Published online Apr 22, 2024. doi: 10.4291/wjgp.v15.i1.91237
Peer-review started: December 25, 2023
First decision: February 24, 2024
Revised: March 8, 2024
Accepted: March 22, 2024
Article in press: March 22, 2024
Published online: April 22, 2024
Processing time: 115 Days and 13.8 Hours
Recent studies have shown that the tumor microenvironment significantly inf
To establish the relationships between TB, DR, and TILs in patients with GAC and to assess their influence on prognosis.
Our study group comprised 130 patients diagnosed with GAC. The definition of TB was established based on the International TB Consensus Conference. The DR was categorized into three groups according to the level of tumor stroma matu
A significant correlation between peritumoral budding (PTB) and intratumoral budding (ITB) was noted (r = 0.943). Tumors with high PTBs and ITBs had a greater incidence of immature DRs and low TILs (P < 0.01). PTB and ITB were associated with histological subtype, lymph node metastasis (LNM), and stage (P < 0.01). ITB, PTB, LNM, DR, and stage were significant risk factors associated with poor prognosis. The multivariate Cox regression analysis identified ITB, PTB, and LNM as independent prognostic variables (P < 0.05). In intestinal-type adenocarcinomas, a positive correlation between PTB and ITB was noted (r = 0.972). While univariate analysis revealed that LNM, stage, PTB, ITB, and DR were strong parameters for predicting survival (P < 0.05), only PTB and ITB were found to be independent prognostic factors (P < 0.001).
TB may be a potential prognostic marker in GAC. However, further studies are needed to delineate its role in pathology reporting protocols and the predictive effects of DR and TILs.
Core Tip: This study investigated the relationships between tumor budding, desmoplastic reaction (DR), and tumor-infiltrating lymphocytes (TILs) in patients with gastric adenocarcinomas (GAC) and assessed their influence on prognosis. Our results demonstrated that TB is a promising prognostic factor in GAC. While it could also be valuable in determining survival in patients with unresectable tumors, further studies are needed to draw a conclusion. Although the DR and TILs were not observed as independent parameters, their close association with TB in patients with GAC suggests their value in predicting tumor behavior merits further research to clarify their roles better.
- Citation: Yavuz A, Simsek K, Alpsoy A, Altunay B, Gedik EO, Unal B, Bassorgun CI, Tatli AM, Elpek GO. Prognostic significance of tumor budding, desmoplastic reaction, and lymphocytic infiltration in patients with gastric adenocarcinoma. World J Gastrointest Pathophysiol 2024; 15(1): 91237
- URL: https://www.wjgnet.com/2150-5330/full/v15/i1/91237.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v15.i1.91237
Gastric adenocarcinoma (GAC), the sixth most common tumor in the world, are among the most lethal types of cancer worldwide and exhibit significant rates of recurrence even after curative surgical procedures[1,2]. While the tumor-node-metastasis classification is often preferred for predicting high risk, heterogeneity in the survival of patients at the same stage has necessitated the search for new prognostic indicators to better determine tumor behavior[3-5]. In recent years, much evidence has shown that epithelial-mesenchymal transition (EMT) plays a vital role in the aggressiveness of many cancers[6-8]. In this context, tumor budding (TB), which reflects EMT in particular, has been used in routine reporting protocols as an independent prognostic parameter in colorectal cancer (CRC) patients[9-11]. In GAC, although there is evidence that TB is associated with tumor behavior[12-14], the data do not reach an agreement[15-17]. Besides, different studies use different methods to evaluate TB, which limits the determination of the importance of this parameter in these tumors.
Recently, studies have demonstrated that the tumor microenvironment (TME) plays a more active role in tumor progression, contrasting with previous opinions that consider the formation of excessive fibrous or connective tissue, or, in other words, desmoplasia (DR), around a tumor as a simple host-related factor[18-20]. Therefore, the DR has been noted to be a determinant of tumor behavior in solid cancers, including CRC[21-23]. However, studies evaluating this parameter in GAC are rare[24-26].
Moreover, immune cells that constitute a part of the TME, especially lymphocytes infiltrating the tumor, may play a role in determining tumor behavior in GAC, as noted in other organ tumors[27].
Recently, few studies in GAC have pointed to the association of high TB with immature stroma and tumor-infiltrating lymphocytes (TILs)[26,28]. Nonetheless, in patients with GAC, the interplay between these parameters and their efficiency in determining tumor behavior and survival have yet to be compared.
Therefore, this study aimed to investigate the relationships among DR, TB, TILs, clinicopathological parameters, and prognosis in GAC.
This retrospective study included patients diagnosed at the Department of Pathology, Akdeniz University Medical School, Antalya, Türkiye, who underwent total or partial gastrectomy for GAC between 2004 and 2019. One hundred thirty patients were selected after excluding patients with other cancers, who underwent neoadjuvant therapy, or who had incomplete clinicopathological data. All patient-related data were collected and revised. Follow-up data were retrieved from patient records from the Department of Oncology of our institution. Tumor subtyping was performed according to the Lauren classification[29]. All patients were staged based on the eighth edition of the American Joint Committee on Cancer manual[30].
The study protocol was based on the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethical Committee of Akdeniz University.
Hematoxylin and eosin (H&E)-stained slides from tumor blocks were reevaluated using light microscopy, and slides with low maturation of the tumor stroma, high tumor bud density, and high lymphocytic infiltration were selected for further analysis.
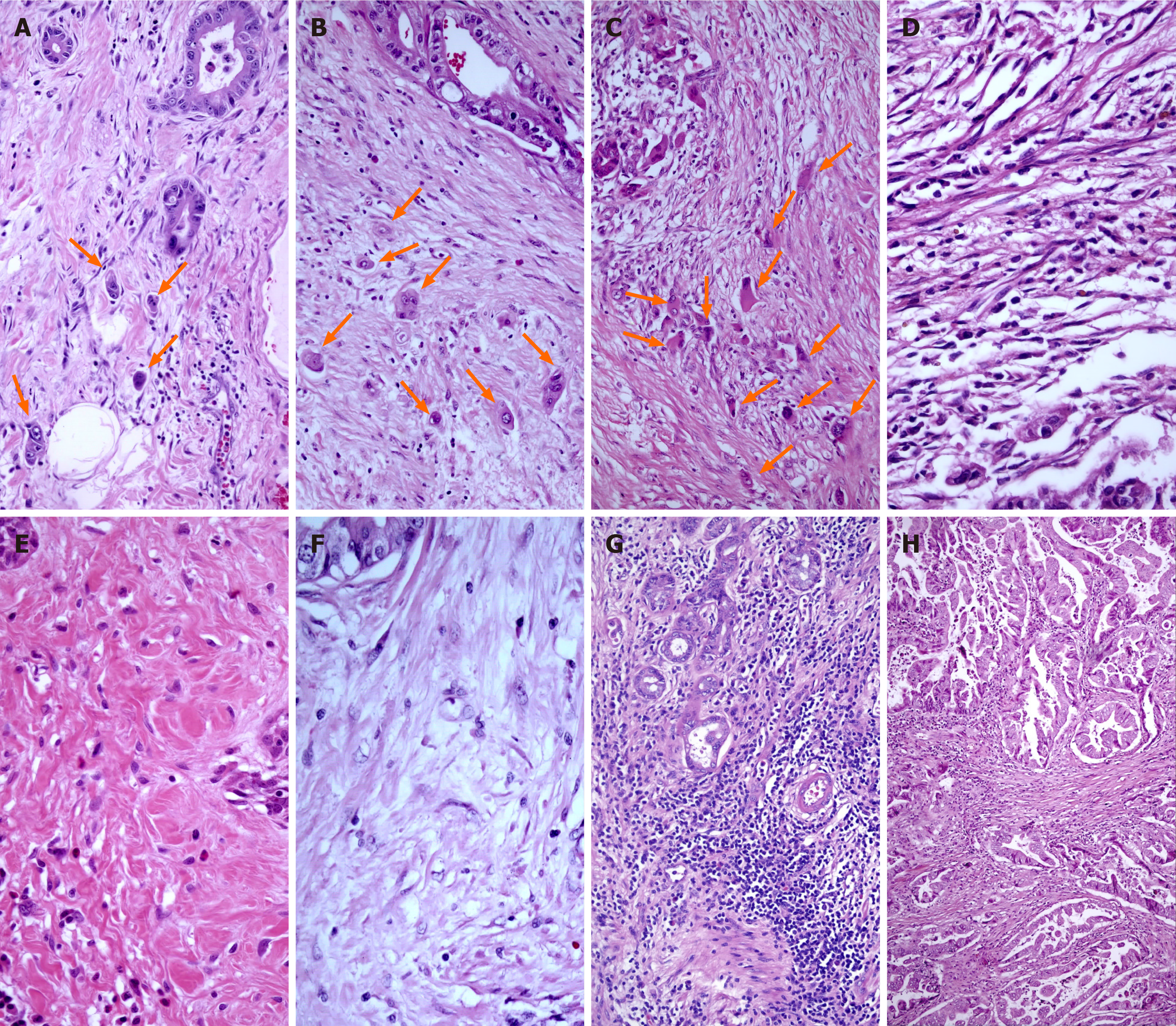
The assessment of peritumoral budding (PTB) and intratumoral budding (ITB) in this study followed the International TB Consensus Conference (ITBCC) guidelines[31]. In brief, a single tumor cell or a cluster of up to four tumor cells at the invasive front and within the primary tumor body were considered PTB and ITB, respectively. The count was determined in a standardized field area of 0.785 mm² at 200 × total magnification, and both PTB and ITB were categorized into three grades: grade 1 (0-4 TB), grade 2 (5-9 TB), and grade 3 (> 10 TB) (Figure 1).
DRs were evaluated and classified into three groups based on the maturation of the tumor stroma, as described by Ueno et al[32]. Mature-type DR comprised fine collagen fibers in multiple layers (DR1). While intermediate-type DR contained keloid-like collagen (DR2), immature-type DR constituted from the myxoid stroma (DR3) and occupied more than a 40 × objective lens field on slides (Figure 1).
The evaluation of TILs was performed semiquantitatively based on a 5% cutoff value on H&E-stained slides at a magnification of 200 ×[33]. Lymphatic infiltrates outside the tumor borders were excluded from the evaluation (Figure 1).
The data were analyzed with SPSS 27.0. Spearman’s correlation test was used to evaluate the relationship between PTB and the ITB. The categorical data were examined by the chi-square test. Univariate survival analysis was performed with the Kaplan-Meier method, and the log-rank test was used to compare survival rates. A multivariate Cox proportional hazards regression model was applied to predict parameters influencing patient prognosis[34]. A P value < 0.05 indicated statistical significance. Furthermore, similar tests were also performed in patients with intestinal-type GAC, which allowed the application of these analyses (100 patients).
The clinicopathological characteristics of the patients in the study group are presented in Table 1. In brief, the mean age was 62.14 years ± 12.00 years (range 28 years to 89 years), and 53 females and 77 males were included. Patients were categorized into two groups for further analysis based on their mean age and mean tumor diameter (1.86 cm ± 1.02 cm, range 1.0 cm to 6.8 cm); regarding the level of invasion, a great majority of patients were classified as having tumors limited to the subserosa (40.0%), followed by tumors limited to the muscularis propria (30.0%), and tumors with invasion beyond the serosa and adjacent organs (24.6%). Invasion of the mucosa and submucosa was observed in 5.4% of the patients. Lymph node metastasis (LNM) was observed in 39.6% of the patients. The median follow-up period was 39 months (2-120 months, mean 42.44 months).
| Parameters | n | PTB1 (%) | PTB2 (%) | PTB3 (%) | ITB1 (%) | ITB2 (%) | ITB3 (%) | DR1 (%) | DR2 (%) | DR3 (%) | TILs (%) | TILs (%) |
| Age, yr | ||||||||||||
| < 62.14 ± 12.00 | 64 | 11 (42.3) | 25 (59.5) | 28 (45.2) | 15 (48.4) | 14 (56.0) | 35 (47.3) | 29 (50.0) | 20 (52.6) | 15 (44.1) | 34 (50.0) | 30 (48.4) |
| ≥ 62.14 ± 12.00 | 66 | 15 (57.7) | 17 (40.5) | 34 (54.8) | 16 (51.6) | 11 (44.0) | 39 (52.7) | 29 (50.0) | 18 (47.4) | 19 (55.9) | 34 (50.0) | 32 (51.6) |
| Gender | ||||||||||||
| Male | 77 | 16 (61.5) | 26 (61.9) | 35 (56.5) | 18 (58.1) | 19 (76.0) | 40 (54.1) | 43 (74.1) | 23 (60.5) | 11 (32.4)a | 36 (52.9) | 41 (66.1) |
| Female | 53 | 10 (38.5) | 16 (38.1) | 27 (43.5) | 13 (41.9) | 6 (24.0) | 34 (45.9) | 15 (25.9) | 15 (39.5) | 23 (67.6) | 32 (47.1) | 21 (33.9) |
| Diameter | ||||||||||||
| < 1.86 ± 1.02 | 84 | 16 (61.5) | 29 (69.0) | 39 (62.9) | 16 (51.6) | 19 (76.0) | 49 (66.2) | 34 (58.6) | 28 (73.7) | 22 (64.7) | 47 (69.1) | 37 (59.7) |
| ≥ 1.86 ± 1.02 | 46 | 10 (38.5) | 13 (31.0) | 23 (37.1) | 15 (48.4) | 6 (24.0) | 25 (33.8) | 24 (41.4) | 10 (26.3) | 12 (35.3) | 21 (30.9) | 25 (40.3) |
| Invasion | ||||||||||||
| T1 | 7 | 5 (19.2) | 2 (4.8) | 0b | 4 (12.9) | 1 (4.0) | 2 (2.7) | 3 (5.2) | 3 (7.9) | 1 (2.9) | 4 (5.9) | 3 (4.8) |
| T2 | 39 | 4 (15.4) | 15 (35.7) | 20 (32.3) | 12 (38.7) | 8 (32.0) | 19 (25.7) | 22 (37.9) | 9 (23.7) | 8 (23.5) | 18 (26.5) | 21 (33.9) |
| T3 | 52 | 11 (42.3) | 15 (35.7) | 26 (41.9) | 10 (32.3) | 6 (24.0) | 36 (48.6) | 25 (43.1) | 16 (42.1) | 11 (32.4) | 27 (39.7) | 25 (40.3) |
| T4 | 32 | 6 (23.1) | 10 (23.8) | 16 (25.8) | 5 (16.1) | 10 (40.0) | 17 (23.0) | 8 (13.8) | 10 (26.3) | 14 (41.2) | 19 (27.9) | 13 (21.0) |
| LNM | ||||||||||||
| Absent | 82 | 23 (88.5) | 29 (69.0) | 30 (48.4)a | 29 (93.5) | 16 (64.0) | 37 (50.0)a | 43 (74.1) | 23 (60.5) | 16 (47.1)c | 38 (55.9) | 44 (71.0) |
| Present | 48 | 3 (11.5) | 13 (31.0) | 32 (51.6) | 2 (6.5) | 9 (36.0) | 37 (50.0) | 15 (25.9) | 15 (39.5) | 18 (52.9) | 30 (44.1) | 18 (29.0) |
| Metastasis | ||||||||||||
| Absent | 106 | 26 (100.0) | 31 (73.8) | 49 (79.0)c | 21 (67.7) | 23 (92.0) | 62 (83.8) | 49 (84.5) | 33 (86.8) | 24 (70.6) | 55 (80.9) | 51 (82.3) |
| Present | 24 | - | 11 (26.2) | 13 (21.0) | 10 (32.3) | 2 (8.0) | 12 (16.2) | 9 (15.5) | 5 (13.2) | 10 (29.4) | 13 (19.1) | 11 (17.7) |
| Stage | ||||||||||||
| I | 21 | 11 (42.3) | 9 (21.4) | 1 (1.6)a | 10 (32.3) | 6 (24.0) | 5 (6.8)a | 13 (22.4) | 6 (15.8) | 2 (5.9) | 13 (19.1) | 8 (12.9) |
| II | 38 | 6 (23.1) | 12 (28.6) | 20 (32.3) | 8 (25.8) | 4 (16.0) | 26 (35.1) | 20 (34.5) | 11 (28.9) | 7 (20.6) | 18 (26.5) | 20 (32.3) |
| III | 48 | 8 (30.8) | 11 (26.2) | 29 (46.8) | 3 (9.7) | 14 (56.0) | 31 (41.9) | 16 (27.6) | 15 (39.5) | 17 (50.0) | 24 (35.3) | 24 (38.7) |
| IV | 23 | 1 (3.8) | 10 (23.8) | 12 (19.4) | 10 (32.3) | 1 (4.0) | 12 (16.2) | 9 (15.5) | 6 (15.8) | 8 (23.5) | 13 (19.1) | 10 (16.1) |
| Subtype | ||||||||||||
| Intestinal | 100 | 24 (92.4) | 39 (92.9) | 37 (59.7)a | 30 (96.8) | 21 (84.0) | 49 (66.2)b | 47 (81.0) | 28 (73.7) | 25 (73.5) | 52 (76.5) | 48 (77.4) |
| Diffuse | 19 | 1 (3.8) | 3 (7.1) | 15 (24.2) | 1 (3.2) | 2 (8.0) | 16 (21.6) | 8 (13.8) | 4 (10.5) | 7 (20.6) | 10 (17.7) | 9 (14.5) |
| Mixed | 11 | 1 (3.8) | 0 | 10 (16.1) | 0 | 2 (8.0) | 9 (12.2) | 3 (5.2) | 6 (15.8) | 2 (5.9) | 6 (8.8) | 5 (8.1) |
| LVI | ||||||||||||
| Absent | 89 | 14 (53.8) | 28 (66.7) | 47 (75.8) | 16 (51.6) | 19 (76.0) | 54 (73.0) | 39 (67.2) | 24 (63.2) | 26 (76.5) | 45 (66.2) | 44 (71.0) |
| Present | 41 | 12 (46.2) | 14 (33.3) | 15 (24.2) | 15 (48.4) | 6 (24.0) | 20 (27.0) | 19 (32.8) | 14 (36.8) | 8 (23.5) | 23 (33.8) | 18 (29.0) |
| PNI | ||||||||||||
| Absent | 94 | 16 (61.5) | 28 (66.7) | 50 (80.6) | 18 (58.1) | 19 (76.0) | 57 (77.0) | 43 (74.1) | 25 (65.8) | 26 (76.5) | 52 (76.5) | 42 (67.7) |
| Present | 36 | 10 (38.5) | 14 (33.3) | 12 (19.4) | 13 (49.0) | 6 (24.0) | 11 (23.0) | 15 (25.9) | 13 (34.2) | 8 (23.5) | 16 (23.5) | 20 (32.3) |
| Survival | ||||||||||||
| Deceased | 100 | 9 (34.6) | 29 (69.0) | 62 (100.0)a | 8 (25.8) | 20 (80.0) | 72 (97.3)a | 40 (69.0) | 29 (76.3) | 31 (91.2) | 56 (82.4) | 44 (71.0) |
| Alive | 30 | 17 (65.4) | 13 (31.0) | 0 | 23 (74.2) | 5 (20.0) | 2 (2.7) | 18 (31.0) | 9 (23.7) | 3 (8.8) | 12 (17.6) | 18 (29.0) |
| PTB | ||||||||||||
| PTB1 | 26 | - | - | - | 17 (54.8) | 3 (12.0) | 6 (8.1)a | 15 (25.9) | 8 (21.1) | 3 (8.8)d | 10 (14.7) | 16 (25.8)b |
| PTB2 | 42 | - | - | - | 14 (45.2) | 18 (72.0) | 10 (13.5) | 24 (41.4) | 12 (31.6) | 6 (17.7) | 17 (25.0) | 25 (40.3) |
| PTB3 | 62 | - | - | - | 0 | 4 (16.0) | 58 (78.4) | 19 (32.8) | 18 (47.4) | 25 (73.5) | 41 (60.3) | 21 (33.9) |
| ITB | ||||||||||||
| ITB1 | 31 | - | - | - | - | - | - | 21 (36.2) | 8 (21.1) | 2 (5.9)d | 9 (13.2) | 22 (35.5)d |
| ITB2 | 25 | - | - | - | - | - | - | 13 (22.4) | 6 (15.8) | 6 (17.6) | 13 (19.2) | 12 (19.4) |
| ITB3 | 74 | - | - | - | - | - | - | 24 (41.4) | 24 (63.2) | 26 (76.5) | 46 (67.6) | 28 (45.1) |
| DR | ||||||||||||
| DR1 | 58 | - | - | - | - | - | - | - | - | - | 22 (32.4) | 36 (58.0)b |
| DR2 | 38 | - | - | - | - | - | - | - | - | - | 24 (35.2) | 14 (22.6) |
| DR3 | 34 | - | - | - | - | - | - | - | - | - | 22 (32.4) | 12 (19.4) |
The patients were divided into three groups according to their PTB status, which resulted in 26 patients (20.0%) being classified as PTB1, 42 patients (32.3%) as PTB2, and 62 patients (47.7%) as PTB3. The ITB groups were categorized as follows: 31 patients (23.8%) were classified as ITB1, 25 patients (19.2%) as ITB2, and 74 patients (56.9%) as ITB3.
According to the DR classification, a total of 58 (44.6%) patients were classified as DR1, 38 (29.2%) patients as DR2, and 34 (26.2%) patients as DR3. The number of patients with TILs less than the cutoff value (68 patients, 52.3%) outnumbered that with higher lymphocytic infiltration (62 patients, 47.7%).
The relationships between clinicopathological parameters and PTB, ITB, DR, and TILs are presented in Table 1. There was a positive correlation between PTB and invasion and distant metastasis (P < 0.05). Higher PTB and ITB were more frequently observed in patients with LNM (P < 0.001). Similarly, both parameters were associated with the disease stage (P < 0.001). Compared with those with intestinal carcinomas, patients with higher PTB and ITB were more likely to have diffuse and mixed subtypes (P < 0.01).
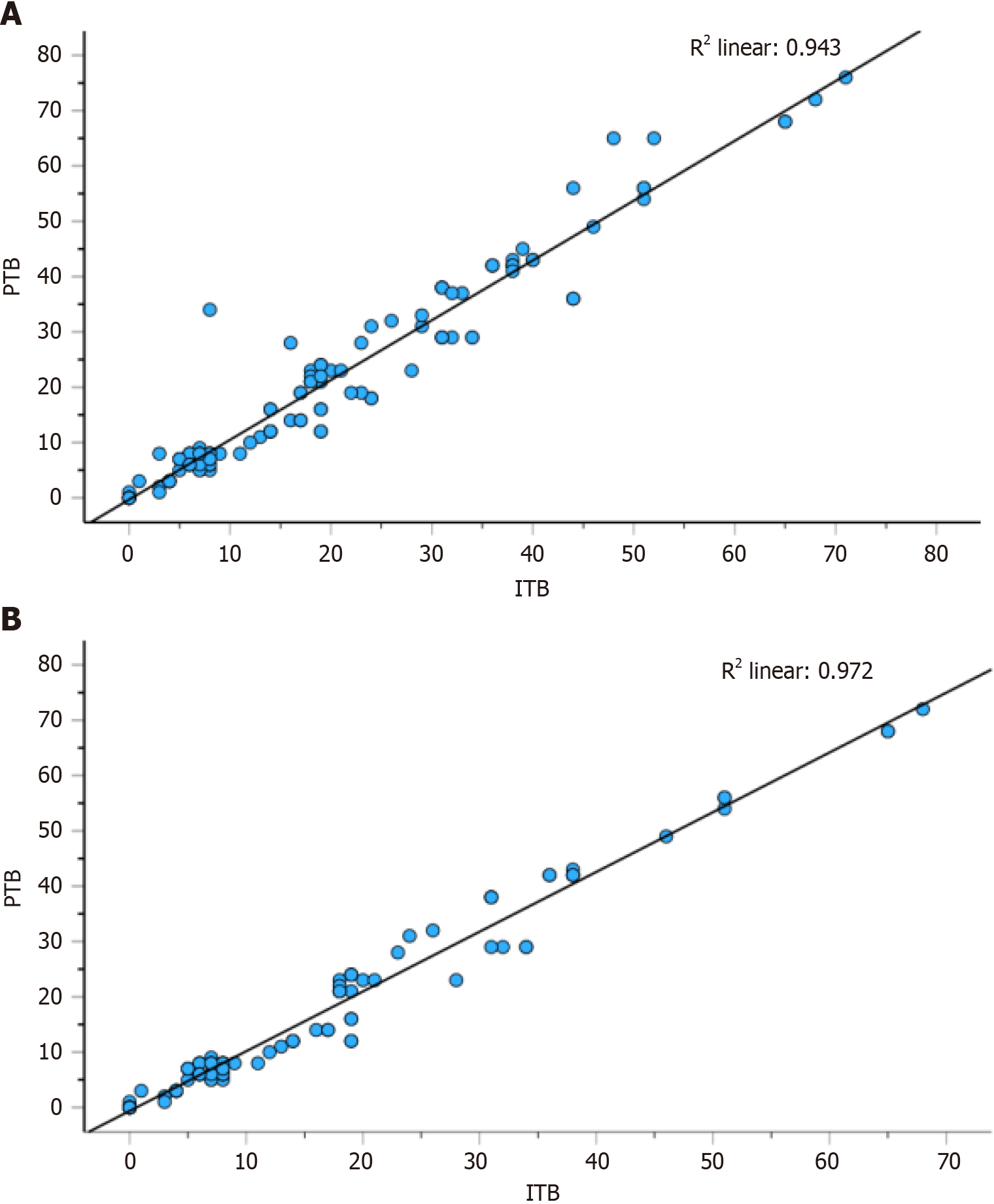
Spearman correlation analysis revealed a strong correlation between PTB and ITB (r = 0.943, Figure 2). In patients with either PTB or ITB, immature stroma (DR3) and low TILs were more frequent (P < 0.01) (Table 1).
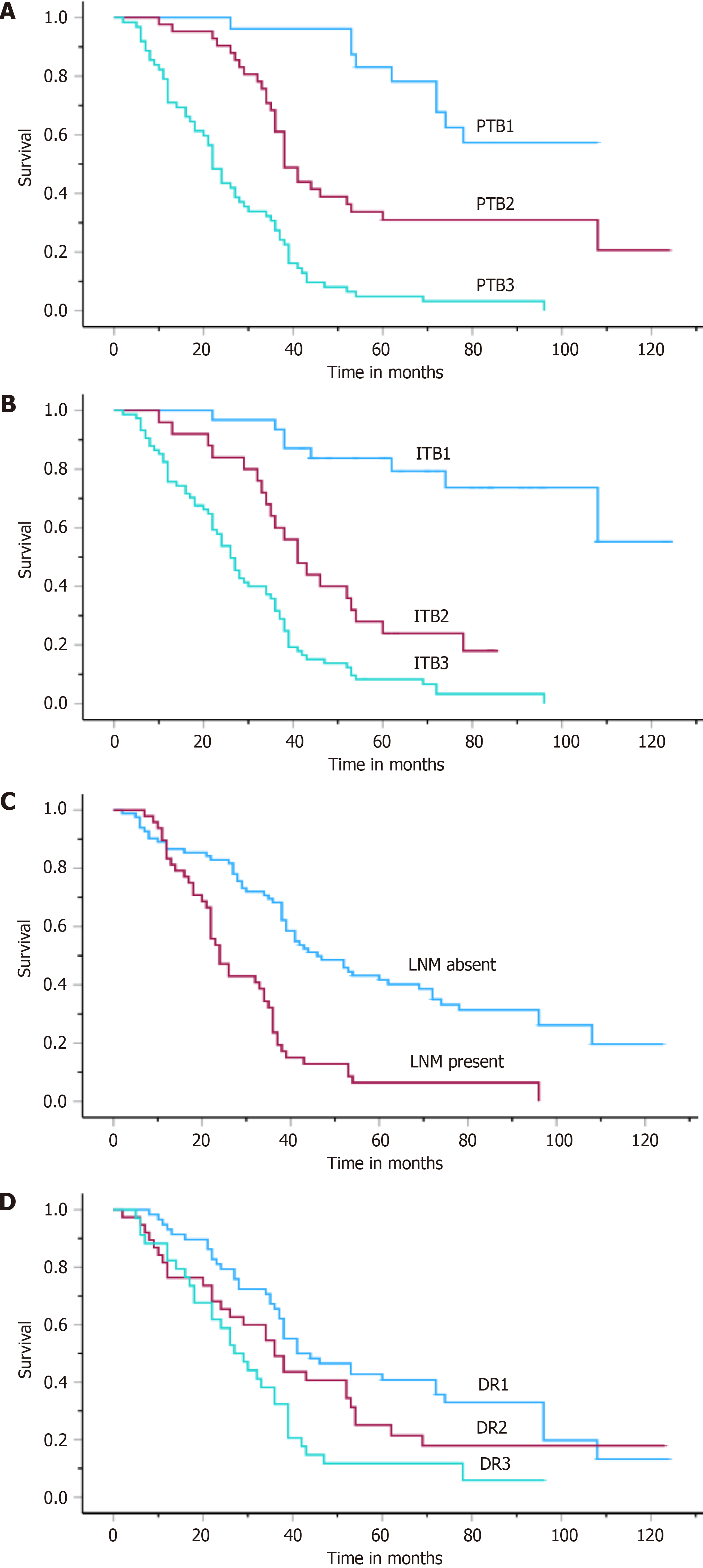
In the total cohort, the median OS was 36.5 ± 14.26 (ranging from 2 to 120 months). According to the univariate analysis, histologic subtype, ITB, PTB, LNM, DR, and stage were identified as risk factors for poor prognosis (P < 0.01) (Table 2, Figure 3). The relationships between age, sex, and tumor diameter and these features and outcomes were not significantly different (P > 0.05).
| Parameters | All cases | Intestinal tumors | ||
| mean ± SE (95%CI) | Median ± SE (95%CI) | mean ± SE (95%CI) | Median ± SE (95%CI) | |
| Age | ||||
| < 62.14 ± 12.00 | 48.2 ± 4.5 (39.4-56.9) | 37.0 ± 1.1 (34.8-39.1) | 53 ± 5.9 (41.4-64.5) | 36.0 ± 1.2 (33.4-38.5) |
| ≥ 62.14 ± 12.00 | 50.8 ± 4.8 (41.3-60.3) | 39.0 ± 9.5 (20.1-57.8) | 55.7 ± 5.7 (44.4-66.9) | 60.0 ± 16.2 (28.2-91.7) |
| Gender | ||||
| Male | 50.1 ± 4.7 (41.3-59.0) | 36.0 ± 2.6 (30.8-41.1) | 54.7 ± 5.5 (43.7-65.6) | 38.0 ± 3.3 (31.5-44.5) |
| Female | 44.9 ± 5.2 (39.1-58.7) | 38.0 ± 1.6 (34.9-41.0) | 54.5 ± 6.3 (42-67.1) | 38.0 ± 9.6 (19.1-56.8) |
| Diameter | ||||
| < 1.86 ± 1.02 | 47.3 ± 3.7 (40.0-54.6) | 38.0 ± 1.8 (3.4-41.5) | 50.2 ± 4.5 (41.3-59.1) | 38.0 ± 2.7 (32.6-43.3) |
| ≥ 1.86 ± 1.02 | 52.3 ± 6.3 (39.8-64.7) | 36.0 ± 4.6 (26.8-45.1) | 61.4 ± 8.2 (45.2-77.6) | 38.0 ± 13.4 (11.6-64.3) |
| Invasion | ||||
| T1 | 65.1 ± 7.1 (51.2-79.1) | 78.0 ± 22.1 (34.5-121.4) | 65.1 ± 7.1 (51.1-79.1) | 78.0 ± 22.1 (34.5-121.4) |
| T2 | 54.5 ± 6.6 (41.5-67.5) | 38.0 ± 2.6 (32.9-43.0) | 63.5 ± 8.6 (46.6-80.4) | 52.0 ± 14.4 (23.7-80.2) |
| T3 | 44.8 ± 4.8 (35.4-54.3) | 36.0 ± 4.3 (27.5-44.5) | 46.4 ± 5.7 (35-57.7) | 35.0 ± 5.5 (241.0-45.8) |
| T4 | 44.0 ± 5.4 (32.3-53.6) | 30.0 ± 2.8 (24.4- 35.5) | 48.7 ± 7.0 (35-62.5) | 36.0 ± 3.1 (29.7-42.2) |
| LNM | ||||
| Absent | 51.6 ± 4.8 (52.1-71.0) | 46.0 ± 5.5 (35.1-56.8)a | 66.1 ± 5.5 (55.3-77.1) | 62.0 ± 13.2 (35.8-88.1)a |
| Present | 30.6 ± 3.0 (24.6-36.6) | 24.0 ± 2.2 (19.5-28.4) | 32.1 ± 4.5 (23.3-40.8) | 24.0 ± 7.7 (8.7-39.2) |
| Metastasis | ||||
| Absent | 48.3 ± 3.2 (41.9-54.8) | 38.0 ± 1.5 (34.9-41.0) | 53.4 ± 4.1 (45.2-61.6) | 39.0 ± 4.8 (29.4-48.5) |
| Present | 51.7 ± 9.7 (32.6-70.7) | 24.0 ± 8.3 (7.6-40.3) | 55.1 ± 10.8 (33.8-76.5) | 36.0 ± 12.0 (12.4-59.5) |
| Stage | ||||
| I | 69.1 ± 6.9 (55.5-82.7) | 72.0 ± 11.1 (50.3-93.6)b | 71.5 ± 6.8 (58.1-85.1) | 72.0 ± 10.9 (50.5-93.4)c |
| II | 45.1 ± 5.3 (34.7-55.5) | 37.0 ± 2.2 (32.6-41.3) | 52.3 ± 7.2 (38.1-66.5) | 39.0 ± 2.5 (34.0-43.9) |
| III | 38.6 ± 3.9 (30.8-46.4) | 33.0 ± 3.4 (26.2-39.7) | 40.0 ± 5.8 (28.5-51.4) | 29.0 ± 4.9 (19.2-38.7) |
| IV | 31.3 ± 10.8 (37.1-59.5) | 30.0 ± 12.3 (11.8-60.1) | 38.3 ± 10.8 (27.1-48.7) | 30.0 ± 12.3 (29.8-42.1) |
| Subtype | ||||
| Intestinal | 55.1 ± 4.4 (46.6-63.7) | 38.0 ± 2.7 (32.9-42.1)d | - | - |
| Not intestinal | 32.5 ± 2.2 (18.8-36.7) | 30.0 ± 1.2 (6.2-17.7) | - | - |
| Grade | ||||
| Low | - | - | 61.9 ± 5.7 (50.6-73.2) | 52.0 ± 11.6 (29.1-74.8) |
| Moderate | - | - | 49.9 ± 10.6 (29.0-70.8) | 32.0 ± 4.7 (22.7-41.2) |
| High | - | - | 43.2 ± 6.8 (29.9-56.5) | 29.0 ± 12.3 (4.7-53.2) |
| LVI | ||||
| Absent | 54.3 ± 5.8 (43.0-65.7) | 41.0 ± 2.2 (36.5-45.4) | 59.0 ± 6.9 (45.4-72.7) | 41.0 ± 7.0 (27.0-54.9) |
| Present | 46.6 ± 4.0 (38.6-54.6) | 36.0 ± 3.4 (29.1-42.8) | 51.4 ± 5.1 (41.2-61.5) | 36.0 ± 2.9 (30.2-41.7) |
| PNI | ||||
| Absent | 55.2 ± 6.5 (42.5-67.9) | 38.0 ± 3.9 (30.2-45.7) | 60.4 ± 7.7 (45.3-75.5) | 38.0 ± 9.8 (18.6-57.3) |
| Present | 46.9 ± 3.8 (39.4-54.4) | 36.0 ± 2.2 (31.6-40.3) | 51.7 ± 4.8 (42.3-61.2) | 38.0 ± 2.5 (33-42.9) |
| PTB | ||||
| PTB1 | 88.3 ± 5.2 (78.0-98.6) | 73.5 ± 12.7 (71.6-92.5)a | 92.4 ± 4.7 (83.3-101.9) | 88.2 ± 7.6 (56.9-91.6)a |
| PTB2 | 61.8 ± 6.4 (48.6-73.6) | 38.0 ± 2.8 (33.5-42.5) | 63.0 ± 6.5 (50.2-75.8) | 41.0 ± 3.1 (34.8-47.1) |
| PTB3 | 26.7 ± 2.4 (21.9-31.5) | 22.0 ± 1.5 (19.1-24.9) | 22.7 ± 3.6 (15.6-29.8) | 16.0 ± 2.1 (33.8-42.1) |
| ITB | ||||
| ITB1 | 101.1 ± 6.7 (87.8-114.4) | 91.2 ± 5.2 (81.4-112.5)a | 103.8 ± 6.4 (91.1-116.4) | 63.0 ± 8.3 (88.7-97.4)a |
| ITB2 | 47.7 ± 4.6 (38.6-56.7) | 41.0 ± 4.2 (32.8-49.2) | 50.1 ± 5.2 (39.9-60.2) | 42.0 ± 6.3 (29.0-52.9) |
| ITB3 | 29.9 ± 2.4 (25.3-34.6) | 26.0 ± 1.9 (22.2-29.8) | 28.5 ± 3.4 (21.8-35.2) | 22.0 ± 4.0 (13.9-30.0) |
| DR | ||||
| DR1 | 60.4 ± 5.4 (49.8-70.9) | 41.0 ± 7.9 (25.4-56.6)d | 67.2 ± 6.2 (55.1-79.3) | 72.0 ± 18.1 (36.4-107.5)a |
| DR2 | 48.0 ± 6.6 (35.1-61.0) | 36.0 ± 4.5 (27.3-44.8) | 53.5 ± 8.4. (36.9-70.1) | 38.0 ± 9.1 (20.0-55.9) |
| DR3 | 27.0 ± 4.1 (18.8-35.2) | 18.0 ± 3.6 (10.8-25.1) | 31.8 ± 5.3 (21.3-42.2) | 24.0 ± 6.7 (10.9-37.0) |
| TILs | ||||
| TILs | 53.8 ± 4.3 (45.3-62.4) | 39.0 ± 4.8 (29.4-48.5) | 59.6 ± 5.2 (49.3-70.0) | 53.0 ± 14.7 (24.0-81.9) |
| TILs | 43.9 ± 4.6 (34.9-52.9) | 35.0 ± 4.5 (26.2-43.7) | 47.8 ± 5.7 (36.5-59.1) | 35.0 ± 5.1 (24.9-45.0) |
According to the multivariate Cox regression analysis, ITB, PTB, and LNM were found to be independent prognostic factors (P < 0.05, Table 3).
| Parameters | All group | P value | Intestinal tumors | P value | ||||
| HR | Lower (95%CI) | Upper (95%CI) | HR | Lower (95%CI) | Upper (95%CI) | |||
| ITB | 2.06 | 1.40 | 3.01 | < 0.001 | 3.32 | 2.34 | 4.72 | < 0.001 |
| PTB | 1.83 | 1.29 | 2.59 | < 0.001 | 2.01 | 1.32 | 3.05 | < 0.001 |
| LNM | 1.53 | 1.00 | 2.33 | 0.04 | 1.09 | 0.59 | 2.04 | 0.760 |
| Stage | 1.06 | 0.81 | 1.40 | 0.63 | 1.12 | 0.80 | 1.58 | 0.480 |
| Subtype | 0.76 | 0.50 | 1.17 | 0.22 | - | - | - | - |
| DR | 1.11 | 0.87 | 1.42 | 0.39 | 1.17 | 0.86 | 1.60 | 0.290 |
In this cohort, higher PTB, higher ITB and immature stroma were more common in patients with LNM (P < 0.003). DR was also associated with male predominance (Table 4).
| Parameters | n | PTB1 (%) | PTB2 (%) | PTB3 (%) | ITB1 (%) | ITB2 (%) | ITB3 (%) | DR1 (%) | DR2 (%) | DR3 (%) | TILs (%) | TILs (%) |
| Age, yr | 24 | 39 | 37 | 30 | 21 | 49 | 47 | 28 | 25 | 52 | 48 | |
| < 62.14 ± 12.00 | 47 | 10 (41.7) | 24 (61.5) | 13 (35.1) | 14 (46.7) | 11 (52.4) | 22 (44.9) | 21 (44.7) | 15 (53.6) | 11 (44.0) | 25 (48.1) | 22 (45.8) |
| ≥ 62.14 ± 12.00 | 53 | 14 (58.3) | 15 (38.5) | 24 (63.9) | 16 (53.3) | 10 (47.6) | 27 (55.1) | 26 (55.3) | 13 (46.4) | 14 (56.0) | 27 (51.9) | 26 (54.2) |
| Gender | ||||||||||||
| Male | 60 | 15 (62.5) | 24 (61.5) | 21 (56.8) | 18 (60.0) | 15 (71.4) | 27 (55.1) | 34 (72.3) | 16 (57.1) | 10 (40.0)a | 27 (51.9) | 33 (68.8) |
| Female | 40 | 9 (37.5) | 15 (38.5) | 16 (43.2) | 12 (40.0) | 6 (28.6) | 22 (44.9) | 13 (27.7) | 12 (42.9) | 15 (60.0) | 25 (48.1) | 15 (31.3) |
| Diameter | ||||||||||||
| < 1.86 ± 1.02 | 67 | 16 (66.7) | 26 (66.7) | 25 (67.6) | 15 (50.0) | 16 (76.2) | 36 (73.5) | 28 (59.6) | 22 (78.6) | 17 (68.0) | 38 (73.1) | 29 (60.4) |
| ≥ 1.86 ± 1.02 | 33 | 8 (33.3) | 13 (33.3) | 12 (32.4) | 15 (50.0) | 5 (23.8) | 13 (26.5) | 19 (40.4) | 6 (21.4) | 8 (32.0) | 14 (26.9) | 19 (39.6) |
| Invasion | ||||||||||||
| T1 | 7 | 5 (20.8) | 2 (5.1) | 0 | 4 (13.3) | 1 (4.8) | 2 (4.1) | 3 (6.4) | 3 (10.7) | 1 (4.0) | 4 (7.7) | 3 (6.3) |
| T2 | 28 | 4 (16.7) | 15 (38.5) | 9 (24.3) | 12 (40.0) | 7 (33.3) | 9 (18.4) | 14 (29.8) | 9 (32.1) | 5 (20.0) | 12 (23.1) | 16 (33.3) |
| T3 | 42 | 10 (41.7) | 13 (33.3) | 19 (51.4) | 9 (30.0) | 6 (28.6) | 27 (55.1) | 23 (48.9) | 12 (42.9) | 7 (28.0) | 23 (44.2) | 19 (39.6) |
| T4 | 23 | 5 (20.8) | 9 (23.1) | 9 (24.3) | 5 (16.7) | 7 (33.3) | 11 (22.4) | 7 (14.9) | 4 (14.3) | 12 (48.0) | 13 (25.0) | 10 (20.8) |
| LNM | ||||||||||||
| Absent | 69 | 22 (91.7) | 29 (74.4) | 18 (48.6)b | 29 (96.7) | 14 (66.7) | 26 (53.1)b | 39 (83.0) | 19 (67.9) | 11 (44.0)c | 32 (61.5) | 37 (77.1) |
| Present | 31 | 2 (8.3) | 10 (25.6) | 19 (51.4) | 1 (3.3) | 7 (33.3) | 23 (46.9) | 8 (17.0) | 9 (32.1) | 14 (56.0) | 20 (38.5) | 11 (22.9) |
| Metastasis | ||||||||||||
| Absent | 79 | 24 (100.0) | 29 (74.4) | 26 (70.3)d | 21 (70.0) | 20 (95.2) | 38 (77.6) | 39 (83.0) | 23 (82.1) | 17 (68.0) | 40 (76.9) | 39 (81.3) |
| Present | 21 | 0 | 10 (25.6) | 11 (29.7) | 9 (30.0) | 1 (4.8) | 11 (22.4) | 8 (17.0) | 5 (17.9) | 8 (32.0) | 12 (23.1) | 9 (18.7) |
| Stage | ||||||||||||
| I | 20 | 11 (45.8) | 9 (23.2) | 0 | 10 (33.3) | 5 (23.8) | 5 (10.2)c | 12 (25.5) | 6 (21.4) | 2 (8.0) | 12 (23.1) | 8 (16.7) |
| II | 26 | 6 (25.0) | 10 (25.6) | 10 (27.0) | 7 (23.3) | 4 (19.0) | 15 (30.6) | 15 (31.9) | 9 (32.1) | 2 (8.0) | 12 (23.1) | 14 (29.3) |
| III | 32 | 6 (25.0) | 10 (25.6) | 16 (43.2) | 3 (10.0) | 11 (52.4) | 18 (36.7) | 12 (25.5) | 7 (25.0) | 13 (52.0) | 15 (28.8) | 17 (35.2) |
| IV | 22 | 1 (4.2) | 10 (25.6) | 11 (29.7) | 10 (33.4) | 1 (4.8) | 11 (22.4) | 8 (17.0) | 6 (21.4) | 8 (32.0) | 13 (25.0) | 9 (18.8) |
| Grade | ||||||||||||
| Low | 54 | 19 (79.2) | 21 (53.8) | 14 (37.8)a | 22 (73.3) | 12 (57.1) | 20 (40.8)a | 28 (59.6) | 16 (57.1) | 10 (40.0) | 29 (55.8) | 25 (52.1) |
| Moderate | 29 | 2 (8.3) | 13 (33.4) | 14 (37.8) | 3 (10.0) | 6 (28.6) | 20 (40.8 | 11 (23.4) | 10 (35.7) | 8 (32.0) | 15 (28.8) | 14 (29.2) |
| High | 17 | 3 (12.5) | 5 (12.8) | 9 (24.3) | 5 (16.7) | 3 (14.3) | 9 (18.4) | 8 (17.0) | 2 (7.2) | 7 (28.0) | 8 (15.4) | 9 (18.7) |
| LVI | ||||||||||||
| Absent | 67 | 13 (54.2) | 26 (66.7) | 28 (75.7) | 15 (50.0) | 15 (71.4) | 37 (75.5) | 34 (72.3) | 14 (50.0) | 19 (76.0) | 32 (61.5) | 35 (72.9) |
| Present | 33 | 11 (45.8) | 13 (33.3) | 9 (24.3) | 15 (50.0) | 6 (28.6) | 12 (24.5) | 13 (27.7) | 14 (50.0) | 6 (24.0) | 20 (38.5) | 13 (27.1) |
| PNI | ||||||||||||
| Absent | 71 | 14 (58.3) | 26 (66.7) | 31 (83.8) | 18 (60.0) | 15 (71.4) | 38 (77.6) | 34 (72.3) | 16 (57.1) | 21 (84.0) | 37 (71.2) | 34 (70.8) |
| Present | 29 | 10 (41.7) | 13 (33.3) | 6 (16.2) | 12 (40.0) | 6 (28.6) | 11 (22.4) | 13 (27.7) | 12 (42.9) | 4 (16.0) | 15 (28.8) | 14 (29.2) |
| Survival | ||||||||||||
| Deceased | 71 | 7 (29.2) | 27 (69.2) | 37 (100.0)b | 7 (23.3) | 16 (76.2) | 48 (98.0)b | 29 (61.7) | 20 (71.4) | 22 (88.0) | 41 (78.8) | 30 (62.5) |
| Alive | 29 | 17 (70.8) | 12 (30.8) | 0 | 23 (76.7) | 5 (23.8) | 1 (2.0) | 18 (38.3) | 8 (28.6) | 3 (12.0) | 11 (21.2) | 18 (37.5) |
| PTB | ||||||||||||
| PTB1 | 24 | - | - | - | 17 (56.7) | 3 (14.3) | 4 (8.2)b | 14 (29.8) | 8 (28.6) | 2 (8.0)c | 10 (19.2) | 14 (29.2)d |
| PTB2 | 39 | - | - | - | 13 (43.3) | 18 (85.7) | 8 (16.3) | 23 (48.9) | 11 (39.3) | 5 (20.0) | 16 (30.8) | 23 (47.9) |
| PTB3 | 37 | - | - | - | 0 | 0 | 37 (75.5) | 10 (21.3) | 9 (32.1) | 18 (72.0) | 26 (50.0) | 11 (22.9) |
| ITB | ||||||||||||
| ITB1 | 30 | - | - | - | - | - | - | 21 (44.7) | 8 (28.6) | 1 (4.0)c | 9 (17.3) | 21 (43.8)c |
| ITB2 | 21 | - | - | - | - | - | - | 12 (25.5) | 3 (10.7) | 6 (24.0) | 9 (17.3) | 12 (25.0) |
| ITB3 | 49 | - | - | - | - | - | - | 14 (29.8) | 17 (60.7) | 18 (72.0) | 34 (65.4) | 15 (31.2) |
| DR | ||||||||||||
| DR1 | 47 | - | - | - | - | - | - | - | - | - | 17 (32.7) | 30 (62.5)d |
| DR2 | 28 | - | - | - | - | - | - | - | - | - | 17 (32.7) | 11 (22.9) |
| DR3 | 25 | - | - | - | - | - | - | - | - | - | 18 (34.6) | 7 (14.6) |
There was a positive correlation between PTB and ITB with the stage and grade (P < 0.01). In addition, PTB was related to invasion (P < 0.05). While PTB was positively associated with DR, an inverse relationship was observed between higher TILs and these parameters (P < 0.006, Table 4). Besides, Spearman correlation analysis revealed a strong correlation between PTB and ITB (r = 0.972, Figure 2).
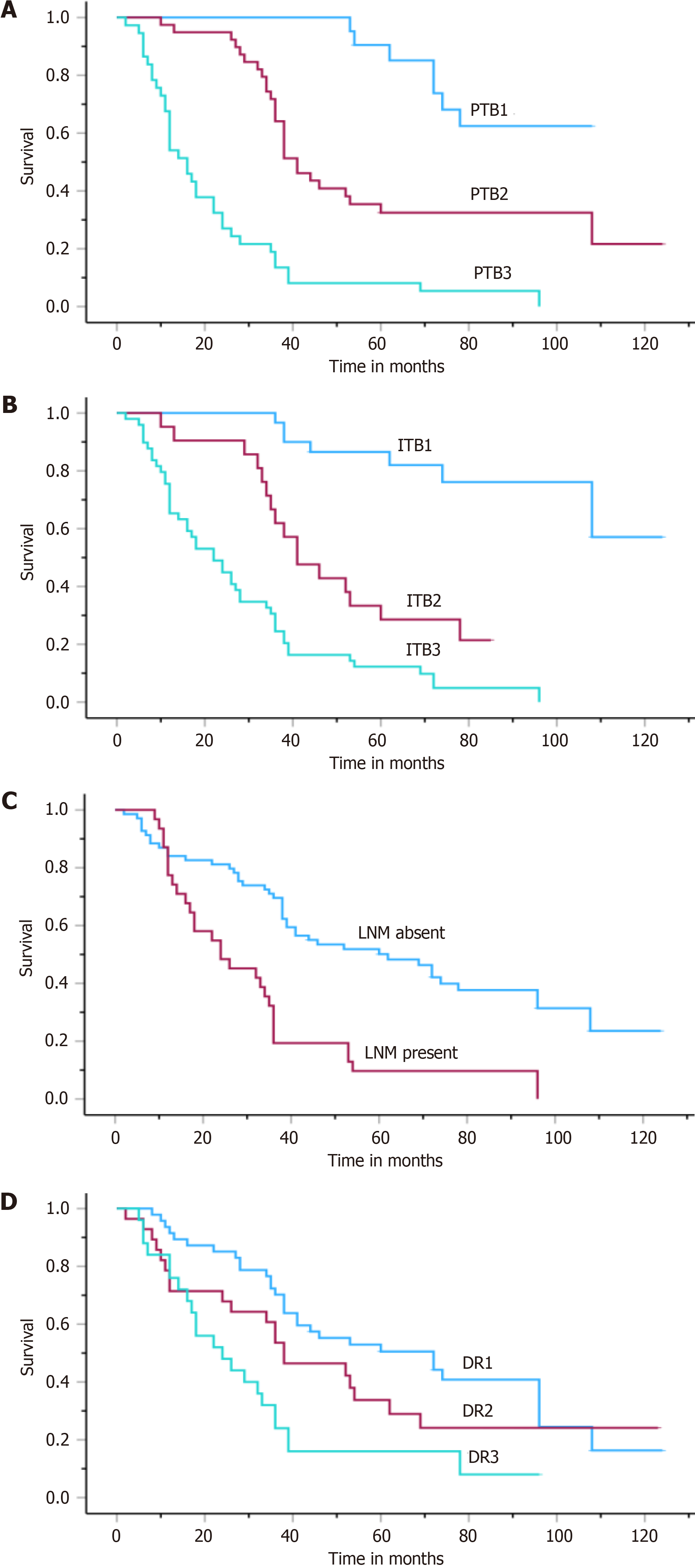
In this group, the median survival ranged from 33.8 to 42.1 months (median: 38.0 ± 2.1). Kaplan-Meier analysis revealed that LNM (P < 0.001), stage (P < 0.04), PTB (P < 0.001), ITB (P < 0.001), and DR (P < 0.001) were powerful indicators of the disease course (Table 4, Figure 4). According to the multivariate analysis, PTB and ITB were found to be independent prognostic parameters (P < 0.001, Table 3).
TB has been investigated in numerous studies of CRC and is currently used in pathological reporting protocols due to its prognostic importance in low-grade tumors[31,35]. However, TB has yet to be studied extensively in GAC. This may be because GAC is less frequently observed than CRC, especially in Western countries[26,36]. Moreover, a standard evaluation method for this variable has yet to be determined. For example, studies investigating the role of TB in predicting LNM in early gastric carcinoma (EGC) patients have indicated that detecting the presence of TB may be effective[37-39]. Yim et al[40] recently observed a strong association between TB and LNM metastasis with three different evaluation methods in EGC. However, only the presence of TB was an independent prognostic factor. The limited number of EGC patients in our series did not allow a separate analysis of this group. However, these results suggest that the presence of TB is an effective marker for predicting LNM metastasis and patient prognosis, at least in EGC.
Recently, in studies that included gastric cancer (GC) patients of all stages and histopathological subtypes, TB was observed to be an independent prognostic factor, which is consistent with our findings[12-14,41,42]. Interestingly, although different categorizations were used in the statistical analysis to determine the predictive role of TB in the course of the disease, the evaluation methods applied in most of these studies were based on the ITBCC, similar to our research[13,14,28,42]. In our study, the survival of patients with TB3 was significantly lower than that of patients with TB2 or TB1. Taken together, these data point to the value of the ITBCC-recommended evaluation of TB in GAC patients.
When adenocarcinoma subtypes in GC were considered separately, TB was observed to be associated with tumor behavior in the intestinal type of GAC but not in diffuse tumors. Although no further analysis of this subtype could be performed in our study group due to the limited number of patients with nonintestinal tumors, TB was observed to be related to survival in patients with intestinal-type GAC according to the log-rank analysis. Moreover, multivariate analysis revealed that the TB score is an independent prognostic parameter. Although TB incidence has been correlated with intestinal-type GAC behavior and survival in many studies, the results of multivariate analyses have yet to be consistent. While in some studies, the evaluation of TB was observed to be a decisive parameter in determining the course of the disease[13,42,43], such an effect was not noted in others[15,16,26]. These different findings may be due to diversity in the number of cases and data categorization among studies. Our findings are consistent with those of studies in which TB was observed to be a strong prognostic parameter in intestinal-type GAC patients and emphasize the need for additional research to establish the value of TB in GAC reporting guidelines.
Another notable finding of our study was that in addition to the whole cohort, PTB and ITB were found to be independent prognostic factors for the intestinal subtype, and their correlation with each other was strong. To our knowledge, only one study has evaluated TB separately in intestinal GAC patients. Qi et al[43] observed a strong association between ITB and PTB; both were found to be independent prognostic parameters for predicting survival. Although these findings need to be supported by further studies, the independent prognostic value of TB in both topographic areas support the idea that TB can be evaluated to stratify patients with intestinal-type GAC for prognosis[4,5]. Furthermore, given the substantial correlation between the ITB and PTB, TB could be used as a predictive parameter for determining tumor behavior, especially in patients who are unsuitable for surgical resection.
Although DR in GAC was associated with survival according to univariate analysis in this study, it was not an independent prognostic factor when other parameters related to tumor behavior and prognosis were analyzed. To our knowledge, very few studies have investigated the effectiveness of DR in determining the survival of patients with GAC[24-26]. In these tumors, examination of the thickness of collagen fibers by second-generation harmonic imaging indicated that the presence of large desmoplastic collagen fibers was associated with poor prognosis[24]. In an elegant study in which DR was categorized into two groups (mature and immature), Kemi et al[25] reported that DR was an independent parameter for determining the course of disease in patients with GAC. They also noted that DR was associated with 5-year survival in the intestinal subgroup, whereas no such association was observed for diffuse carcinomas.
On the other hand, Pun et al[26] did not detect such a relationship in intestinal-type adenocarcinomas. In both studies, DR was evaluated both in the invasive tumor area and in the main tumor mass. In our study, we investigated DR only on invasive edges according to the method applied in the assessment of DR in many studies, and we found that DR was not an effective prognostic parameter in either the whole group or intestinal tumors. These results emphasize that a different method should be applied to investigate the role of DR in GAC. Recently, Hacking et al[44] suggested a different approach for evaluating stromal maturity in patients with CRC. However, the prognostic impact of DR in GAC remains to be investigated via this method. In brief, further studies comparing different evaluation methods and categorizations in large patient series are needed to determine the value of DR as a parameter in pathology protocols for these tumors.
We observed a strong positive relationship between DR and TB in the study group. In parallel with these data, a recent study demonstrated the association of high TB with immature stroma in GAC[26]. Moreover, our research revealed an inverse correlation between DR and TILs. To our knowledge, no study has investigated the relationship between these three parameters in patients with GAC. Our findings support studies highlighting the importance of DR in the TME. Although it has not been determined to be an independent prognostic marker, further studies are needed to determine the potential of DR as a marker in GAC.
TILs, an essential component of the tumor environment, have been studied extensively in GAC, but the results are still controversial, even when evaluating lymphocyte subsets by immunohistochemistry. In this study, we did not observe TILs to be a significant predictive parameter for GAC prognosis. There are studies in which TILs were semiquantitatively investigated by H&E staining, similar to our method. A substantial correlation between TILs and survival has been noted[45,46]. Unfortunately, the topographical differences in the evaluation of TILs (intratumoral vs stromal) in these studies and the investigation of different types of GACs, such as EBV-associated GCs, limit the comparison of our data[47,48].
Regarding immunohistochemical studies on TILs in GAC, while one study linked higher CD8+ T-cell density in GAC to poor prognosis[49], another noted that higher numbers of CD8+ T cells and TILs improved overall survival (OS)[50]. Similarly, there is disagreement over the predictive importance of CD4+ T-cell tumor infiltration[27,51]. Different data were also obtained in past meta-analyses of GAG[51-53]. The presence of CD3+ lymphocytes was the highest predictive factor for OS (HR = 0.52)[51]. A significant relationship between CD8+ TILs and survival was demonstrated in another analysis[53]. The results also indicated that high intratumoral T-cell infiltration levels were associated with improved survival in GAC patients, and a high density of intratumoral FOXP3+ T cells was not closely associated with poor prognosis[28].
In our study, the strong association between TILs and TB suggested the potential role of TILs in tumor behavior in GAC. Parallel to this observation, in a recent study, Zhang et al[28], by double immunohistochemical staining, noted an inverse correlation between TILs and TB, predicting a favorable outcome. On the other hand, we did not observe TILs to be a significant predictive factor. The present study suggests that the method employed for assessing TILs has certain limitations. In other words, it is essential to emphasize that the finding that TILs were unrelated to survival in our study does not exclude the importance of recent research that has primarily investigated various lymphocyte subtypes by immunohistochemistry.
To our knowledge, the relationship between TILs and DR has yet to be described in GAC, and the present study revealed the inverse relationship between TILs and DR, suggesting that DR is an important component of tumor immune surveillance. Moreover, these data merit further investigations into the association of DR with different subsets of lymphocytes to better understand its role in the prediction of survival in GAC.
This study has several limitations. It is conducted within a single center, limiting the sample size to remain relatively small, which might restrict the power to detect more nuanced associations or differences, particularly when stratifying the analysis by adenocarcinoma subtypes or evaluating the interaction between different prognostic factors. Moreover, potential selection biases cannot be excluded due to the retrospective nature of the study, limiting the generalizability of the results to other populations and settings. Therefore, multicenter prospective studies and external validation are needed to confirm the findings.
Another limitation is the need for a standardized evaluation method for assessing TB, DR, and TILs in GAC, which might lead to variability in the results. Although we have employed methods consistent with current literature and guidelines, the need for universally accepted criteria for these histopathological features may affect the reproducibility and comparison of our findings with those of other studies. Additionally, the heterogeneous behavior of GAC necessitates a multifactorial analysis incorporating a wide range of potential prognostic markers. Our study focused on a select few, which, while important, do not encompass all the factors that could influence patient outcomes.
Despite these limitations, our study contributes valuable insights into the prognostic significance of TB, DR, and TILs in GAC, supporting the need for their consideration in future research and potential inclusion in pathological reporting protocols.
The findings support that the assessment of TB based on the ITBCC criteria can be used to categorize patients with GAC for treatment and prognosis. Although the strong relationship between PTB and ITB also suggests that these two variables can be used in determining the course of the disease in patients for whom surgical resection is not feasible, especially for those with the intestinal subtype, further studies are needed to delineate their role.
Although DR was related to TB in our series, it was not an independent parameter for predicting survival, suggesting that its value in determining GAC behavior merits further research.
Within the context of our findings, despite the emergence of recent discoveries, we did not notice TILs to be a significant predictive component in GAC. The present study suggested that the method employed for assessing TILs in these tumors has certain limitations. However, it is essential to note that this does not diminish the importance of recent research investigating various lymphocyte subtypes.
The relationships among TB, DR, and TILs in the tumor area observed in our study warrant further investigations with a more extensive patient cohort to determine the role of a scoring system consisting of these three parameters in determining the behavior of GC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: European Society of Pathology, No. 11959.
Specialty type: Pathology
Country/Territory of origin: Türkiye
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang TJ, China S-Editor: Chen YL L-Editor: A P-Editor: Zhao YQ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64648] [Article Influence: 16162.0] [Reference Citation Analysis (176)] |
| 2. | Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, Lieto E, Ciardiello F, De Vita F. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 461] [Cited by in RCA: 483] [Article Influence: 43.9] [Reference Citation Analysis (5)] |
| 3. | Luu C, Thapa R, Woo K, Coppola D, Almhanna K, Pimiento JM, Chen DT, Marquez DD, Hodul PJ. Does histology really influence gastric cancer prognosis? J Gastrointest Oncol. 2017;8:1026-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 5. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1086] [Article Influence: 271.5] [Reference Citation Analysis (0)] |
| 6. | Song K, Ma C, Gu B, Wang B, Ma H, Deng X, Chen H. Molecular mechanism underlying epithelial-mesenchymal transformation and cisplatin resistance in esophageal squamous cell carcinoma. Thorac Cancer. 2023;14:3069-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Kozak J, Forma A, Czeczelewski M, Kozyra P, Sitarz E, Radzikowska-Büchner E, Sitarz M, Baj J. Inhibition or Reversal of the Epithelial-Mesenchymal Transition in Gastric Cancer: Pharmacological Approaches. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Lu J, Kornmann M, Traub B. Role of Epithelial to Mesenchymal Transition in Colorectal Cancer. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 10. | Haddad TS, Lugli A, Aherne S, Barresi V, Terris B, Bokhorst JM, Brockmoeller SF, Cuatrecasas M, Simmer F, El-Zimaity H, Fléjou JF, Gibbons D, Cathomas G, Kirsch R, Kuhlmann TP, Langner C, Loughrey MB, Riddell R, Ristimäki A, Kakar S, Sheahan K, Treanor D, van der Laak J, Vieth M, Zlobec I, Nagtegaal ID. Improving tumor budding reporting in colorectal cancer: a Delphi consensus study. Virchows Arch. 2021;479:459-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Zlobec I, Lugli A. Tumour budding in colorectal cancer: molecular rationale for clinical translation. Nat Rev Cancer. 2018;18:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Che K, Zhao Y, Qu X, Pang Z, Ni Y, Zhang T, Du J, Shen H. Prognostic significance of tumor budding and single cell invasion in gastric adenocarcinoma. Onco Targets Ther. 2017;10:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Szalai L, Jakab Á, Kocsmár I, Szirtes I, Kenessey I, Szijártó A, Schaff Z, Kiss A, Lotz G, Kocsmár É. Prognostic Ability of Tumor Budding Outperforms Poorly Differentiated Clusters in Gastric Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Dao TV, Nguyen CV, Nguyen QT, Vu HTN, Phung HT, Bui OT, Nguyen DK, Luong BV, Tran TV. Evaluation of Tumor Budding in Predicting Survival for Gastric Carcinoma Patients in Vietnam. Cancer Control. 2020;27:1073274820968883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Tanaka K, Shimura T, Kitajima T, Kondo S, Ide S, Okugawa Y, Saigusa S, Toiyama Y, Inoue Y, Araki T, Uchida K, Mohri Y, Kusunoki M. Tropomyosin-related receptor kinase B at the invasive front and tumour cell dedifferentiation in gastric cancer. Br J Cancer. 2014;110:2923-2934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Olsen S, Jin L, Fields RC, Yan Y, Nalbantoglu I. Tumor budding in intestinal-type gastric adenocarcinoma is associated with nodal metastasis and recurrence. Hum Pathol. 2017;68:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 17. | Ulase D, Heckl S, Behrens HM, Krüger S, Röcken C. Prognostic significance of tumour budding assessed in gastric carcinoma according to the criteria of the International Tumour Budding Consensus Conference. Histopathology. 2020;76:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Patriarca C, Pini GM, Conti G. Invasion and metastasis: a historical perspective. Pathologica. 2020;112:229-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Chan TS, Shaked Y, Tsai KK. Targeting the Interplay Between Cancer Fibroblasts, Mesenchymal Stem Cells, and Cancer Stem Cells in Desmoplastic Cancers. Front Oncol. 2019;9:688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Kong BT, Fan QS, Wang XM, Zhang Q, Zhang GL. Clinical implications and mechanism of histopathological growth pattern in colorectal cancer liver metastases. World J Gastroenterol. 2022;28:3101-3115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (5)] |
| 21. | Hu Q, Wang Y, Yao S, Mao Y, Liu L, Li Z, Chen Y, Zhang S, Li Q, Zhao Y, Fan X, Cui Y, Zhao K, Liu Z. Desmoplastic Reaction Associates with Prognosis and Adjuvant Chemotherapy Response in Colorectal Cancer: A Multicenter Retrospective Study. Cancer Res Commun. 2023;3:1057-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Yoshida Y, Nakanishi Y, Mitsuhashi T, Yamamoto H, Hayashi MO, Oba M, Nitta T, Ueno T, Yamada T, Ono M, Kuwabara S, Hatanaka Y, Hirano S. Postoperative Prognosis According to Pathologic Categorization of Desmoplastic Reaction in Patients with Extrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2023;30:7348-7357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Sato H, Hara T, Meng S, Tsuji Y, Arao Y, Saito Y, Sasaki K, Kobayashi S, Doki Y, Eguchi H, Ishii H. Multifaced roles of desmoplastic reaction and fibrosis in pancreatic cancer progression: Current understanding and future directions. Cancer Sci. 2023;114:3487-3495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 24. | Zhou ZH, Ji CD, Xiao HL, Zhao HB, Cui YH, Bian XW. Reorganized Collagen in the Tumor Microenvironment of Gastric Cancer and Its Association with Prognosis. J Cancer. 2017;8:1466-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Kemi NA, Eskuri M, Pohjanen VM, Karttunen TJ, Kauppila JH. Histological assessment of stromal maturity as a prognostic factor in surgically treated gastric adenocarcinoma. Histopathology. 2019;75:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Pun C, Luu S, Swallow C, Kirsch R, Conner JR. Prognostic Significance of Tumour Budding and Desmoplastic Reaction in Intestinal-Type Gastric Adenocarcinoma. Int J Surg Pathol. 2023;31:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Díaz Del Arco C, Ortega Medina L, Estrada Muñoz L, García Gómez de Las Heras S, Fernández Aceñero MJ. Is there still a place for conventional histopathology in the age of molecular medicine? Laurén classification, inflammatory infiltration and other current topics in gastric cancer diagnosis and prognosis. Histol Histopathol. 2021;36:587-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Zhang N, Wang D, Duan Y, Ayarick VA, Cao M, Wang Y, Zhang G. The special immune microenvironment of tumor budding and its impact on prognosis in gastric adenocarcinoma. Pathol Res Pract. 2020;216:152926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4325] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 30. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4402] [Article Influence: 550.3] [Reference Citation Analysis (4)] |
| 31. | Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 729] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 32. | Ueno H, Kanemitsu Y, Sekine S, Ishiguro M, Ito E, Hashiguchi Y, Kondo F, Shimazaki H, Mochizuki S, Kajiwara Y, Shinto E, Yamamoto J. Desmoplastic Pattern at the Tumor Front Defines Poor-prognosis Subtypes of Colorectal Cancer. Am J Surg Pathol. 2017;41:1506-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | Zhang D, He W, Wu C, Tan Y, He Y, Xu B, Chen L, Li Q, Jiang J. Scoring System for Tumor-Infiltrating Lymphocytes and Its Prognostic Value for Gastric Cancer. Front Immunol. 2019;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 34. | Green SB. How Many Subjects Does It Take To Do A Regression Analysis. Multivariate Behav Res. 1991;26:499-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1663] [Cited by in RCA: 1259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 35. | Jesinghaus M, Schmitt M, Lang C, Reiser M, Scheiter A, Konukiewitz B, Steiger K, Silva M, Tschurtschenthaler M, Lange S, Foersch S, Becker KF, Saur D, Friess H, Halfter K, Engel J, Boxberg M, Pfarr N, Wilhelm D, Weichert W. Morphology Matters: A Critical Reappraisal of the Clinical Relevance of Morphologic Criteria From the 2019 WHO Classification in a Large Colorectal Cancer Cohort Comprising 1004 Cases. Am J Surg Pathol. 2021;45:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483-4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 265] [Cited by in RCA: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 37. | Gulluoglu M, Yegen G, Ozluk Y, Keskin M, Dogan S, Gundogdu G, Onder S, Balik E. Tumor Budding Is Independently Predictive for Lymph Node Involvement in Early Gastric Cancer. Int J Surg Pathol. 2015;23:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Du M, Chen L, Cheng Y, Wang Y, Fan X, Zhang Y, Zhou X, Guo L, Xu G, Zou X, Huang Q. Tumor Budding and Other Risk Factors of Lymph Node Metastasis in Submucosal Early Gastric Carcinoma: A Multicenter Clinicopathologic Study in 621 Radical Gastrectomies of Chinese Patients. Am J Surg Pathol. 2019;43:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Yao G, Fang Y, Fu Y, Xu J, Song H, Zhu H, Gu M, Ding X. Tumor budding as an indicator for lymph node metastasis and prognosis of early gastric cancer. J Cancer Res Clin Oncol. 2023;149:5603-5616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Yim K, Jang WM, Lee SH. Modified Tumor Budding as a Better Predictor of Lymph Node Metastasis in Early Gastric Cancer: Possible Real-World Applications. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Gabbert HE, Meier S, Gerharz CD, Hommel G. Tumor-cell dissociation at the invasion front: a new prognostic parameter in gastric cancer patients. Int J Cancer. 1992;50:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Kemi N, Eskuri M, Ikäläinen J, Karttunen TJ, Kauppila JH. Tumor Budding and Prognosis in Gastric Adenocarcinoma. Am J Surg Pathol. 2019;43:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 43. | Qi B, Liu L, Pan Y, Xu S, Li J. Prognostic significance of peritumoural and intratumoural budding in intestinal-type gastric adenocarcinoma. Arab J Gastroenterol. 2020;21:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 44. | Hacking S, Ebare K, Angert M, Lee L, Vitkovski T, Thomas R, Chavarria H, Jin C, Nasim M. Immature Stroma and Prognostic Profiling in Colorectal Carcinoma: Development and Validation of Novel Classification Systems. Pathol Res Pract. 2020;216:152970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Kang BW, Kim JG, Lee IH, Bae HI, Seo AN. Clinical significance of tumor-infiltrating lymphocytes for gastric cancer in the era of immunology. World J Gastrointest Oncol. 2017;9:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Zhang N, Zhang G, Wang D, Liu H, Zhang Y, Ayarick VA, Han X, Lv Y, Wang Y. The relationship of the tertiary lymphoid structures with the tumor-infiltrating lymphocytes and its prognostic value in gastric cancer. Arch Med Sci. 2024;20:255-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 47. | Kang BW, Seo AN, Yoon S, Bae HI, Jeon SW, Kwon OK, Chung HY, Yu W, Kang H, Kim JG. Prognostic value of tumor-infiltrating lymphocytes in Epstein-Barr virus-associated gastric cancer. Ann Oncol. 2016;27:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 48. | Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003;16:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, Anders RA, Kelly RJ. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 363] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 50. | Yu PC, Long D, Liao CC, Zhang S. Association between density of tumor-infiltrating lymphocytes and prognoses of patients with gastric cancer. Medicine (Baltimore). 2018;97:e11387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Zhang N, Cao M, Duan Y, Bai H, Li X, Wang Y. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis and experimental validation. Arch Med Sci. 2020;16:1092-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Zheng X, Song X, Shao Y, Xu B, Chen L, Zhou Q, Hu W, Zhang D, Wu C, Tao M, Zhu Y, Jiang J. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget. 2017;8:57386-57398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Lee JS, Won HS, Sun S, Hong JH, Ko YH. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e11769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |