Copyright
©The Author(s) 2016.
World J Gastrointest Pharmacol Ther. May 6, 2016; 7(2): 179-189
Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.179
Published online May 6, 2016. doi: 10.4292/wjgpt.v7.i2.179
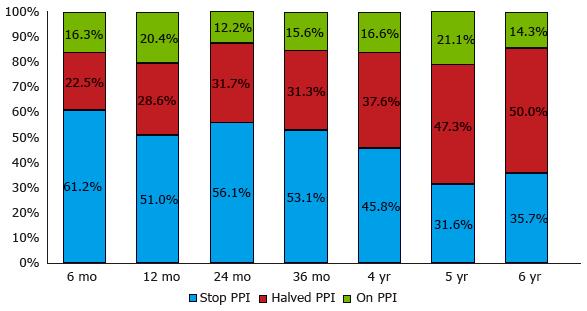
Figure 8 Symptomatic responses six months and 1-6 years after transoral incisionless fundoplication with Esophyx® device, classified according to proton pump inhibitor use.
Patients were grouped as complete responders [who completely stopped using proton pump inhibitor (PPI)] or partial responders (who halved the previous PPI dose) and non-responders (who still used the pre-TIF PPI dose): 12 mo vs 6 mo after TIF P = 0.8; 24 mo vs 12 mo, P = 0.4; 36 mo vs 24 mo, P = 0.7; 4 years vs 36 mo, P = 1.0; 5 years vs 4 years, P = 1.0; 6 years vs 5 years, P = 1.0.
- Citation: Testoni PA, Mazzoleni G, Testoni SGG. Transoral incisionless fundoplication for gastro-esophageal reflux disease: Techniques and outcomes. World J Gastrointest Pharmacol Ther 2016; 7(2): 179-189
- URL: https://www.wjgnet.com/2150-5349/full/v7/i2/179.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i2.179