Published online Aug 10, 2013. doi: 10.5306/wjco.v4.i3.75
Revised: May 23, 2013
Accepted: June 1, 2013
Published online: August 10, 2013
Processing time: 136 Days and 23.8 Hours
Angioimmunoblastic T-cell lymphoma (AITL) is a unique type of peripheral T-cell lymphoma with a constellation of clinical symptoms and signs, including weight loss, fever, chills, anemia, skin rash, hepatosplenomegaly, lymphadenopathy, thrombocytopenia and polyclonal hypergammaglobulinemia. The histological features of AITL are also distinctive. Pure red cell aplasia is a bone marrow failure characterized by progressive normocytic anemia and reticulocytopenia without leucopenia or thrombocytopenia. However, AITL with abdominal pain and pure red cell aplasia has rarely been reported. Here, we report a rare case of AITL-associated pure red cell aplasia with abdominal pain. The diagnosis was verified by a biopsy of the enlarged abdominal lymph nodes with immunohistochemical staining.
Core tip: Angioimmunoblastic T-cell lymphoma (AITL)-associated pure red cell aplasia is a unique type of peripheral T-cell lymphoma with a constellation of clinical symptoms and signs; the histological features of AITL are also distinctive. AITL-associated pure red cell aplasia with abdominal pain has rarely been reported. Here, we report a rare case of AITL-associated pure red cell aplasia with abdominal pain. The diagnosis was verified by a biopsy of the enlarged abdominal lymph nodes with immunohistochemical staining.
- Citation: Tao J, Zheng FP, Tian H, Lin Y, Li JZ, Chen XL, Chen JN, Shao CK, Wu B. Angioimmunoblastic T-cell lymphoma-associated pure red cell aplasia with abdominal pain. World J Clin Oncol 2013; 4(3): 75-81
- URL: https://www.wjgnet.com/2218-4333/full/v4/i3/75.htm
- DOI: https://dx.doi.org/10.5306/wjco.v4.i3.75
Angioimmunoblastic T-cell lymphoma (AITL) is a unique type of peripheral T-cell lymphoma; AITL is rare, accounting for approximately 2%-5% of all non-Hodgkin lymphomas[1]. It is an uncommon type of non-Hodgkin’s lymphoma and has a constellation of clinical symptoms and signs, including weight loss, fever, chills, anemia, skin rash, hepatosplenomegaly, lymphadenopathy, thrombocytopenia and polyclonal hypergammaglobulinemia[2,3]. The histological features of AITL are also distinctive. The lymph node is characterized by a polymorphic infiltrate, a marked proliferation of high endothelial venules, and a dense meshwork of dendritic cells, and the neoplastic cells display an atypical minimal cell. The neoplastic cells account for only a fraction of the infiltrate and are admixed with a reactive population of small lymphocytes, eosinophils, histiocytes, plasma cells and a large lymphoid, sometimes composed of Reed-Sternberg-like B cells that are often infected with Epstein-Barr virus (EBV). It is believed that AITL derives from a follicular helper T-cell subset[4,5]. This subset of T-cells is located at the boundary between the germinal center light zone and the mantle zone. The tumor cells usually express CD3, CD10, CD21, CD23 and CXCL13, a phenotype that is unique among T-cell lymphomas. Pure red cell aplasia is a bone marrow failure characterized by progressive normocytic anemia and reticulocytopenia without leucopenia or thrombocytopenia. It is associated with various diseases, including thymoma, myeloproliferative disorders, infection and autoimmune diseases[6]. Compared with other non-Hodgkin lymphomas, AITL with abdominal pain and pure red cell aplasia is rare, and the prognosis is poor.
A 71-year-old woman presented with abdominal distension and fatigue that had developed two months previously. She also had a twenty-year medical history of hypertension but did not take medicine regularly. The patient suffered from cholecystolithiasis with cholecystitis for over ten years, and the cholecystitis with abdominal pain was relieved by antibiotic treatment. She had received a blood transfusion three years earlier because of severe anemia. Histories of hepatitis, infection, tuberculosis, wounds and surgery were denied. Two months prior to admission, the patient developed symptoms of abdominal pain with fullness not related to eating or posture, which sometimes showed spontaneous remission. She complained of a progressively poor appetite, fatigue, low-grade fever and nausea. On physical examination, severe pallor was observed, enlarged supraclavicular and inguinal lymph nodes were identified, and the liver and spleen were enlarged.
Laboratory blood examinations showed the following indexes (normal range in parentheses): hemoglobin, 50 g/L (120-140 g/L); peripheral white cell count, 4.61 × 109/L (5-10 × 109/L); neutrophils, 51.7% (40%-60%); reticulocyte count, 0.1% (0.5%-1.5%); platelet count, 229×109/L (100-300×109/L); C-reactive protein, 67.3 mg/L (0-6.0 mg/L); erythrocyte sedimentation rate, 53 mm/h (0-20 mm/h); albumin, 30.7 mg/L (36-51 mg/L); total immunoglobulin (Ig), 38.3 mg/L (25.0-35.0 mg/L); lactate dehydrogenase, 208 U/L (71-231 U/L); total bilirubin, 10.58 μmol/L (4-23.9 μmol/L); alkaline phosphatase, 143 U/L (35-125 U/L); c-glutamyl transpeptidase, 104U/L (7-50 U/L); aspartate aminotransferase, 16 U/L (14-40 U/L); alanine aminotransferase, 22 U/L (5-35 U/L); creatinine, 76 μmol/L (31.8-91.0 μmol/L), and blood urine nitrogen, 33 g/L (31.8-91.0 g/L). A routine urine and stool test were normal. Autoimmune-related indicators and tuberculosis (TB)-related antibodies were not found in the blood, and the TB purified protein derivative (PPD) skin test was negative. Hepatitis B and C markers were also negative. The levels of CA-125, CA 19-9, CEA and AFP were normal.
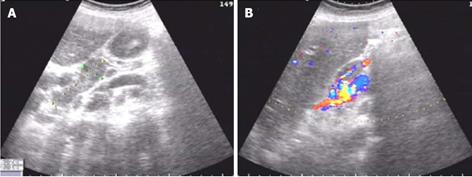
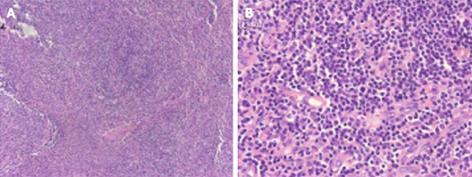
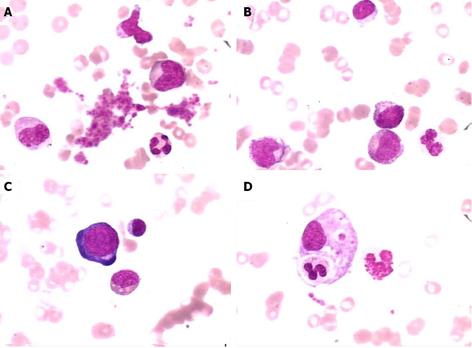
A gastroscopy performed at another hospital indicated gastritis, and gastric polyp were removed by electrocision. Helicobacter pylori were negative. The histopathology of the gastric polyp showed a hyperplastic polyp. During the first two weeks of hospitalization, the patient received a blood transfusion of 800 mL, and the patient’s symptoms were partly relieved. However, after two weeks, the patient experienced drastic upper abdominal pain, and abdominal ultrasonography demonstrated an acute onset of chronic cholecystitis and enlarged porta lymph nodes (Figure 1). An abdominal computed tomography (CT) scan revealed multiple enlarged coeliac and retroperitoneal lymph nodes, hepatosplenomegaly, and cholecystolithiasis with chronic cholecystitis (data not shown). Biopsies of the right cervical and left inguinal lymph nodes were performed at different times; the results revealed an inflammatory reaction only without lymphoma (Figure 2). On the sixth day of hospitalization, a bone marrow biopsy was performed; the bone marrow examination showed that the granulocyte series and megakaryocytes series were actively proliferating, whereas the erythrocyte series was marked by aplasia. The result demonstrated pure red cell aplasia with anemia (Figure 3). The patient accepted treatment with antibiotics and spasmolytics over one week, but the abdominal pain still worsened progressively.
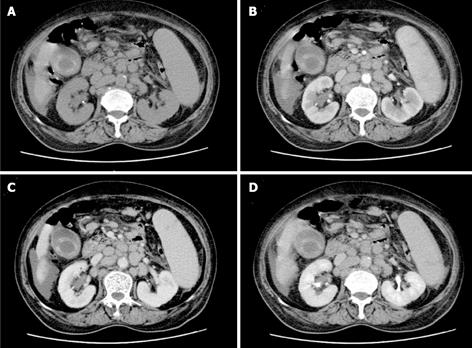
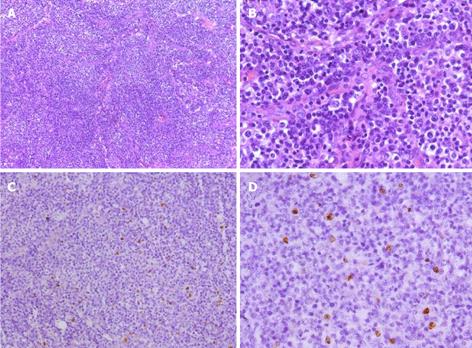
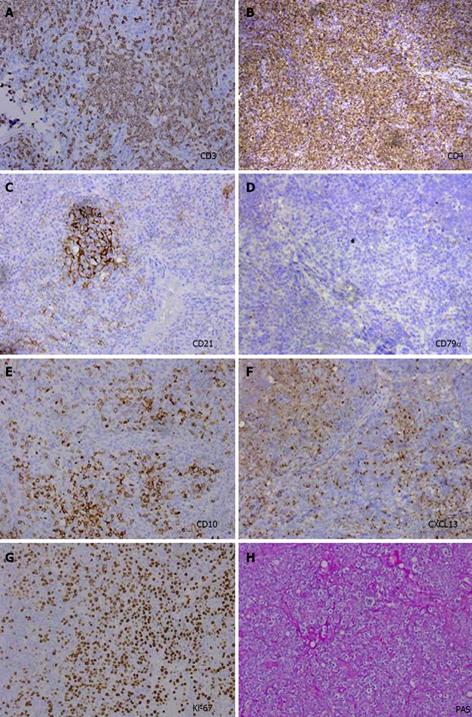
The patient was examined again by abdominal CT scan at the sixth week. The images revealed that the number of lymph nodes had markedly increased and that the lymph node size was dramatically enlarged compared to the size observed six weeks earlier; the peritoneal cavity fat gap was fuzzy, and the peritoneum was thickening (Figure 4). To identify the cause of disease, the patient underwent a biopsy of the enlarged lymph nodes at both the porta hepatis and greater omentum with a cholecystectomy; a pathological examination and immunohistochemical staining showed only chronic inflammation without lymphomatous cells. However, the abdominal pain was not significantly alleviated after the cholecystectomy. Microscopically, the biopsy specimens revealed that the normal architecture was lost, except for the presence of a few depleted follicles with concentrically arranged follicular dendritic cells, and the architecture was effaced by some polymorphic infiltrate with marked vascular proliferation (Figures 5A and B). In addition, in situ hybridization showed a weakly positive nuclear labeling for Epstein-Barr virus-encoded small nuclear RNA (Figures 5C and D). The results of the immunohistochemical staining are shown in Figure 6. Evident positive staining was observed for CD3, CD4, CD21, CD10, CXCL13, Ki-67 and PAS, and marked follicular dendritic cell proliferation was also found around small vessels. However, the staining showed negative staining for CD79α. Simultaneously, evident, positive CD23 staining was observed, whereas CD8 showed negative staining (data not shown).
Based on the pathological characteristics, the diagnosis of AITL was made. However, the patient was increasingly weak following deterioration and a consequential severe infection two weeks later, and the family requested that the patient return to a hometown hospital for sequential therapy.
AITL is a rare subtype of lymphoma and accounts for approximately 2%-5% of non-Hodgkin lymphomas. No consistent risk factors for development of the disease have been reported and to date, no etiological agent has been identified. Epstein-Barr virus is observed in most cases, and the virus has been found in the reactive B-cells that participate in the polymorphous infiltrate of this disease and in the neoplastic T-cells[7]. In our case, in situ hybridization showed a weakly positive nuclear labeling of Epstein-Barr virus-encoded small nuclear RNA. Immunodeficiency is also observed in this disease, but it may simply be a parallel state rather than a precipitating factor.
AITL is an age-related disease, with most patients presenting AITL within their sixth and seventh decades[8]. The presenting features of AITL span a spectrum ranging from asymptomatic lymphadenopathy to a syndrome associated with severe systemic symptoms. Nonetheless, the constellation of symptoms at diagnosis often involves diverse constitutional indicators, including fevers, night sweats, arthralgias or arthritis, and weight loss, but abdominal pain is rare. In the present case, though the patient had a ten-year medical history of cholecystolithiasis and cholecystitis, and her abdominal pain was not relieved after antibiotic treatment and gradually worsened; this outcome indicated that the abdominal pain was connected to the AITL but not the cholecystolithiasis and cholecystitis. The clinical syndrome of AITL overlaps with a wide range of inflammatory and neoplastic processes, and the changes in both peripheral blood and bone marrow are usually non-specific. AITL diagnosis can only be achieved by a biopsy with histological examination of the enlarged lymph nodes, through which the characteristic morphological features can be best appreciated.
AITL is usually accompanied by various immunologic and hematologic diseases, such as autoimmune hemolytic anemia, vasculitis, and autoimmune thyroid disease[9]. Pure red cell aplasia (PRCA), an autoimmune disorder resulting in selective aplasia of the erythroid series, is a bone marrow failure characterized by progressive normocytic anemia and reticulocytopenia without leucopenia or thrombocytopenia. PRCA is associated with various diseases, such as thymoma, lymphoma and myelo-proliferative disorders, autoimmune diseases, infection, and drugs, but PRCA is rarely associated with AITL. The pathogenesis has not been elucidated yet. Some reports suggest that the mechanism of lymphoma-associated PRCA is heterogeneous and that the durable maintenance-free remission of anemia can be obtained in some patients; moreover, some humoral factors causing inhibition of red cell precursors might play an important role in the pathogenesis of pure red cell aplasia associated with AITL[10,11] . Some researchers have noted that the patients had a dose-dependent inhibitor of colony-forming unit-erythroid but not of colony-forming unit-granulocyte and macrophage from a normal bone marrow cultures. Patients with AITL are known to produce antibodies against several autologous epitopes, and the most well-characterized mechanism of pure red cell aplasia involves antibodies to red cell precursors[12].
The diagnosis of AITL is difficult, and it is sometimes misdiagnosed as an infectious or other hematological disease. In particular, the histological appearance of extra nodal involvement, such as bone, skin spleen, marrow, and lung, is usually non-specific[13]. In our case, the diagnosis was made by biopsy of the enlarged lymph nodes at the porta hepatis and greater omentum with immunohistochemical staining but not of the peripheral lymph nodes because the lymphadenopathies existed only in the intraabdominal area. We and other groups have shown that CD10, CD21 and CXCL13 are expressed by neoplastic cells of AITL and that these specific immunohistochemical stainings contribute to AITL diagnosis.
Some researchers have found pertinent prognostic factors for AITL, such as male sex, mediastinal lymphadenopathy and anemia. However, these factors are not specific to AITL diagnosis. Few reports have attempted to investigate histological prognostic features, but none of them has been proven of clinical value[14,15]. Some studies have tried to identify the potential relationship between the increase in large cells and the disease prognosis, but these studies also show no definite outcome for the prognosis.
In summary, AITL- associated abdominal pain with pure red cell aplasia is a very rare disease and may be related to immune disorders. The diagnosis of AITL should rely on a combination of classical pathological criteria together with clinical and biological features; specific immunohistochemical study is especially pivotal. Because of gerontism and poor physical condition, the majority of patients cannot endure chemotherapy, the five-year survival rate is less than 20%, and the cause of death is usually severe infection.
P- Reviewer Tsuji K S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909-3918. [PubMed] |
| 2. | Iannitto E, Ferreri AJ, Minardi V, Tripodo C, Kreipe HH. Angioimmunoblastic T-cell lymphoma. Crit Rev Oncol Hematol. 2008;68:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Mourad N, Mounier N, Brière J, Raffoux E, Delmer A, Feller A, Meijer CJ, Emile JF, Bouabdallah R, Bosly A. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Krenacs L, Schaerli P, Kis G, Bagdi E. Phenotype of neoplastic cells in angioimmunoblastic T-cell lymphoma is consistent with activated follicular B helper T cells. Blood. 2006;108:1110-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | de Leval L, Rickman DS, Thielen C, Reynies Ad, Huang YL, Delsol G, Lamant L, Leroy K, Brière J, Molina T. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952-4963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 448] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 6. | Djaldetti M, Blay A, Bergman M, Salman H, Bessler H. Pure red cell aplasia--a rare disease with multiple causes. Biomed Pharmacother. 2003;57:326-332. [PubMed] |
| 7. | Anagnostopoulos I, Hummel M, Finn T, Tiemann M, Korbjuhn P, Dimmler C, Gatter K, Dallenbach F, Parwaresch MR, Stein H. Heterogeneous Epstein-Barr virus infection patterns in peripheral T-cell lymphoma of angioimmunoblastic lymphadenopathy type. Blood. 1992;80:1804-1812. [PubMed] |
| 8. | Attygalle A, Al-Jehani R, Diss TC, Munson P, Liu H, Du MQ, Isaacson PG, Dogan A. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 237] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematol. 2003;121:681-691. [PubMed] |
| 10. | Hirokawa M, Sawada K, Fujishima N, Kawano F, Kimura A, Watanabe T, Arai A, Matsui T, Nakao S, Urabe A. Acquired pure red cell aplasia associated with malignant lymphomas: a nationwide cohort study in Japan for the PRCA Collaborative Study Group. Am J Hematol. 2009;84:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Choi JH, Oh YH, Park IK. A case of pure red cell aplasia associated with angioimmunoblastic T-cell lymphoma. Cancer Res Treat. 2010;42:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Lynch JW, Elfenbein GJ, Noyes WD, Braylan RC, Gross MA, Weiner RS. Pure red cell aplasia associated with angioimmunoblastic lymphadenopathy with dysproteinemia. Am J Hematol. 1994;46:72-78. [PubMed] |
| 13. | Cho YU, Chi HS, Park CJ, Jang S, Seo EJ, Huh J. Distinct features of angioimmunoblastic T-cell lymphoma with bone marrow involvement. Am J Clin Pathol. 2009;131:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Went P, Agostinelli C, Gallamini A, Piccaluga PP, Ascani S, Sabattini E, Bacci F, Falini B, Motta T, Paulli M. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24:2472-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |