Copyright
©The Author(s) 2016.
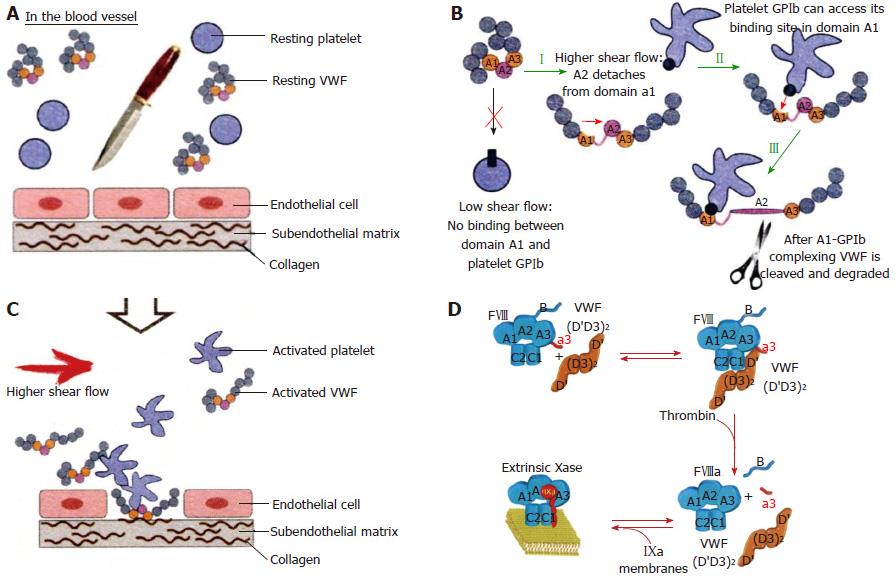
Figure 4 In the equilibrium state, with intact endothelial cells and no injured vessel, resting von Willebrand factor circulates as globules with resting platelets in blood (A).
In this state, VWF is incapable of mediating platelet adhesion. After an injury of the endothelial cells, the activated VWF: Von Willebrand factor (VWF) interacts with exposed collagen via vWF domains A1 and A3 (orange parts) and triggers the adhesion of activated platelets via VWF domain A1 (B, C). At low shear there is no binding between VWF domain A1 and platelet GPIb. At high shear rate the VWF globules elongate and made the VWF A1 domain accessible by the dissociation of domain A1 from A2. High shear flow detaches the A2 domain from domain A1 (I). Binding between GPIb of activated platelet to the GPIb receptor of VWF (II), which is immediately followed by cleavage of VWF in the A2 domain by ADAMTS13 (III) (B). Courtesy of Dr Sandra Posch. Insitute of Biophysics. Linz, Austria: sandra.posch@jku.at. FVIII:C is a heterodimer with a domain structure of A1-A2-B-A3-C1-C2 (upper left, Blue). FVIII:C circulates in complex with VWF through binding to the D’D3 domain, the FVIIIbinding site on VWF. Thrombin cleavage of FVIII liberates the a3 peptide and the B domain of FVIII (D), resulting in the dissociation of VWF from FVIII[6].
- Citation: Michiels JJ, Batorova A, Prigancova T, Smejkal P, Penka M, Vangenechten I, Gadisseur A. Changing insights in the diagnosis and classification of autosomal recessive and dominant von Willebrand diseases 1980-2015. World J Hematol 2016; 5(3): 61-74
- URL: https://www.wjgnet.com/2218-6204/full/v5/i3/61.htm
- DOI: https://dx.doi.org/10.5315/wjh.v5.i3.61