Copyright
©The Author(s) 2016.
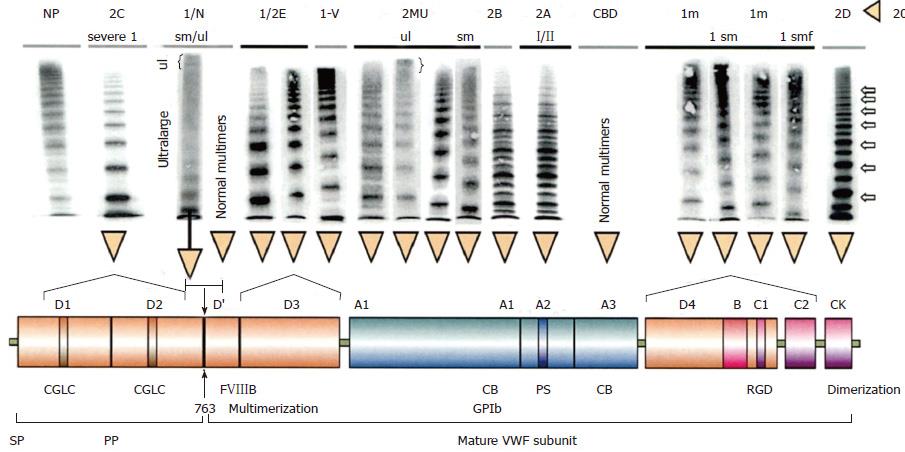
Figure 8 Structure and function of normal von Willebrand factor protein[20].
Mutations in the D1D2 domain prohibit the cleavage of VWFpp from mature VWF leading to a severe secretion and multimerization defects in recessive VWD 2C[16,31,33]. FVIII binding defects in the VWF D’ domain either homozygous or double heterozygous causes recessive VWD 2N[34,35]. Dominant VWD type 2E due to heterozygous missense mutations in the D3 leads to a secretion clearance multimerization defect, VWD 2E[20,38,39]. Loss of function mutations in the VWF GpIb of the A1 domain induce dominant VWD 2M[18,36,37]. Dominant VWD 2A due to mutations in the A2 domain makes the mutant VWF hypersensitive to the VWF cleavage protease ADAMTS13 at the VWF cleavage site (1605-1606)[40-44]. Immediately after secretion the 2A mutated VWF is proteolysed with loss of large VWF multimers and typical triplet structure of each VWF band. Dominant VWD 2B due to gain of function mutation in the A1 domain accelerates the interaction of platelet-GpIb and VWF A1 followed by VWF proteolysis by ADAMTS 13 interaction[17,46]. This process starts immediately after secretion of the 2B mutated VWF and causes VWD 2B with loss of large multimers and typical triplet structure of each VWF band. A new category of VWD type 1 secretion defect (SD) is due to mutations in the D4,B1-3,C1-2[39,49] domains relabelled as the C1, C2, C3, C4, C5 and C6 domains of the VWF gene/protein[3-5]. Heterozygous mutations in the D4, C1-C6 domains result in VWD type 1 SD and have either normal multimers or abnormal multimers. Homozygous or double heterozygous mutations in the D4, C1-C6 domains are associated with severe VWD type 1[26-29]. Cysteine mutations in the CK dimerization domain, either heterozygous and homozygous or double heterozygous, are associated with VWD 2D[30]. VWD: Von Willebrand disease; VWF: Von Willebrand factor; CBD: Collagen binding defect.
- Citation: Michiels JJ, Batorova A, Prigancova T, Smejkal P, Penka M, Vangenechten I, Gadisseur A. Changing insights in the diagnosis and classification of autosomal recessive and dominant von Willebrand diseases 1980-2015. World J Hematol 2016; 5(3): 61-74
- URL: https://www.wjgnet.com/2218-6204/full/v5/i3/61.htm
- DOI: https://dx.doi.org/10.5315/wjh.v5.i3.61