Published online May 10, 2011. doi: 10.5306/wjco.v2.i5.229
Revised: March 8, 2011
Accepted: March 15, 2011
Published online: May 10, 2011
In Japan, the use of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) for some malignant tumors came to be covered by the National Health Insurance in 2002. In 2010, the health insurance coverage was expanded to all types of malignant tumors. However, since PET examination requires a large amount of capital investment, facilities at which PET is available are still limited. On the other hand, PET equipment has rapidly been introduced in large hospitals and in the diagnostic imaging centers of major cities during the past few years. Although numerous middle-sized and small hospitals cannot afford to perform PET, physicians can refer their patients to facilities where PET is available. Therefore, it is essential for general physicians to gain accurate knowledge on PET, including the appropriate indications for PET, in order to select patients for referral to PET facilities. PET is not always a useful tool, especially for lesions of the pancreas and hepatobiliary system, which is the main topic of this review. The indications of PET for lesions in these organs vary depending on the purpose of the examination. In this article, we review the indications for PET (or PET/computed tomography [CT]) using FDG of the liver, biliary tract, and pancreas.
- Citation: Murakami K. FDG-PET for hepatobiliary and pancreatic cancer: Advances and current limitations. World J Clin Oncol 2011; 2(5): 229-236
- URL: https://www.wjgnet.com/2218-4333/full/v2/i5/229.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i5.229
Liver cancer can be classified as hepatocellular carcinoma (HCC), cholangiocellular carcinoma (CCC) or metastatic hepatic carcinoma, and the degree of 18F-fluorodeoxyglucose(FDG) uptake and clinical usefulness of FDG-positron emission tomography (PET) differ according to the histological type.
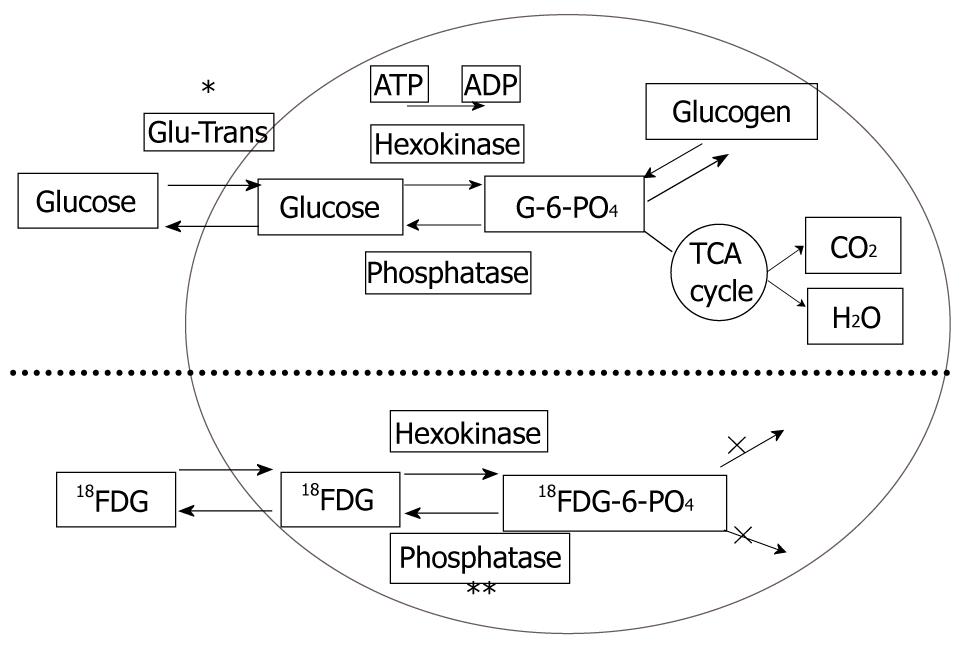
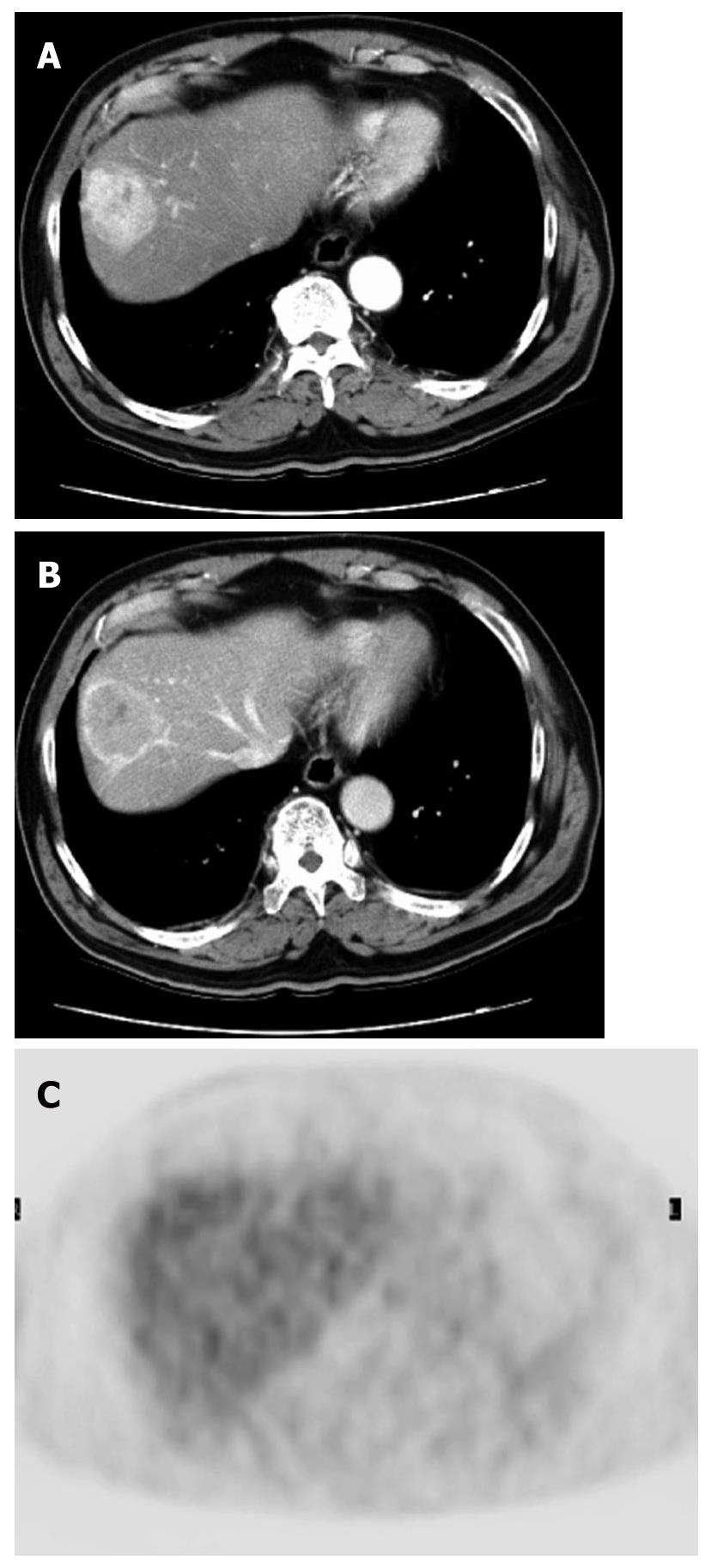
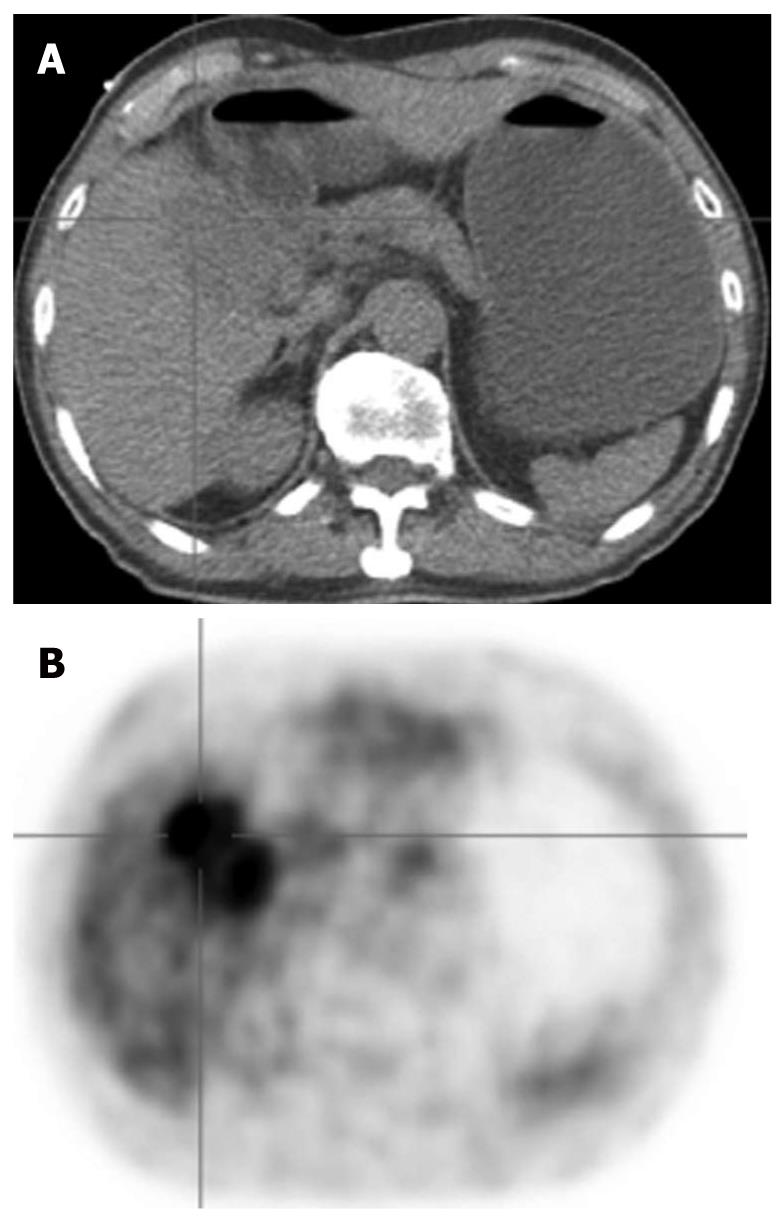
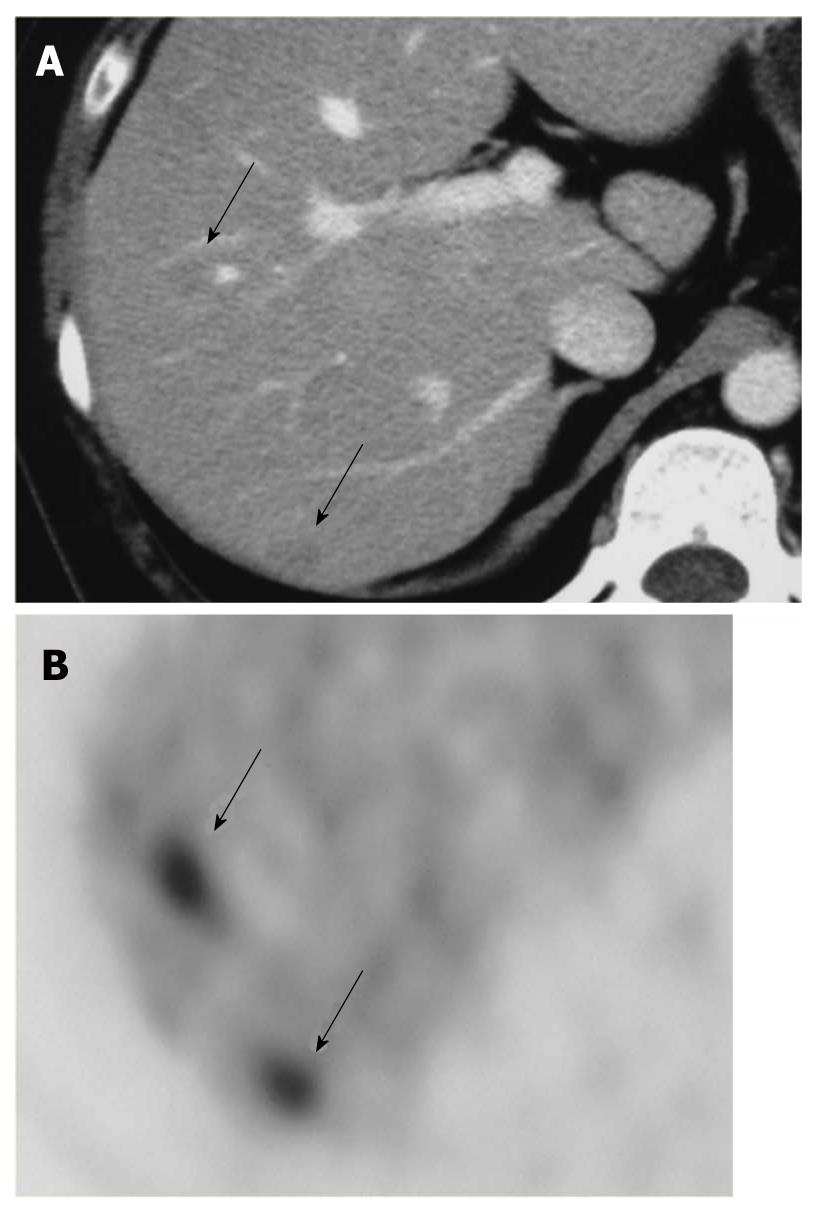
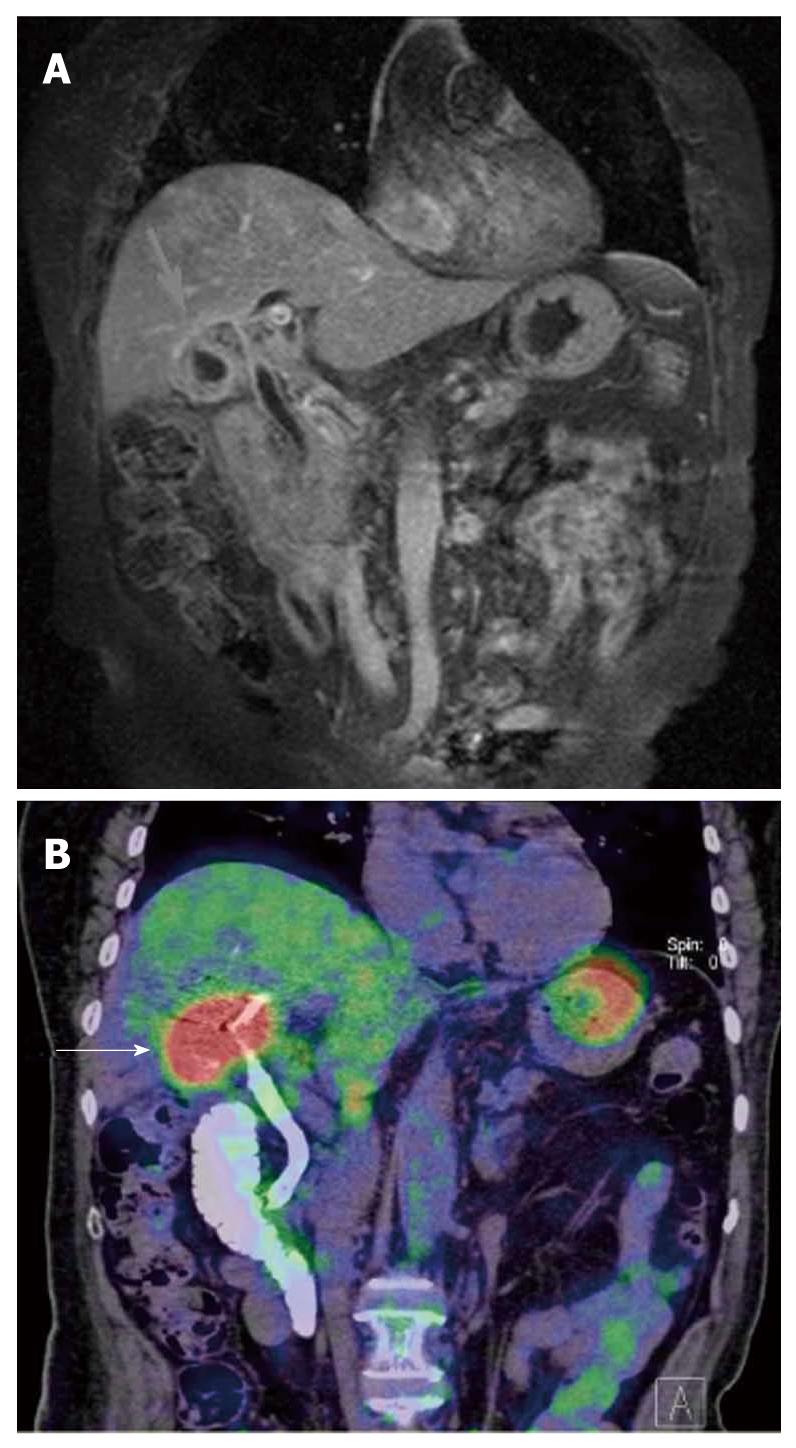
HCC is known to show a faint FDG uptake. This can be explained based on the mechanism of FDG uptake in tumors. FDG is an analogue of glucose, and when injected into the body, it is taken up by the cells and phosphorylated in the same pathway as glucose. The metabolic process of FDG is the same as that of glucose up to this point, but the reactions of FDG do not proceed further (Figure 1). In other words, the FDG remains in the cells. On the other hand, because dephosphorylating enzyme activity is higher in normal liver cells than in other tissues, it is likely that the glucose accumulated by normal cells is dephosphorylated again and excreted out of the cells. Since such enzyme activity is retained in well-differentiated HCC, equilibrium is reached when the FDG in the cells is also excreted out of the cells. Moreover, the activity of the glucose transporter is known to be weak as compared with that in other types of malignant tumors. Therefore, HCC shows relatively weak FDG uptake (Figure 2). On the other hand, since the enzyme activity is correlated with the degree of differentiation of HCC, poorly-differentiated HCC shows weak enzyme activity and strong FDG uptake[1,2]. Since the FDG uptake appears to vary with the degree of differentiation of HCC, we may be able to predict, to some extent at least, the degree of differentiation of HCC by the degree of FDG uptake, even though FDG-PET is still not very useful for the diagnosis of HCC: the lower the degree of histological differentiation of HCC, the higher the FDG uptake level. Furthermore, since poorly differentiated HCC is frequently associated with metastasis and recurrence, FDG/PET is useful for detecting such metastasis/recurrence, as it has the merit of imaging the whole body (Figures 3 and 4)[3]. Moreover, the degree of histological differentiation is thought to be correlated with prognosis, and the poorer the degree of differentiation of the HCC, the poorer the prognosis. Thus, FDG-PET may be a promising and useful tool in the future for predicting the prognosis of HCC[4,5]. On the other hand, some reports have mentioned the usefulness of non-FDG radiopharmaceuticals such as choline[6] and acetate[7]. Although the efficacy and the role of these drugs in HCC are not yet established, it is possible that non-FDG PET may also be a promising tool in the future.
CCC is histologically classified as adenocarcinoma, and usually shows increased FDG uptake (Figure 5)[8,9]. However, since both poorly differentiated HCC and metastatic hepatic carcinoma show marked FDG uptake, as stated above, it is difficult to differentiate between the two types of cancer based on the uptake of FDG alone. Thus, other morphological diagnostic imaging techniques such as CT or magnetic resonance imaging (MRI) are indispensable for reference.
Moreover, diagnostic “high-resolution” imaging tools, such as direct contrast radiography, endoscopic ultrasound (EUS), intraductal ultrasound (IDUS), contrast-enhanced CT and MRI, are sufficient for diagnosing the stage of primary lesions, thus, the clinical significance of PET is of little value for diagnosis of the T factor in CCC. On the other hand, PET may be used as a complementary diagnostic tool for the diagnosis of lymph node metastases when lesions are around 10 mm, when they are difficult to assess by CT alone. However, it is difficult to detect microscopic metastases by PET; thus, PET remains a diagnostic imaging procedure with “high specificity but low sensitivity”. As in the case of other cancers, PET is expected to be useful for detecting the presence/absence of distant metastases and diagnosing recurrent disease in CCC. In particular, PET is useful for the diagnosis of distant metastasis, as demonstrated by a study which showed that the treatment policy was determined by PET in 17% of cases[10], and another study which showed that PET was helpful in changing the treatment policy in 30% of cases[11]. PET is excellent for diagnosing recurrent disease, which is difficult to detect after hepatic resection or bile duct resection, due to its excellent contrast resolution. However, FDG uptake is reduced even in cases of CCC when recurrent cancer cells grow only gradually; thus, one of the pitfalls of FDG-PET is its low detection rate of recurrence.
The visualization of liver metastases may depend on the histological features of the primary lesion. In general, when the primary lesion shows marked FDG uptake, the metastases also show increased FDG uptake. However, the visualization of metastases on PET is also influenced by other factors, e.g., tumor-related factors, such as tumor size, cell density, presence of bleeding and necrosis, and external factors, such as blood glucose level and respiratory movements during data acquisition. Thus, the FDG uptake may differ between the primary and metastatic lesions depending on the aforementioned factors.
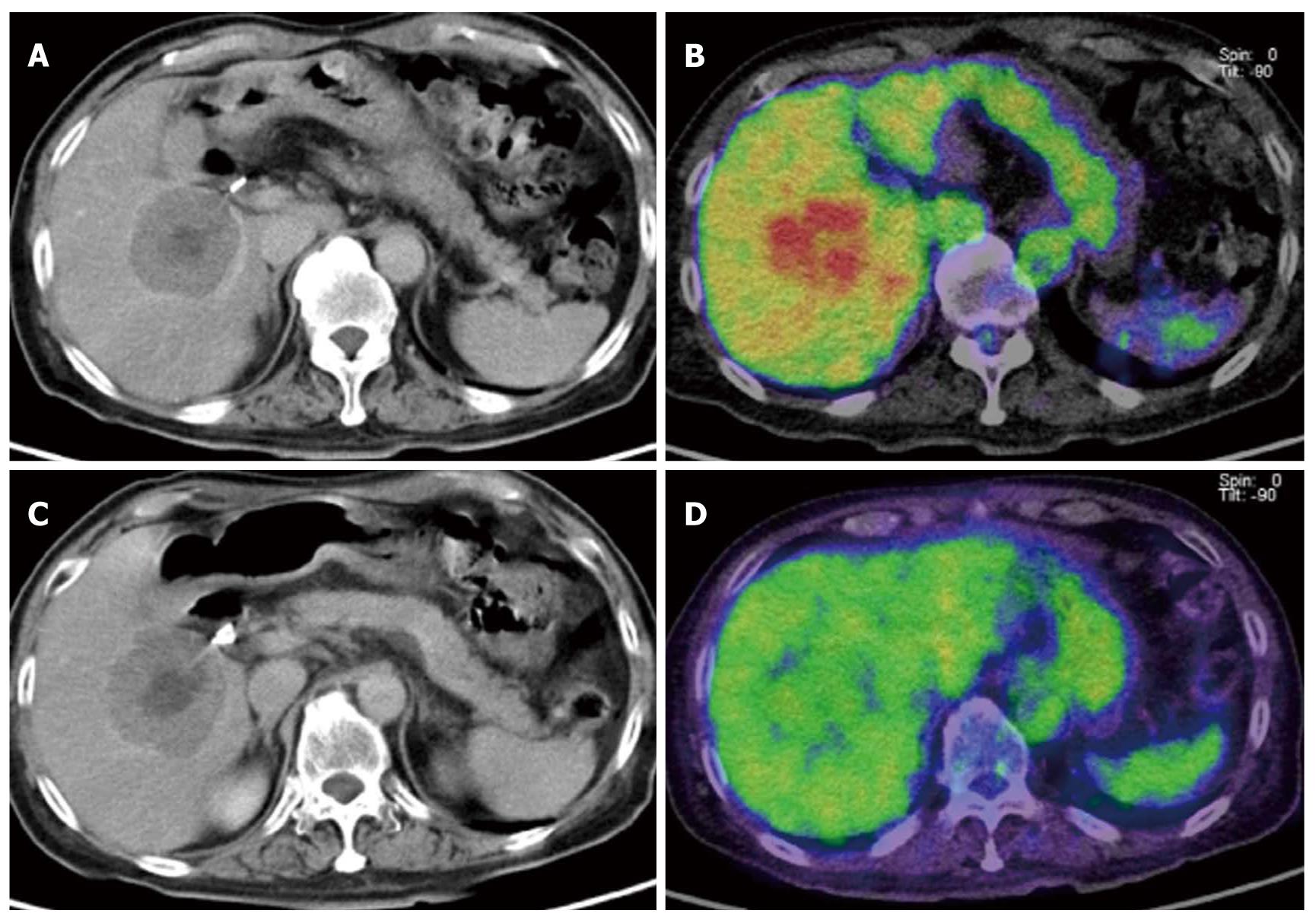
As compared with other imaging modalities, PET is not the most suitable for detecting small lesions because of its poor spatial resolution. Even if liver tumors show FDG avidity, tumor uptake of FDG must be stronger than the physiological liver uptake to be clearly recognized. It is evident that the contrast resolution of PET is superior to that offered by plain or contrast-enhanced CT (Figure 6). However, contrast-enhanced dynamic CT performed at an appropriate contrast timing using multi-detector row CT may allow the detection of small lesions that measure φ5 mm or less. MRI, which offers a good balance of both contrast resolution and spatial resolution, can also be an excellent diagnostic tool for visualizing liver metastases. Ruers et al[12] focused on the usefulness of PET for the detection of metastatic lesions in addition to primary hepatic tumors. Another report also emphasized the merit of FDG-PET to identify restaging disease, and FDG-PET has additional clinical value in the management of solitary liver metastases[13].
CT alone is sometimes inadequate for differentiating liver tumors, such as small cysts from hemangiomas or hepatic metastases. However, FDG-PET is useful for differentiating malignant from benign tumors because of its high specificity. Thus, a combination of modalities, i.e., CT with high sensitivity and PET with high specificity, may be the most effective combination for the diagnosis of liver metastases. As plain CT alone is inadequate for detecting liver metastases, we sometimes perform PET/contrast-enhanced CT at our facility to avoid performing contrast-enhanced CT and PET separately.
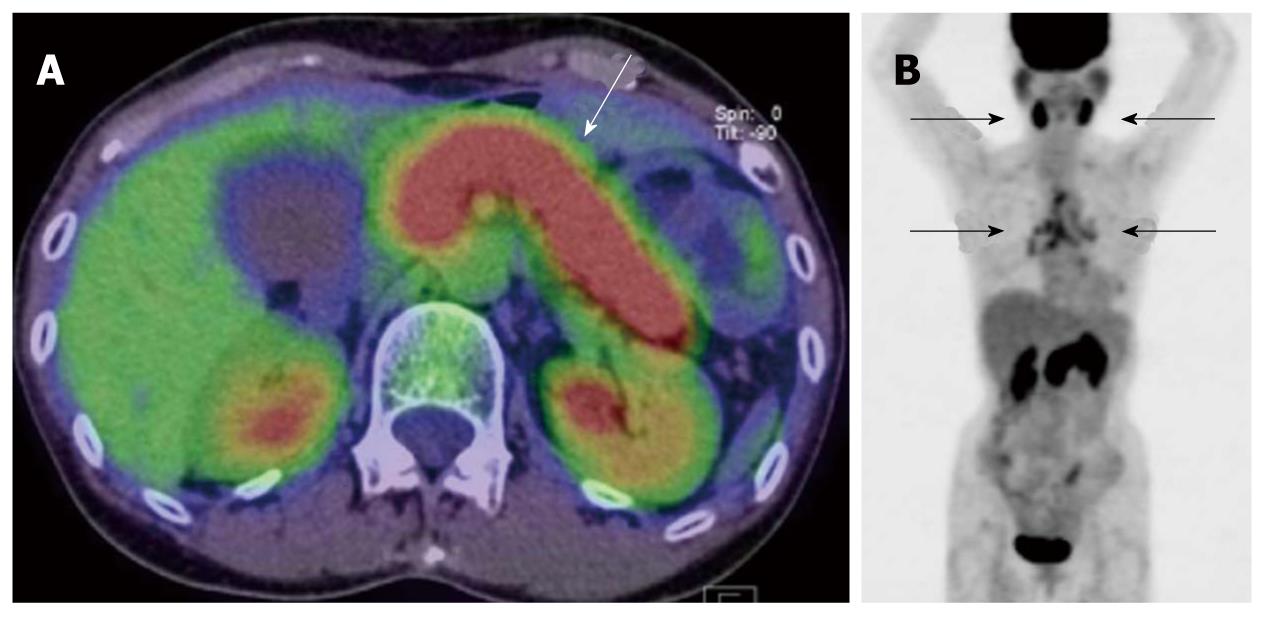
PET is expected to play an important role in the future for the assessment of therapeutic response to molecular-targeted drugs. Molecular-targeted drugs have been reported to be less effective in decreasing tumor size compared to conventional anticancer drugs. Consequently, the findings of PET have attracted attention as surrogate markers for the effects of molecular-targeted drugs. At present, molecular-targeted drugs are widely used in the treatment of lung cancer, breast cancer and gastrointestinal stromal tumors, which frequently occur with liver metastases. Since PET allows detection of not only liver metastases but also metastases elsewhere in the body, it is expected to play a more important role in the future for surrogate markers (Figure 7)[14].
PET examination for extrahepatic bile duct cancer: According to the report of Petrowsky et al[10] the diagnostic accuracy of FDG-PET was 53% for extrahepatic bile duct cancer, indicative of a poor diagnostic performance. The flat “infiltrating” type, which is the most common type of extrahepatic bile duct cancer, is characterized by tubular adenocarcinoma with abundant fibrosis and endoluminal extension. Such histological and morphological features are major reasons for the apparently reduced uptake of FDG in these tumors.
On the other hand, the papillary type (one of the minor subtypes of bile duct cancer) which is characterized by a massive form and protruding growth into the lumen sometimes shows increased uptake of FDG. PET has been shown to have high sensitivity for the detection of this histological type of bile duct cancer[11,15].
It is desirable that PET examination for bile duct cancer be performed prior to the insertion of a PTCD tube, because stimulation due to the tip of the inserted tube causes cholangitis. It may cause a pseudo-positive result.
Although FDG also accumulates due to lymph node metastases of extrahepatic bile duct cancer, it is incapable of revealing microscopic metastases. In other words, FDG-PET is not useful for the detection of lymph node metastases from extrahepatic bile duct cancer because of its low sensitivity[15]. Thus, FDG-PET appears to have limited usefulness in the diagnosis of bile duct cancer.
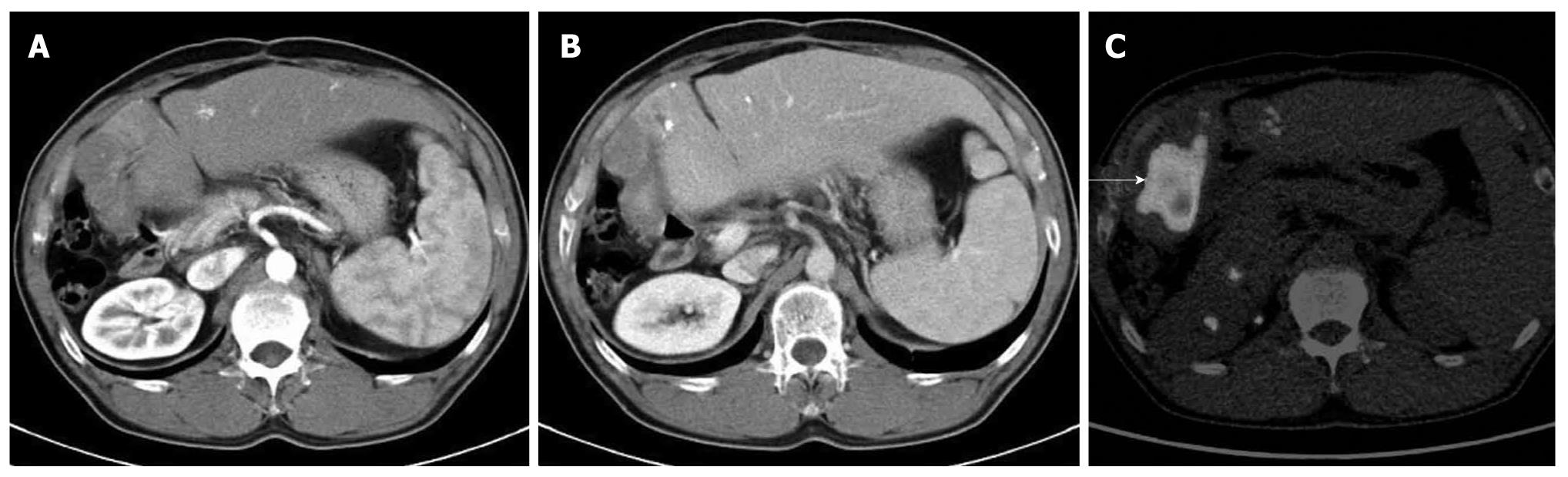
FDG-PET has a sensitivity of 75-100% and specificity of 80-89% for the detection of primary gallbladder cancer as mentioned in the literature (Figure 8). However, ultrasound, MRI, and contrast-enhanced CT are better for the detection of this cancer because of their high spatial resolution. FDG-PET is reported to be useful for differentiating benign from malignant gallbladder tumors[16], although acute cholecystitis and mass-forming xanthogranulomatous cholecystitis may also show marked FDG uptake (Figure 9). Thus, the ability of this modality to differentiate these tumors remains controversial. Moreover, FDG-PET appears to be a poor tool for detecting early gallbladder cancer because of its poor spatial resolution. For gallbladder cancer, the primary aim of performing FDG-PET is to identify distant metastases and recurrence.
PET examination of the pancreas is covered by the National Health Insurance for “differentiating pancreatitis from pancreatic cancer.” In 2006, the health insurance coverage was expanded to the diagnosis of metastasis and recurrence.
Conventionally, it has been thought that FDG-PET would be useful for differentiating pancreatic cancer from tumor-forming pancreatitis, as cancers show more marked FDG uptake as compared to pancreatitis. Chronic pancreatitis can be differentiated from cancer due to its lower FDG uptake compared to that of cancer. However, inflammatory cells also show increased FDG uptake because of accelerated glucose metabolism, therefore, the differentiation between acute pancreatitis and cancer is difficult. Accordingly, positive findings obtained in patients who have clinical symptoms of pancreatitis or biochemical evidence of inflammation should be interpreted with caution. Imdahl A et al[17] reported that delayed PET imaging is useful for the differentiation of cancer from acute pancreatitis as cancer shows increased deposits in the delayed phase. However, a controversial study has reported that FDG uptake is enhanced in the delayed phase even in cases of inflammation. Thus, FDG-PET cannot be regarded as a reliable imaging tool for differentiating between acute pancreatitis and cancer even when delayed images are obtained.
A possible diagnosis of pancreatitis can be made when a tumor shows gradually decreasing FDG uptake within a short interval.
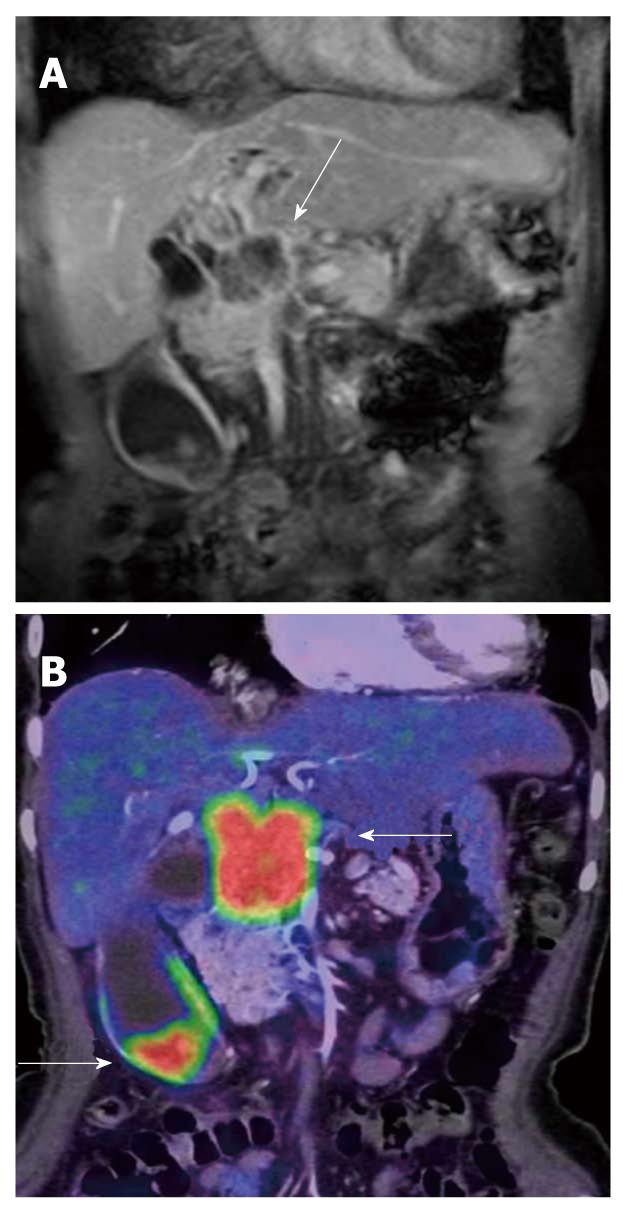
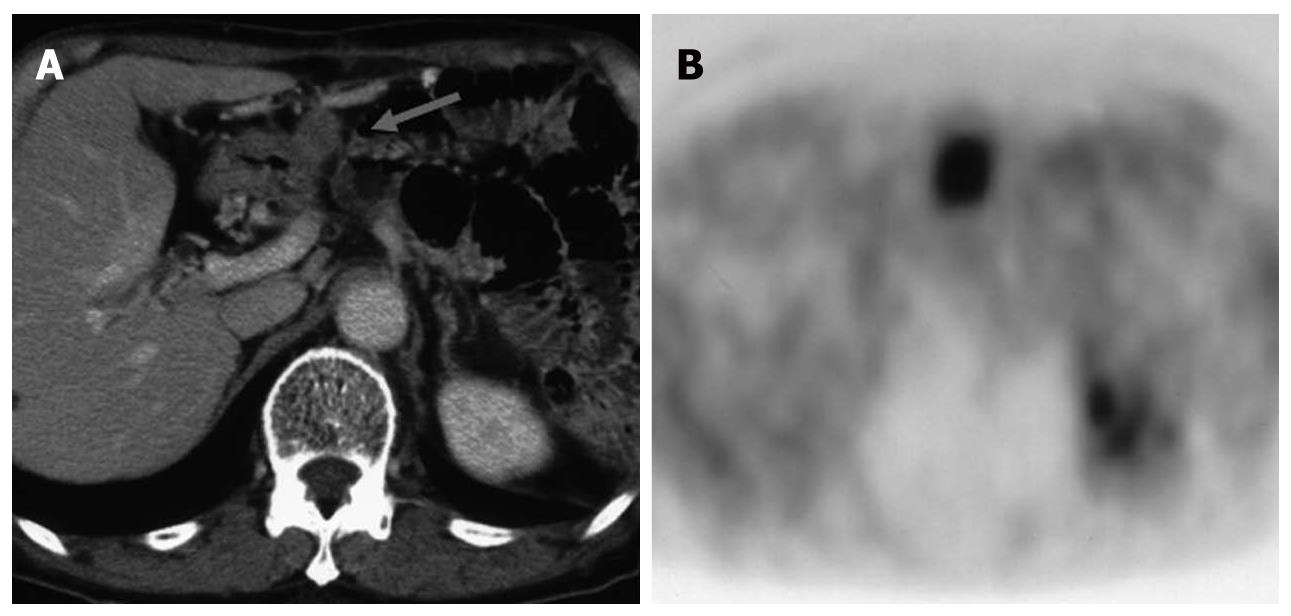
FDG-PET has been reported to play a significant role in the differentiation of IgG4-related pancreatitis among cases of pancreatitis. This disease entity has been widely recognized in recent years, and an increasing number of patients are diagnosed with IgG4-related pancreatitis. This disease has been defined as a systemic disease complicated by inflammation in various organs other than the pancreas. FDG-PET is reported to be an effective tool for evaluating these lesions[18] because various organs, such as the salivary glands, hilar lymph nodes, lungs (interstitial pneumonia), kidney (nephritis) and retroperitoneum are sometimes involved simultaneously. In other words, abnormal FDG uptake other than in the pancreas may raise suspicion of IgG4-related pancreatitis rather than pancreatic cancer (Figure 10).
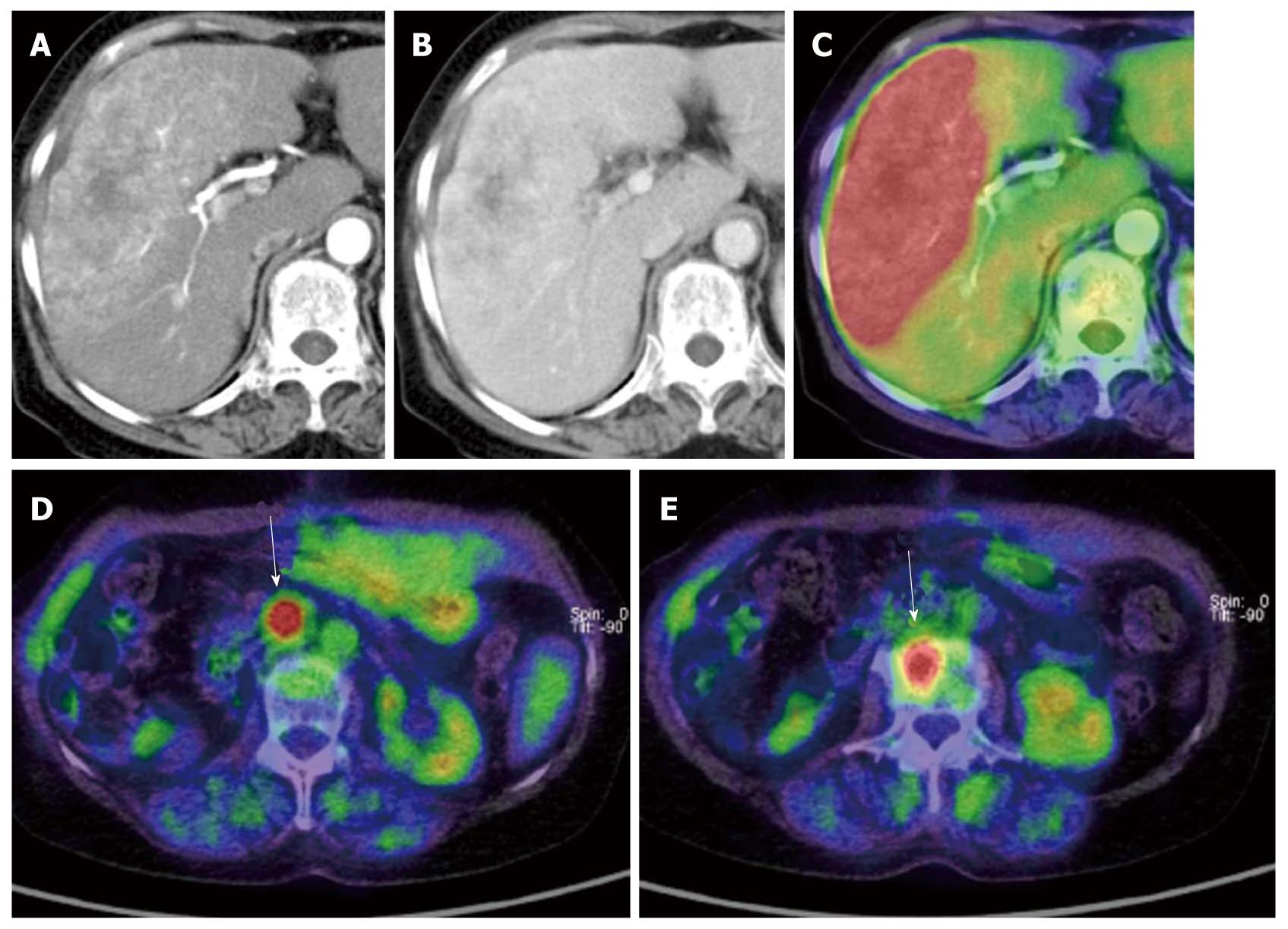
In cases of pancreatic cancer, PET is most useful for identifying distant metastasis and recurrence. Local recurrence is sometimes difficult to evaluate by conventional morphological imaging alone because it is associated with treatment-related morphological changes, such as fibrosis, hemorrhage, etc. Moreover, as pancreatic cancer has poor vascularity, it is difficult to evaluate the tumor based on the dynamic contrast study. Under these circumstances, PET may be of significant value for visualizing the lesion due to its high contrast resolution.
Another reason for the difficulty in detecting distant metastasis based on conventional imaging is that it is hard to predict the site of metastasis. PET whole body imaging is of great value particularly when recurrence is suspected by clinical symptoms such as the development of pain or increased serum levels of tumor markers, etc. (Figure 11). Ruf et al[19] performed PET, CT and MRI in 23 patients with clinically suspected recurrence of pancreatic cancer based on the development of postoperative pain, decreased body weight and increased serum levels of tumor markers, and confirmed recurrence by PET in 22 patients (96%) on PET, but in only 9 patients (39%) by CT/MRI.
Even PET alone has been shown to be superior to CT in previous publications. However, it is difficult to differentiate between physiological and pathological accumulation in the ureter, bladder and intestinal tract by PET alone because of a lack of anatomical information. To resolve this issue, a PET/CT system was developed. PET/CT can offer combined images of PET with CT to add anatomical information to FDG uptake. PET/CT may replace dedicated PET scanners in the near future.
In this review, we have outlined the usefulness and limitations of PET for the evaluation of lesions in the liver, gallbladder, and pancreas. Ultrasound and dynamic CT are the simplest and most economical imaging modalities for the diagnosis of lesions in these organs. In addition, many other imaging tools, such as MRI, EUS and IDUS, are also available for detailed evaluation of these organs. All of these methods are used as “high-resolution” diagnostic imaging tools for visualizing “locoregional areas,” and PET is unlikely to play an important role in the local diagnosis of these lesions. In contrast, PET (PET/CT) involves whole-body imaging and is useful for visualizing distant metastases and unexpected recurrences. Therefore, PET/CT appears to be of significance in evaluation of the whole body in cases with advanced or atypical tumors. Since PET/CT began to be covered by the National Health Insurance in 2002, we perform PET/contrast-enhanced CT in cases of advanced cancer for evaluation of the presence of distant metastases, for evaluation of therapeutic outcomes, and for the early diagnosis of recurrence. I have also recommended performing “PET/contrast-enhanced CT scans first” for examination of the whole body (except for the head). Simultaneous PET and contrast-enhanced CT scanning appears to be an efficient method with improved diagnostic accuracy, and it is unnecessary to perform PET and contrast-enhanced CT separately.
Peer reviewer: Domenico Rubello, MD, Professor, Director of the Department of Nuclear Medicine, PET/CT Centre, Radiology, Medical Physics, Santa Maria della Misericordia Hospital; Via Tre Martiri 140, ZIP 45100, Rovigo, Italy
S- Editor Tian L L- Editor Webster JR E- Editor Tian L
| 1. | Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, Zeuzem S. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314-3319. |
| 2. | Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, Tanaka A, Yamaoka Y, Yamamoto K, Konishi J. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995;36:1811-1817. |
| 3. | Iwata Y, Shiomi S, Sasaki N, Jomura H, Nishiguchi S, Seki S, Kawabe J, Ochi H. Clinical usefulness of positron emission tomography with fluorine-18-fluorodeoxyglucose in the diagnosis of liver tumors. Ann Nucl Med. 2000;14:121-126. |
| 4. | Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, Fujii H, Shimahara Y. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg. 2006;30:1736-1741. |
| 5. | Sugiyama M, Sakahara H, Torizuka T, Kanno T, Nakamura F, Futatsubashi M, Nakamura S. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39:961-968. |
| 6. | Talbot JN, Gutman F, Fartoux L, Grange JD, Ganne N, Kerrou K, Grahek D, Montravers F, Poupon R, Rosmorduc O. PET/CT in patients with hepatocellular carcinoma using [(18)F]fluorocholine: preliminary comparison with [(18)F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2006;33:1285-1289. |
| 7. | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213-221. |
| 8. | Keiding S, Hansen SB, Rasmussen HH, Gee A, Kruse A, Roelsgaard K, Tage-Jensen U, Dahlerup JF. Detection of cholangiocar cinoma in primary sclerosing cholangitis by positron emission tomography. Hepatology. 1998;28:700-706. |
| 9. | Kim YJ, Yun M, Lee WJ, Kim KS, Lee JD. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur J Nucl Med Mol Imaging. 2003;30:1467-1472. |
| 10. | Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, Clavien PA. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43-50. |
| 11. | Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90-97. |
| 12. | Ruers TJ, Langenhoff BS, Neeleman N, Jager GJ, Strijk S, Wobbes T, Corstens FH, Oyen WJ. Value of positron emission tomography with [F-18]fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20:388-395. |
| 13. | Grassetto G, Fornasiero A, Bonciarelli G, Banti E, Rampin L, Marzola MC, Massaro A, Galeotti F, Del Favero G, Pasini F. Additional value of FDG-PET/CT in management of “solitary” liver metastases: preliminary results of a prospective multicenter study. Mol Imaging Biol. 2010;12:139-144. |
| 14. | Heinicke T, Wardelmann E, Sauerbruch T, Tschampa HJ, Glasmacher A, Palmedo H. Very early detection of response to imatinib mesylate therapy of gastrointestinal stromal tumours using 18fluoro-deoxyglucose-positron emission tomography. Anticancer Res. 2005;25:4591-4594. |
| 15. | Kato T, Tsukamoto E, Kuge Y, Katoh C, Nambu T, Nobuta A, Kondo S, Asaka M, Tamaki N. Clinical role of (18)F-FDG PET for initial staging of patients with extrahepatic bile duct cancer. Eur J Nucl Med Mol Imaging. 2002;29:1047-1054. |
| 16. | Koh T, Taniguchi H, Yamaguchi A, Kunishima S, Yamagishi H. Differential diagnosis of gallbladder cancer using positron emission tomography with fluorine-18-labeled fluoro-deoxyglucose (FDG-PET). J Surg Oncol. 2003;84:74-81. |
| 17. | Imdahl A, Nitzsche E, Krautmann F, Högerle S, Boos S, Einert A, Sontheimer J, Farthmann EH. Evaluation of positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose for the differentiation of chronic pancreatitis and pancreatic cancer. Br J Surg. 1999;86:194-199. |
| 18. | Nakajo M, Jinnouchi S, Noguchi M, Uozumi K, Tanabe H, Tateno R, Nakajo M. FDG PET and PET/CT monitoring of autoimmune pancreatitis associated with extrapancreatic autoimmune disease. Clin Nucl Med. 2007;32:282-285. |
| 19. | Ruf J, Lopez Hänninen E, Oettle H, Plotkin M, Pelzer U, Stroszczynski C, Felix R, Amthauer H. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5:266-272. |