Copyright
©The Author(s) 2016.
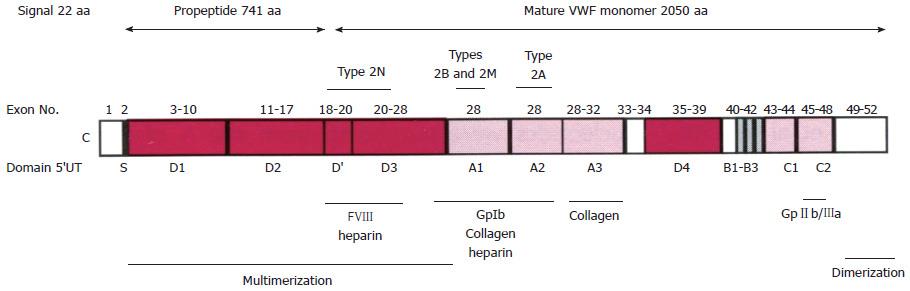
Figure 1 Structure and function relationship of the von Willebrand factor domains[1].
The VWF is synthesized in endothelial cells as a large protein of 2813 amino acid (aa): signal prepetide 22 aa, propeptide 741 aa, and the mature VWF monomer 2050 aa. D1-D2 pro-peptide is cleaved off at the furin cleavage site at time of secretion. VWF circulates bound to the FVIII at the D’ FVIII binding domain. Below the figure are the areas of VWF involved in binding specific factors. VWF circulates as large multimers as a function of the D3 multimerization and CK dimerization domains. Source: Goodeve and Peake[1]. VWD: Von Willebrand disease; VWF: Von Willebrand factor.
- Citation: Michiels JJ, Batorova A, Prigancova T, Smejkal P, Penka M, Vangenechten I, Gadisseur A. Changing insights in the diagnosis and classification of autosomal recessive and dominant von Willebrand diseases 1980-2015. World J Hematol 2016; 5(3): 61-74
- URL: https://www.wjgnet.com/2218-6204/full/v5/i3/61.htm
- DOI: https://dx.doi.org/10.5315/wjh.v5.i3.61