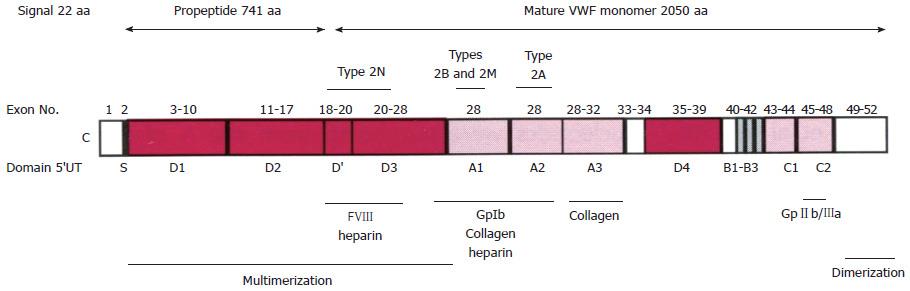
Figure 1 Structure and function relationship of the von Willebrand factor domains[1].
The VWF is synthesized in endothelial cells as a large protein of 2813 amino acid (aa): signal prepetide 22 aa, propeptide 741 aa, and the mature VWF monomer 2050 aa. D1-D2 pro-peptide is cleaved off at the furin cleavage site at time of secretion. VWF circulates bound to the FVIII at the D’ FVIII binding domain. Below the figure are the areas of VWF involved in binding specific factors. VWF circulates as large multimers as a function of the D3 multimerization and CK dimerization domains. Source: Goodeve and Peake[1]. VWD: Von Willebrand disease; VWF: Von Willebrand factor.
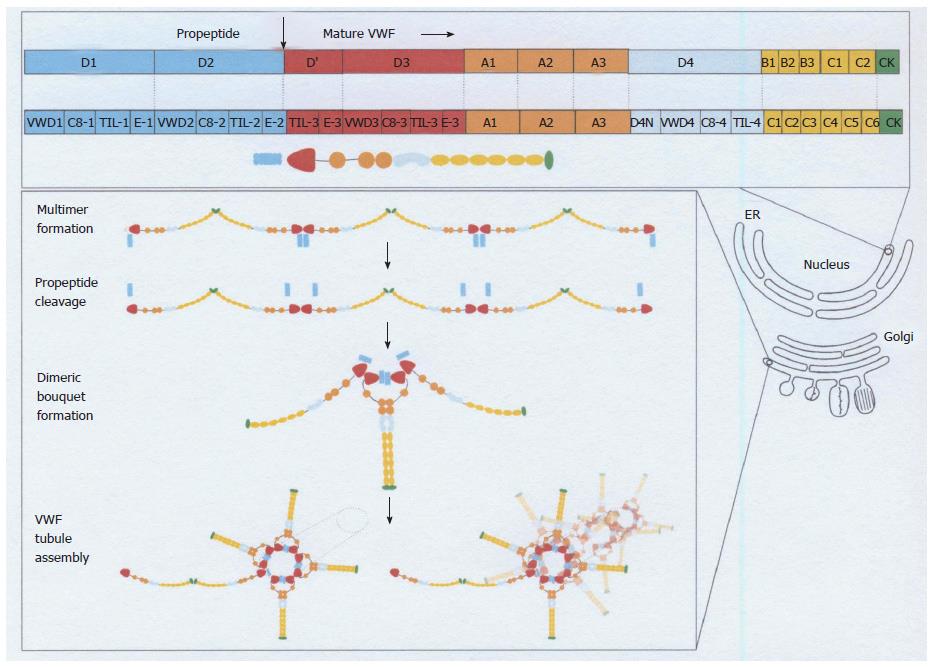
Figure 2 Von Willebrand factor domain structure and assembly throughout the biosynthetic pathways in endothelial cells[3-5].
The top panel shows the different domains of VWF as it is synthesized in the ER[4]. The arrow between the D2 domain and the D’ domain indicates the furin cleavage site at 764 leading to the production of the VWF propeptide (VWFpp) D1-D2 (blue) and the mature VWD protein with the domains D’, D3, A1, A2, D4, C1-6 and the cysteine knot (CK). The lower panel shows the assembly of VWF into multimers in the Golgi compartment, the cleavage VWFpp (blue), and the assembly of VWF into the dimeric bouquet at the trans-Golgi network (TNG). During the translocation of proVWF to the ER the signal peptide is cleaved off, and the proVWF forms dimers in a tail-to-tail fashion through cysteines in its carboxyterminal cysteine knot domain. ProVWF dimers transit to the Golgi apparatus to assemble into multimers in a “head-to-head” fashion through the formation of intermolecular disulphide bonds between cysteine residues in the D3 (multimerization) domain[4]. This is followed by the assembly of VWF in the Golgi network. ER: Endoplasmatic reticulum; VWD: Von Willebrand disease; VWF: Von Willebrand factor. Source: Valentijn and Eikenboom 2013[4].
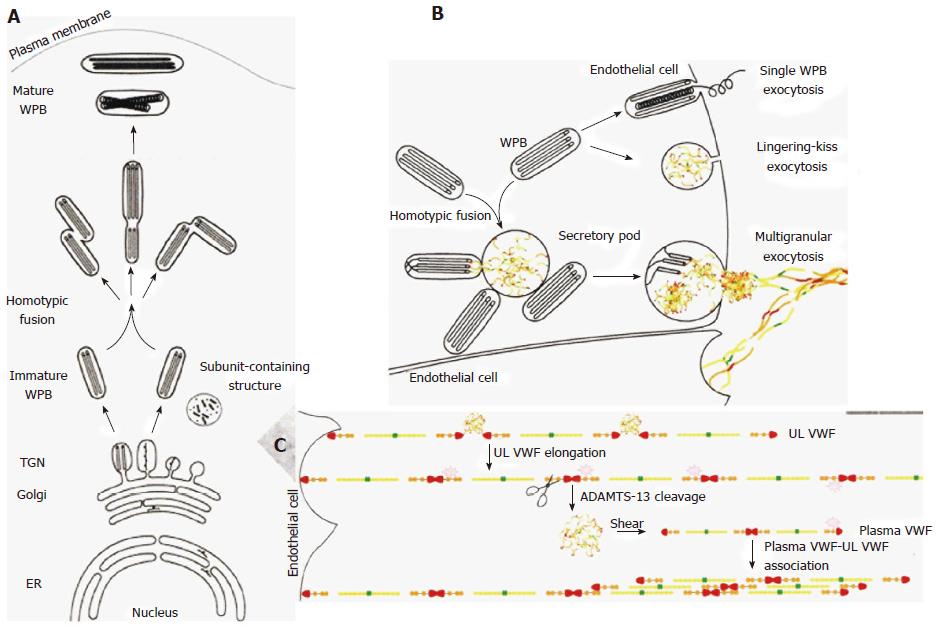
Figure 3 A left biosynthesis pathway of Weibel-Palade body[4].
A, B: The different steps in WPB synthesis of von Willebrand factor (VWF) assembly at the level of endoplasmatic reticulum (ER), at the trans-Golgi network (TGN) level (A), and VWF tubules are assembled and packed into budding vesicles prior to immature WPB formation. Homotypic fusion of WPB gives rise to the formation of WPB with different shapes. As WPB mature they became more electron-dense and reach the plasma membrane. B: Different modes of WPB exocytosis and VWF string formation on endothelial cells. In single WPB exocytosis mode, a single WPB fuses with the plasma membrane and ultra large VWF multimers (MM) are secreted. In lingering-kiss exocytosis mode (B), WPB round up and a small pore is formed with the plasma membrane, allowing the secretion of ultra large VWF MM. In multigranular exocytosis mode (B), WPB undergo homotypic fusion leading to the formation of a secretory pod that permits pooling of ultra large VWF MM prior to secretion[4]. After release, the ultra large vWF strings stick to the endothelial cell surface, attract platelets through platelet GpIb ligand and VWF GpIb receptor interaction, thereby activating the VWF cleavage site to be cleaved by ADAMTS13 at high shear stress in the endarterial circulation (C). WPB: Weibel-palade body. Source: Valentijn and Eikenboom 2013[4].
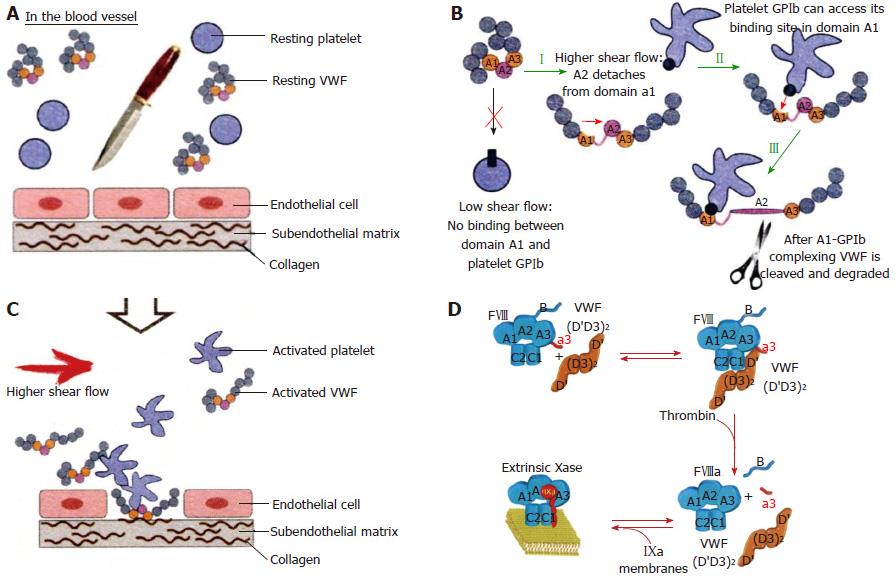
Figure 4 In the equilibrium state, with intact endothelial cells and no injured vessel, resting von Willebrand factor circulates as globules with resting platelets in blood (A).
In this state, VWF is incapable of mediating platelet adhesion. After an injury of the endothelial cells, the activated VWF: Von Willebrand factor (VWF) interacts with exposed collagen via vWF domains A1 and A3 (orange parts) and triggers the adhesion of activated platelets via VWF domain A1 (B, C). At low shear there is no binding between VWF domain A1 and platelet GPIb. At high shear rate the VWF globules elongate and made the VWF A1 domain accessible by the dissociation of domain A1 from A2. High shear flow detaches the A2 domain from domain A1 (I). Binding between GPIb of activated platelet to the GPIb receptor of VWF (II), which is immediately followed by cleavage of VWF in the A2 domain by ADAMTS13 (III) (B). Courtesy of Dr Sandra Posch. Insitute of Biophysics. Linz, Austria: sandra.posch@jku.at. FVIII:C is a heterodimer with a domain structure of A1-A2-B-A3-C1-C2 (upper left, Blue). FVIII:C circulates in complex with VWF through binding to the D’D3 domain, the FVIIIbinding site on VWF. Thrombin cleavage of FVIII liberates the a3 peptide and the B domain of FVIII (D), resulting in the dissociation of VWF from FVIII[6].
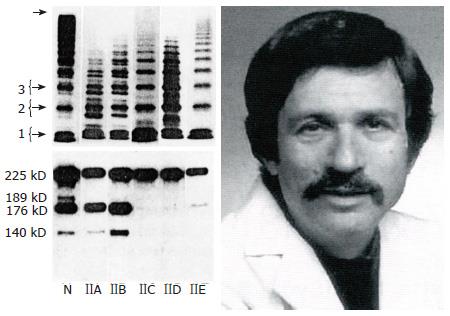
Figure 5 The 1986 Zimmerman Classification of Von Willebrand disease type IIA, IIB, IIC, IID and IIE[9] and Dr.
Ted Zimmerman (1937-1988). SDS-agarose multimeric analysis of plasma VWF in normal plasma (N) and in VWD type IIA, IIB, IIC, IIE and IID. Left lower part: Immunoblots of VWF proteolytic degradation products show increased proteolysis in VWD type IIA and IIB, but not or even absent in VWD type IIC, IIE and IID (N = normal plasma). VWD: Von Willebrand disease; VWF: Von Willebrand factor.
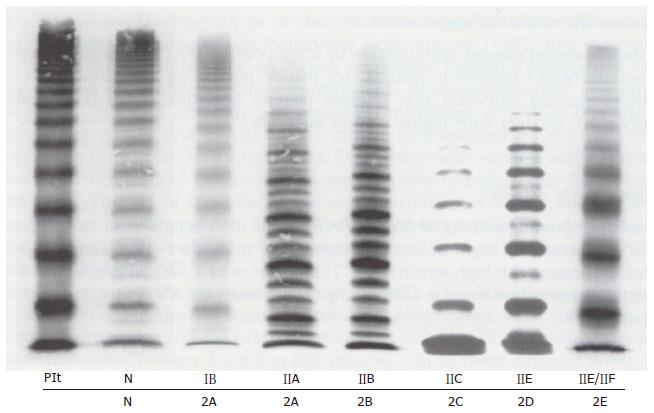
Figure 6 Multimeric pattern.
VWF from plasma of patients with von Willebrand disease classified according to the ISTH criteria for VWD type IIA, IIB, IIC and IIE and the translation into the 2001 Hamburg criteria for 1B = 2M (not 2A), 2A, 2B, 2C, 2D and 2E anno 2001 compared to a normal control in high resolution gel concentration (1.5%) according to Schneppenheim et al[10]. Dominant 1B relatively lacking large VWF multimers (MM) = 2M (Michiels). Dominant IIA = 2A lack of large molecular weight MM and the outer sub-bands of the individual triplets are markedly pronounced indicating increased proteolysis as the cause of 2A. Dominant 2B cannot be distinguished from 2A by MM alone. Recessive IIC = 2C lack of large MM and absence of triplets. Low MM and especially the first band, which probably reflects protomer (dimer) and a tetramer, is markedly pronounced. IID = 2D intervening VWF band and an odd number of MM. 2E lack or relative decrease of large MM and absence of the outer sub-bands of the normal triplet structure. Triplets are lacking in 2C, 2D, 2E and 1B = 2M are lacking indicating the absence of proteolysis. VWD: Von Willebrand disease; VWF: Von Willebrand factor. Plt: Platelet VWF; N: Normal.
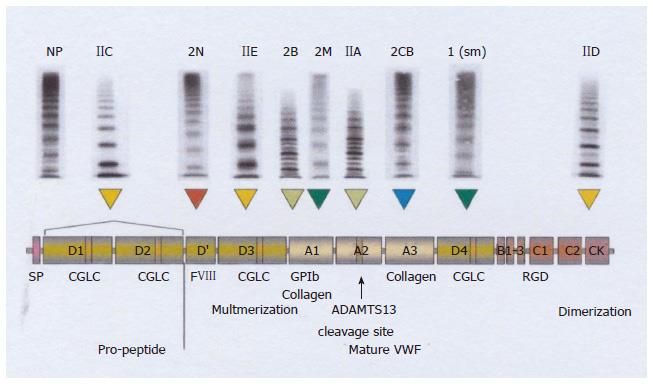
Figure 7 Translation and integration of the 2006 International Society on Thrombosis and Haemostasis and the 2009 Hamburg classification.
VWD type 2 variants[20] (recessive IIC, recessive 2N, dominant IIE, 2B, 2M, IIA, 2CB 1 (sm) and IID related to clustered distribution of VWF gene mutations in the D1-D2 propeptide, D’, D3, A1, A1, A2, A3, D4 and CK domains respectively. For explanation see Figure 8: from left to right recessive 2N, recessive IIC → 2C, and dominant → IIE → 2E, 2B, 2M, IIA → 2A, 2 CB (collagen binding defect), 1 smeary pattern (sm) and IID → 2D (from ref.[20]). VWD: Von Willebrand disease; VWF: Von Willebrand factor.
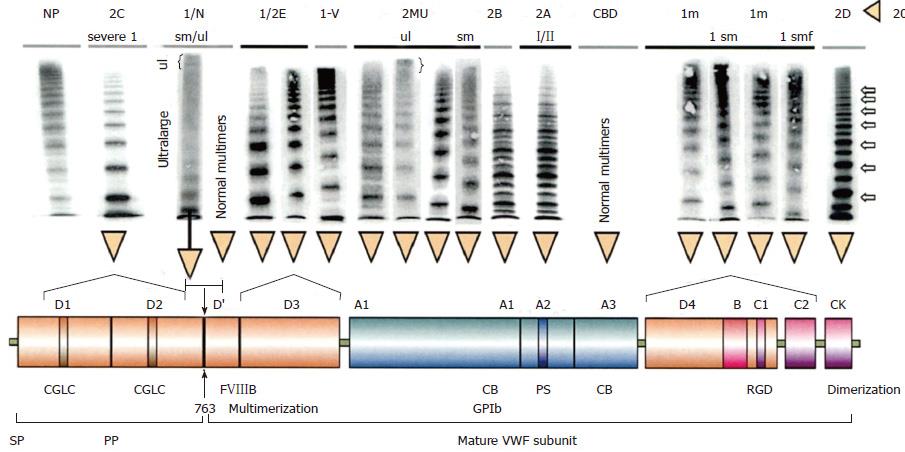
Figure 8 Structure and function of normal von Willebrand factor protein[20].
Mutations in the D1D2 domain prohibit the cleavage of VWFpp from mature VWF leading to a severe secretion and multimerization defects in recessive VWD 2C[16,31,33]. FVIII binding defects in the VWF D’ domain either homozygous or double heterozygous causes recessive VWD 2N[34,35]. Dominant VWD type 2E due to heterozygous missense mutations in the D3 leads to a secretion clearance multimerization defect, VWD 2E[20,38,39]. Loss of function mutations in the VWF GpIb of the A1 domain induce dominant VWD 2M[18,36,37]. Dominant VWD 2A due to mutations in the A2 domain makes the mutant VWF hypersensitive to the VWF cleavage protease ADAMTS13 at the VWF cleavage site (1605-1606)[40-44]. Immediately after secretion the 2A mutated VWF is proteolysed with loss of large VWF multimers and typical triplet structure of each VWF band. Dominant VWD 2B due to gain of function mutation in the A1 domain accelerates the interaction of platelet-GpIb and VWF A1 followed by VWF proteolysis by ADAMTS 13 interaction[17,46]. This process starts immediately after secretion of the 2B mutated VWF and causes VWD 2B with loss of large multimers and typical triplet structure of each VWF band. A new category of VWD type 1 secretion defect (SD) is due to mutations in the D4,B1-3,C1-2[39,49] domains relabelled as the C1, C2, C3, C4, C5 and C6 domains of the VWF gene/protein[3-5]. Heterozygous mutations in the D4, C1-C6 domains result in VWD type 1 SD and have either normal multimers or abnormal multimers. Homozygous or double heterozygous mutations in the D4, C1-C6 domains are associated with severe VWD type 1[26-29]. Cysteine mutations in the CK dimerization domain, either heterozygous and homozygous or double heterozygous, are associated with VWD 2D[30]. VWD: Von Willebrand disease; VWF: Von Willebrand factor; CBD: Collagen binding defect.
Figure 9 Restricted response of von Willebrand factor parameters to desmopressin.
Pronounced dominant VWD type 1 secretion defect (high FVIII:C/VWF:Ag ratio) with restricted response of VWF parameters to DDAVP as compared to completely normal responses of FVIII (high FVIII:C/VWF:Ag ratio) is indicative for VWD type 1 secretion defect (Left). Diagnostic differentiation of pronounced 1 VWD 1 secretion defect with normal VWF multimers (VWF MM according to Budde) and restricted decreased response to DDAVP of all VWF parameters in two members of one family (proband and her brother) vs pronounced case of dominant VWD 2M (Right) with normal VWF multimers before and after DDAVP[18], a poor response of VWF:RCo to DDAVP and fairly good responses to DDAVP of FVIII:C, VWF:Ag and VWF:CB followed by shortened half life times of FVIII:C, VWF:Ag and VWF:CB indicative for rapid clearance defect. Dominant VWD type 2M (Michiels) is featured by loss of function mutation in the A1 domain, normal multimers, decreased to zero RIPA, low VWF:RCo activity, a secretion defect and rapid clearance[18]. VWD: Von Willebrand disease; VWF: Von Willebrand factor; DDAVP: Desmopressin; NP: Normal plasma; P: Patient.
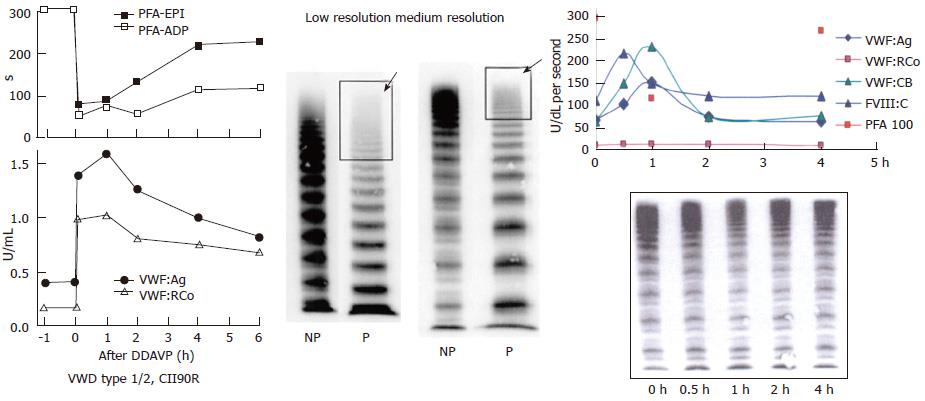
Figure 10 Von Willebrand disease 2E (left) and von Willebrand disease 2M (right).
Left: Dominant VWD type 2E: multimerization defect with loss of large VWF multimers to W1120S mutation in the A3 domain. DDAVP induced transient correction of PFA-100 closure time and restricted increase of VWF parameters from around 0.20-0.40 U/mL to around 1.0 U/mL. In VWD type 2E, VWF multimeric pattern is characterized by a lack or relative decrease of large multimers and the absence of the outer sub-band of the normal triplet structure. Medium resolution gel according to Budde et al[12]. Right: VWD 2M: Poor response of VWF:RCo to DDAVP, normal VWF multimers before and after DDAVP and good responses of FVIII, VWF:Ag and VWF:CB followed by shortened half-life time indicating rapid clearance defect of the FVIII-VWF complex on top of loss of VWF:RCo function in VWD 2M[20]. Medium resolution gel according to Budde et al[12]. VWD: Von Willebrand disease; VWF: Von Willebrand factor; DDAVP: Desmopressin; PFA-EPI: Platelet function analyzer, epinephrin; PFA-ADP: Platelet function analyser adenosine di-phosphate; NP: Normal plasma; P: Patient.
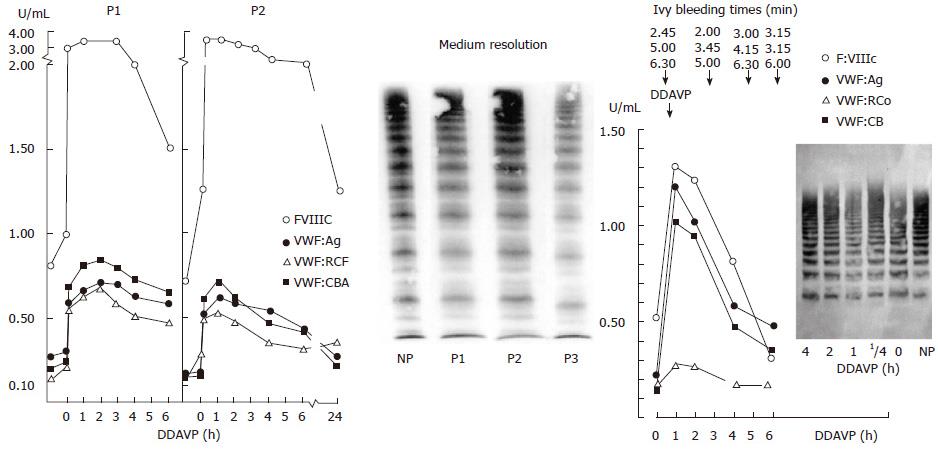
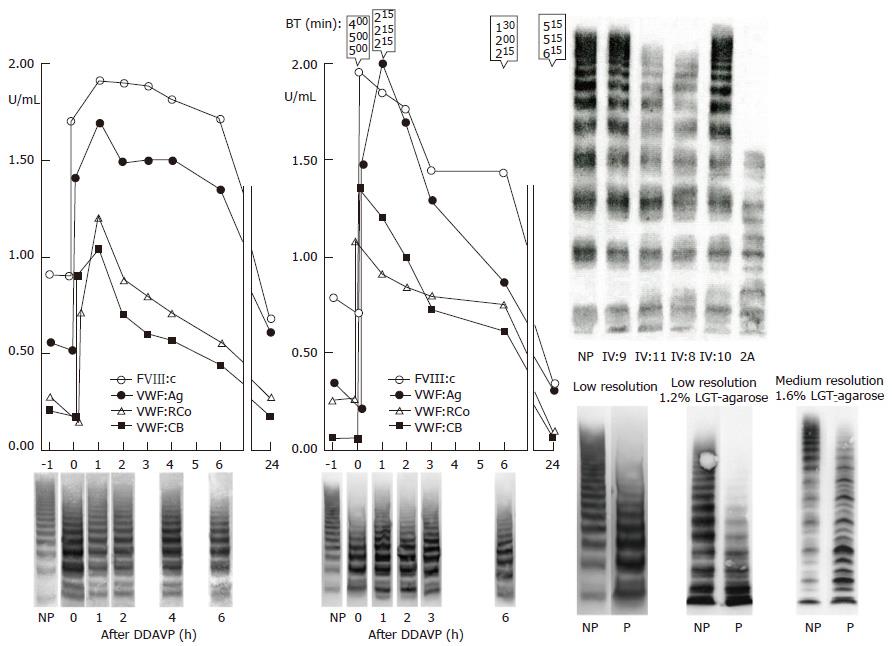
Figure 11 Dominant von Willebrand disease type 2A mutation V1499E is featured by a normal ristocetin-induced platelet aggregation assay.
The loss of largest VWF multimers and increase of intermediate and small VWF multimers in low resolution gels (VWF multimeric pattern before DDAVP, lower left)[43,44]. The responses to DDAVP of FVIII:C and von Willebrand factor antigen (VWF:Ag) are normal. The responses to DDAVP of the functional VWF:RCF and VWF:CB are restricted to about 1 U/mL 1 h post-DDAVP with transient correction of Ivy bleeding times and transient reappearance of large VWF multimers in two cases of moderate dominant VWD type 2A (mutation V1499E). As compared to VWF:Ag and FVIII:C, the half life times of VWF:RCo and VWF:CB are shortened due to increased proteolysis of VWF multimers (Left). Lower right: Please note that the VWF multimers in low resolution gels in the Rotterdam laboratory and in the Hamburg Laboratory (Budde, middle lanes) clearly show the absence of large VWF multimers and no triplet of the individual VWF bands. The typical triplet structure of the individual VWF bands diagnostic for VWD type 2A was only seen in the medium resolution gels (right lanes) according to Budde. Upper right: The multimeric analysis of VWF from affected patients from the large Dutch family with dominant V1499E mutated VWD 2A in a third laboratory (Amsterdam)[43] show the loss of the largest VWF multimers as shown for 2 affected cases (IV:8 and IV:11) as compared to normal (NP) and 2 non-affected family members (IV:9 and IV:10). The loss of large multimers in V1499E mutated VWD patients was less pronounced as compared to a case of typical VWD 2A with the loss of large and some of the intermediate VWF multimers and a typical triplet structure of each VWF band in that laboratory[43]. VWD: Von Willebrand disease; VWF: Von Willebrand factor; DDAVP: Desmopressin; NP: Normal plasma; P: Patient.
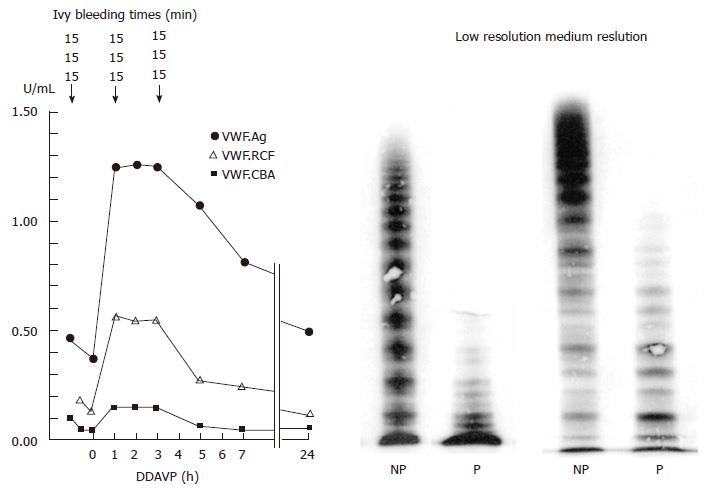
Figure 12 Absence of large and intermediate von Willebrand factor multimers in severe dominant von Willebrand disease type 2 A, with absence of ristocetin-induced platelet aggregation and strongly prolonged Ivy bleeding times in a case with the S1506L mutation[44].
The responses of VWF parameters to DDAVP are very poor with no correction of Ivy bleeding times (BT) and no re-appearance of large VWF multimers in this case with dominant severe VWD 2A Group I indicating impaired assembling, transport and proteolysis of intracellar VWF multimers caused by the mutation S1506L near to the VWF cleavage site in the A2 domain of the VWF gene. VWD: Von Willebrand disease; VWF: Von Willebrand factor; DDAVP: Desmopressin; NP: Normal plasma; P: Patient.
- Citation: Michiels JJ, Batorova A, Prigancova T, Smejkal P, Penka M, Vangenechten I, Gadisseur A. Changing insights in the diagnosis and classification of autosomal recessive and dominant von Willebrand diseases 1980-2015. World J Hematol 2016; 5(3): 61-74
- URL: https://www.wjgnet.com/2218-6204/full/v5/i3/61.htm
- DOI: https://dx.doi.org/10.5315/wjh.v5.i3.61