Published online Jun 5, 2025. doi: 10.4292/wjgpt.v16.i2.103971
Revised: March 13, 2025
Accepted: March 26, 2025
Published online: June 5, 2025
Processing time: 174 Days and 5 Hours
Cyclic vomiting syndrome (CVS) and its effect on nutritional status has not been well described.
To describe the clinical characteristics, treatment and outcomes of children with CVS in Singapore.
Retrospective cohort study of pediatric patients aged 1 to 18 years old with CVS diagnosed at KK Women’s and Children’s Hospital in Singapore from 2011 to 2021.
Thirty-two children (69% female) with CVS were included in the study, with mean age of onset of symptoms at 7 (± 4) years and mean follow up duration of 5 years. Forty percent (12/32) of patients were underweight at diagnosis with no other identifiable organic cause, with a median body mass index (BMI) z score -3.2 (range -2 to -7.5). The incidence of systemic hypertension was 10% (3/32). The overall mean frequency of exacerbations in this cohort of patients was 4 (± 4) episodes per year. In total, 16 (50%) patients, who had mean baseline frequency of 6 (± 5) attacks per year, were commenced on prophylactic treatment. Twelve patients (75%) responded to first-line therapy, whereas 4 (25%) required escalation to second-line treatment. With prophylactic treatment, there was an overall improvement in the frequency of attacks with a mean reduction of 5 (± 3) attacks per year. Also, there was improvement in the BMI z score of these patients from a median of -2.9 to -0.9.
Prophylactic treatment is effective in improving nutritional status as well as reducing symptom frequency and should be considered for patients with complications such as growth failure and significant hypertension.
Core Tip: Cyclic vomiting syndrome (CVS) in children can be debilitating. However, there are prophylactic medications available. The nutritional status of children with CVS before and after starting prophylactic medications is unknown. The incidence of CVS associated hypertension has also not been well described. The improvement in nutritional status after starting prophylactic medications should be a consideration for providers to propose prophylactic medications for poor nutritional status.
- Citation: Goh L, Ho JMD, Chiou FK. Disease characteristics and long-term outcomes of cyclic vomiting syndrome in Singaporean children. World J Gastrointest Pharmacol Ther 2025; 16(2): 103971
- URL: https://www.wjgnet.com/2150-5349/full/v16/i2/103971.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i2.103971
Cyclic vomiting syndrome (CVS) is a chronic gastrointestinal disorder characterized by recurrent episodes of nausea, retching and intense vomiting lasting for hours to days[1]. Many patients experience associated symptoms such as pallor, anorexia, abdominal pain, listlessness, and headache. The episodes of vomiting are separated by symptom-free periods[2,3]. Although it was described more than 100 years ago, the cause of CVS is still unknown. Several theories have been suggested, including autonomic dysfunction, hypothalamic-pituitary-adrenal axis dysfunction, gut-brain axis dysregulation, mitochondrial DNA mutations and other genetic polymorphisms[4,5]. CVS is considered by some to be a migraine variant, given that many children with CVS develop migraine as adolescents[6]. In addition, migraine therapies such as tricyclic antidepressants, triptans and topiramate have been shown to have varying degrees of efficacy in CVS[7].
Although CVS is defined as a functional disorder, it can lead to frequent exacerbations requiring multiple, repeated hospitalizations and interruption of food intake, which may in turn result in impairment in nutrition and growth. The effect of CVS on growth and nutritional status has not been previously described. Some children experience episodic hypertension (the Sato subtype) and may require intensive care for invasive monitoring and titration of intravenous (IV) anti-hypertensive medications[8]. To our knowledge, no other studies of pediatric CVS have described nutritional status or the incidence of hypertension.
Prophylactic therapy is recommended for patients with frequent, severe exacerbations, however therapeutic response and side effects can be unpredictable. The aim of this study is describe the clinical characteristics, treatment and outcomes of children with CVS in Singapore.
Retrospective data was collected from pediatric patients aged 1 to 18 years old with CVS diagnosed at KK Women’s and Children’s Hospital in Singapore from 2011 to 2021. The diagnosis of CVS was based on the Rome IV criteria[9] namely recurrent stereotypic episodic attacks with return to baseline health between episodes, with the exclusion of any medical or surgical disorder that could result in vomiting. All patients underwent extensive investigations including metabolic work up with liver function test, plasma acyl-carnitine, amino acids, ammonia, lactate, amylase, lipase and urine organic acids, as well as esophagogastroduodenoscopy, abdominal ultrasound and/or barium meal performed. All patients had brain imaging, either with magnetic resonance imaging or computer tomography as part of routine diagnostic work-up. Patients with hypertension (defined as persistent systolic/diastolic blood pressure > 95th percentile for gender, age and height) underwent additional electrocardiogram, echocardiogram, renal arteries Doppler ultrasound, urine analysis, renal function and thyroid function tests to exclude possible secondary causes of hypertension. CVS-related hypertension, which defines the Sato subset, was confirmed if an underlying cardiac, renal or endocrine pathology had been excluded.
With regards to nutritional assessment, the weight, height, and body mass index (BMI) were recorded at diagnosis and subsequent clinical encounters. Based on WHO criteria, underweight was defined as weight and/or BMI z -score < -2 for age and short stature (stunting) was defined as height z -score of < -2 for age.
Acute exacerbations of CVS were treated with IV anti-emetics such as ondansetron and diphenhydramine, sometimes with the addition of IV lorazepam in refractory cases. The prophylactic management of CVS was adapted from the 2008 consensus statement by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) using cyproheptadine for children < 5 years old and amitriptyline for children > 5 years old as first-line options, and propranolol or phenobarbital for refractory cases[10].
Data was analyzed using SPSS Statistics for Windows Version 19. All patients with CVS were included and clinical data gathered included gender, age of onset, concomitant incidence of migraine headaches, hypertension, developmental delay, height, weight, BMI and response to acute treatment as well as prophylaxis. Ethics approval was obtained from the Institutional Review Board (2021/2657).
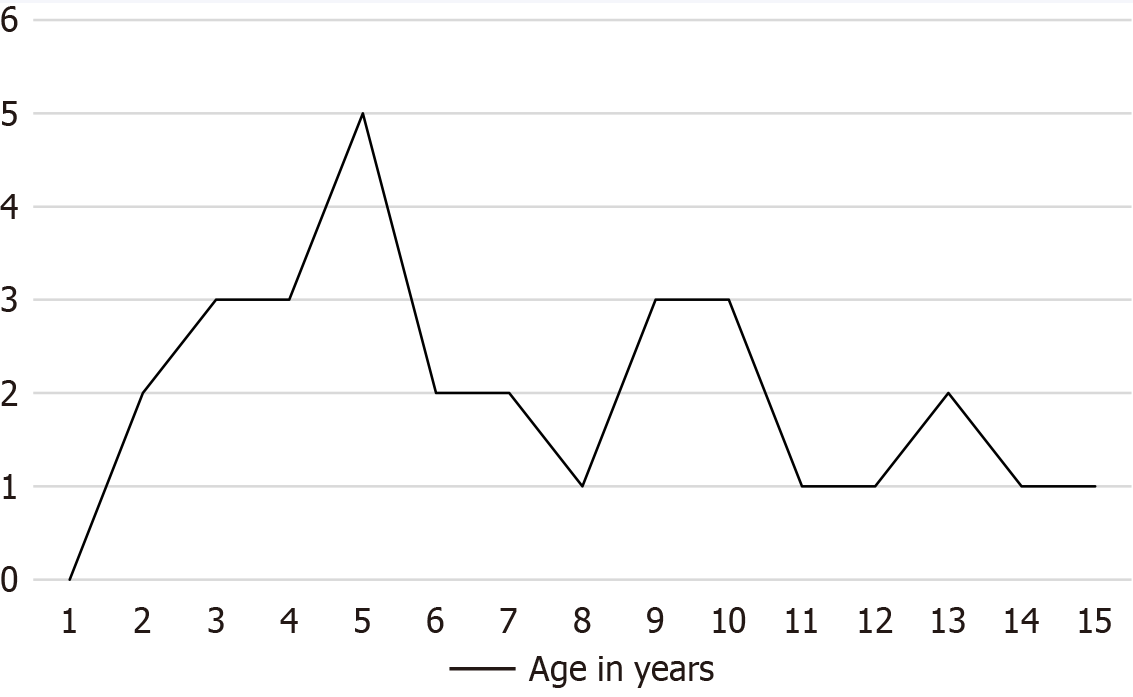
Thirty-two children (69% female) with CVS were included in the study, with mean age of onset of symptoms at 7 (± 4) years old and mean follow up duration of 5 years. The peak age of presentation was 5 years old, with the overall distribution of age at presentation shown in Figure 1.
Forty percent (12/32) of patients were underweight at diagnosis with no other identifiable organic cause, with a median BMI z score -3.2 (range -2 to -7.5). Patients who were underweight had a mean frequency of 5.5 exacerbations per year as compared to 3.8 exacerbations per year in those who were not underweight.
Amongst the underweight patients, 10 (80%) were started on prophylaxis. Eight of them (80%) had improvement in BMI corresponding to reduction in frequency of exacerbations to a mean of less than one episode per year, while 2 patients remained underweight and unresponsive to prophylactic medication. Both patients who were refractory to treatment had underlying conditions namely autism spectrum disorder and Fanconi anemia respectively. Out of the 2 patients who declined prophylaxis, one remained underweight and one showed spontaneous clinical improvement in symptoms as well as BMI.
The overall mean frequency of exacerbations in this cohort of patients was 4 (± 4) episodes per year. In total, 16 (50%) patients, who had mean baseline frequency of 6 (± 5) attacks per year, were commenced on prophylactic treatment. Twelve patients (75%) responded to first-line therapy, cyproheptadine or amitriptyline, while 4 (25%) were escalated to the second line of treatment, namely propranolol (n = 2) sodium valproate (n = 2). Sertraline (n = 1) and olanzapine (n = 1) were used as third-line agents in 2 patients.
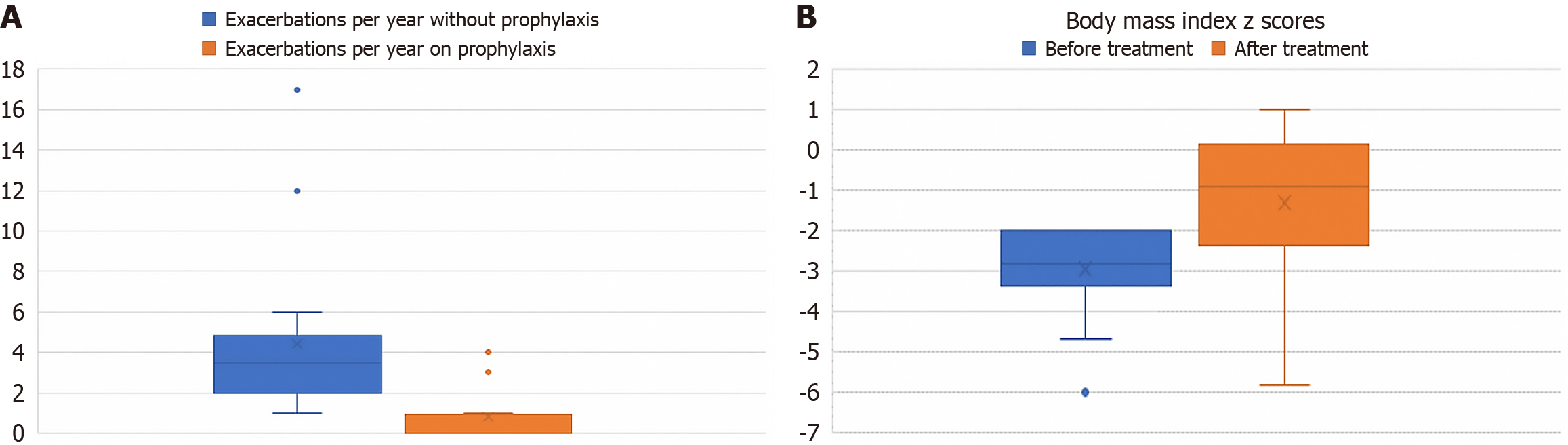
With prophylactic treatment, there was an overall improvement in the frequency of attacks with patients having a mean reduction of 5 (± 3) attacks per year (Figure 2A). There were no severe adverse side effects reported from use of prophylactic medication other than 2 patients who reported that the medications made them drowsy. There was a notable improvement in the BMI z score of patients who received prophylactic medication from a median of -2.9 to -0.9 (Figure 2B).
There was a history of migraines present in 7% (2/32) and the incidence of the Sato subset with systemic hypertension was 10% (3/32). The mean interval from symptom onset to obtaining a diagnosis of CVS was 1 (± 1) year. There were no identifiable food triggers in our cohort. The baseline characteristics and growth parameters are summarized in Table 1.
| Total number of patients (n = 32) | |
| Female | 22 (69) |
| Developmental delay/autism spectrum disorder | 5 (16) |
| Started on prophylaxis medications | 16 (50) |
| Incidence of hypertension (Sato subset) | 3 (10) |
| History of migraines | 2 (7) |
| Identifiable food trigger | 0 (0) |
| Growth parameters | |
| Median weight-for-age z score | -1.5 (range -3.8 to 2) |
| Median BMI z score | -1 (range -7.5 to 1.9) |
| Weight z score < -2 | 12 (40) |
| BMI z score < -2 | 12 (40) |
| Height z score < -2 | 5 (16) |
With regards to the 3 patients with CVS associated hypertension, all were developmentally normal females aged 4, 5 and 10 years old who presented with hypertension during exacerbations of cyclic vomiting with normal blood pressures recorded during well periods. Significantly, one patient had hypertensive urgency, namely a sudden and abrupt elevation of blood pressure from baseline to > 20 mmHg above the 95th centile without evidence of end organ dysfunction[11]. With blood pressure persistently > 20 mmHg above the 99th centile associated with giddiness, she required IV phentolamine treatment up to the maximum dose of 6 mcg/kg/min with PO Amlodipine 5 mg and PO Nifedipine 7.5 mg q 4 hourly in the intensive care unit to reduce her blood pressure safely over 24 hours. All patients had subsequent normalization of blood pressure during symptom free intervals but required oral antihypertensive agents during exacerbations of CVS.
Three patients were refractory to both 1st and 2nd line prophylactic treatment with no reduction in frequency of vomiting episodes. Of note, drug toxicity was observed in 1 patient who developed prolonged QTC from the use of ondansetron and diphenhydramine that persisted despite discontinuation of medications.
At the last clinic visit, 12 patients (37.5%) with mean age of 10 years old had no vomiting episode for > 2 years. None of the patients had essential hypertension on long term follow up.
Our study has shown that although first-line prophylaxis medications are effective in majority of the patients with CVS, leading to reduced frequency in attacks and improved growth. Those who are refractory continue to be difficult to manage even on second line medications such as anti-epileptics, anti-psychotics and anti-depressants.
Most studies on prophylactic therapy for CVS were based on retrospective case series or cohort studies. A cohort of Taiwanese children with CVS were found to have 2 (± 2) exacerbations per year pre-prophylaxis and 0.6 (± 1) exacerbations per year post-prophylaxis[12]. In comparison, Andersen et al[13] reported that 94% treated with amitriptyline and 83% treated with cyproheptadine showed > 50% reduction in number of attacks. A study from Iran in 2018[14] reported that 66% in the amitriptyline group and 50% in the cyproheptadine group had > 50% reduction in number of attacks. This is comparable to our cohort where 80% of patients started on prophylactic medication had a reduction in the number of CVS attacks per year.
The only randomized clinical trial done to compare the efficacy of prophylactic amitriptyline and cyproheptadine in pediatric CVS showed no superiority of one medication over the other[15]. Nevertheless, cyproheptadine has shown to be useful for treating other functional gastrointestinal disorders in children as well, such as functional abdominal pain and irritable bowel syndrome[16,17]. It has serotonin (5HT2) and calcium channel antagonist effects and common side effects include increase in appetite and weight gain.
From our experience, the use of prophylactic medications has been safe with no severe side effects reported and the treatment was well tolerated. In most of our cases, no obvious triggers are identified and hence food elimination or avoidance alone would not be effective.
The incidence of CVS associated hypertension in our cohort albeit low (10%), is associated with significant morbidity as seen in our patient with hypertensive crisis. Although infrequent, the incidence of CVS associated hypertension requires close monitoring for development of complications such as hypertensive urgency or emergency. A recent case report of CVS induced hypertension causing posterior reversible encephalopathy syndrome demonstrates the danger of this complication[18]. Hence, it is imperative to recognize CVS associated hypertension in patients with acute exacerbations of CVS and to treat accordingly.
The poor nutritional status of patients with CVS can be attributed to the fact that most patients were only able to be diagnosed a year after their first presentation. This is likewise in other countries as CVS is commonly misdiagnosed, with some institutions reporting a delay of 2-4 years before the diagnosis of CVS is obtained[19]. While cyptoheptadine is known to induce an increase in appetite and weight gain, based on our data, the reduction in frequency of CVS exacerbations following initiation of prophylactic treatment may also be linked with a corresponding improvement in BMI. Hence, we propose growth failure as an important indication for initiation of prophylactic therapy.
Although it has been reported that some patients with CVS have concomitant pervasive developmental delay such as autistic spectrum disorder with a reported prevalence of 5%[6], this was higher in our local cohort with 16% of patients having such comorbidities. This group was found to have a higher number of exacerbations per year as compared to those without developmental issues, with a mean frequency of 7 episodes in contrast with 4 episodes (P = 0.07).
The pediatric cohort studies that have been described across the world are summarized in Table 2, with the latest data from this study added. CVS in children was first reported by Abu-Arafeh et al[20] in 1995. The largest cohort was characterized in 1999 by Li et al[21], showing a strong association of CVS with migraine and this was also similarly shown in the Turkish population[22].
| Ref. | Cohort size | Mean age of onset | Overall sex ratio (male:female) | Concomitant incidence of migraine (%) | Mean frequency of attacks (before prophylaxis) | Response to prophylaxis |
| Abu-Arafeh et al[20] | 34 children | 5 years | 1:1 | 21 | 8 episodes/year | Not described |
| Li et al[21] | 214 children | 6 years | 1:3 | 30 | Not described | Not described |
| Ertekin et al[22] | 24 children | 7 years | 1:2 | 25 | Not described | Not described |
| Haghighat et al[14] | 181 children | 5 years | 1:1 | 20 | 6 episodes/year | Not described |
| Liao et al[12] | 24 children | 7 years | 2:1 | Not described | 2 episodes/year | 1 episode/year |
| Foreman et al[23] | 42 children | 4 years | 1:1 | 4 | 12 episodes/year | Not described |
| Dipasquale et al[24] | 57 children | 8 years | 1.3:1 | Not described | Not described | Not described |
| Singapore | 32 children | 7 years | 1:2 | 7 | 4 episodes/year | 1 episode/year |
The preponderance of female children with CVS as described by NASPGHAN[10] is echoed in our local population with a statistically significant female disposition (P = 0.03). This was also described amongst Turkish children[22] but contrastingly the opposite was seen in the Taiwanese cohort[12]. Elsewhere, the gender distribution of children with CVS seems to be the same.
This study is one of the few studies to report the reduction in frequency of exacerbations per year after starting prophylactic medications, further analysis can be done to calculate the cost effectiveness. Nonetheless, the healthcare costs associated with CVS are significant, estimated to be USD $17000 per year[23]. Notwithstanding the monetary costs, the improvement of the patient’s and family’s quality of life after starting prophylactic medications needs to be looked at as well[24].
The main limitation of this study was the small cohort size as well as the inherent bias associated with retrospective data collection. While we acknowledge that CVS is a rare condition, we were able to follow-up our patients over a long study period with minimal drop-out rates and provide comprehensive data on the medium to long-term outcomes of children with CVS in our Asian cohort. Future studies could include prospective multi-center studies to increase the cohort size as well as incorporating biomarker and genetic analyses to delve further into the underlying mechanisms.
Prophylactic treatment is effective in improving nutritional status as well as reducing symptom frequency and should be considered for patients with complications such as growth failure and significant hypertension.
We would like to acknowledge all the patients and family who were involved in this study.
| 1. | Li BU. Cyclic vomiting: the pattern and syndrome paradigm. J Pediatr Gastroenterol Nutr. 1995;21 Suppl 1:S6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Hoyt CS, Stickler GB. A study of 44 children with the syndrome of recurrent (cyclic) vomiting. Pediatrics. 1960;25:775-780. [PubMed] |
| 3. | Pfau BT, Li BU, Murray RD, Heitlinger LA, McClung HJ, Hayes JR. Differentiating cyclic from chronic vomiting patterns in children: quantitative criteria and diagnostic implications. Pediatrics. 1996;97:364-368. [PubMed] |
| 4. | Chelimsky TC, Chelimsky GG. Autonomic abnormalities in cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr. 2007;44:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Ye Z, Xue A, Huang Y, Wu Q. Children with cyclic vomiting syndrome: phenotypes, disease burden and mitochondrial DNA analysis. BMC Gastroenterol. 2018;18:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 6. | Boles RG, Powers AL, Adams K. Cyclic vomiting syndrome plus. J Child Neurol. 2006;21:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Gui S, Patel N, Issenman R, Kam AJ. Acute Management of Pediatric Cyclic Vomiting Syndrome: A Systematic Review. J Pediatr. 2019;214:158-164.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 8. | Sato T, Igarashi N, Minami S, Okabe T, Hashimoto H, Hasui M, Kato E. Recurrent attacks of vomiting, hypertension and psychotic depression: a syndrome of periodic catecholamine and prostaglandin discharge. Acta Endocrinol (Copenh). 1988;117:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1077] [Article Influence: 56.7] [Reference Citation Analysis (6)] |
| 10. | Li BU, Lefevre F, Chelimsky GG, Boles RG, Nelson SP, Lewis DW, Linder SL, Issenman RM, Rudolph CD; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition consensus statement on the diagnosis and management of cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr. 2008;47:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Chandar J, Zilleruelo G. Hypertensive crisis in children. Pediatr Nephrol. 2012;27:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Liao KY, Chang FY, Wu LT, Wu TC. Cyclic vomiting syndrome in Taiwanese children. J Formos Med Assoc. 2011;110:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Andersen JM, Sugerman KS, Lockhart JR, Weinberg WA. Effective prophylactic therapy for cyclic vomiting syndrome in children using amitriptyline or cyproheptadine. Pediatrics. 1997;100:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Haghighat M, Rafie SM, Dehghani SM, Fallahi GH, Nejabat M. Cyclic vomiting syndrome in children: experience with 181 cases from southern Iran. World J Gastroenterol. 2007;13:1833-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (9)] |
| 15. | Badihian N, Saneian H, Badihian S, Yaghini O. Prophylactic Therapy of Cyclic Vomiting Syndrome in Children: Comparison of Amitriptyline and Cyproheptadine: A Randomized Clinical Trial. Am J Gastroenterol. 2018;113:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Madani S, Cortes O, Thomas R. Cyproheptadine Use in Children With Functional Gastrointestinal Disorders. J Pediatr Gastroenterol Nutr. 2016;62:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Krasaelap A, Madani S. Cyproheptadine: A Potentially Effective Treatment for Functional Gastrointestinal Disorders in Children. Pediatr Ann. 2017;46:e120-e125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Beckman M, Lendner N, Sferra TJ, Moses J. A Case of Cyclic Vomiting Syndrome-Induced Hypertension Causing Posterior Reversible Encephalopathy Syndrome. JPGN Rep. 2023;4:e294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Sunku B. Cyclic Vomiting Syndrome: A Disorder of All Ages. Gastroenterol Hepatol (N Y). 2009;5:507-515. [PubMed] |
| 20. | Abu-Arafeh I, Russell G. Cyclical vomiting syndrome in children: a population-based study. J Pediatr Gastroenterol Nutr. 1995;21:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 3.8] [Reference Citation Analysis (3)] |
| 21. | Li BU, Murray RD, Heitlinger LA, Robbins JL, Hayes JR. Is cyclic vomiting syndrome related to migraine? J Pediatr. 1999;134:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Ertekin V, Selimoğlu MA, Altnkaynak S. Prevalence of cyclic vomiting syndrome in a sample of Turkish school children in an urban area. J Clin Gastroenterol. 2006;40:896-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Foreman MS, Camp T. Cyclic Vomiting Syndrome. Pediatr Rev. 2018;39:100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Dipasquale V, Falsaperla R, Bongiovanni A, Ruggieri M, Romano C. Clinical features and long-term outcomes in pediatric cyclic vomiting syndrome: A 9-year experience at three tertiary academic centers. Neurogastroenterol Motil. 2022;34:e14224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |