Published online Jul 18, 2025. doi: 10.5312/wjo.v16.i7.107045
Revised: April 14, 2025
Accepted: May 27, 2025
Published online: July 18, 2025
Processing time: 125 Days and 23.4 Hours
Degenerative disc disease (DDD) is characterized by the loss of nucleus pulposus cells (NPCs). Inducing differentiation of bone marrow mesenchymal stem cells (MSCs) into NPCs has emerged as a novel therapeutic strategy for DDD. Ho
To investigate the role and mechanism of miR-365 in promoting the differentiation of MSCs into NPCs for DDD treatment.
In vitro, the effects of miR-365 on MSC proliferation, apoptosis, and differentiation were assessed by cell counting kit-8 assay, flow cytometry, and quantitative real-time polymerase chain reaction (qRT-PCR). In vivo, the expression levels of miR-365, HIF-1α, Sox9, Kdm6a, and HOXA9 in the spinal cord of rats with spinal cord injury were determined by qRT-PCR and Western blot.
In vitro, miR-365 significantly promoted MSC proliferation and inhibited MSC apoptosis. The expression levels of glycosaminoglycans, proteoglycan, and type 2 collagen were significantly increased with miR-365 ectopic expression. In vivo, the expression levels of miR-365, HIF-1α, Sox9, and Kdm6a were significantly increased, whereas HOXA9 was remarkably decreased. Mechanically, miR-365 inhibited HOXA9 expression by directly binding to its 3’ untranslated region. HOXA9 could inhibit HIF-1α expression by binding to the Hif-1α promoter, thereby affecting the expression levels of Sox9 and Kdm6a. Moreover, HOXA9 knockdown significantly reversed the differentiation of MSCs into NPCs induced by miR-365.
miR-365 promotes HOXA9-mediated differentiation of MSCs into NPCs by interacting with HIF-1α and may serve as a potential target for DDD treatment.
Core Tip: This study investigates the role of miR-365 in promoting the differentiation of bone marrow mesenchymal stem cells (MSCs) into nucleus pulposus cells (NPCs) for degenerative disc disease (DDD) treatment. We reveal that miR-365 enhances MSC proliferation and inhibits apoptosis, while promoting NPC-related marker expression. Mechanistically, miR-365 targets HOXA9, which in turn regulates HIF-1α, Sox9, and Kdm6a. This finding highlights miR-365 as a potential therapeutic target for DDD, offering a novel strategy to address the loss of NPCs.
- Citation: Zhi ZZ, Liu T, Kang J, Zhou FC, Liu XD, He ZM. miR-365 promotes HOXA9-mediated differentiation of mesenchymal stem cells to nucleus pulposus cells by interacting with HIF-1α. World J Orthop 2025; 16(7): 107045
- URL: https://www.wjgnet.com/2218-5836/full/v16/i7/107045.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i7.107045
Intervertebral disc (IVD) degeneration is a prevalent phenomenon associated with the aging process. Degenerative disc disease (DDD), which frequently accompanies IVD degeneration, has emerged as one of the principal etiologies of chronic low back pain[1]. This condition is increasingly recognized as a significant socioeconomic burden. The overall prevalence of DDD is estimated to be around 10% in men by the age of 50, and this figure can rise to as high as 50% by the age of 70[1]. Even more concerning, some studies suggest that the proportion of the population affected by DDD could be as high as 90%, with many individuals remaining asymptomatic[2].
The onset of DDD is a complex phenomenon influenced by a variety of factors, including genetic predispositions, mechanical stress, and environmental exposures. These diverse pathways converge on a common outcome: An imbalance in the extracellular matrix (ECM) anabolic and catabolic processes, favoring catabolism[3]. This imbalance ultimately leads to disc bulging, a reduction in the nucleus pulposus (NP) and its water content, and a subsequent decrease in disc height[4]. The NP, a highly hydrated tissue that forms the inner core of the IVD, is particularly susceptible to dege
MicroRNAs (miRNAs), approximately 22 nucleotides (nt) in length, exert a pivotal influence on gene regulation. These small non-coding RNA molecules typically bind to the 3’ untranslated region (3’UTR) of target messenger RNA (mRNA) molecules, thereby inhibiting their translation or inducing degradation[8]. These small non-coding RNAs are integral to a wide array of biological pathways. Their dysregulation has been linked to numerous pathological conditions, including cancer, cardiovascular diseases, osteoarthritis, and IVD degeneration[9]. Notably, an increasing body of evidence suggests that miRNAs are frequently dysregulated during the progression of DDD[10]. Furthermore, miRNAs are known to be involved in the precise modulation of gene expression during the differentiation of osteoblasts and chondrocytes[11]. However, there is a relative scarcity of research focusing on the role of miRNAs in the differentiation of mesenchymal stem cells (MSCs) into NPCs.
Here we investigated the function and underlying mechanisms of miR-365 in DDD therapy, and we found that miR-365 promotes HOXA9-mediated differentiation of MSCs into NPCs by interacting with HIF-1α. The development of miR-365-based therapeutic strategies holds considerable potential for the treatment of DDD, suggesting a promising avenue for future research and clinical applications.
All experimental procedures involving animals were rigorously executed in full compliance with the protocols stipulated by the Animal Care and Use Committee. Ethical approval for this study was granted under the committee's approval number WTPZ20230612001. To establish a rat model of DDD, we utilized a modified Allen's percussion method, which induces spinal cord injury (SCI) via epidural impingement[12]. Following the experimental protocols, the mice were humanely euthanized at specified time points within 2 weeks as the recovery from the injury tends to stabilize[13]. Their spinal cord segments were carefully isolated for further analysis.
The number of samples in this study was decided upon by referring to earlier research and conducting a statistical power analysis. Research on SCI models in the past has pointed out that, in order to spot significant differences in motor function and histological results with a power of 80% and a significance level set at 0.05, a minimum of six animals per group are needed[14]. Accordingly, we chose to use six rats per group in our study, which we believe is enough to provide the statistical power needed to identify significant differences between the SCI and sham groups. Additionally, we carried out preliminary experiments to confirm that this sample size can ensure the reliability and reproducibility of our findings.
Human bone MSCs, sourced from the National Collection of Authenticated Cell Cultures (CAS, China), were cultured in MesenPRO RS medium (Thermo Fisher Scientific) under hypoxic conditions to induce differentiation. Human NPCs, obtained from Sunncell, China, were maintained in NPC culture media (Sunncell). Co-culture of MSCs and NPCs was performed in transwell system with NPCs in the upper compartment under hypoxic conditions (3% O2) for 48 hours.
The coding sequence of the Hoxa9 gene was amplified and then subcloned into the pCMV-tag 3A vector (Thermo Fisher Scientific) at the BamHI and HindIII restriction sites, thereby constructing the Hoxa9 overexpression plasmid. The potential binding sites between miR-365 and Hoxa9 were identified through bioinformatics analysis using the TargetScan website (www.targetscan.org). Fragments of the human Hoxa9 3’-UTR, encompassing the miR-365 binding sites, were amplified by PCR in both wild type and mutant forms and then cloned into the pGL3-Basic vector for subsequent studies.
For the transfection experiments, MSCs were plated in a 12-well plate and allowed to grow until they achieved 70%–80% confluence. The transfection was performed using the Lipofectamine 3000 reagent (Thermo Fisher Scientific) following the manufacturer’s protocol. Initially, the MSCs were transfected with the indicated miRNA mimic, miRNA antisense, siRNA (listed in Table 1), or plasmid. The RNA molecules were applied at a concentration of 20 nm, whereas the plasmid DNA was utilized at a concentration of 0.5 μg per well. The Lipofectamine 3000 reagent was combined with either the RNA or the plasmid and subsequently added to the cells to initiate the transfection process. Following a 48-hour incubation period post-transfection, the cells were harvested for a range of downstream analyses. These analyses encompassed quantitative polymerase chain reaction (qPCR) to evaluate gene expression levels, immunoblotting to assess protein expression, and staining with annexin-V and propidium iodide (PI) to determine cell viability and apoptosis.
| qPCR primer | Sequence |
| miR-365 forward primer | TAATGCCCCTAAAAATCCTTAT |
| miR-365 reverse primer | CAAGCAGAAGACGGCATACG |
| Sox9 forward primer | AGAATGAGAGCGGCGGAGACAA |
| Sox9 reverse primer | CTCTTTCTCCAGTTCCAGGGTC |
| Kdm6a forward primer | AGCGCAAAGGAGCCGTGGAAAA |
| Kdm6a reverse prime | GTCGTTCACCATTAGGACCTGC |
| Hoxa9 forward primer | AGAATGAGAGCGGCGGAGACAA |
| Hoxa9 reverse primer | CTCTTTCTCCAGTTCCAGGGTC |
| Hif-1α forward primer | TATGAGCCAGAAGAACTTTTAGGC |
| Hif-1α reverse primer | CACCTCTTTTGGCAAGCATCCTG |
| β-actin forward primer | CACCATTGGCAATGAGCGGTTC |
| β-actin reverse primer | AGGTCTTTGCGGATGTCCACGT |
| siRNA mimics | Sequence |
| NC-mimic-sense | UUUGUACUACACAAAAGUACUG |
| NC-mimic-antisense | CAGUACUUUUGUGUAGUACAAA |
| miR-365-mimic-sense | UAUUCCUAAAAAUCCCCGUAAU |
| miR-365-mimic-antisense | AUUACGGGGAUUUUUAGGAAUA |
| siRNAs | Sequence (dT, deoxythymidine) |
| siNC-sense | GGCUCUAGAAAAGCCUAUGC (dTdT) |
| siNC-antisense | GCAUAGGCUUUUCUAGAGCC (dTdT) |
| siHoxa9-sense | AGAAUGAGAGCGGCGGAGAC (dTdT) |
| siHoxa9-antisense | GUCUCCGCCGCUCUCAUCU (dTdT) |
Isolation and purification of total RNA from the samples were accomplished utilizing Trizol reagent (Invitrogen). The purified RNA was then dissolved in RNase-free H2O. The integrity and quantity of the extracted RNA were determined by diluting 1 μL of the RNA sample and measuring its absorbance at 260 nm and 280 nm via spectrophotometry. Based on these absorbance readings, the RNA concentration and purity were computed and appraised. For the reverse transcription procedure, 2 μg of total RNA was employed. Utilizing M-MLV Reverse Transcriptase (Promega) along with random hexamer primers, the RNA was converted into single-stranded cDNA. This cDNA was subsequently utilized as the template for qPCR, which was performed using the FastStart Universal SYBR Green Master (Roche) on the Applied Biosystems 7900 Real-Time PCR System. The relative expression levels of the target gene were normalized to the expression level of the housekeeping gene actin. The comparative Ct method (2−△△Ct) was employed to determine the fold change in gene expression. The sequences of the qPCR primers are provided in Table 1.
MSCs were seeded into a 24-well plate at a density of 5 × 104 cells per well. After the respective treatments were applied, the cells' proliferative activity was evaluated at the end of the indicated experimental periods using the CCK-8 assay kit (Beyotime Biotechnology), following the manufacturer's instructions. The absorbance of each well was then measured at 450 nm using a microplate reader (Thermo Fisher Scientific).
A total of 1 × 105 cells per well were plated into a 6-well plate and allowed to adhere overnight. Following the designated treatments, the cells were harvested and subjected to staining with the annexin-V and PI staining kit (Beyotime Biotechnology), following the manufacturer's protocol. The stained cells were then analyzed via flow cytometry using a BD FACSAria III instrument.
To analyze the protein expression levels, total proteins were extracted and purified using RIPA reagent (Beyotime Biotechnology). The concentration of purified proteins was determined using BCA Protein Assay Kit (Thermo Fisher Scientific). For the Western blot analysis, 20 μg of each protein was loaded onto an SDS-polyacrylamide gel. The proteins were then separated by molecular weight through electrophoresis. Following electrophoresis, the proteins were transferred onto a PVDF membrane (Millipore). The membrane was blocked to reduce non-specific binding by incubating it with 5% bovine serum albumin (BSA), followed by incubation with primary antibodies against type 2 collagen (1:2000), HOXA9 (1:2000) from Abcam, aggrecan (1:1000) from Thermo Fisher Scientific, HIF-1α (1:2000) from Cell Signaling Technology, and Actin (1:10000) from Sigma-Aldrich, and then incubation with AffiniPure-conjugated corresponding secondary antibody (Sigma-Aldrich) (1:1000-5000). Finally, the proteins of interest were visualized on the membrane using an enhanced ECL kit (Thermo Fisher Scientific). The expression level of Actin, a housekeeping protein, was used as an internal control to normalize the protein expression levels.
Glycosaminoglycans (GAGs) were determined by colorimetric method with Total GAGs Assay Kit (NEB) according to the manufacturer’s protocol.
MSCs were cultured in 10 cm dishes until they achieved the desired confluence. Before UV irradiation, the cells were thoroughly washed twice with ice-cold PBS to eliminate any residual culture medium. After removing the PBS, the cells were subjected to UV irradiation at a wavelength of 254 nm and a dosage of 400 mJ/cm² using a Stratalinker 1800 (Stratagene), while being maintained on ice to mitigate heat-induced damage. Subsequently, 500 μL of lysis buffer was added. The buffer consisted of 0.5 mmol/L DTT, 137 mmol/L NaCl, 1% Nonidet P-40, 20 mmol/L Tris-HCl (pH 7.5), 2 mmol/L EDTA, 100 U/mL ribonuclease inhibitor (Takara), and 1 × proteinase inhibitor cocktail (Roche). The mixture was incubated on ice for 10 minutes to facilitate cell lysis. Subsequently, the lysates were centrifuged at 12000 g for 10 minutes at 4 °C, and the resulting supernatant was collected for further processing.
For the immunoprecipitation process, 20 μL of protein A/G magnetic bead suspension (Thermo Fisher Scientific) was initially cleansed with lysis buffer to eliminate any impurities. Following this, the purified beads were combined with 2 μg of specific antibodies—either anti-HOXA9 or anti-Pol II (Santa Cruz Biotechnology)—in a 200 μL volume of lysis buffer, and the mixture was allowed to incubate at 4 °C for a duration of 4 hours. Once the incubation was complete, the beads, now bound with antibodies, underwent three successive washes with lysis buffer to strip away any proteins that were not specifically bound. Subsequently, the beads were incubated with the cell lysate supernatant for an extended period at 4 °C, aiming to capture DNA fragments that were associated with the proteins of interest. To ensure specificity, the beads were subjected to three additional washes with lysis buffer to remove any nonspecifically adhered substances, thereby finalizing their preparation for the subsequent DNA extraction step. To facilitate DNA extraction, the beads were re-suspended in a buffer consisting of 400 μL 100 mmol/L Tris-HCl (pH 7.5), 50 mmol/L NaCl, and 10 mmol/L EDTA, supplemented with 10 mg/mL protease K (Millipore). This suspension was incubated at 37 °C for 30 minutes to degrade proteins. Subsequent to protease K digestion, DNA was purified using the phenol-chloroform extraction method. The purified DNA, derived from both input and ChIP samples, was then employed for quantitative PCR analysis, utilizing primers designed to amplify specific promoter regions of the Hif-1α gene (forward primer, 5’-TACTTGAGTTCATATCACCTG-3’; reverse primer, 5’-CGCATTTCATTCACTCATCAT-3’).
Statistical analyses were conducted using GraphPad Prism software. Measurement data are presented as the median. The statistical significance of differences was evaluated using the two-tailed unpaired Student’s t-test. A P-value of less than 0.05 was considered to indicate statistical significance.
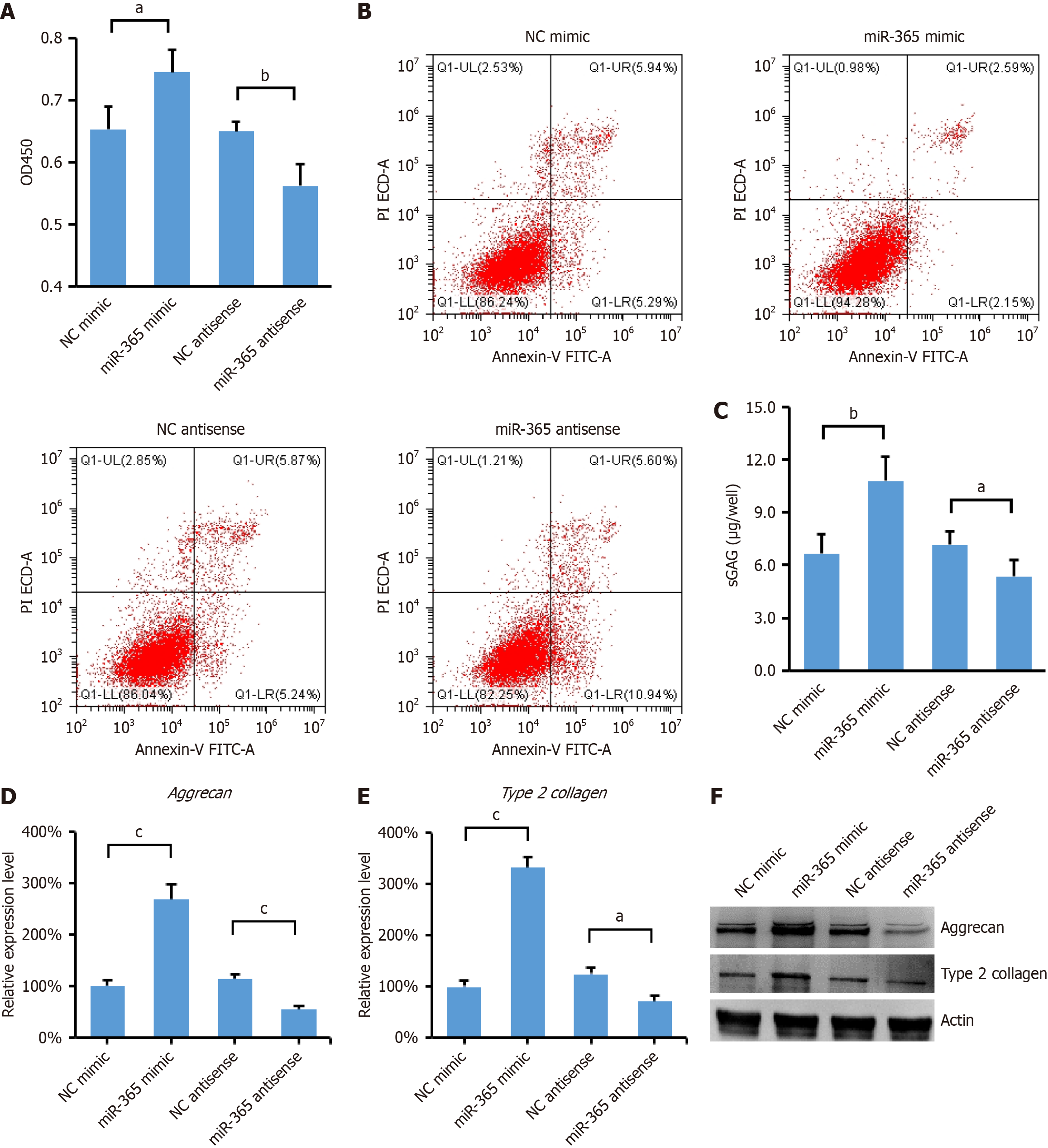
miR-365 has been recognized as a critical regulator in the context of chondrocyte hypertrophy and differentiation[15], but the function of miR-365 in MSCs remains to be elucidated. To explore this, we conducted experiments where MSCs were transfected with miR-365 mimic or antisense inhibitor to overexpress or knock down miR-365, respectively. As shown in Figure 1A, overexpression of miR-365 Led to a significant increase in the proliferation of MSCs, whereas downregulation of miR-365 with antisense inhibitor resulted in inhibition of cell proliferation. Moreover, we observed that the overexpression of miR-365 also significantly reduced cell apoptosis. In contrast, treatment with miR-365 antisense inhibitor led to an increase in apoptotic cells (Figure 1B).
It has been reported that NPC conditioned medium promotes differentiation of MSCs into NPCs, particularly under hypoxic conditions[16]. To investigate the role of miR-365 in this process, we examined the production of GAGs, which are key components of the ECM and are indicative of the differentiation ability of MSCs towards an NPC phenotype. As shown in Figure 1C, overexpression of miR-365 significantly enhanced the production of GAGs, suggesting a pro-differentiation effect. Conversely, inhibition of miR-365 expression resulted in a decrease in GAGs, indicating that miR-365 is crucial for the differentiation process. Given the critical role of the ECM in the mechanical functionality of the IVD, we further focused on the expression of aggrecan and type II collagen, two major components of the IVD ECM. The results show that miR-365 overexpression upregulated the mRNA and protein levels of aggrecan and type2 collagen (Figure 1D-F). These data indicate that miR-365 plays a pivotal role in promoting the differentiation of MSCs into NPCs in a coculture system under hypoxic conditions.
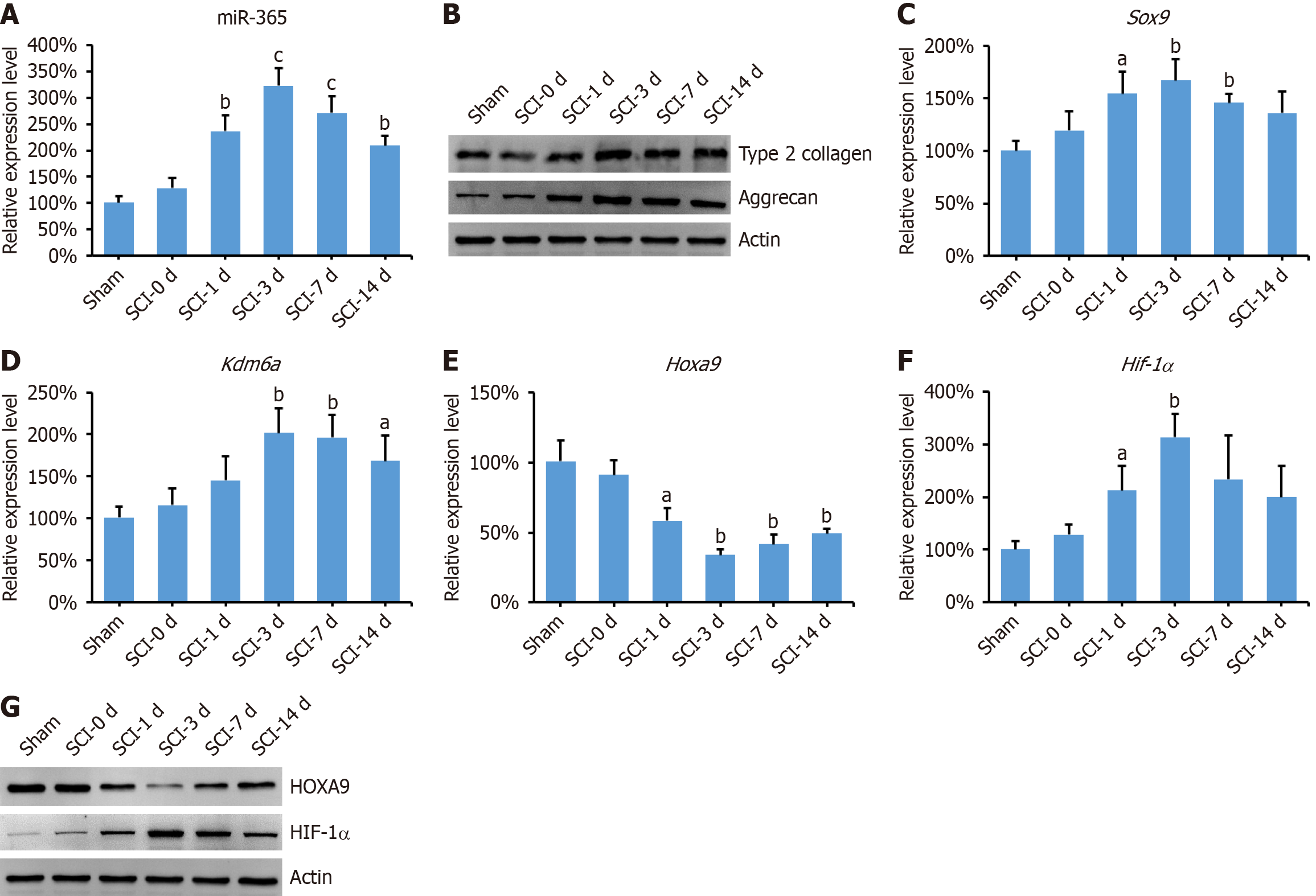
Next, we analyzed the expression levels of miR-365 in the spinal cord of rats subjected to SCI. As shown in Figure 2A, miR-365 exhibited a significant increase following SCI, with peak expression observed at day 3 post-injury. This upregulation of miR-365 was accompanied by an upregulation in the expression levels of aggrecan and type2 collagen (Figure 2B). Furthermore, we investigated the expression of Sox9 and Kdm6a, both of which are known to play pivotal roles in the chondrogenic differentiation of MSCs[17]. Our findings revealed a remarkable increase in the expression levels of both Sox9 and Kdm6a in the spinal cord of SCI rats (Figure 2C and D). In addition, HOXA9—a molecule directly targeted by miR-365—exhibited notable downregulation post-SCI, with its levels inversely correlating with those of miR-365 (Figure 2E-G). The transcription factor HIF-1α is acknowledged for its significant influence on cellular hypoxia adaptation and its involvement in the differentiation of MSCs[18]. Here we found that the HIF-1α was significantly increased in the spinal cord of SCI rats (Figure 2F and G).
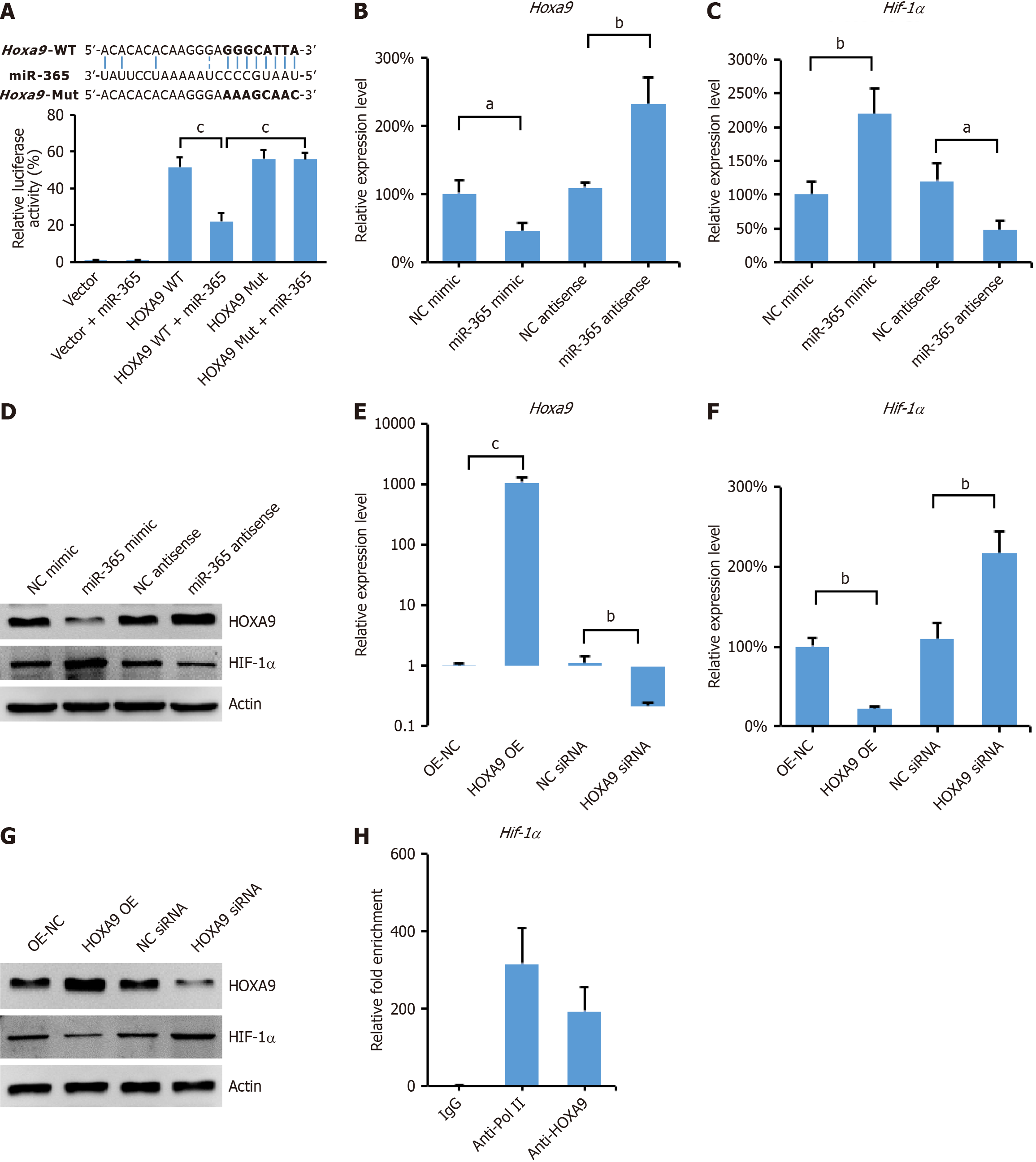
To elucidate the molecular mechanism by which miR-365 regulates the differentiation of MSCs into NPCs, we utilized bioinformatics tools from the TargetScan website to predict potential targets of miR-365. To validate this interaction, we cloned the Hoxa9 3'-UTR sequence, including the predicted miR-365 binding site, into a luciferase reporter vector. This construct was designated as Hoxa9-3'UTR-WT (wild type). Additionally, a mutated version of the Hoxa9 3'-UTR, termed Hoxa9-3'UTR-Mut, was created to disrupt the miR-365 binding site. Both constructs were used in a dual-luciferase reporter assay system (Figure 3A). The results indicated that transfection with a miR-365 mimic substantially decreased luciferase activity in cells harboring the Hoxa9-3'UTR-WT reporter. However, this effect was not observed with the Hoxa9-3'UTR-Mut reporter in MSCs (Figure 3A). This finding provides strong evidence that miR-365 directly binds to the 3'-UTR of Hoxa9 mRNA, thereby inhibiting its expression. Moreover, we observed that overexpression of miR-365 led to a decrease in both the mRNA and protein levels of HOXA9, while simultaneously increasing the levels of HIF-1α in MSCs (Figure 3B-D). Conversely, the expression of a miR-365 inhibitor had the opposite effect, further supporting the role of miR-365 in regulating HOXA9 and HIF-1α expression (Figure 3B-D).
It has been reported that HOXA9 can repress the expression of HIF-1α by directly binding to its promoter regions, as observed in cutaneous squamous cell carcinoma[19]. Consistent with this, our findings revealed an inverse correlation between the expression levels of HOXA9 and HIF-1α (Figure 3B-D), suggesting a potential regulatory interaction between these two genes. To further dissect the regulatory function of HOXA9 and HIF-1α, we constructed a plasmid to overexpress HOXA9 and designed small interfering RNA (siRNA) to knockdown its expression (Figure 3E). Our results showed that overexpression of HOXA9 led to a decrease in both the mRNA and protein levels of HIF-1α, whereas knockdown of HOXA9 resulted in an increase in HIF-1α expression (Figure 3F and G). To validate the direct interaction between HOXA9 and the Hif-1α promoter, we performed ChIP assays followed by qPCR. The ChIP assays confirmed the binding of HOXA9 protein to the promoter region of the Hif-1α gene in MSCs (Figure 3H). These data indicate that miR-365 functions as a negative regulator of HOXA9 expression. By modulating HOXA9, miR-365 indirectly regulates the expression of HIF-1α in MSCs, thereby potentially influencing the differentiation of MSCs into NPCs under hypoxic conditions.
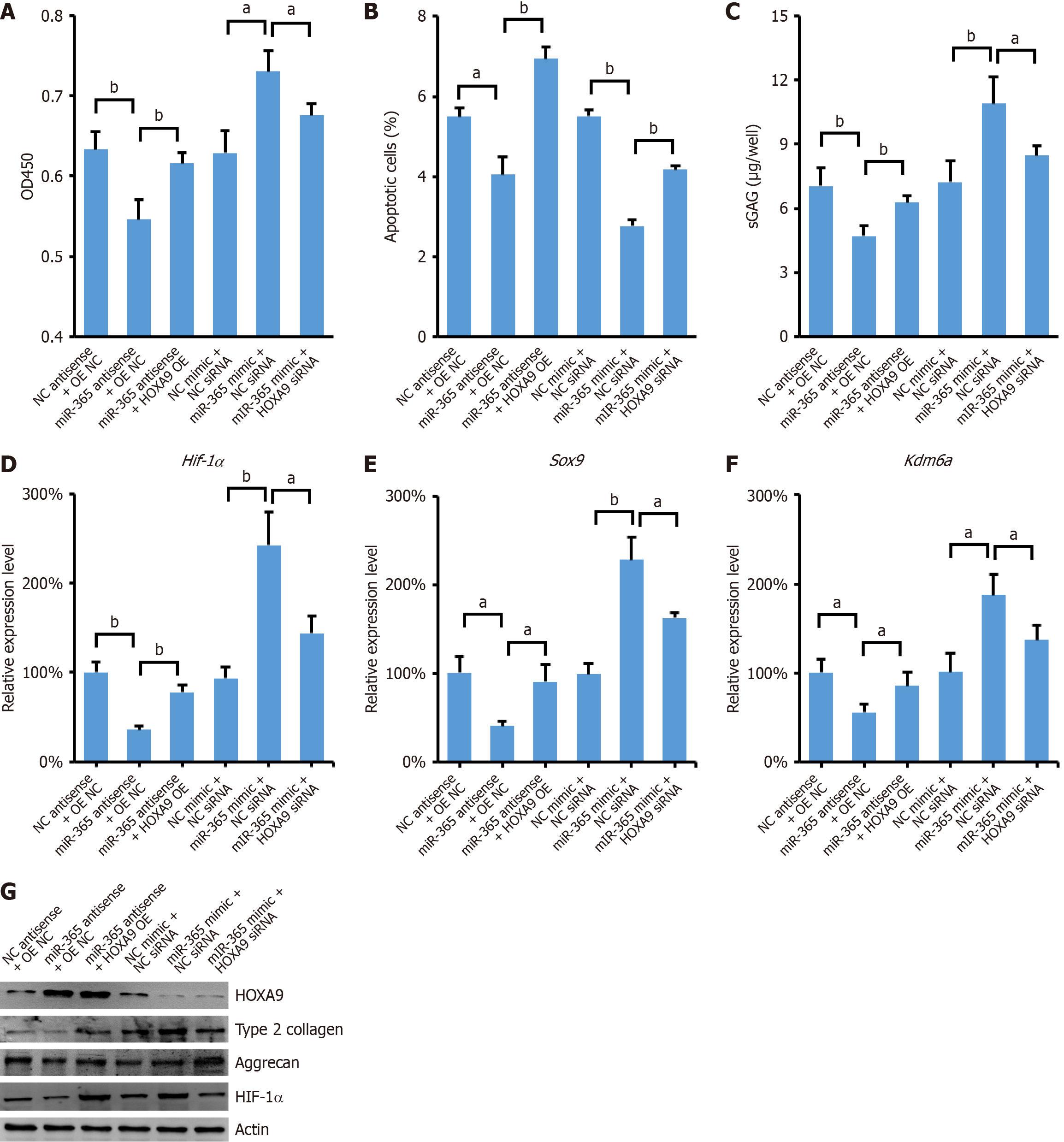
To further validate the role of miR-365/HOXA9/HIF-1α axis in differentiation of MSCs to NPCs, we conducted experiments to assess the impact of miR-365 overexpression in MSCs that were also treated with Hoxa9 siRNA. Our findings demonstrated that the overexpression of miR-365 significantly promoted cell proliferation in MSCs. However, this proliferative effect was abrogated by the knockdown of HOXA9 (Figure 4A). Conversely, the suppression of proliferation observed with the expression of miR-365 antisense inhibitor was rescued by the overexpression of HOXA9 (Figure 4A). Additionally, the increase in apoptotic cells, typically associated with reduced proliferation, was also reversed by the ectopic expression of HOXA9 (Figure 4B). In line with these observations, the expression levels of GAGs, HIF-1α, Sox9, and Kdm6a were coordinately regulated as miR-365/HOXA9/HIF-1α axis (Figure 4C-G) (Supplementary material).
In this study, we have elucidated a critical role for miR-365 in promoting differentiation of MSCs into NPCs. Our research has uncovered a novel molecular mechanism by which miR-365 exerts its effects. We demonstrated that miR-365 directly targets the 3’UTR of Hoxa9 mRNA, leading to the inhibition of HOXA9 expression. The downregulation of HOXA9, in turn, relieves its transcriptional repression of HIF-1α. Moreover, HOXA9 knockdown significantly reversed the differentiation of MSCs into NPCs induced by miR-365 overexpression. Our findings reveal a previously unknown function of miR-365 in the regulation of MSC differentiation, highlighting its potential as a therapeutic target for interventions aimed at modulating the behavior of MSCs, particularly in the context of IVD and other related conditions.
Currently, the therapeutic landscape for restoring degenerated IVDs is limited, primarily consisting of pain management, reduced physical activities, and surgical interventions. There is a pressing need to develop regenerative medicine-based strategies that can effectively heal and repair injured discs. In this context, cellular therapy has emerged as a promising alternative for IVD management. A key factor in disc degeneration is believed to be a decrease in the number of NPCs. Transplanting autologous NPCs has shown potential in inhibiting further disc degeneration in vivo[20], suggesting that replenishing the depleted NPC population could be a viable approach. Studies have demonstrated that NPC transplantation can partially restore disc height, indicating a positive impact on the structural integrity of the IVD[21]. Moreover, the transplantation of allogenic NPCs has been shown to attenuate IVD degeneration by inhibiting apoptosis and promoting cell migration, further supporting the therapeutic potential of cellular interventions in this area[22]. The use of NPCs in 3D tissue engineering complexes represents an innovative strategy for IVD repair, leveraging the cells' natural ability to regenerate and maintain the disc's ECM[23]. However, the clinical application of NPCs faces significant challenges, primarily due to the difficulty in retrieving a sufficient number of NPCs and the complexities associated with their in vitro expansion and culture[24]. These hurdles have limited the number of studies that have been able to assess the therapeutic efficacy of NPCs in the treatment of disc degeneration[25].
Despite these challenges, the potential of cellular therapy, particularly NPC transplantation, remains a compelling area of research. Continued advancements in cell isolation techniques, culture methods, and tissue engineering strategies could pave the way for more effective treatments for IVD degeneration.
MSCs, which can be isolated from the autologous source, are increasingly considered as an alternative treatment opinion for DDD. This interest stems from their extensive renewal potential, multilineage differentiation capacity, and immunosuppressive properties[26]. These characteristics make MSCs a promising cell source for regenerative therapies in the context of IVD degeneration. Several in vivo studies have demonstrated that the transplantation of MSCs into IVDs can lead to the regeneration of disc cells. Notably, autogenous MSCs derived from the vertebral body have been shown to enhance IVD regeneration through paracrine interactions with NPCs or annulus fibrosus cells[27], suggesting that MSCs can integrate into the disc microenvironment and stimulate a regenerative response. Pre-conditioning MSCs under hypoxic culture conditions has also been identified as a crucial factor. This approach has been shown to increase MSC proliferation, enhance their plasticity, and extend their survival upon transplantation into the IVD[28]. A pilot study assessing the feasibility and safety of MSC treatment in patients with DDD showed promising results. Patients exhibited rapid improvement in pain and disability, with 85% of maximum improvement achieved within three months, approaching 71% of optimal efficacy[29]. Moreover, co-culture systems involving MSCs and NP cells have demonstrated that direct cell-to-cell contact is essential for guiding MSC differentiation towards an NP-like phenotype[30]. Type 2 collagen and aggrecan, key components of the IVD ECM, play significant roles in the communication between NPCs and MSCs in co-culture[31]. Our findings also indicate that co-culturing MSCs with NPCs can effectively elevate the expression of type II collagen and aggrecan in MSCs, thereby inducing MSC differentiation into NPCs. This effect is particularly pronounced with the ectopic expression of miR-365, suggesting a synergistic role of miR-365 in facilitating MSC-to-NPC differentiation.
Although miR-365 has been recognized for its role in promoting oncogenesis and metastasis in certain cancers[32,33], its function in the differentiation of MSCs remains less explored. Recent studies have begun to shed light on this miRNA's potential in the chondrogenic differentiation of MSCs. For instance, miR-365 expression is activated upon chondrogenic induction, and its transfection into osteoarthritis-derived MSCs has been shown to induce the expression of chondrogenic and osteogenic genes[34]. Guan et al[35] further elucidated the mechanistic role of miR-365 in chondrocyte differentiation, demonstrating that it directly targets histone deacetylase 4 (Hdac4) by binding to its 3’UTR region. Another study corroborated these findings, showing that overexpression of miR-365 can promote cartilage regeneration from MSCs by inhibiting Hdac4 expression[15]. In this study, we have extended these insights to identify miR-365 as an efficient non-coding RNA that regulates the differentiation of MSCs into NPCs. We found that miR-365 targets Hoxa9, leading to the disinhibition of HIF-1α. This regulatory axis is particularly significant as HOXA9 has been shown to inhibit HIF-1α-mediated glycolysis and repress the development of cutaneous squamous cell carcinoma by interacting with the cAMP-responsive element-binding protein 2[19]. Our results discovered a new miR-365/HOXA9/HIF-1α axis for MSC differentiation and subsequently DDD cell therapy. A recent study also highlighted the role of miR-365 in IVD degeneration, but via a different mechanism involving EFNA3[36]. In that study, miR-365 was found to regulate the expression of EFNA3, which subsequently affected the differentiation and function of NPCs. This finding underscores the multifaceted role of miR-365 in IVD biology.
Our study suggests that miR-365 may be a candidate target for DDD treatment. However, the delivery of miRNA to the musculoskeletal system is challenging due to the need for efficient and targeted delivery systems[37]. Recent advances in the use of lipid nanoparticles (LNPs) for RNA delivery offer a promising approach to overcome these challenges. LNPs have been successfully used to deliver siRNAs and miRNA to various tissues, including those in the musculoskeletal system[38,39]. The development of LNP-based delivery systems for miR-365 could potentially enhance the therapeutic efficacy of miR-365 in treating DDD. Such delivery systems would need to be designed to specifically target the IVD tissue, ensuring that miR-365 is delivered to the site of degeneration. Future studies should focus on optimizing LNP formulations for miR-365 delivery, evaluating their safety and efficacy in preclinical models, and exploring the potential for clinical translation.
In conclusion, we have found that miR-365 promotes HOXA9-mediated differentiation of MSCs into NPCs by interacting with HIF-1α. Advancements in the development of efficient stem cell techniques, including the ectopic expression of miR-365, have shown promise in directing stem cells toward specific fates. These developments are crucial for the adoption of a stem cell-based regenerative approach to treating DDD.
| 1. | Kos N, Gradisnik L, Velnar T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med Arch. 2019;73:421-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 2. | Teraguchi M, Yoshimura N, Hashizume H, Muraki S, Yamada H, Minamide A, Oka H, Ishimoto Y, Nagata K, Kagotani R, Takiguchi N, Akune T, Kawaguchi H, Nakamura K, Yoshida M. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage. 2014;22:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 3. | Scarcia L, Pileggi M, Camilli A, Romi A, Bartolo A, Giubbolini F, Valente I, Garignano G, D'Argento F, Pedicelli A, Alexandre AM. Degenerative Disc Disease of the Spine: From Anatomy to Pathophysiology and Radiological Appearance, with Morphological and Functional Considerations. J Pers Med. 2022;12:1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 4. | Hemanta D, Jiang XX, Feng ZZ, Chen ZX, Cao YW. Etiology for Degenerative Disc Disease. Chin Med Sci J. 2016;31:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Li G, Song Y, Liao Z, Wang K, Luo R, Lu S, Zhao K, Feng X, Liang H, Ma L, Wang B, Ke W, Yin H, Zhan S, Li S, Wu X, Zhang Y, Yang C. Bone-derived mesenchymal stem cells alleviate compression-induced apoptosis of nucleus pulposus cells by N6 methyladenosine of autophagy. Cell Death Dis. 2020;11:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Muresanu C, Somasundaram SG, Neganova ME, Bovina EV, Vissarionov SV, Ofodile ONFC, Fisenko VP, Bragin V, Minyaeva NN, Chubarev VN, Klochkov SG, Tarasov VV, Mikhaleva LM, Kirkland CE, Aliev G. Updated Understanding of the Degenerative Disc Diseases - Causes Versus Effects - Treatments, Studies and Hypothesis. Curr Genomics. 2020;21:464-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Sivakamasundari V, Lufkin T. Stemming the Degeneration: IVD Stem Cells and Stem Cell Regenerative Therapy for Degenerative Disc Disease. Adv Stem Cells. 2013;2013:724547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Shang R, Lee S, Senavirathne G, Lai EC. microRNAs in action: biogenesis, function and regulation. Nat Rev Genet. 2023;24:816-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 394] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 9. | Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451-5465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1431] [Cited by in RCA: 1334] [Article Influence: 222.3] [Reference Citation Analysis (0)] |
| 10. | Wang C, Cui L, Gu Q, Guo S, Zhu B, Liu X, Li Y, Liu X, Wang D, Li S. The Mechanism and Function of miRNA in Intervertebral Disc Degeneration. Orthop Surg. 2022;14:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Iaquinta MR, Lanzillotti C, Mazziotta C, Bononi I, Frontini F, Mazzoni E, Oton-Gonzalez L, Rotondo JC, Torreggiani E, Tognon M, Martini F. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics. 2021;11:6573-6591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 12. | Wang XH, Jiang C, Zhang YY, Chen Z, Wang ZY, Yang H, Hao DJ. Analysis and comparison of a spinal cord injury model with a single-axle-lever clip or a parallel-moving clip compression in rats. Spinal Cord. 2022;60:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Zhang M, Bai Y, Xu C, Lin J, Jin J, Xu A, Lou JN, Qian C, Yu W, Wu Y, Qi Y, Tao H. Novel optimized drug delivery systems for enhancing spinal cord injury repair in rats. Drug Deliv. 2021;28:2548-2561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Wu X, Wei H, Wu JQ. Coding and long non-coding gene expression changes in the CNS traumatic injuries. Cell Mol Life Sci. 2022;79:123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Chen J, Wu X. Cyclic tensile strain promotes chondrogenesis of bone marrow-derived mesenchymal stem cells by increasing miR-365 expression. Life Sci. 2019;232:116625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Sinkemani A, Wang F, Xie Z, Chen L, Zhang C, Wu X. Nucleus Pulposus Cell Conditioned Medium Promotes Mesenchymal Stem Cell Differentiation into Nucleus Pulposus-Like Cells under Hypoxic Conditions. Stem Cells Int. 2020;2020:8882549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Wang P, Li Y, Meng T, Zhang J, Wei Y, Meng Z, Lin Y, Liu D, Sui L. KDM6A promotes chondrogenic differentiation of periodontal ligament stem cells by demethylation of SOX9. Cell Prolif. 2018;51:e12413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Jiang C, Sun J, Dai Y, Cao P, Zhang L, Peng S, Zhou Y, Li G, Tang J, Xiang J. HIF-1A and C/EBPs transcriptionally regulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia. Stem Cell Res Ther. 2015;6:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Zhou L, Wang Y, Zhou M, Zhang Y, Wang P, Li X, Yang J, Wang H, Ding Z. HOXA9 inhibits HIF-1α-mediated glycolysis through interacting with CRIP2 to repress cutaneous squamous cell carcinoma development. Nat Commun. 2018;9:1480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN Jr. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine (Phila Pa 1976). 2002;27:1626-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Chaofeng W, Chao Z, Deli W, Jianhong W, Yan Z, Cheng X, Hongkui X, Qing H, Dike R. Nucleus pulposus cells expressing hBMP7 can prevent the degeneration of allogenic IVD in a canine transplantation model. J Orthop Res. 2013;31:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Wang W, Deng G, Qiu Y, Huang X, Xi Y, Yu J, Yang X, Ye X. Transplantation of allogenic nucleus pulposus cells attenuates intervertebral disc degeneration by inhibiting apoptosis and increasing migration. Int J Mol Med. 2018;41:2553-2564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Stich S, Stolk M, Girod PP, Thomé C, Sittinger M, Ringe J, Seifert M, Hegewald AA. Regenerative and immunogenic characteristics of cultured nucleus pulposus cells from human cervical intervertebral discs. PLoS One. 2015;10:e0126954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Kushioka J, Kaito T, Chijimatsu R, Okada R, Ishiguro H, Bal Z, Kodama J, Takenaka S, Makino T, Sakai Y, Yoshikawa H. A novel and efficient method for culturing mouse nucleus pulposus cells. Spine J. 2019;19:1573-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Williams RJ, Tryfonidou MA, Snuggs JW, Le Maitre CL. Cell sources proposed for nucleus pulposus regeneration. JOR Spine. 2021;4:e1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Drazin D, Rosner J, Avalos P, Acosta F. Stem cell therapy for degenerative disc disease. Adv Orthop. 2012;2012:961052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Shim EK, Lee JS, Kim DE, Kim SK, Jung BJ, Choi EY, Kim CS. Autogenous Mesenchymal Stem Cells from the Vertebral Body Enhance Intervertebral Disc Regeneration via Paracrine Interaction: An in Vitro Pilot Study. Cell Transplant. 2016;25:1819-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Samanta A, Lufkin T, Kraus P. Intervertebral disc degeneration-Current therapeutic options and challenges. Front Public Health. 2023;11:1156749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 29. | Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 30. | Strassburg S, Hodson NW, Hill PI, Richardson SM, Hoyland JA. Bi-directional exchange of membrane components occurs during co-culture of mesenchymal stem cells and nucleus pulposus cells. PLoS One. 2012;7:e33739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Lehmann TP, Jakub G, Harasymczuk J, Jagodziński PP. Transforming growth factor β mediates communication of co-cultured human nucleus pulposus cells and mesenchymal stem cells. J Orthop Res. 2018;36:3023-3032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Zhou M, Liu W, Ma S, Cao H, Peng X, Guo L, Zhou X, Zheng L, Guo L, Wan M, Shi W, He Y, Lu C, Jiang L, Ou C, Guo Y, Ding Z. A novel onco-miR-365 induces cutaneous squamous cell carcinoma. Carcinogenesis. 2013;34:1653-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Zhan B, Lu D, Luo P, Wang B. Prognostic Value of Expression of MicroRNAs in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Clin Lab. 2016;62:2203-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Hu N, Gao Y, Jayasuriya CT, Liu W, Du H, Ding J, Feng M, Chen Q. Chondrogenic induction of human osteoarthritic cartilage-derived mesenchymal stem cells activates mineralization and hypertrophic and osteogenic gene expression through a mechanomiR. Arthritis Res Ther. 2019;21:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Guan YJ, Yang X, Wei L, Chen Q. miR-365: a mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 2011;25:4457-4466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Jiang C, Liu Y, Zhao W, Yang Y, Ren Z, Wang X, Hao D, Du H, Yin S. microRNA-365 attenuated intervertebral disc degeneration through modulating nucleus pulposus cell apoptosis and extracellular matrix degradation by targeting EFNA3. J Cell Mol Med. 2024;28:e18054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Vorrius B, Qiao Z, Ge J, Chen Q. Smart Strategies to Overcome Drug Delivery Challenges in the Musculoskeletal System. Pharmaceuticals (Basel). 2023;16:967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 38. | Hassett KJ, Benenato KE, Jacquinet E, Lee A, Woods A, Yuzhakov O, Himansu S, Deterling J, Geilich BM, Ketova T, Mihai C, Lynn A, McFadyen I, Moore MJ, Senn JJ, Stanton MG, Almarsson Ö, Ciaramella G, Brito LA. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol Ther Nucleic Acids. 2019;15:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 543] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 39. | Khan T, Weber H, DiMuzio J, Matter A, Dogdas B, Shah T, Thankappan A, Disa J, Jadhav V, Lubbers L, Sepp-Lorenzino L, Strapps WR, Tadin-Strapps M. Silencing Myostatin Using Cholesterol-conjugated siRNAs Induces Muscle Growth. Mol Ther Nucleic Acids. 2016;5:e342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |