Published online Nov 18, 2012. doi: 10.5312/wjo.v3.i11.167
Revised: October 25, 2012
Accepted: November 1, 2012
Published online: November 18, 2012
Osteoclast differentiation depends on receptor activator of nuclear factor-κB (RANK) signaling, which can be divided into triggering, amplifying and targeting phases based on how active the master regulator nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) is. The triggering phase is characterized by immediate-early RANK signaling induced by RANK ligand (RANKL) stimulation mediated by three adaptor proteins, tumor necrosis factor receptor-associated factor 6, Grb-2-associated binder-2 and phospholipase C (PLC)γ2, leading to activation of IκB kinase, mitogen-activated protein kinases and the transcription factors nuclear factor (NF)-κB and activator protein-1 (AP-1). Mice lacking NF-κB p50/p52 or the AP-1 subunit c-Fos (encoded by Fos) exhibit severe osteopetrosis due to a differentiation block in the osteoclast lineage. The amplification phase occurs about 24 h later in a RANKL-induced osteoclastogenic culture when Ca2+ oscillation starts and the transcription factor NFATc1 is abundantly produced. In addition to Ca2+ oscillation-dependent nuclear translocation and transcriptional auto-induction of NFATc1, a Ca2+ oscillation-independent, osteoblast-dependent mechanism stabilizes NFATc1 protein in differentiating osteoclasts. Osteoclast precursors lacking PLCγ2, inositol-1,4,5-trisphosphate receptors, regulator of G-protein signaling 10, or NFATc1 show an impaired transition from the triggering to amplifying phases. The final targeting phase is mediated by activation of numerous NFATc1 target genes responsible for cell-cell fusion and regulation of bone-resorptive function. This review focuses on molecular mechanisms for each of the three phases of RANK signaling during osteoclast differentiation.
- Citation: Kuroda Y, Matsuo K. Molecular mechanisms of triggering, amplifying and targeting RANK signaling in osteoclasts. World J Orthop 2012; 3(11): 167-174
- URL: https://www.wjgnet.com/2218-5836/full/v3/i11/167.htm
- DOI: https://dx.doi.org/10.5312/wjo.v3.i11.167
Osteoclasts are bone-resorbing cells derived from hematopoietic precursor cells[1-3]. Macrophage-colony stimulating factor (M-CSF) stimulation up-regulates expression of receptor activator of nuclear factor-κB (RANK, encoded by Tnfrsf11a) in the osteoclast precursor cell[4]. RANK, a type I transmembrane receptor with a C-terminal cytosolic tail, is responsible for osteoclast differentiation and function. RANK signaling is induced by RANK ligand (RANKL, encoded by Tnfsf11), which is a type II transmembrane protein (i.e., with a cytoplasmic N-terminus and an extracellular C-terminus). Mice with genetic deletion of Tnfrsf11a or Tnfsf11 lack osteoclasts and exhibit severe osteopetrosis[5,6]. In humans, mutations in genes encoding RANK or RANKL are associated with osteoclast poor, autosomal recessive osteopetrosis[7,8]. RANK signaling is also modified by osteoprotegerin (encoded by Tnfrsf11b), a soluble decoy receptor of RANK that blocks RANKL binding to RANK[9,10].
RANK is a member of the tumor necrosis factor receptor (TNFR) superfamily consisting of 616 and 625 amino acid residues in human and mouse, respectively[11]. RANKL is produced by osteoblasts and osteocytes[12,13] and binds in a trimeric form to RANK, initiating signaling[14,15]. Like other TNFR superfamily members, RANK lacks intrinsic enzymatic activity and transduces intracellular signals by recruiting adaptor proteins including TNFR-associated factors (TRAFs), activating nuclear factor (NF)-κB and downstream mitogen activated protein kinase (MAPK) and Akt signaling[16-18]. RANK exhibits one of the longest cytoplasmic tails of any TNFR superfamily protein, and this domain is responsible for the osteoclast-specific signaling pathway[19,20].
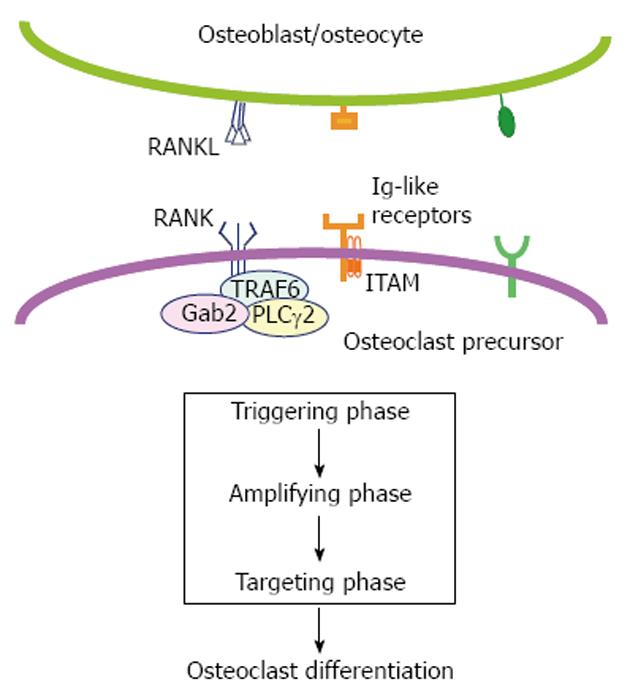
Spatio-temporal control of signaling downstream of RANK[21] is divided into three phases in this review (Figure 1). In the triggering phase, NF-κB, activator protein-1 (AP-1), and MAPKs are rapidly activated within an hour of RANKL stimulation in a culture system[22]. Then, during the amplifying phase, nuclear factor of activated T-cells cytoplasmic 1 (NFATc1, encoded by Nfatc1) begins to accumulate approximately 24 h after RANKL stimulation as cytosolic Ca2+ levels begin to oscillate[23]. Finally, in the targeting phase, RANK signaling regulates multinucleation and bone resorptive function mainly through activation of NFATc1 target genes. Concerted action of RANK and its adaptor proteins as well as immunoreceptors and other co-stimulatory molecules drive these phases. Here we review literature relevant to the molecular mechanism of RANK signaling at each phase during osteoclast differentiation.
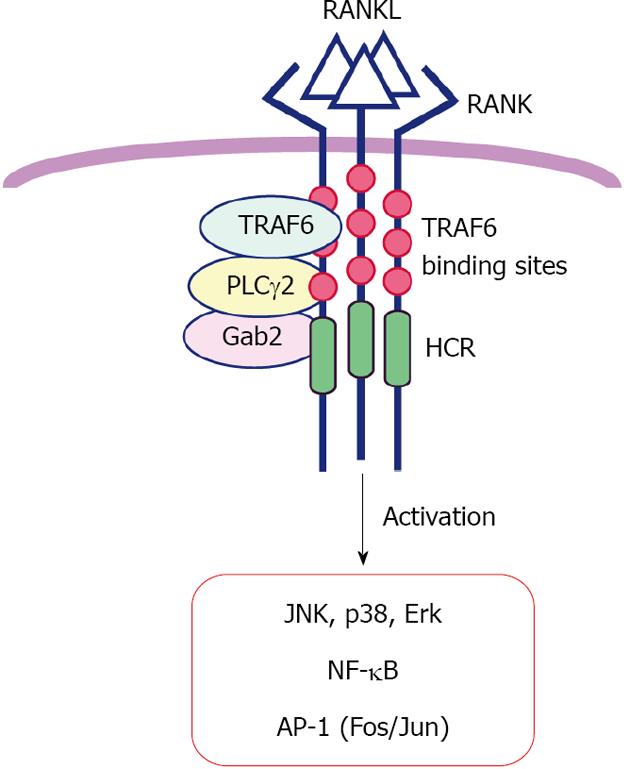
Once homotrimeric RANKL forms complex with its receptor RANK[14,15], a cascade of downstream signaling is initiated. RANK recruits adaptor proteins to specific motifs in its C-terminal cytoplasmic tail, which contains three TRAF6 binding sites near the transmembrane domain, the a highly conserved domain in RANK (HCR) motif, and two binding sites for TRAF2 or TRAF5 near the C-terminus[20] (Figure 2). These motifs have been analyzed using various mutant RANK proteins[16,22]. Inoue and colleagues generated a CD40/RANK chimeric receptor carrying the N-terminal extracellular domain of human CD40 (TNFRSF5) and the cytoplasmic tail of mouse RANK (Tnfrsf11a), which can be specifically activated by anti-CD40 antibody and found that TRAF6 binding sites, but not the HCR, are essential for RANK signaling in the immediate-early phase[16,22]. At least three molecules, TRAF6, Grb-2-associated binder-2 (Gab2) and phospholipase C (PLC)γ2, function as adaptor molecules for RANK. TRAF6 is a really interesting new gene (RING) E3 ubiquitin ligase and Lys63-linked auto-ubiquitination is necessary for the signal transduction to activate IκB kinase and NF-κB during osteoclast differentiation[24]. Mutational analysis of PLCγ2 revealed that catalytic activity of PLCγ2 is dispensable at the triggering phase but necessary for the amplifying phase of RANK signaling[25] (see below). These adaptor proteins activate diverse signaling molecules, phosphatidylinositol-3 kinase, Akt kinase, and MAPKs including c-Jun N-terminal kinase, p38, and extracellular-regulated kinase, leading to activation of the dimeric transcription factors NF-κB and AP-1[20,25,26]. Production of reactive oxygen species via a RANKL-TRAF6-Rac1-nicotinamide adenine dinucleotide phosphate oxidase-dependent pathway is also required for MAPK activation and osteoclastogenesis[27]. In osteoclast lineage cells, NF-κB and AP-1 are composed of two molecules among p65, RelB, p50 and p52 for NF-κB, and c-Fos (also Fra-1, Fra-2 or FosB) and c-Jun (also JunB or JunD) for AP-1. Double knockout mice lacking both p50 and p52 and single knockout mice lacking c-Fos lack osteoclasts and exhibit severe osteopetrosis[28-32]. Mice overexpressing dominant negative c-Jun also develop osteopetrosis[33]. These studies demonstrate the importance of NF-κB and AP-1 activation by RANK signaling in osteoclast differentiation.
The transition from triggering to amplifying phase requires induction of Nfatc1 transcription, which allows cooperation with signaling downstream of immune receptors. NFAT was first identified in nuclear extracts of activated T-cells as a transcription factor that binds to the interleukin-2 (IL-2) promoter[34]. NFAT regulates not only differentiation and activation of immune cells but also the development of tissues such as skeletal muscle, cardiac valve, and bone[35]. Since the promoter of the osteoclast-specific tartrate-resistant acid phosphatase (TRAP) gene carries an evolutionarily conserved AP-1/NFAT binding element similar to the cooperative AP-1/NFAT binding site in the IL-2 promoter, it was hypothesized that c-Fos/AP-1 is required for NFAT function in osteoclasts[36]. It was demonstrated that Nfatc1 itself is a major c-Fos target gene during osteoclast differentiation[37-39]. In cells lacking c-Fos, NF-κB activity is unexpectedly elevated[40], supporting the idea that Fos and Nfatc1 induction is downstream of NF-κB p50 and p52 activation in RANK signaling[41]. It is likely that NF-κB, c-Fos/AP-1 and NFATc2 mediate basal expression of Nfatc1 in preparation for the amplification phase[42].
In concert with RANK signaling, immunoglobulin-like receptors such as osteoclast-associated receptor (OSCAR) and the triggering receptor expressed in myeloid cells (TREM)-2 transduce Nfatc1 induction signals[43,44]. Both are associated with adaptor proteins containing the immunoreceptor tyrosine-based activation motif (ITAM), such as DNAX-activation protein 12 or the Fc receptor common γ subunit[45]. After ITAM tyrosine phosphorylation, a complex containing the tyrosine kinases Bruton’s tyrosine kinase and Tec and the adaptor molecules B cell linker protein and Src homology 2 domain-containing leukocyte protein of 76 kD may facilitate cooperation between RANK and ITAM signaling (Figure 3)[46]. This combined signaling selectively leads to PLCγ phosphorylation, suggesting that integration of RANK and ITAM signaling is required for efficient activation of PLCγ during the amplifying phase. Furthermore, following elevation of intracellular Ca2+ levels, prior to the beginning of Ca2+ oscillation, Nfatc1 transcription is enhanced by Ca2+/calmodulin-dependent kinase IV, which phosphorylates the cAMP response element-binding protein, inducing Fos expression[47].
During the amplifying phase starting approximately 24 h after RANKL stimulation in osteoclastogenic cultures, intracellular Ca2+ levels oscillate, and activate the Ca2+/calmodulin-dependent phosphatase calcineurin, which dephosphorylates NFATc1 and induces its nuclear translocation. On the HCR of the RANK C-terminal tail, PLCγ2 forms a complex with the TRAF6 and Gab2 adapter proteins in a stimulation-dependent manner[20]. An HCR deletion mutant of CD40/RANK chimeric receptor does not alter NF-κB and MAPK activation in the triggering phase but abolishes Ca2+ oscillation, indicating that HCR-mediated signaling is indispensable for continuous PLCγ2 activation.
Both HCR-dependent RANK signaling and ITAM signaling lead to long-term induction of PLCγ2 catalytic activity. PLCγ2 increases intracellular Ca2+ levels by producing inositol-1,4,5-trisphosphate (IP3). Since Ca2+ oscillation during osteoclast differentiation is abolished in IP3 receptor (IP3R) knockout cells, Ca2+ release from endoplasmic reticulum (ER) via IP3Rs is required to generate Ca2+ oscillation[48]. The PLCγ family consists of PLCγ1, which is widely distributed, and PLCγ2, which is primarily limited to hematopoietic cells[49]. PLCγ2 null mice exhibit an osteopetrotic phenotype[25], indicating that PLCγ2, independent of PLCγ1, is required for osteoclastogenesis.
Intracellular Ca2+ levels (approximately 100 nmol/L) are 20 000-fold lower than outside the cell (approximately 2 mmol/L)[50]. Ca2+ oscillation in osteoclasts is tightly controlled by the regulator of G-protein signaling 10 (RGS10)[51]. RGS10 is competitively bound by phosphatidylinositol 3, 4, 5-trisphosphate (PIP3) and Ca2+/calmodulin, and intracellular Ca2+ concentration shifts the balance between RGS10-PIP3 and RGS10-Ca2+/calmodulin complexes[51] (Figure 3). PIP3 is required for membrane localization and subsequent activation of PLCγ2. As the first peak formation of Ca2+ oscillation, PLCγ2 activation induces transient release of Ca2+ from the ER, elevating intracellular Ca2+ concentration (Figure 3, arrows 1 and 2). RGS10 forms a complex with the Ca2+/calmodulin complex and increases levels of free PIP3, further activating PLCγ2 until the intracellular Ca2+ level reaches its peak (Figure 3, arrow 1 and 3). Empty ER Ca2+ stores reload through sooth endoplasmic reticular Ca2+ ATPase, decreasing intracellular Ca2+, increasing RGS10-PIP3, and reducing PLCγ2 activity (Figure 3, arrows 4-6). A repeat of these processes may generate Ca2+ oscillation through oscillatory regulation of PLCγ2 activation[51]. RGS10 knockout mice exhibit severe osteopetrosis caused by a defect in osteoclasts in vivo, indicating that Ca2+ oscillation is a crucial mechanism underlying NFATc1 activation and amplification during osteoclast differentiation[51].
NFATc1 is also activated by an osteoblast-induced Ca2+ oscillation-independent pathway. When osteoclast precursors are co-cultured with osteoblasts, osteoblasts increase NFATc1 levels in osteoclast precursors, and promote osteoclast differentiation even in the presence of the calcineurin inhibitor FK506. Furthermore, wild-type osteoblasts induce differentiation of osteoclast precursors derived from IP3R type 2 and type 3 double knockout mice without detectable RANKL-induced Ca2+ oscillation[48]. Indeed, Cot (cancer osaka thyroid) serine/threonine kinase, also known as tumor progression locus 2, is activated by cell-cell interaction of osteoclasts with osteoblasts and promotes Ca2+ oscillation/calcineurin-independent osteoclastogenesis[52]. Furthermore, Cot increases NFATc1 protein levels through phosphorylation-dependent protein stabilization thereby amplifying NFATc1 activity in the absence of Ca2+ oscillation. Cot likely phosphorylates residues that differ from those targeted by calcineurin-mediated dephosphorylation required for nuclear translocation. At present, the identity of osteoblast-derived molecules that activate Cot in osteoclasts is unknown, but Cot-mediated NFATc1 stabilization clearly contributes to osteoclastogenesis in vivo. Collectively, NFATc1 amplification is achieved by both upregulated expression and enhanced stability.
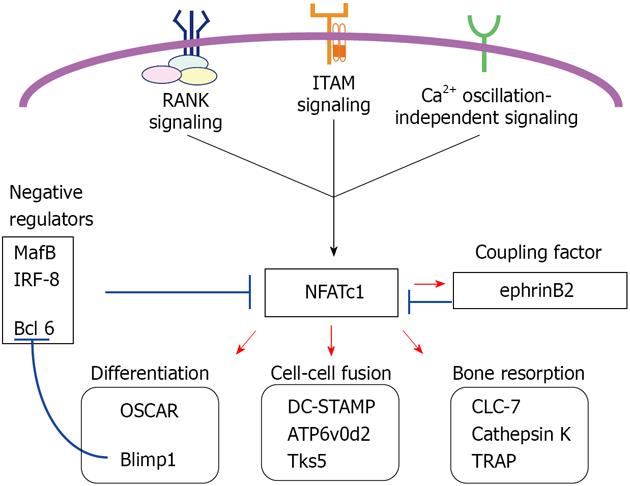
NFATc1 induction and amplification regulate mRNA levels of target genes driving osteoclast differentiation, fusion and function. While forced NFATc1 expression directs osteoclast differentiation, NFATc1-deficient embryonic stem cells fail to differentiate into osteoclasts following RANKL-stimulation[23,42].
In osteoclast differentiation, the immunoglobulin-like receptor OSCAR, but not TREM-2, is an NFATc1 target gene[53,54]. During differentiation, positive regulators of NFATc1 are enhanced while negative regulators are suppressed. The transcriptional repressor B-lymphocyte-induced maturation protein-1 (Blimp1) is induced by RANKL-stimulation and down-regulates three negative regulators: the v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B; interferon regulatory factor-8; and B cell lymphoma 6. All of these proteins repress Nfatc1 transcription[55-58] (Figure 4). Evidence showing that Blimp1 is a direct NFATc1 target[55] suggests that NFATc1 maintains expression of itself via NFATc1/Blimp1 signaling.
NFATc1 target genes encode proteins crucial for osteoclast cell-cell fusion such as a dendritic cell-specific transmembrane protein (DC-STAMP), vacuolar proton pump subunit Atp6v0d2 and the c-Src substrate Tks5 (tyrosine kinase substrate with five SH3 domains)[59-62]. Tks5 appears to be required not only for fusion but for circumferential podosome (actin ring or sealing ring) formation. Following Tks5 knockdown in osteoclasts, multinucleation is abolished although mononuclear osteoclasts still express and amplify NFATc1 in the presence of M-CSF and RANKL[62]. Furthermore, defects of c-Src knockout osteoclasts can be partially rescued by expression of a form of Tks5 carrying glutamate substitutions that mimic constitutive phosphorylation at c-Src phosphorylation target tyrosines[62]. The c-Src-Tks5 axis illustrates an additional signaling pathway induced by RANK signaling beyond NFATc1. In conjunction with ITAM-bearing proteins, c-Src also phosphorylates the tyrosine kinase Syk when integrin αVβ3 is activated by adhesion to bone matrix, in particular, vitronectin[63,64]. In these c-Src-Syk signaling components, integrin β3 and c-Src are NFATc1 target gene products[65,66], suggesting that NFATc1 target genes include those critical for osteoclast-adhesion.
To resorb bone, osteoclasts secrete acid hydrogen chrolide and various hydrolases. Several NFATc1 target genes encode proteins required for acidification and proteolysis, such as the CLC-7 chloride channel (Clc7)[66], a late endosomal/lysosomal chloride channel localizing in ruffled borders, cathepsin K[67], which degrades collagens, and TRAP[23,39], which dephosphorylates the bone matrix phosphoproteins osteopontin and bone sialoprotein. Mice lacking Clc7 or the V0-ATPase subunit a3 show severe osteopetrosis reminiscent of osteoclast-rich osteopetrosis in humans[68,69]. These mice show TRAP-positive osteoclasts with apparently normal NFATc1 amplification. Expression of the calcitonin receptor depends on NFATc1[23,39,53,70], and calcitonin receptor signaling inhibits both osteoclast formation and function independently of transcriptional regulation by RANK signaling[71].
Finally, NFATc1 induces transcription of ephrinB2[72]. Eph receptors and ephrin ligands are increasingly recognized as important in bone biology[73]. Reverse signaling into ephrinB2-expressing osteoclast lineage cells suppresses osteoclast differentiation by downregulating c-Fos and NFATc1, while forward signaling into receptor EphB4-expressing osteoblast lineage cells enhances osteoblastic differentiation and bone formation. Therefore, ephrinB2 is considered as a coupling factor inducible by RANK signaling[73].
In conclusion, RANK signaling appears to be a straightforward transcriptional cascade of “NF-κB/c-Fos induces NFATc1 induces target genes”. Numerous signaling molecules including receptors, adaptors, kinases and lipases reinforce this cascade. Oscillation of intracellular Ca2+ levels drives the cascade, but a Ca2+ oscillation-independent mechanism also contributes to amplification of NFATc1 activity. RANK signaling stimulates the cell-cell fusion machinery (specifically, DC-STAMP and Tks5) and activates proteins located on or secreted from the osteoclast ruffled border (CLC-7 and cathepsin K, respectively). Numerous questions remain unanswered about RANK signaling, such as whether and how RANK signaling is connected to microRNA control[74-77] or to long noncoding RNAs (such as competing endogenous RNAs, or ceRNAs)[78]. Components of the RANK signaling pathway will continue to provide not only topics for investigation but novel therapeutic targets to prevent osteoporosis and other bone loss diseases.
We thank Tsukasa Oikawa, Ichiro Takada and Elise Lamar for critical reading of the manuscript.
Peer reviewer: Shao-Hung Hung, MD, PhD, Associate Professor, Department of Orthopedic Surgery, Fooyin University Hospital, No. 5, Chun-Sun Rd., Tungkang PingTung 928, Taiwan, China
S- Editor Huang XZ L- Editor A E- Editor Xiong L
| 1. | Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2794] [Cited by in RCA: 2791] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 2. | Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1106] [Cited by in RCA: 1106] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 3. | Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1069] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 4. | Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 533] [Cited by in RCA: 542] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412-2424. [PubMed] |
| 6. | Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1300] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 7. | Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop N. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet. 2007;39:960-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 8. | Guerrini MM, Sobacchi C, Cassani B, Abinun M, Kilic SS, Pangrazio A, Moratto D, Mazzolari E, Clayton-Smith J, Orchard P. Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet. 2008;83:64-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 9. | Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309-319. [PubMed] |
| 10. | Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597-3602. [PubMed] |
| 11. | Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1647] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 12. | Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 1281] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 13. | Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 940] [Cited by in RCA: 994] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 14. | Douni E, Rinotas V, Makrinou E, Zwerina J, Penninger JM, Eliopoulos E, Schett G, Kollias G. A RANKL G278R mutation causing osteopetrosis identifies a functional amino acid essential for trimer assembly in RANKL and TNF. Hum Mol Genet. 2012;21:784-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Liu C, Walter TS, Huang P, Zhang S, Zhu X, Wu Y, Wedderburn LR, Tang P, Owens RJ, Stuart DI. Structural and functional insights of RANKL-RANK interaction and signaling. J Immunol. 2010;184:6910-6919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J Biol Chem. 1998;273:28355-28359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 367] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Galibert L, Tometsko ME, Anderson DM, Cosman D, Dougall WC. The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-kappaB, a member of the TNFR superfamily. J Biol Chem. 1998;273:34120-34127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 471] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 19. | Gohda J, Akiyama T, Koga T, Takayanagi H, Tanaka S, Inoue J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005;24:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Taguchi Y, Gohda J, Koga T, Takayanagi H, Inoue J. A unique domain in RANK is required for Gab2 and PLCgamma2 binding to establish osteoclastogenic signals. Genes Cells. 2009;14:1331-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1355] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 22. | Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 386] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1914] [Cited by in RCA: 2031] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 24. | Lamothe B, Besse A, Campos AD, Webster WK, Wu H, Darnay BG. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. J Biol Chem. 2007;282:4102-4112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 305] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 25. | Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Wada T, Nakashima T, Oliveira-dos-Santos AJ, Gasser J, Hara H, Schett G, Penninger JM. The molecular scaffold Gab2 is a crucial component of RANK signaling and osteoclastogenesis. Nat Med. 2005;11:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 752] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 28. | Wang ZQ, Ovitt C, Grigoriadis AE, Möhle-Steinlein U, Rüther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 685] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 29. | Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 979] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 30. | Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482-3496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 819] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 31. | Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 785] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 32. | Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet. 2000;24:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 263] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 33. | Ikeda F, Nishimura R, Matsubara T, Tanaka S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest. 2004;114:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 744] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 35. | Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1497] [Cited by in RCA: 1553] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 36. | Matsuo K, Tonko M, Wagner EF. Why do c-Fos deficient mice lack osteoclasts? J Bone Miner Res. 1996;11 Suppl 1:M377. |
| 37. | Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem. 2002;277:41147-41156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 319] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 38. | Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 543] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 39. | Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279:26475-26480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 445] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 40. | Ray N, Kuwahara M, Takada Y, Maruyama K, Kawaguchi T, Tsubone H, Ishikawa H, Matsuo K. c-Fos suppresses systemic inflammatory response to endotoxin. Int Immunol. 2006;18:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245-18253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 348] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 42. | Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 703] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 43. | Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198:645-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 45. | Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 645] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 46. | Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med. 2006;12:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 48. | Kuroda Y, Hisatsune C, Nakamura T, Matsuo K, Mikoshiba K. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc Natl Acad Sci U S A. 2008;105:8643-8648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Wilde JI, Watson SP. Regulation of phospholipase C gamma isoforms in haematopoietic cells: why one, not the other? Cell Signal. 2001;13:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Clapham DE. Calcium signaling. Cell. 2007;131:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2811] [Cited by in RCA: 3164] [Article Influence: 186.1] [Reference Citation Analysis (0)] |
| 51. | Yang S, Li YP. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev. 2007;21:1803-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Kuroda Y, Hisatsune C, Mizutani A, Ogawa N, Matsuo K, Mikoshiba K. Cot kinase promotes Ca2+ oscillation/calcineurin-independent osteoclastogenesis by stabilizing NFATc1 protein. Mol Cell Biol. 2012;32:2954-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem. 2005;280:32905-32913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Kim K, Kim JH, Lee J, Jin HM, Lee SH, Fisher DE, Kook H, Kim KK, Choi Y, Kim N. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem. 2005;280:35209-35216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 55. | Nishikawa K, Nakashima T, Hayashi M, Fukunaga T, Kato S, Kodama T, Takahashi S, Calame K, Takayanagi H. Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proc Natl Acad Sci USA. 2010;107:3117-3122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 56. | Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109:3253-3259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 57. | Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med. 2009;15:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 58. | Miyauchi Y, Ninomiya K, Miyamoto H, Sakamoto A, Iwasaki R, Hoshi H, Miyamoto K, Hao W, Yoshida S, Morioka H. The Blimp1-Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med. 2010;207:751-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 59. | Yagi M, Ninomiya K, Fujita N, Suzuki T, Iwasaki R, Morita K, Hosogane N, Matsuo K, Toyama Y, Suda T. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J Bone Miner Res. 2007;22:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 61. | Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrinol. 2008;22:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 62. | Oikawa T, Oyama M, Kozuka-Hata H, Uehara S, Udagawa N, Saya H, Matsuo K. Tks5-dependent formation of circumferential podosomes/invadopodia mediates cell-cell fusion. J Cell Biol. 2012;197:553-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 63. | Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, Ginsberg MH, Ross FP, Teitelbaum SL. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 64. | Chambers TJ, Fuller K. How are osteoclasts induced to resorb bone? Ann N Y Acad Sci. 2011;1240:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP. NFATc1 regulation of the human beta3 integrin promoter in osteoclast differentiation. Gene. 2006;372:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Song I, Kim JH, Kim K, Jin HM, Youn BU, Kim N. Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett. 2009;583:2435-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, Hisatake K, Nogi Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem. 2004;279:45969-45979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 348] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 68. | Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000;25:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 466] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 69. | Scimeca JC, Franchi A, Trojani C, Parrinello H, Grosgeorge J, Robert C, Jaillon O, Poirier C, Gaudray P, Carle GF. The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone. 2000;26:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Anusaksathien O, Laplace C, Li X, Ren Y, Peng L, Goldring SR, Galson DL. Tissue-specific and ubiquitous promoters direct the expression of alternatively spliced transcripts from the calcitonin receptor gene. J Biol Chem. 2001;276:22663-22674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Granholm S, Lundberg P, Lerner UH. Calcitonin inhibits osteoclast formation in mouse haematopoetic cells independently of transcriptional regulation by receptor activator of NF-{kappa}B and c-Fms. J Endocrinol. 2007;195:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 570] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 73. | Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr. 2012;6:148-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 74. | Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 470] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 75. | Sugatani T, Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J Biol Chem. 2009;284:4667-4678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 76. | Mizoguchi F, Izu Y, Hayata T, Hemmi H, Nakashima K, Nakamura T, Kato S, Miyasaka N, Ezura Y, Noda M. Osteoclast-specific Dicer gene deficiency suppresses osteoclastic bone resorption. J Cell Biochem. 2010;109:866-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 77. | Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood. 2011;117:3648-3657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 78. | Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1874] [Cited by in RCA: 2163] [Article Influence: 154.5] [Reference Citation Analysis (0)] |