Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.179
Peer-review started: October 17, 2015
First decision: November 24, 2015
Revised: December 24, 2015
Accepted: February 23, 2016
Article in press: February 24, 2016
Published online: May 6, 2016
Processing time: 186 Days and 22.2 Hours
Gastro-esophageal reflux disease (GERD) is a very common disorder that results primarily from the loss of an effective antireflux barrier, which forms a mechanical obstacle to the retrograde movement of gastric content. GERD can be currently treated by medical therapy, surgical or endoscopic transoral intervention. Medical therapy is the most common approach, though concerns have been increasingly raised in recent years about the potential side effects of continuous long-term medication, drug intolerance or unresponsiveness, and the need for high dosages for long periods to treat symptoms or prevent recurrences. Surgery too may in some cases have consequences such as long-lasting dysphagia, flatulence, inability to belch or vomit, diarrhea, or functional dyspepsia related to delayed gastric emptying. In the last few years, transoral incisionless fundoplication (TIF) has proved an effective and promising therapeutic option as an alternative to medical and surgical therapy. This review describes the steps of the TIF technique, using the EsophyX® device and the MUSETM system. Complications and their management are described in detail, and the recent literature regarding the outcomes is reviewed. TIF reconfigures the tissue to obtain a full-thickness gastro-esophageal valve from inside the stomach, by serosa-to-serosa plications which include the muscle layers. To date the procedure has achieved lasting improvement of GERD symptoms (up to six years), cessation or reduction of proton pump inhibitor medication in about 75% of patients, and improvement of functional findings, measured by either pH or impedance monitoring.
Core tip: Transoral incisionless fundoplication (TIF) has recently emerged as an effective and promising therapeutic option in alternative to medical and surgical therapy for gastro-esophageal reflux disease (GERD). A number of prospective observational studies for TIF using the EsophyX® device have been published but there is still only limited data for TIF with the MUSETM system. This review describes the techniques for TIF with both these devices, and is intended to consolidate the current literature, clarifying better the outcomes of TIF in patients with GERD.
- Citation: Testoni PA, Mazzoleni G, Testoni SGG. Transoral incisionless fundoplication for gastro-esophageal reflux disease: Techniques and outcomes. World J Gastrointest Pharmacol Ther 2016; 7(2): 179-189
- URL: https://www.wjgnet.com/2150-5349/full/v7/i2/179.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i2.179
Gastro-esophageal reflux disease (GERD) is a very common disorder that can be currently treated by medical therapy, surgical or endoscopic transoral intervention. Medical therapy with proton pump inhibitors (PPIs) is the most common approach: However, concerns have increasingly been voiced in recent years regarding the potential PPI-related side effects, intolerance or unresponsiveness, and in some cases the need for a long-term therapy with high dosages to relieve symptoms or prevent recurrences. Surgical therapy too may in some cases have consequences such as long-lasting dysphagia, flatulence, inability to belch or vomit, diarrhea, or functional dyspepsia related to delayed gastric emptying[1-4]. Even for interventions done in centers of excellence, incisional hernias at the site of trocar insertion have been reported in up to 3% of cases[5].
For these reasons several transoral endoscopic techniques have been proposed in the last 15 years as alternatives to medical and surgical therapy; however most of them had disappointing outcomes and have been abandoned.
In the last few years, transoral incisionless fundoplication (TIF) has proved to be an effective and promising therapeutic alternative to medical and surgical therapy; the procedure achieves lasting improvement of GERD symptoms (up to six years) and functional findings, and cessation or reduction of PPI medication in about 75% of patients. TIF reconfigures the tissue to obtain a full-thickness gastro-esophageal valve from inside the stomach, by serosa-to-serosa plications which include the muscle layers; the new valve boosts the barrier function of the LES with potentially fewer procedure-related side effects than surgery.
TIF can be done using the EsophyX® device (EndoGastric Solutions, Redmond, WA, United States) or the Medigus Ultrasonic Surgical Endostapler system (MUSE™, Medigus Ltd., Omer; Israel). EsophyX® device constructs an omega-shaped valve 3-5 cm long, in a 250°-300° circumferential pattern around the gastro-esophageal junction, by deploying non-absorbable polypropylene fasteners through the two layers (esophagus and stomach) under endoscopic vision of the operator. The MUSE™ system staples the fundus of the stomach to the esophagus below the diaphragm using multiple sets of metal stitches placed under an ultrasound-guided technique and creates an anterior fundoplication functionally similar to the standard surgical Dor-Thal operation. In a patient with sliding hiatal hernia, the procedure can be done only if the hernia can be reduced below the diaphragm.
Publications on TIF with the EsophyX® device report the persistence of the newly created valve at six months in all studies and for up to six years in one study, with satisfactory outcomes, assessed by 24-h pH and/or impedance monitoring[6-27]. There is less information so far for TIF with the MUSE™ system: One animal study found the technique safe and feasible and two trials in humans reported good clinical and functional results at six-month and up to five-year follow-up[28-30].
This review describes the techniques for TIF, using the EsophyX® device and the MUSETM system, pre- and post-procedure patients’ management, and complications. Outcomes are reported in detail, and a revision of the literature was performed to assess the efficacy of TIF in patients with GERD. Manuscripts were identified by searching PubMed, Embase and The Cochrane Library databases, using the following key words “gastro-esophageal reflux disease”, “transoral incisionless fundoplication”, “anterior fundoplication”, “medigus ultrasonic surgical endostapler”, “EsophyX”, “MUSE”, and “surgical fundoplication”.
Preoperative upper gastrointestinal endoscopy must be done to assess the distance between the incisor teeth and the esophago-gastric junction (EGJ), and the transverse dimension of the diaphragmatic hiatus. With the current TIF technique only a hiatal hernia not more than 3.0 cm long can be reduced below the diaphragm, while a hiatus larger than 3.0 cm may facilitate a cranial displacement of the plication up in the thorax, making ineffective the newly created valve.
Prior to the intervention all the patients should be examined by esophageal manometry to exclude primary motility disorders, and by 24-h pH-impedance monitoring to exclude a functional heartburn. If the MUSE™ system is used, barium swallow should be done to assess the reducibility of the hernia, since irreducibility is a contraindication to the procedure.
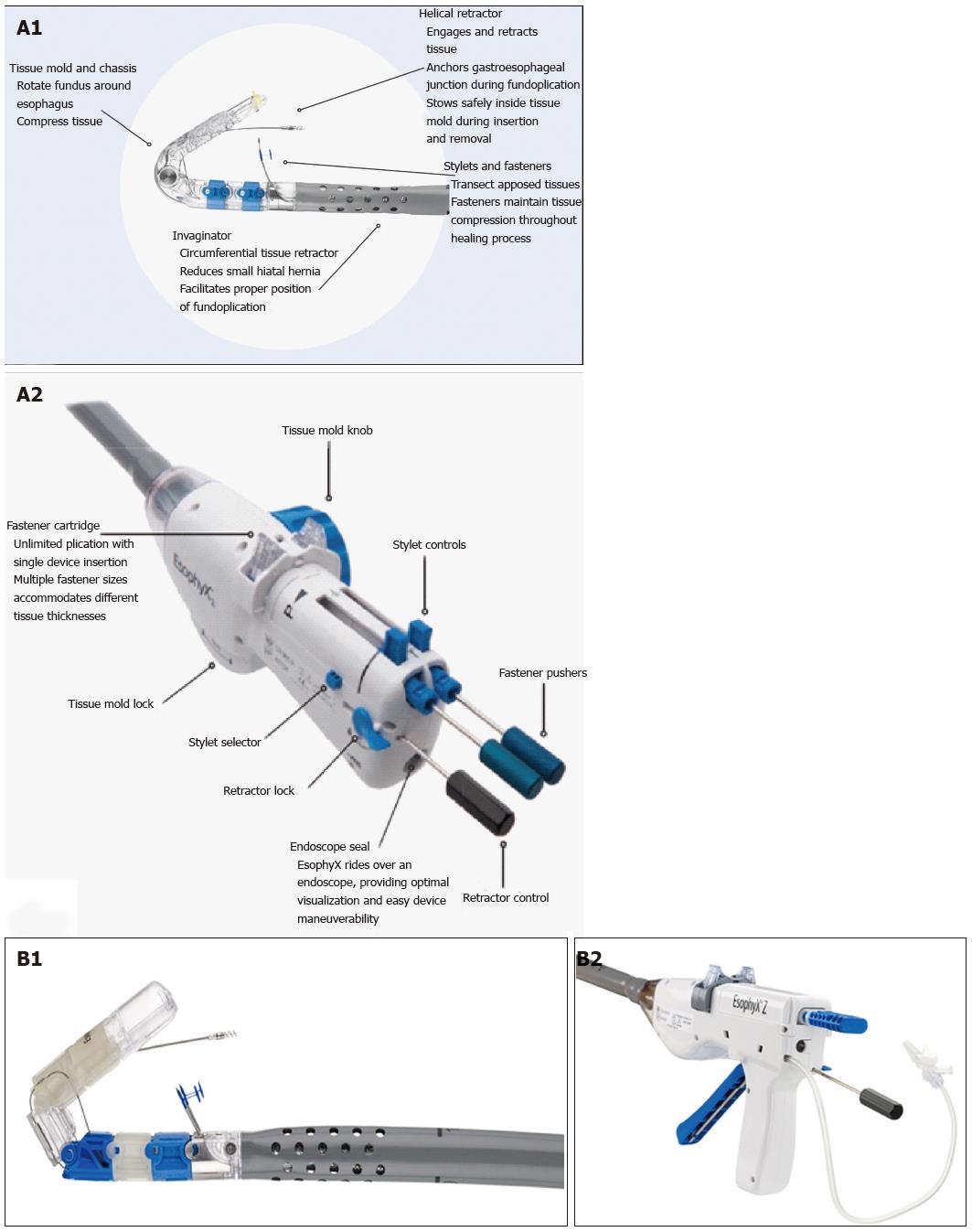
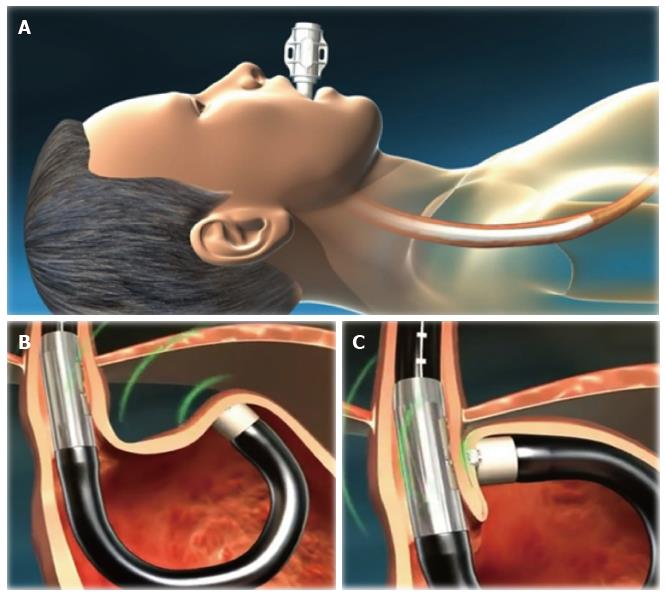
The EsophyX® device is composed of: (1) a handle that houses the controls; (2) an 18-mm diameter chassis that includes operative channels through which a front-view 9-mm diameter endoscope can be inserted; (3) the tissue invaginator, provided by side holes on the distal part of the chassis, to which external suction can be applied; (4) the tissue mold, which can be brought into retroflection and pushes tissue against the shaft of the device; (5) a helical screw, which is advanced into the tissue so the tissue between the tissue mold and the shaft can be retracted; (6) two stylets, which pass through the plicated tissue and the tissue mold, and H-shaped polypropylene fasteners can be deployed over them; and (7) a cartridge containing 20 fasteners. The device has been recently updated and improved in a new generation instrument: The EsophyX® Z device. The fastener deployment is similar to a surgical stapler firing mechanism with a reduction of control complexity and dual fastener deployment, and is improved by managing trailing leg. The crossing profile has been reduced with elimination of tissue mold elbow and increase of tissue mold lateral stiffness; the tissue mold tip covers stylets during deployment.
Details of the first and second generation devices are illustrated in the Figure 1.
The procedure requires two operators: One handles the device and the other the endoscope.
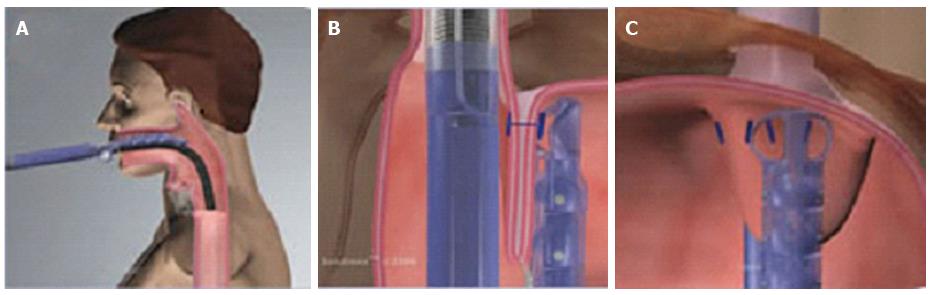
The device is introduced transorally with the patient in the left lateral or supine position, under general anesthesia. In cases with difficult insertion, the device can be gently rotated during the introduction: This maneuver allows to easily pass the upper esophageal sphincter. In this phase, there is a risk of hypopharyngeal perforation if the instrument is inserted without caution.
During the procedure, air or CO2 is insufflated to distend the gastric cavity and permit adequate vision of the fundus and EGJ; CO2 is preferable because it reduces the patient’s discomfort and is safer in case of perforation.
With the patient placed in left decubitus and endoscope positioned in retroflexed view, the lesser curve is located at the 12 o’clock position and the greater curve at 6 o’clock. The tissue mold is retroflexed and closed against the Esophyx device; then it is rotated to 11 or 1 o’clock (lesser curve) and pulled back, to have its tip just inside the esophageal lumen. At this point: (1) the helical retractor is advanced to engage tissue under direct vision just below the Z-line; (2) the tissue mold is opened and the helical screw cable is pulled back to retract the tissue; (3) in this phase of the procedure the stomach is being desufflated to engage an adequate amount of tissue for fundoplication; (4) once such a maneuver has been completed, with both the helical retractor and tissue mold locked in place, suction is applied to the tissue invaginator and the device is then advanced into the stomach, which has been re-insufflated. This permits to create the esophago-gastric plication in an intra-abdominal position and reduces any hiatal hernia.
Plication is performed by deploying multiple H-shaped polypropylene fasteners advanced over the two stylets, starting on the far posterior and anterior sides of the esophago-gastric junction; then additional fasteners are deployed along the greater curvature part of the valve by rotating the tissue mold axially to slide the stomach over the esophagus. This maneuver results in circumferential tightening and a new valve circumference of > 240°. In general 14 fasteners are needed to construct an adequate circumferential valve; however, the more fasteners are deployed the more continent is the valve.
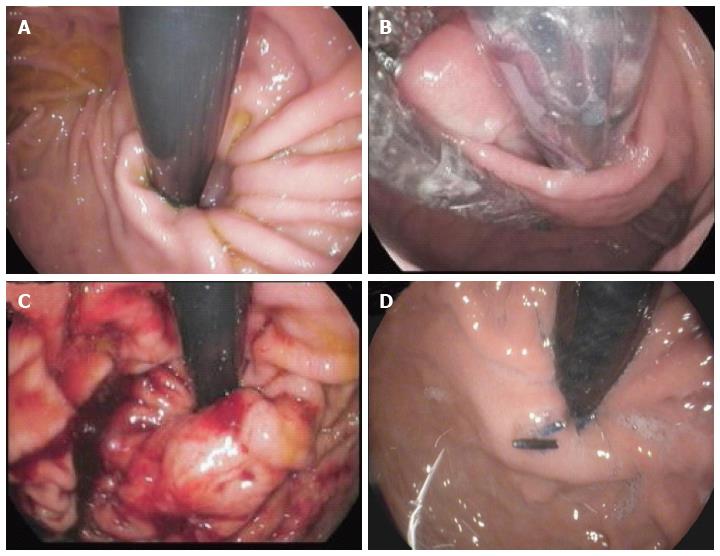
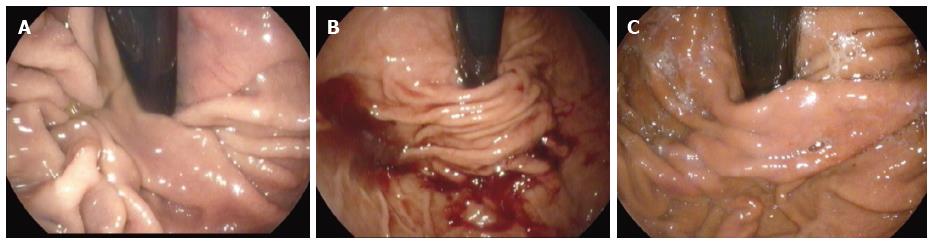
Details of the EsophyX® technique are shown in Figure 2. Endoscopic pre- and post-procedural findings are reported in Figure 3.
Beside the standard procedure, two modified techniques have been described over time to create the fundoplication.
The one we use, engages the tissue below the Z-line at 11 and 1 o’clock positions; then a torque is applied by rotating the locked tissue mold clockwise and counter-clockwise before inserting the stylet. By this maneuver, part of the fundus is rotated around the esophageal wall and more tissue is engaged by the stylet. Four fasteners for each site are deployed at 1 and 11 o’clock, and two for each site in the middle part of the valve, at 4, 6, and 8 o’clock, to reinforce the plication. This technique increased by 30% the success rate of the procedure, achieving the complete elimination of PPI use at 12 mo in 14/22 patients (63.6%), while with the standard technique only 11/27 patients (40.7%) completely stopped PPIs.
Bell et al[19] have developed a so called rotational fundoplication. The helical retractor is engaged at 12 o’clock and the tissue mold is placed at 6 o’clock. Then the tissue mold locked is rotated toward the lesser curve by a radial motion of the handle of the device, to the 12 o’clock position. This maneuver rolls the fundus over and around the distal esophagus to the 1 o’clock position.
At the end of the plication, endoscopy is done to examine the pharynx, esophageal lumen and the gastric fundus, and the fundoplication.
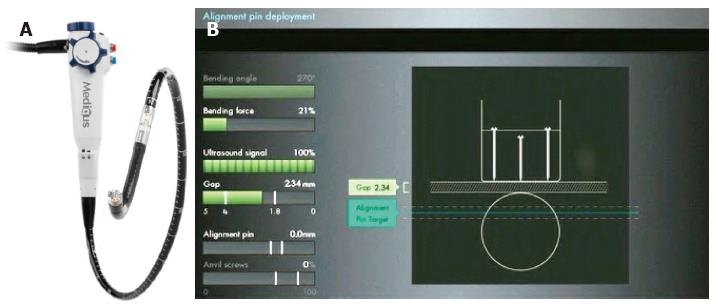
The MUSE™ system includes the endostapler and a console connected with it, containing a controller for the camera, ultrasonic range finder and various sensors, a pump for insufflation and irrigation, a suction system, power and controls for the LED.
The endostapler has: (1) a handle, housing the controls; (2) an insertion tube 15.5 mm in diameter, 66 cm long, containing the suction, insufflation/irrigation channels, and electrical and mechanical cables to operate the device; (3) a rigid section 66 mm long containing the cartridge. Each cartridge holds five standard 4.8-mm titanium staples, the ultrasound mirror, one alignment pin funnel, and two anvil screw funnels; and (4) the distal tip, similar to that of an endoscope, for suction, irrigation, illumination (with a LED) and visualization (with a miniature camera). The anvil, alignment pin, anvil screw and ultrasound are all designed to ensure proper alignment and positioning of the device during stapling. The distal tip can be articulated in one direction to align with the rigid section and cartridge, with a bending radius of 26 and 40 mm. Details of the device are illustrated in Figure 4.
The whole procedure can be done by one operator in experienced hands. The patient is placed in the supine position, under general anesthesia with tracheal intubation. Positive end-expiratory pressure of at least 5 mmHg (7.5 cmH2O) is provided. After a preliminary endoscopic assessment of the esophagus and stomach and, as long as once no contraindications are found, an overtube is placed. The endostapler is then inserted transorally through the overtube and gently advanced into the stomach under direct vision; passing the rigid section across the pharyngo-esophageal junction it may encounter some resistance. To avoid having to apply excessive force and risk injury the esophagus, the overtube may be withdrawn about 5 cm and then advanced with the endostapler as a unit. This maneuver can be repeated until the system reaches the esophageal midbody. Flexing the neck may make passage easier.
Once in the stomach, distended by insufflation of air or CO2, the stapler is advanced until the tip is approximately 5 cm past the EGJ and then retroflexed 180° to obtain adequate vision of the gastric fundus and EGJ so as to select the stapling location. The most important location is the left-most, and is typically done first. This is the anchoring point for the fundus, and should be as far to the left of the esophagus as possible. Sometimes, depending on the anatomy, it may be easier to do the first stapling in a more central position.
Subsequent staplings should be within 60°-180° as long as the right-most stapling is not be on the lesser curve, where it may attach the antrum to the esophagus and open the esophago-gastric junction rather than close it. Further staplings may be placed between the left-most and right-most.
Once the correct locations for stapling have been identified, the rest of the procedure is done under ultrasound guidance. Subsequent phases include clamping tissue, deploying the alignment pin, advancing the anvil screw, stapling, and retrieving anvil screws.
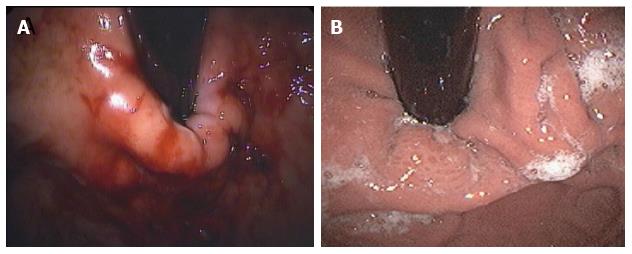
Details of the MUSE™ technique are shown in Figure 5. Endoscopic pre- and post-procedural findings after TIF with this device are reported in Figure 6.
Antiemetic prophylaxis with at least two drugs (according to the ASA recommendations for interventions with high risk of post-procedural nausea and vomiting) and full muscle relaxation throughout the procedure are mandatory for TIF. Antiemetic prophylaxis is maintained intravenously for 24 h, and broad-spectrum antibiotic therapy is continued intravenously for 48 h, then orally for five days.
A transient pharyngeal irritation occurs in most patients, as a result of insertion and manipulation of the device; some patients suffer from a mild to moderate epigastric pain in the six hours after the intervention. If pain persists longer, an esophageal or gastric leak should be considered; in these cases a CT scan and hydrosoluble contrast X-ray investigation should be done. A transient slight elevation of white blood cells count may occur in the 24 h after the intervention.
Patients must follow a liquid diet for the first two weeks and a soft diet for the next four weeks. They are also asked to refrain from vigorous exercise for four weeks. PPIs can be discontinued seven days after the procedure.
The overall complication rates reported so far for TIF with the EsophyX® device range from 3% to 10%. Major complications arose rarely and were bleeding, mucosal tears or perforation requiring endoscopic intervention or surgery, pneumothorax, and mediastinal abscesses. Bleeding requiring transfusions has been reported in about 3%-5% of cases. Mediastinal abscesses have been reported in less than 2% of cases. No procedure-related deaths have occurred.
Among the two studies so far published on TIF with the MUSE™ system, only one reported complications[28]. Minor side effects such as chest pain, sore throat, transient atelectasia, shoulder pain and belching were reported by 5.5% to 22% of patients. Major complications occurred in 6.2% of cases (4 out of 64 patients): Pneumothorax, pneumothorax and esophageal leak, pneumomediastinum, and severe bleeding. Patients with pneumothorax and esophageal leak and with bleeding required intervention. All major complications occurred in the first 24 patients.
No late complications or lasting side effects have occurred with either TIF technique.
To date, 21 prospective studies (3 randomized, controlled) and one retrospective study have been published for TIF using Esophyx® device. Most studies were observational and carried out in limited series, with one to three years follow-up. One study reported outcomes up to six years after the procedure. Sixteen studies assessed symptoms using the GERD health-related quality of life (HRQL) questions; 11 assessed pre- and post-procedure pH ± impedance recordings. A multicenter prospective study compared the efficacy of TIF vs omeprazole in a randomized controlled trial.
In all, 16 studies found TIF enabled patients to discontinue anti-reflux medications or markedly reduce their doses; four voiced concerns about the effectiveness of the procedure. In successful studies, 6- and 12-mo outcomes after TIF showed that 75%-93% and 72%-85% of patients had either discontinued PPI or halved the dose. Normalization of esophageal acid exposure, in terms of total acidic refluxes, number of refluxates, and De Meester score was reported in 37%-89% of patients. By 24 mo after TIF, daily high-dosage PPI dependence had been eliminated in 75%-93%[8,21,22].
Endoscopic findings comparing fundoplication immediately after the procedure and two years later are reported in Figure 7. In the two series reporting three-year outcomes lasting discontinuation of daily PPI ranged from 74%-84% of cases[22,24].
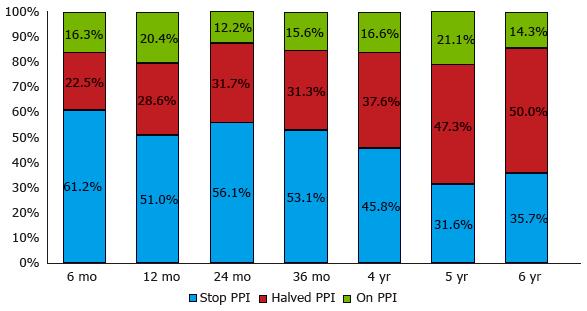
In the only study that followed patients for six years after TIF (14 out of 50), high-dosage PPI dependence was eliminated in 86% and approximately half completely stopped PPI. Unsuccessful outcomes mainly occurred between 6 and 12 mo after the intervention; results did not change substantially between 12 and 36 mo. The six-year results were similar to those at 36 mo[24], providing evidence of the lasting efficay of TIF (Figure 8).
These findings show that the patient selection is determinant to achieve clinical success and confirm that failures occur within the first 6-12 mo after the procedure in most patients.
The operator’s experience is also important in the outcomes. All TIF failures in our series were in patients who underwent the procedure early in the operator’s learning curve. A retrospective study in 124 unselected patients in two community hospitals, reported respectively 75% and 80% of patients free of GER symptoms over a mean follow-up of seven months, confirming that operator’s experience markedly affects outcomes[20].
Only three prospective randomized controlled trials have been published so far. Two compared the six-month efficacy of TIF or omeprazole: One found TIF more effective than PPI in treating regurgitation and extra-esophageal symptoms (97% vs 50% of patients, respectively, P = 0.006)[26]; in the second one intention-to-treat analysis indicated TIF was more effective than PPI in eliminating GERD symptoms (67% vs 45%, P = 0.023)[27]. These discrepancies require additional randomized studies to clarify the efficacy of TIF in treating GERD. The third study compared 3- and 12-mo results of TIF and Nissen fundoplication, showing TIF as effective and safe as the Nissen method but with significantly shorter hospital stays (2.9 ± 0.8 d vs 6.4 ± 0.7 d, P < 0.0001)[31]. Symptomatic responses up to six years after TIF with EsophyX® device, in terms of PPI abolition or 50% reduction, in published series (20 studies) are reported in Table 1. Outcomes up to five years after TIF by the MUSETM system, as regards the effects on PPI use, in published series (two studies) are reported in Table 2.
| Ref. | 6 mo | 12 mo | 24 mo | 36 mo | 6 yr |
| Cadière et al[6], 2008 | - | 85% | - | - | - |
| Cadière et al[8], 2009 | - | - | 93% | - | - |
| Testoni et al[9], 2010 | 82% | 76% | - | - | - |
| Velanovich et al[11], 2010 | 79% | - | - | - | - |
| Repici et al[12], 2010 | 55% | 47% | - | - | - |
| Demyttenaere et al[10], 2010 | - | 53% | - | - | - |
| Hoppo et al[13], 2010 | - | 42% | - | - | - |
| Barnes et al[20], 2011 | 93% | - | - | - | - |
| Bell et al[14], 2011 | 75% | - | - | - | - |
| Ihde et al[15], 2011 | 76% | - | - | - | - |
| Trad et al[18], 2012 | - | 82% | - | - | - |
| Testoni et al[21], 2012 | - | - | 75% | 75% | - |
| Petersen et al[17], 2012 | 58% | - | - | - | - |
| Bell et al[23], 2012 | 86% | - | - | - | - |
| Muls et al[22]2013 | - | 77% | - | 65% | - |
| Bell et al[34], 2013 | - | 82% | - | - | - |
| Bell et al[25], 2014 | - | - | 77%-80% | - | - |
| Trad et al[26], 2015 | 93% | - | - | - | - |
| Hunter et al[27], 2015 | - | 72% | - | - | - |
| Testoni et al[24], 2015 | 84% | 80% | 88% | 84% | 86% |
Unsuccessful outcomes after TIF were reported in three studies. Two series found worsening of distal esophageal acid exposure in 66.7% of cases and persistent GER symptoms in 68% of cases, in small series with a short follow-up (12 mo)[12,13]. A trial comparing TIF with Nissen fundoplication in PPI-refractory GERD patients reported symptom remission and normalization of gastro-esophageal acid reflux in 30% and 100% of patients after TIF and 50% and 100% after surgical fundoplication[16]. These data suggest that in patients unresponsive to PPIs Nissen fundoplication seems more effective than TIF by EsophyX®.
When TIF fails surgical fundoplication is still feasible, with no technical difficulties or increased morbidity. Surgical revision after TIF failure was reported in 8.1%-18.0% of cases[21,22,32,33]. In two studies (9 and 11 patients) Nissen fundoplication achieved the complete disappearance of symptoms in all cases of TIF failure[32,33]. In our series, however, only one of the four patients who required Nissen fundoplication for persisting GERD symptoms after TIF stopped using acid-suppressive therapy[21]. This may depend on the fact that the patients who underwent TIF in our series had only mild impairment of the gastro-esophageal junction and suffered from GERD-related symptoms that could derive from several mechanisms, including increased esophageal sensitivity to refluxate.
On the other hand, re-intervention after laparoscopic fundoplication has been reported in up to 14% of cases[1] and TIF has been found effective after failed surgery[34].
Only two studies so far have reported outcomes after TIF with the MUSETM technique (anterior fundoplication): A pilot study with a five-year follow-up and a multicenter prospective study. The pilot study examined GERD-related symptoms and PPI use up to five years after the procedure in 13 patients: The GERD-related symptom score returned to normal in 92% of cases, PPI use was stopped or halved in 77% (54% stopped PPI completely)[29].
Another study reported outcomes after TIF using the MUSE technique in a multicenter, prospective international trial enrolling 66 patients with a six-month follow-up[28]. GERD-related symptoms scores improved by more than 50% in 73% of patients and 64.6% were no longer taking daily PPIs. Among patients who continued to take PPI, 56.5% cut the dose by more than half. At 24-h pH-recording the total time with esophageal pH < 4.0 dropped significantly from baseline. There were none of the post-procedure side effects commonly seen after laparoscopic fundoplication such as gas bloating, inability to belch or vomit, dysphagia or diarrhea.
An important issue regarding all new interventional procedures introduced in clinical practice is the recognition of technique- or patient-related factors that can affect the outcomes. Factors affecting TIF outcomes have been reported to date only in EsophyX studies.
In our series, from the technical point of view, the number of fasteners deployed and the rotational technique were associated with a better outcome; a larger number of fasteners increased by four folds the success rate[21]. Another study too reported that the number of fasteners plays a key role for the success of the procedure[19]. The rotational technique increased by half the probability of being a responder, according with other reports[19,23].
Patient-related factors affecting post-operative outcomes in our series were pre-operative Hill grades III and IV, hiatal hernia larger than 2 cm, and ineffective esophageal motility, which were associated with a higher rate of unsuccessful results. An impaired esophageal clearance may induce epithelial sensitization and reflux-related symptoms, even in presence of a low-volume reflux[35].
Univariate and multivariate analysis of preoperative factors influencing symptomatic outcomes of TIF with EsophyX® device was done on data from 158 consecutive patients identified[25]. Predictors of successful outcomes for patients with typical symptoms was age 50 years or more, GERD health-related quality of life score (GERD-HRQL) on PPIs 15 or more, a reflux symptom index > 13 on PPIs, and the gastroesophageal reflux symptom score 18 or more on PPIs. Age and GERD-HRLQ remained significant predictors also in multivariate analysis. For patients with atypical GER symptoms only a GERD-HRQL score 15 or more on PPIs was associated with successful outcomes.
In the last few years TIF has only been done in clinical trials enrolling patients with typical gastro-esophageal reflux symptoms responsive or partially responsive to PPI therapy, without or with only small hiatal hernia (< 3 cm), who refused long-term medication, or were intolerant to PPIs, or required high doses of antisecretory maintenance therapy. Patients with grade C and D esophagitis, according to Los Angeles classification, and Barrett’s esophagus were excluded from these studies. The majority of studies used the EsophyX® device, which was effective in the short term in approximately 75% of patients, eliminating their daily dependence on PPIs in half the PPI-responsive GERD patients and markedly reducing the overall dose in the other cases. Similar results were obtained more recently for TIF with the MEDIGUS endostapler, but there are only a few studies.
These results were confirmed in the few studies with follow-up up to three years, and in the single study with up to six years follow-up. None of these reported any troublesome procedure-related persisting side-effects.
Overall outcomes showed that the TIF procedure can be an effective and safe alternative therapeutic option to surgery in selected patients, like those recruited in the published studies. In the series with three- to six-year follow-up, TIF resulted slightly inferior to Nissen fundoplication, but similar to partial posterior (Toupet) or anterior (Dor-Thal) fundoplication[36,37], without surgery-related side effects.
Currently, on the basis of the clinical results, TIF may be offered as an alternative to surgery in patients suffering from gastro-esophageal reflux disease and grade A-B esophagitis, if present, with the sole limitation of the length and reducibility of any hiatal hernia, which at present is the only limiting factor. TIF may also be offered to patients who have some risk of persistent post-surgical side effects. To date, data supporting the efficacy of TIF in the treatment of severe grades of esophagitis or symptoms associated with oro-pharingeal reflux are lacking.
However, as for any new intervention, despite the encouraging short- and medium-term outcomes, the long-term efficacy of TIF needs to be further assessed, mainly for the MUSETM technique. Therefore, randomized controlled trials are now needed to establish the role of TIF in the management of GERD, and whether one or other of the two techniques is likely to be more effective and safe. Preoperative anatomical and functional findings and technical procedural aspects that will help select patients and predict a successful outcome still need to be identified, too.
P- Reviewer: Dehghani SM, Nagaya M, Teramoto-Matsubara OT S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Liedman B, Hatlebakk JG, Julkonen R, Levander K, Carlsson J, Lamm M. Continued (5-year) followup of a randomized clinical study comparing antireflux surgery and omeprazole in gastroesophageal reflux disease. J Am Coll Surg. 2001;192:172-179; discussion 179-181. [PubMed] |
| 2. | Draaisma WA, Rijnhart-de Jong HG, Broeders IA, Smout AJ, Furnee EJ, Gooszen HG. Five-year subjective and objective results of laparoscopic and conventional Nissen fundoplication: a randomized trial. Ann Surg. 2006;244:34-41. [PubMed] |
| 3. | Smith CD. Surgical therapy for gastroesophageal reflux disease: indications, evaluation, and procedures. Gastrointest Endosc Clin N Am. 2009;19:35-48, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Broeders JA, Draaisma WA, Bredenoord AJ, Smout AJ, Broeders IA, Gooszen HG. Impact of symptom-reflux association analysis on long-term outcome after Nissen fundoplication. Br J Surg. 2011;98:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Bowrey DJ, Blom D, Crookes PF, Bremner CG, Johansson JL, Lord RV, Hagen JA, DeMeester SR, DeMeester TR, Peters JH. Risk factors and the prevalence of trocar site herniation after laparoscopic fundoplication. Surg Endosc. 2001;15:663-666. [PubMed] |
| 6. | Cadière GB, Buset M, Muls V, Rajan A, Rösch T, Eckardt AJ, Weerts J, Bastens B, Costamagna G, Marchese M. Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg. 2008;32:1676-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 7. | Bergman S, Mikami DJ, Hazey JW, Roland JC, Dettorre R, Melvin WS. Endolumenal fundoplication with EsophyX: the initial North American experience. Surg Innov. 2008;15:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Cadière GB, Van Sante N, Graves JE, Gawlicka AK, Rajan A. Two-year results of a feasibility study on antireflux transoral incisionless fundoplication using EsophyX. Surg Endosc. 2009;23:957-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Testoni PA, Corsetti M, Di Pietro S, Castellaneta AG, Vailati C, Masci E, Passaretti S. Effect of transoral incisionless fundoplication on symptoms, PPI use, and ph-impedance refluxes of GERD patients. World J Surg. 2010;34:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Demyttenaere SV, Bergman S, Pham T, Anderson J, Dettorre R, Melvin WS, Mikami DJ. Transoral incisionless fundoplication for gastroesophageal reflux disease in an unselected patient population. Surg Endosc. 2010;24:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Velanovich V. Re: “Endoscopic, endoluminal fundoplication for gastroesophageal reflux disease: initial experience and lessons learned” by Vic Velanovich, MD, Surgery 2010; 148: 646-653. Surgery. 2011;149:595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Repici A, Fumagalli U, Malesci A, Barbera R, Gambaro C, Rosati R. Endoluminal fundoplication (ELF) for GERD using EsophyX: a 12-month follow-up in a single-center experience. J Gastrointest Surg. 2010;14:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Hoppo T, Immanuel A, Schuchert M, Dubrava Z, Smith A, Nottle P, Watson DI, Jobe BA. Transoral incisionless fundoplication 2.0 procedure using EsophyX™ for gastroesophageal reflux disease. J Gastrointest Surg. 2010;14:1895-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Bell RC, Freeman KD. Clinical and pH-metric outcomes of transoral esophagogastric fundoplication for the treatment of gastroesophageal reflux disease. Surg Endosc. 2011;25:1975-1984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ihde GM, Besancon K, Deljkich E. Short-term safety and symptomatic outcomes of transoral incisionless fundoplication with or without hiatal hernia repair in patients with chronic gastroesophageal reflux disease. Am J Surg. 2011;202:740-746; discussion 746-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Frazzoni M, Conigliaro R, Manta R, Melotti G. Reflux parameters as modified by EsophyX or laparoscopic fundoplication in refractory GERD. Aliment Pharmacol Ther. 2011;34:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Petersen RP, Filippa L, Wassenaar EB, Martin AV, Tatum R, Oelschlager BK. Comprehensive evaluation of endoscopic fundoplication using the EsophyX™ device. Surg Endosc. 2012;26:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Trad KS, Turgeon DG, Deljkich E. Long-term outcomes after transoral incisionless fundoplication in patients with GERD and LPR symptoms. Surg Endosc. 2012;26:650-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Bell RC, Cadière GB. Transoral rotational esophagogastric fundoplication: technical, anatomical, and safety considerations. Surg Endosc. 2011;25:2387-2399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Barnes WE, Hoddinott KM, Mundy S, Williams M. Transoral incisionless fundoplication offers high patient satisfaction and relief of therapy-resistant typical and atypical symptoms of GERD in community practice. Surg Innov. 2011;18:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Testoni PA, Vailati C, Testoni S, Corsetti M. Transoral incisionless fundoplication (TIF 2.0) with EsophyX for gastroesophageal reflux disease: long-term results and findings affecting outcome. Surg Endosc. 2012;26:1425-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Muls V, Eckardt AJ, Marchese M, Bastens B, Buset M, Devière J, Louis H, Rajan A, Daniel MA, Costamagna G. Three-year results of a multicenter prospective study of transoral incisionless fundoplication. Surg Innov. 2013;20:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Bell RC, Mavrelis PG, Barnes WE, Dargis D, Carter BJ, Hoddinott KM, Sewell RW, Trad KS, DaCosta Gill B, Ihde GM. A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg. 2012;215:794-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Testoni PA, Testoni S, Mazzoleni G, Vailati C, Passaretti S. Long-term efficacy of transoral incisionless fundoplication with Esophyx (Tif 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc. 2015;29:2770-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Bell RC, Fox MA, Barnes WE, Mavrelis PG, Sewell RW, Carter BJ, Ihde GM, Trad KS, Dargis D, Hoddinott KM. Univariate and multivariate analyses of preoperative factors influencing symptomatic outcomes of transoral fundoplication. Surg Endosc. 2014;28:2949-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Trad KS, Barnes WE, Simoni G, Shughoury AB, Mavrelis PG, Raza M, Heise JA, Turgeon DG, Fox MA. Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: the TEMPO Randomized Clinical Trial. Surg Innov. 2015;22:26-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Hunter JG, Kahrilas PJ, Bell RC, Wilson EB, Trad KS, Dolan JP, Perry KA, Oelschlager BK, Soper NJ, Snyder BE. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology. 2015;148:324-333.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 28. | Zacherl J, Roy-Shapira A, Bonavina L, Bapaye A, Kiesslich R, Schoppmann SF, Kessler WR, Selzer DJ, Broderick RC, Lehman GA. Endoscopic anterior fundoplication with the Medigus Ultrasonic Surgical Endostapler (MUSE™) for gastroesophageal reflux disease: 6-month results from a multi-center prospective trial. Surg Endosc. 2015;29:220-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Roy-Shapira A, Bapaye A, Date S, Pujari R, Dorwat S. Trans-oral anterior fundoplication: 5-year follow-up of pilot study. Surg Endosc. 2015;29:3717-3721. [PubMed] |
| 30. | Kauer WK, Roy-Shapira A, Watson D, Sonnenschein M, Sonnenschein E, Unger J, Voget M, Stein HJ. Preclinical trial of a modified gastroscope that performs a true anterior fundoplication for the endoluminal treatment of gastroesophageal reflux disease. Surg Endosc. 2009;23:2728-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Svoboda P, Kantorová I, Kozumplík L, Scheer P, Radvan M, Radvanová J, Krass V, Horálek F. Our experience with transoral incisionless plication of gastroesophageal reflux disease: NOTES procedure. Hepatogastroenterology. 2011;58:1208-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Fumagalli Romario U, Barbera R, Repici A, Porta M, Malesci A, Rosati R. Nissen fundoplication after failure of endoluminal fundoplication: short-term results. J Gastrointest Surg. 2011;15:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Furnée EJ, Broeders JA, Draaisma WA, Schwartz MP, Hazebroek EJ, Smout AJ, van Rijn PJ, Broeders IA. Laparoscopic Nissen fundoplication after failed EsophyX fundoplication. Br J Surg. 2010;97:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Bell RC, Hufford RJ, Fearon J, Freeman KD. Revision of failed traditional fundoplication using EsophyX transoral fundoplication. Surg Endosc. 2013;27:761-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Kim KY, Kim GH, Kim DU, Wang SG, Lee BJ, Lee JC, Park DY, Song GA. Is ineffective esophageal motility associated with gastropharyngeal reflux disease? World J Gastroenterol. 2008;14:6030-6035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Nijjar RS, Watson DI, Jamieson GG, Archer S, Bessell JR, Booth M, Cade R, Cullingford GL, Devitt PG, Fletcher DR. Five-year follow-up of a multicenter, double-blind randomized clinical trial of laparoscopic Nissen vs anterior 90 degrees partial fundoplication. Arch Surg. 2010;145:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Broeders JA, Mauritz FA, Ahmed Ali U, Draaisma WA, Ruurda JP, Gooszen HG, Smout AJ, Broeders IA, Hazebroek EJ. Systematic review and meta-analysis of laparoscopic Nissen (posterior total) versus Toupet (posterior partial) fundoplication for gastro-oesophageal reflux disease. Br J Surg. 2010;97:1318-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (3)] |