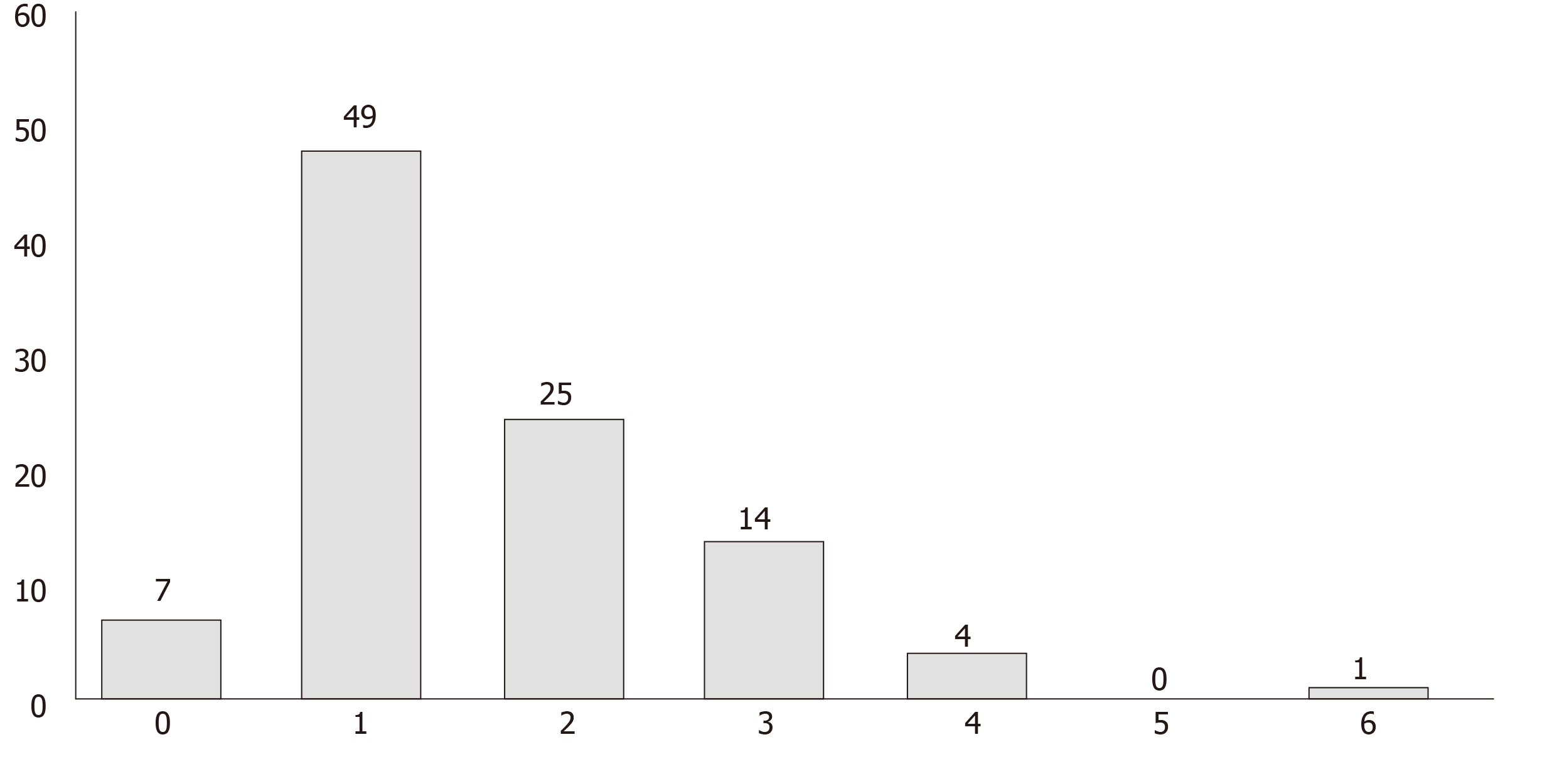Published online May 18, 2020. doi: 10.5312/wjo.v11.i5.252
Peer-review started: December 20, 2019
First decision: February 20, 2020
Revised: March 1, 2020
Accepted: April 4, 2020
Article in press: April 4, 2020
Published online: May 18, 2020
Processing time: 149 Days and 17.2 Hours
Surgical site infections are a major cause of morbidity and mortality following orthopedic surgery. Recent efforts to identify sources of contamination in the operating rooms have implicated mobile phones.
To investigate microbial colonization on the mobile phones of health care professionals in the orthopedic operating room.
We conducted a cross-sectional study involving culture and sensitivity analysis of swabs taken from the mobile phones of orthopedic and anesthesia attendings, residents, technicians and nurses working in the orthopedic operating rooms over a period of two months. Demographic and cell phone related factors were recorded using a questionnaire and the factors associated with contamination were analyzed.
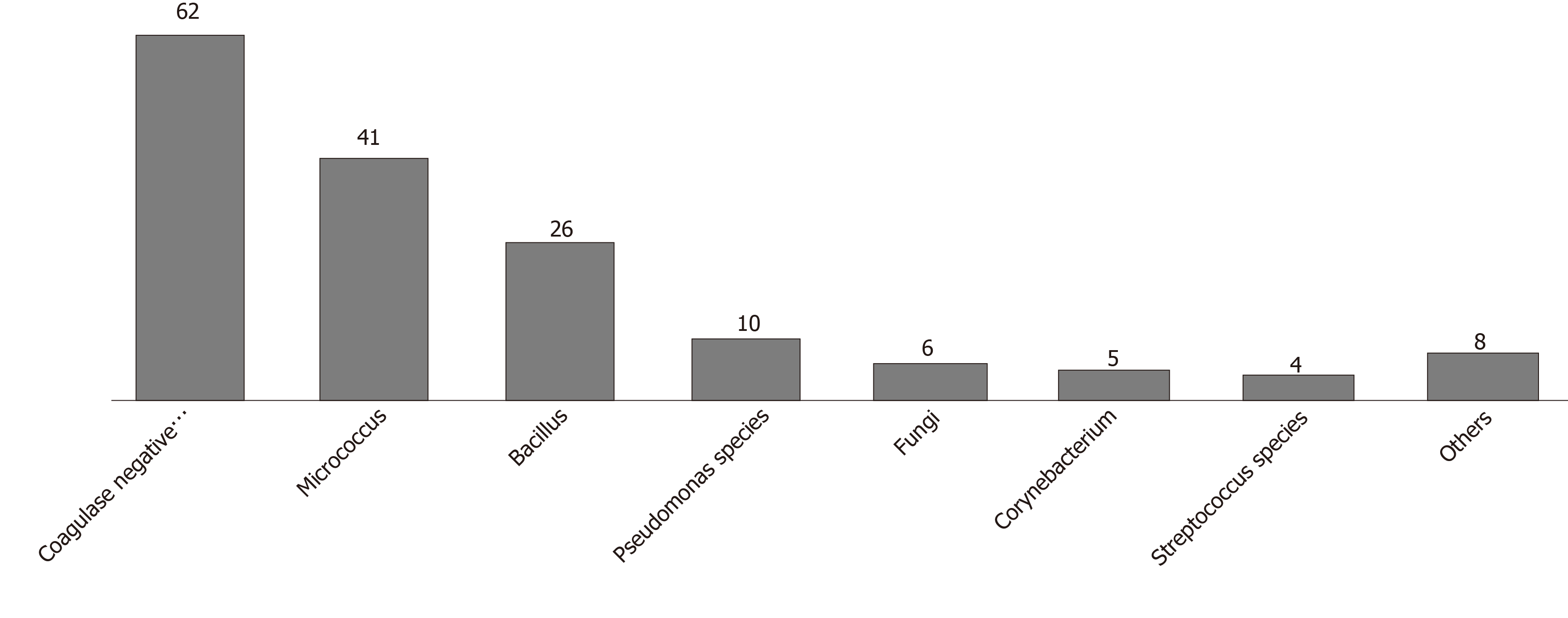
Ninety-three of 100 mobile phones were contaminated. Species isolated were Coagulase-negative Staphylococcus (62%), Micrococcus (41%) and Bacillus (26%). The risk of contamination was increased with mobile covers and cracked screens and decreased by cell phone cleaning.
Mobile phones belonging to health care workers are frequently contaminated with pathogenic bacteria with the potential of transferring drug resistance to nosocomial pathogens. Studies investigating the relationship to surgical site infections need to be conducted. The concept of “mobile hygiene” involving the change of mobile covers, replacement of cracked screens or even wiping the phone with an alcohol swab could yield the cost-effective balance that contaminated cell phones deserve until they are established as a direct cause of surgical site infections.
Core tip: Mobile phones have been implicated in contamination in the operating room. We cultured samples from the phones of 100 health care professionals in orthopedic operating rooms at a tertiary care hospital in Pakistan. Ninety-three percent of the phones were colonized by potentially pathogenic bacteria. The most common cause of implant related infections, Coagulase-negative Staphylococci, were isolated from 62 mobile phones, 54% of which were resistant to oxacillin. The risk of contamination increased with mobile covers and cracked screens and decreased with cleaning using an alcohol swab. These findings indicate the use of “mobile hygiene” until a relationship with surgical site infections is established.
- Citation: Qureshi NQ, Mufarrih SH, Irfan S, Rashid RH, Zubairi AJ, Sadruddin A, Ahmed I, Noordin S. Mobile phones in the orthopedic operating room: Microbial colonization and antimicrobial resistance. World J Orthop 2020; 11(5): 252-264
- URL: https://www.wjgnet.com/2218-5836/full/v11/i5/252.htm
- DOI: https://dx.doi.org/10.5312/wjo.v11.i5.252
Each year, approximately $3.5 to $10 billion is spent on managing surgical site infections (SSIs) in the United States[1]. Data show that the incidence of SSIs is between 2% and 5%, with 60% of them being preventable[2]. Although the incidence can vary greatly as assessed using the National Nosocomial Infections Surveillance risk index[3], orthopedic surgery employs more robust sterile measures and has a lower rate of post-operative SSIs, particularly following elective surgery such as joint arthroplasty and hand surgery[4].
Nevertheless, given the consequent high morbidity, longer hospital stay, readmissions and revision procedures which increase health care costs, SSIs continue to be a cause for concern[5,6]. In 2012, according to the National Surgical Quality Improvement Program of the American College of Surgeons, SSIs were the most common cause of unplanned readmissions in 346 US hospitals[7]. It is estimated that by 2020 there will be at least 70000 total hip and knee arthroplasty revision surgeries due to deep SSIs, at a cost of US $1.62 billion annually[7].
Not surprisingly, these alarming numbers have led to countless efforts by health care professionals to identify and reduce the sources and risk factors of SSIs. Studies have identified numerous sources implicated in the transmission of pathogenic microbes including air[8], hospital surfaces[9], liquid nitrogen freezers[10], computer keyboards[11], stethoscopes[12], staff uniforms[13], tourniquets[14] and even leaving sterile trays open for too long[15].
A common source of contamination in the operating room seems to be the mobile phones used by health care professionals in all areas of the hospital[16,17]. Many studies have demonstrated the colonization of mobile or cell phones by potentially pathogenic organisms such as Coagulase-negative Staphylococcus (CoNS) species, Staphylococcus aureus and Acetinobacter[18-20]. Murgier et al[21] evaluated orthopedic operating rooms and added Corynebacterium and Viridans streptococci to the list.
While the transmission of infection through these contaminated mobile phones via direct and indirect routes is a possibility[18], the use of cell phones by health care professionals is a current and relevant reality, and further data are required to devise a protocol for their use in health care settings[18]. Surveys have revealed that not only are they considered an important work and academic tool by individual professionals[22], but cell phones have been shown to facilitate rapid communication between team members of the health care system[18,23]. Having an application in various clinical and educational scenarios including medical imaging[24], tele-microscopy[25], and improving patient-doctor communication[26,27], mobile phones have become indispensable.
Therefore, the aim of our study was to assess the extent of microbial colonization and drug resistance on the mobile phones of health care professionals in our hospital. Our goal was to estimate the proportion of health care workers including orthopedic and anesthesia attendings, residents, technicians and nursing staff working in the orthopedic operating room of our hospital with contaminated mobile phones. It is hoped that our findings will shed light on the need and cost-effectiveness of decontamination and/or restrictions on the use of mobile phones in operating rooms.
We used the STROBE checklist for observational studies[28] to report the data.
This was a cross-sectional study involving the collection of sample swabs from the mobile phones of hospital staff members and the administration of a structured questionnaire after obtaining informed written consent. Samples were collected in the orthopedic operating rooms and the adjacent recovery room of our university hospital over a period of two months between 0800 and 1700 h for 5-d a week excluding weekends.
All orthopedic and anesthesia attendings, residents and technicians and all nursing staff working in the orthopedic operating room and/or the recovery room met the inclusion criteria. The only exclusion criteria were visiting staff members and medical students. The orthopedic attendings, residents and technicians were directly involved in the surgery and had physical contact with the patient. The anesthesia attendings, residents and technicians also had direct contact at the time of intubation and extubation. The nursing staff was in contact with the patient after surgery in the recovery room.
A team of two researchers collected the data. One researcher obtained consent and administered the questionnaire while the other researcher collected the sample swabs. The questionnaire was structured to obtain brief demographic information and details of mobile phone and hand hygiene.
All study participants provided informed written consent prior to study enrolment. Written consent was obtained after providing both a verbal explanation and written material regarding the study. Participants were ensured of their anonymity and E-mail addresses of the participants who wished to be informed of the results of the culture from the swab sample were recorded separately. Each participant was then asked to place his/her cell phone on a clean surface for collection of the swab while the questionnaire was administered.
The participants were asked to fill out a structured questionnaire typed out in English.
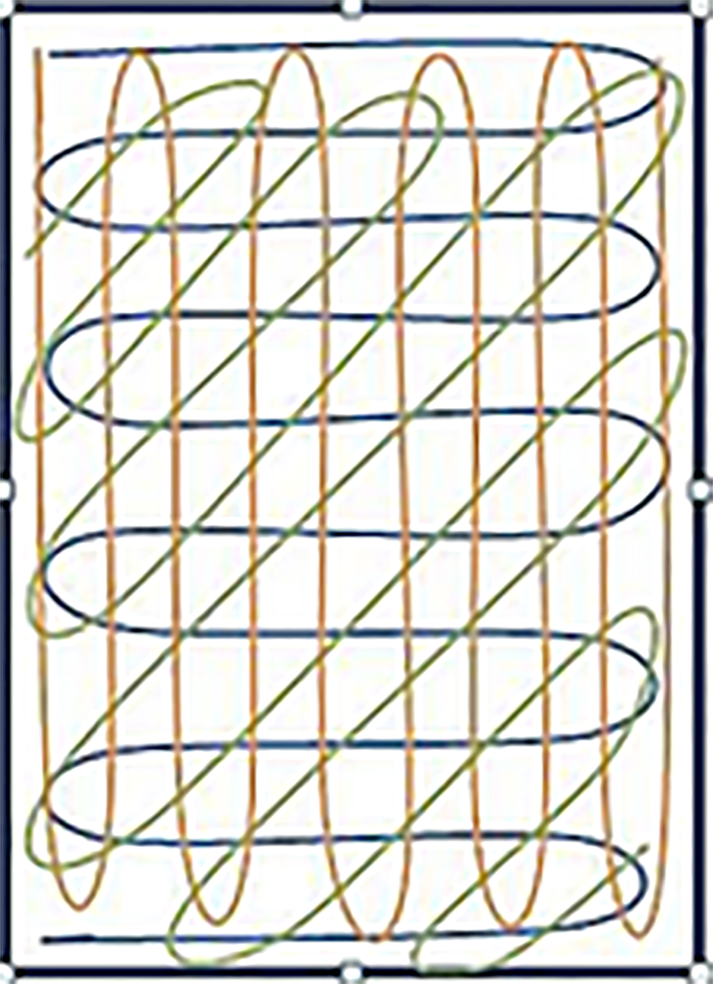
The researchers followed a standardized protocol for sample collection developed through collaboration with the microbiology department of our university hospital. The researcher collecting the swab samples was dressed in surgical scrubs with a surgical cap and mask. After standard scrubbing, the researcher wore examination gloves to retrieve the mobile phone from the clean surface the owner placed it on. The swab was collected in accordance with recommendations from the Centers for Disease Control and Prevention[16]. First, an overlapping “S” pattern was used to cover the entire surface with horizontal strokes using a rolling motion to maximize contact with the surface. The same area was then swabbed again using vertical “S” strokes, and finally the swab was rotated once more using diagonal “S” strokes (Figure 1). Mobile covers were not removed for sample collection and any cracks on the screens were noted. Gloves were discarded after each use and the samples were labeled with a code and delivered to the microbiology lab for processing.
A total of 110 orthopedic and anesthesia attendings, residents and nursing staff working in the orthopedic operating room during the 2-mo study period were requested for a sample. With 10 refusals, 100 samples were obtained for inclusion in our study. Each mobile phone was examined only once.
Data were analyzed using SPSS Version 22. A descriptive analysis was conducted on participant characteristics, the type of isolated organism and resistance to antibiotics. Continuous variables were reported as measures of central tendency and categorical variables as proportions. Based on the results of the Shapiro-Wilk normality test, appropriate measures of central tendency were reported (mean vs median). The Pearson chi-square test or Fisher’s exact test was used to determine the relationship between cell phone contamination and variables such as age, gender, duration since last hand wash, duration since last cell phone cleaning, cracked screen, cell phone cover and cell phone with keypad.
Further analysis was conducted on the most common species isolated to identify the risk factors for contamination. This was done by fitting a binary logistic regression model. The variables were divided into 2 groups; Specialty-related factors such as (orthopedic surgeon, anesthesia attending, nursing staff, resident and technicians) and cell phone-related factors (such as type of cell phone, cracked screen, cell phone cover, duration since last cleaning of the cell phone and duration since last hand-wash). Results were reported as crude and adjusted Odds ratio. The threshold for statistical significance was set at P < 0.25 for univariate analysis and P < 0.05 for multivariable analyses.
During the course of 2 mo, 110 individuals were approached. With 10 refusals to consent, 100 individuals participated in our study. The median age was 30 years with 81 male participants. A total of 43 orthopedic team members, 42 anesthesia team members and 15 members of the nursing staff were included. Only 10 out of the 100 participants had basic phones, with the remainder having smart phones. Sixty-seven participants had mobile covers and 42 had one or more cracks on their screen. 55 participants reported cleaning their cell phone with an alcohol swab with the median time passed since cleaning their cell phone at the time of sample collection being 24 h. Ten participants chose not to answer this question.
Results of the culture showed that 93 of the 100 tested cell phones were colonized by one or more bacterial species. The most common species isolated was the CoNS, found on 62% of the cell phones (Figure 2: Organisms isolated from mobile phones). This was followed by Micrococcus species (41%) and Bacillus (26%) species with the majority having low colony counts (Table 1). Twenty-one of 22 mobile phones (95.5%) belonging to attendings were colonized with potentially pathogenic bacteria. Interestingly, there was 100% and 80% resistance to meropenem among the Enterobacter and Pseudomonas species, respectively, and 54% resistance to oxacillin among CoNS species (Table 2). One phone harbored up to 6 organisms, 44 phones harbored more than 1 organism and 49 phones harbored exactly 1 organism (Figure 3).
| Organism | Cell phones (n)1 | Colony count, n (%) | ||
| Low | Medium | High | ||
| Coagulase-negative Staphylococcus | 62 | 58 (93.5) | 3 (4.9) | 1 (1.6) |
| Micrococcus | 41 | 41 (100) | 0 | 0 |
| Bacillus | 26 | 26 (100) | 0 | 0 |
| Pseudomonas species | 10 | 10 (100) | 0 | 0 |
| Fungi | 6 | 6 (100) | 0 | 0 |
| Corynebacterium | 5 | 4 (80) | 0 | 1 (20) |
| Streptococcus species | 4 | 4 (100) | 0 | 0 |
| Enterobacter | 3 | 0 | 1 (33.3) | 2 (66.7) |
| Acinetobacter | 2 | 0 | 1 (50) | 1 (50) |
| Klebsiella | 1 | 1 (100) | 0 | 0 |
| Pseudomonas aeruginosa | 1 | 1 (100) | 0 | 0 |
| E. coli | 1 | 1 (100) | 0 | 0 |
| Coagulase-negative Staphylococcus (n = 62) | Enterobacter (n = 3) | Pseudomonas species (n = 10) | |||
| Drug | Resistant (%) | Drug | Resistant (%) | Drug | Resistant (%) |
| Clindamycin | 26 (41.3) | Augmentin | 3 (100) | Augmentin | 9 (90) |
| Erythromycin | 40 (63.5) | Gentamycin | 0 | Amikacin | 1 (10) |
| Levofloxacin | 15 (23.8) | Ceftriaxone | 3 (100) | Aztreonam | 10 (100) |
| Penicillin | 62 (98.4) | Ciprofloxacin | 3 (100) | Imipenem | 9 (90) |
| Clotrimazole | 19 (29.7) | Tetramycin | 3 (100) | Piptazobactam | 0 |
| Tetramycin | 12 (19.0) | Meropenem | 3 (100) | Gentamycin | 0 |
| Oxacillin | 34 (54.0) | Ceftriaxone | 10 (100) | ||
| Tetramycin | 1 (10) | ||||
| Ciprofloxacin | 3 (30) | ||||
| Meropenem | 8 (80) | ||||
To understand which factors contribute to an increased risk of colonization by the bacteria identified in the culture, variables recorded from the questionnaire were analyzed with respect to the presence or absence of colonization. Mobile covers and cracked screens were found to be associated with microbial contamination, while cleaning the cell phone, particularly within the last 24 h, were associated with having less or no contamination (Table 3).
| Variable | Total | Bacterial colonization present, n (%) | P value |
| Specialty | |||
| Orthopedics | 43 | 39 (90.7) | 1.000 |
| Anesthesia | 42 | 39 (92.9) | |
| Appointment1 | |||
| Surgeon | 22 | 21 (95.5) | 1.000 |
| Resident | 30 | 27 (90) | 0.425 |
| Technician | 33 | 30 (90.9) | 0.681 |
| Nursing | 15 | 15 (100) | 0.590 |
| Age group (yr) | 1.000 | ||
| ≤ 30 | 52 | 48 (92.3) | |
| > 30 | 48 | 45 (93.8) | |
| Gender | |||
| Male | 81 | 75 (92.6) | 1.000 |
| Female | 19 | 18 (94.7) | |
| Type of cell phone | |||
| Smart phone | 93 | 86 (92.5) | 1.000 |
| Basic | 7 | 7 (100) | |
| Mobile cover | |||
| Present | 67 | 65 (97) | 0.038 |
| Absent | 33 | 28 (84.8) | |
| Keypad | |||
| Present | 10 | 10 (100) | 1.000 |
| Absent | 90 | 83 (92.2) | |
| Cracked screen | |||
| Yes | 42 | 42 (100) | 0.020 |
| No | 58 | 51 (87.9) | |
| Cell phone cleaning | |||
| Yes | 55 | 48 (87.3) | 0.040 |
| No | 45 | 45 (100) | |
| Duration since last cell phone cleaning | |||
| < 24 h | 33 | 26 (78.8) | 0.034 |
| > 24 h | 22 | 22 (100) | |
| Duration since last hand wash | |||
| < 10 min | 46 | 44 (95.7) | 0.666 |
| ≥ 10 min | 42 | 39 (92.9) | |
An important cause of post-operative SSIs in orthopedic surgery, CoNS was isolated on 62% of the mobile phones. To understand this further, we analyzed demographic/job-related and cell phone-related factors to assess their association with microbial contamination (Table 4). The results of univariate analysis showed that the risk of colonization with CoNS was significantly higher in orthopedic surgeons (P = 0.067), technicians (P = 0.185) and anesthesia (P = 0.009) technicians. The presence of a mobile cover (P = 0.001) and more than 24 h since cleaning the cell phone (P = 0.013) were also significant risk factors in univariate analysis. However, multivariable analysis showed that the category of anesthesia technicians (P = 0.043) and the presence of a mobile cover (P = 0.004) were the only significant risk factors for colonization by CoNS.
| Variable | Colonized cell phones (%) | Crude odds ratio | P value | Adjusted odds ratio | P value |
| Demographic/job related factors | |||||
| Orthopedic surgeon | 0.067 | 0.085 | |||
| No | 58.4 | 1 | 1 | ||
| Yes | 90.9 | 7.12 (0.87, 58.01) | 6.47 (0.77, 54.15) | ||
| Anesthesia consultant | 0.906 | ||||
| No | 63.6 | 1 | |||
| Yes | 55 | 0.91 (0.30, 3.97) | |||
| Orthopedic residents | 0.332 | ||||
| No | 51 | 1 | |||
| Yes | 73.3 | 1.83 (0.54, 6.23) | |||
| Anesthesia resident | 0.455 | ||||
| No | 63.5 | 1 | |||
| Yes | 53.3 | 0.66 (0.22, 1.98) | |||
| Orthopedic technician | 0.185 | 0.241 | |||
| No | 59 | 1 | 1 | ||
| Yes | 76.5 | 2.26 (0.68, 7.51) | 2.10 (0.61, 7.28) | ||
| Anesthesia technician | 0.009 | 0.043 | |||
| No | 67.9 | 1 | 1 | ||
| Yes | 31.3 | 0.22 (0.07, 0.68) | 0.29 (0.09, 0.96) | ||
| Nursing staff | 0.455 | ||||
| No | 63.5 | 1 | |||
| Yes | 53.3 | 1.52 (0.50, 4.61) | |||
| Gender | 0.682 | ||||
| Female | 57.9 | 1 | |||
| Male | 63 | 1.24 (0.45, 3.42) | |||
| Age (yr) | 0.951 | ||||
| < 30 | 1 | ||||
| 30-50 | 0.78 (0.17, 3.58) | ||||
| ≥ 50 | 0.82 (0.18, 3.65) | ||||
| Cell phone related factors | |||||
| Type of cell phone | 0.290 | ||||
| Basic phone | 42.9 | 1 | |||
| Smart phone | 63.4 | 2.31 (0.49, 10.96) | |||
| Mobile cover | 0.001 | 0.004 | |||
| No | 39.4 | 1 | 1 | ||
| Yes | 73.1 | 4.19 (1.73, 10.13) | 8.31 (1.95, 35.40) | ||
| Keypad | 0.414 | ||||
| No | 63.3 | 1 | |||
| Yes | 50 | 1.73 (0.47, 6.41) | |||
| Cracked screen | 0.395 | ||||
| No | 65.5 | 1 | |||
| Yes | 57.1 | 0.70 (0.31, 1.59) | |||
| Cleans their cell phone | 0.601 | ||||
| No | 60 | 1 | |||
| Yes | 65.5 | 1.26 (0.53, 3.03) | |||
| Duration since last cell phone cleaning | 0.013 | 0.032 | |||
| < 24 h | 51.5 | 1 | 1 | ||
| ≥ 24 h | 86.4 | 5.82 (1.46, 23.17) | 5.30 (1.16, 24.34) | ||
Despite persistent efforts to minimize the rate of post-operative SSIs, their incidence following orthopedic surgery unfortunately continues to persist[29]. Along with introducing different regimens of antibiotic prophylaxis, optimization of patients’ comorbid conditions and improving operating room sterile practices[30], numerous attempts have been made to identify potential sources of SSIs in the operating room. Among the many suspects is the mobile phone, which nowadays is akin to an electronic appendage of its owner. Considering the importance of mobile phones as an academic and communicative tool[31-33], significant contamination of cell phones with drug resistant bacteria may call for restriction of their use inside the operating room and the patient’s bedside.
The results of our study showed that 93 of 100 samples taken from the mobile phones of health care professionals in the orthopedic operating room were contaminated with potentially pathogenic organisms. Interestingly and of concern, the most common organism causing SSIs following orthopedic surgery[34-38], CoNS was also the most common organism isolated in our study.
The results of similar studies published during the last 2 years show a wide range of colonization between 9%-90%, with a wide variety of organisms including CoNS, Staphylococcus aureus, Acinetobacter species[16,18,39-41]. As these studies are from all around the world, this variation may be the result of differences in geographical and economic status between countries. A meta-analysis published in 2016 showed that the contamination of cell phones of health care professionals was significantly greater in developing countries in comparison to the developed world[42].
In this study, we only investigated the extent of microbial colonization of mobile phones in the orthopedic operating room. Specific investigation into whether this contamination leads to SSIs was not performed in our study. Some researchers have attempted to investigate the relationship between mobile phone contamination and SSIs. Chang et al[20] in 2017 used genotyping to demonstrate the transmission of pathogenic organisms between the hands, nostrils and mobile phones of health care professionals. However, typing of strains responsible for SSIs was not carried out. Another study compared the strains found on the mobile phones and skin of health care professionals with the strains isolated from the skin and blood samples of their patients. A match was found with the strains on the skin of the patients but not with the blood isolates[43].
Theoretically, contaminated cell phones can lead to SSIs in various ways. While direct transmission (contact between the reservoir and the host) is unlikely in this situation, indirect transmission via the hands of medical personnel and cross-contamination of other medical equipment is plausible and is a cause for concern[18]. An incident in Larry Dossey’s “Distracted doctoring and the iPatient” narrates a nurse attending a phone call after washing her hands and forgets to clean them again before administering intravenous antibiotics to her patient[44], serving as an example of how cell phones may unintentionally contribute to a breach of the hand hygiene protocol. A survey conducted at a hospital in Barbados reported 47% of health care professionals using their mobile phones while attending patients with only 3% actually washing their hands after use[45]. Another study proved the ineffectiveness of the practice of using a sterile disposable towel as a barrier for attending phone calls while scrubbed in[2].
Antibiotic resistance among isolated organisms may also be a problem. Our study shows significant resistance to meropenem among Enterobacter and Pseudomonas species. More importantly, 54% of the isolated CoNS species were resistant to oxacillin/methicillin. Studies show that these methicillin resistant strains may act as reservoirs for the genetic material, particularly SCCmec Iva, found in MRSA[46].
In 2010, Roy et al[47] suggested that the concept of implementing highly expensive methods to eradicate bacterial contamination in the hope of reducing the cost of treating SSIs has yet to be proven. They went on to refer to attempts made at erasing contamination that is not documented to cause SSIs as a “trap” of treating only surrogate end-points[47]. While proof of contaminated cell phones causing SSIs may not be concrete, unsafe practices involving contaminated mobile phones and the prevalence of high rates of antibiotic resistance among the isolated organisms also do not make it easy to simply discard the idea of dealing with contaminated cell phones. The balance ultimately lies in using only cheap and convenient methods to reduce contamination until a causal relationship is established.
In light of research findings we evaluated several factors including demographic variables, cell phone characteristics and cleaning practices as they relate to the contamination of mobile phones. Similar to previous studies, our results also showed that demographic variables and hand washing practices do not have any significant impact on the risk of contamination[19,48]. However, cleaning the mobile phone with an alcohol swab did decrease the risk of contamination. This may be due to the ubiquitous use of mobile phones by individuals where the phone is acting as a reservoir. Although our study shows no significant difference in contamination between basic phones and smart phones, previous studies have shown contradicting views on this[48-50].
Our study adds to existing literature by showing a significantly increased risk of contamination on phones with a mobile cover and a cracked screen. Additionally, participants who claimed to have cleaned their cell phone with an alcohol swab in the last 24 h also had significantly less contaminated cell phones. These findings could form the basis of the concept of “mobile hygiene” involving steps as simple as the periodic change of a mobile cover, replacement of a cracked screen or even wiping your phone with an alcohol swab in the morning. With further investigation into factors contributing to the contamination of mobile phones and replication of the findings of our study, the practice of mobile hygiene could yield the perfect cost-effective balance that contaminated cell phones deserve until they are established as a direct cause of SSIs. Caveats in our study include potential attribution bias as the samples continued to be collected over two months rather than in a cross-sectional manner. Furthermore, to contain the costs of sampling, we used examination gloves instead of sterile gloves to collect the culture specimens. Our study investigated only the extent of colonization by potentially pathogenic bacteria on mobile phones in the orthopedic operating room. We did not investigate the link between colonization and SSIs. Further research needs to be conducted to investigate the causation between colonization of mobile phones and SSIs. In addition, when studying the factors contributing to the risk of colonization it is necessary to identify of any source of infection in the owner of the mobile phone.
In conclusion, SSIs following orthopedic surgery are a cause of concern due to their associated morbidity, potential for mortality and phenomenal cost; thus, there is a need to identify and eradicate any sources of pathogenic organisms. Health care workers’ mobile phones are frequently contaminated with pathogenic bacteria. Studies examining the relationship between contaminated cell phones and SSIs need to be conducted. Until such a causation is established, cheap and convenient methods are needed to decrease contamination in the form of simple practices constituting “mobile hygiene”.
Surgical site infections (SSIs) are a major cause of morbidity and mortality following orthopedic surgery. Each year, approximately $3.5 to $10 billion is spent on managing SSIs in the United States. Data show that the incidence of SSIs is between 2% to 5%, with 60% of them being preventable. To decrease the rate of SSIs following orthopedic surgery, potential sources of contamination need to be identified.
Literature has identified numerous sources implicated in the transmission of pathogenic microbes including air, hospital surfaces, liquid nitrogen freezers, computer keyboards, stethoscopes, staff uniforms, tourniquets and even leaving sterile trays open for too long. Mobile phones are used ubiquitously and have academic and clinical uses. Recently, mobile phones have been implicated as a source of contamination in the orthopedic operating rooms.
The purpose of this study was to investigate microbial colonization, risk factors and antibiotic sensitivity patterns on the mobile phones of health care professionals in the orthopedic operating room.
We conducted a cross-sectional study involving culture and sensitivity analysis of swabs taken from mobile phones of orthopedic and anesthesia attendings, residents, technicians and nurses working in the orthopedic operating rooms over a period of 2 mo. Demographic and cell phone related factors were recorded using a questionnaire and the factors associated with contamination were analyzed.
Ninety-three of 100 mobile phones were contaminated. Species isolated were Coagulase negative Staphylococcus (62%), Micrococcus (41%) and Bacillus (26%). The risk of contamination was increased by mobile covers and cracked screens and decreased by cell phone cleaning.
Mobile phones belonging to health care workers are frequently contaminated with pathogenic bacteria with the potential of transferring drug resistance to nosocomial pathogens.
Studies investigating the relationship between mobile phones and SSIs need to be conducted. The concept of “mobile hygiene” involving the change of mobile covers, replacement of cracked screens or even wiping the phone with an alcohol swab could yield the cost-effective balance that contaminated cell phones deserve until they are established as a direct cause of SSIs.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dailiana ZH S-Editor: Gong ZM L-Editor: Webster JR E-Editor: Xing YX
| 1. | Ban KA, Minei JP, Laronga C, Harbrecht BG, Jensen EH, Fry DE, Itani KM, Dellinger EP, Ko CY, Duane TM. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 2017;224:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 656] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 2. | Schirmer A, Swan C, Hughes SJ, Vasilopoulos T, Oli M, Chaudhry S, Gravenstein N, Giordano C. Break Scrub to Take That Phone Call? J Am Coll Surg. 2018;226:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Chauveaux D. Preventing surgical-site infections: measures other than antibiotics. Orthop Traumatol Surg Res. 2015;101:S77-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Li K, Sambare TD, Jiang SY, Shearer EJ, Douglass NP, Kamal RN. Effectiveness of Preoperative Antibiotics in Preventing Surgical Site Infection After Common Soft Tissue Procedures of the Hand. Clin Orthop Relat Res. 2018;476:664-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Yousufuddin S, Latif MA, Samena C, Ali S, Ashraf M, Cornell J. Surgical Site Infection in Hip Fracture Surgery; Reducing the Incidence of Infection and Correlation With co Morbidities and Blood Parameters. Int J Orthop. 2018;5:891-895. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Stambough JB, Nam D, Warren DK, Keeney JA, Clohisy JC, Barrack RL, Nunley RM. Decreased Hospital Costs and Surgical Site Infection Incidence With a Universal Decolonization Protocol in Primary Total Joint Arthroplasty. J Arthroplasty. 2017;32:728-734.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Lu K, Chermside-Scabbo CJ, Marino NE, Concepcion, Yugawa C, Aladegbami B, Paar T, St John TA, Ross W, Clohisy JC, Kirby JP. Accessible Communication Tools for Surgical Site Infection Monitoring and Prevention in Joint Reconstruction: Feasibility Study. JMIR Perioper Med. 2018;1:e1. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Edmiston CE, Seabrook GR, Cambria RA, Brown KR, Lewis BD, Sommers JR, Krepel CJ, Wilson PJ, Sinski S, Towne JB. Molecular epidemiology of microbial contamination in the operating room environment: Is there a risk for infection? Surgery. 2005;138:573-579; discussion 579-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32:687-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 414] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 10. | Fountain D, Ralston M, Higgins N, Gorlin JB, Uhl L, Wheeler C, Antin JH, Churchill WH, Benjamin RJ. Liquid nitrogen freezers: a potential source of microbial contamination of hematopoietic stem cell components. Transfusion. 1997;37:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Schultz M, Gill J, Zubairi S, Huber R, Gordin F. Bacterial contamination of computer keyboards in a teaching hospital. Infect Control Hosp Epidemiol. 2003;24:302-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Bernard L, Kereveur A, Durand D, Gonot J, Goldstein F, Mainardi JL, Acar J, Carlet J. Bacterial contamination of hospital physicians' stethoscopes. Infect Control Hosp Epidemiol. 1999;20:626-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Wiener-Well Y, Galuty M, Rudensky B, Schlesinger Y, Attias D, Yinnon AM. Nursing and physician attire as possible source of nosocomial infections. Am J Infect Control. 2011;39:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Ahmed SM, Ahmad R, Case R, Spencer RF. A study of microbial colonisation of orthopaedic tourniquets. Ann R Coll Surg Engl. 2009;91:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Dalstrom DJ, Venkatarayappa I, Manternach AL, Palcic MS, Heyse BA, Prayson MJ. Time-dependent contamination of opened sterile operating-room trays. J Bone Joint Surg Am. 2008;90:1022-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Shakir IA, Patel NH, Chamberland RR, Kaar SG. Investigation of cell phones as a potential source of bacterial contamination in the operating room. J Bone Joint Surg Am. 2015;97:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 17. | Jeske HC, Tiefenthaler W, Hohlrieder M, Hinterberger G, Benzer A. Bacterial contamination of anaesthetists' hands by personal mobile phone and fixed phone use in the operating theatre. Anaesthesia. 2007;62:904-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Graveto JM, Costa PJ, Santos CI. Cell phone usage by health personnel: preventive strategies to decrease risk of cross infection in clinical context. Texto Contexto Enferm. 2018;27:e5140016. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Chaman R, Nargeseyan S, Jannesar R, Ravangard S, Nikbakht G. Survey of prevalence and types of bacterial contamination of mobile phones of personnel employed in major wards of educational hospitals in Yasuj. J Fundam Appl Sci. 2018;10:449-458. [DOI] [Full Text] |
| 20. | Chang CH, Chen SY, Lu JJ, Chang CJ, Chang Y, Hsieh PH. Nasal colonization and bacterial contamination of mobile phones carried by medical staff in the operating room. PLoS One. 2017;12:e0175811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Murgier J, Coste JF, Cavaignac E, Bayle-Iniguez X, Chiron P, Bonnevialle P, Laffosse JM. Microbial flora on cell-phones in an orthopedic surgery room before and after decontamination. Orthop Traumatol Surg Res. 2016;102:1093-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Freundlich RE, Freundlich KL, Drolet BC. Pagers, Smartphones, and HIPAA: Finding the Best Solution for Electronic Communication of Protected Health Information. J Med Syst. 2017;42:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Martinez R, Rogers AD, Numanoglu A, Rode H. The value of WhatsApp communication in paediatric burn care. Burns. 2018;44:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Granot Y, Ivorra A, Rubinsky B. A new concept for medical imaging centered on cellular phone technology. PLoS One. 2008;3:e2075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Bellina L, Missoni E. Mobile cell-phones (M-phones) in telemicroscopy: increasing connectivity of isolated laboratories. Diagn Pathol. 2009;4:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Boland P. The emerging role of cell phone technology in ambulatory care. J Ambul Care Manage. 2007;30:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Mukund Bahadur KC, Murray PJ. Cell phone short messaging service (SMS) for HIV/AIDS in South Africa: a literature review. Stud Health Technol Inform. 2010;160:530-534. [PubMed] |
| 28. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6805] [Cited by in RCA: 11781] [Article Influence: 654.5] [Reference Citation Analysis (0)] |
| 29. | Hanssen AD, Rand JA. Evaluation and Treatment of Infection at the Site of a Total Hip or Knee Arthroplasty. J Bone Joint Surg - Series A. 1998;80:910-922. |
| 30. | Shohat N, Muhsen K, Gilat R, Rondon AJ, Chen AF, Parvizi J. Inadequate Glycemic Control Is Associated With Increased Surgical Site Infection in Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J Arthroplasty. 2018;33:2312-2321.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Firdouse M, Devon K, Kayssi A, Goldfarb J, Rossos P, Cil TD. Using Texting for Clinical Communication in Surgery: A Survey of Academic Staff Surgeons. Surg Innov. 2018;25:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Fung R, Hyde JH, Davis M. Oiling the gate: a mobile application to improve the admissions process from the emergency department to an academic community hospital inpatient medicine service. J Community Hosp Intern Med Perspect. 2018;8:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Nikolic A, Wickramasinghe N, Claydon-Platt D, Balakrishnan V, Smart P. The Use of Communication Apps by Medical Staff in the Australian Health Care System: Survey Study on Prevalence and Use. JMIR Med Inform. 2018;6:e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;15-23. [PubMed] |
| 35. | Hope PG, Kristinsson KG, Norman P, Elson RA. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J Bone Joint Surg Br. 1989;71:851-855. [PubMed] |
| 36. | Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 37. | De Vecchi E, George DA, Romanò CL, Pregliasco FE, Mattina R, Drago L. Antibiotic sensitivities of coagulase-negative staphylococci and Staphylococcus aureus in hip and knee periprosthetic joint infections: does this differ if patients meet the International Consensus Meeting Criteria? Infect Drug Resist. 2018;11:539-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Lourtet-Hascoët J, Félicé MP, Bicart-See A, Bouige A, Giordano G, Bonnet E. Species and antimicrobial susceptibility testing of coagulase-negative staphylococci in periprosthetic joint infections. Epidemiol Infect. 2018;146:1771-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Sadat-Ali M, Al-Omran AK, Azam Q, Bukari H, Al-Zahrani AJ, Al-Turki RA, Al-Omran AS. Bacterial flora on cell phones of health care providers in a teaching institution. Am J Infect Control. 2010;38:404-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Brady RR, Verran J, Damani NN, Gibb AP. Review of mobile communication devices as potential reservoirs of nosocomial pathogens. J Hosp Infect. 2009;71:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Karkee P, Madhup SK, Humagain P, Thaku N, Timilsina B. Mobile Phone: A Possible Vector of Bacterial Transmission in Hospital Setting. Kathmandu Univ Med J (KUMJ). 2017;15:217-221. [PubMed] |
| 42. | Lin D, Ou Q, Lin J, Peng Y, Yao Z. A meta-analysis of the rates of Staphylococcus aureus and methicillin-resistant S aureus contamination on the surfaces of environmental objects that health care workers frequently touch. Am J Infect Control. 2017;45:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Borer A, Gilad J, Smolyakov R, Eskira S, Peled N, Porat N, Hyam E, Trefler R, Riesenberg K, Schlaeffer F. Cell phones and Acinetobacter transmission. Emerg Infect Dis. 2005;11:1160-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Ross S, Forgie S. Distracted doctoring: smartphones before patients? CMAJ. 2012;184:1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Ramesh J, Carter AO, Campbell MH, Gibbons N, Powlett C, Moseley H Sr, Lewis D, Carter T. Use of mobile phones by medical staff at Queen Elizabeth Hospital, Barbados: evidence for both benefit and harm. J Hosp Infect. 2008;70:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Barbier F, Ruppé E, Hernandez D, Lebeaux D, Francois P, Felix B, Desprez A, Maiga A, Woerther PL, Gaillard K, Jeanrot C, Wolff M, Schrenzel J, Andremont A, Ruimy R. Methicillin-resistant coagulase-negative staphylococci in the community: high homology of SCCmec IVa between Staphylococcus epidermidis and major clones of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2010;202:270-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Roy RC, Brull SJ, Eichhorn JH. Surgical site infections and the anesthesia professionals' microbiome: we've all been slimed! Now what are we going to do about it? Anesth Analg. 2011;112:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Koroglu M, Gunal S, Yildiz F, Savas M, Ozer A, Altindis M. Comparison of keypads and touch-screen mobile phones/devices as potential risk for microbial contamination. J Infect Dev Ctries. 2015;9:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Lee YJ, Yoo CG, Lee CT, Chung HS, Kim YW, Han SK, Yim JJ. Contamination rates between smart cell phones and non-smart cell phones of healthcare workers. J Hosp Med. 2013;8:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Pal P, Roy A, Moore G, Muzslay M, Lee E, Alder S, Wilson P, Powles T, Wilson P, Kelly J. Keypad mobile phones are associated with a significant increased risk of microbial contamination compared to touch screen phones. J Infect Prevent. 2013;14:65-68. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |