Published online Dec 28, 2017. doi: 10.4329/wjr.v9.i12.438
Peer-review started: July 3, 2017
First decision: August 7, 2017
Revised: October 20, 2017
Accepted: November 8, 2017
Article in press: November 8, 2017
Published online: December 28, 2017
Processing time: 176 Days and 12.9 Hours
Lung transplantation has been a method for treating end stage lung disease for decades. Despite improvements in the preoperative assessment of recipients and donors as well as improved surgical techniques, lung transplant recipients are still at a high risk of developing post-operative complications which tend to impact negatively the patients’ outcome if not recognised early. The recognised complications post lung transplantation can be broadly categorised into acute and chronic complications. Recognising the radiological features of these complications has a significant positive impact on patients’ survival post transplantation. This manuscript provides a comprehensive review of the radiological features of post lung transplantations complications over a time continuum.
Core tip: Lung transplantation is a common method of treating end stage lung disease. However, despite advances in surgical techniques, complications are still common and can occur years after lung transplantation. Radiological imaging plays an essential role in characterising many post-transplantation complications. It is crucial for radiologists to identify early signs of common complications on imaging to ensure that appropriate treatments are instituted early.
- Citation: Chia E, Babawale SN. Imaging features of intrathoracic complications of lung transplantation: What the radiologists need to know. World J Radiol 2017; 9(12): 438-447
- URL: https://www.wjgnet.com/1949-8470/full/v9/i12/438.htm
- DOI: https://dx.doi.org/10.4329/wjr.v9.i12.438
Lung transplantation is an accepted treatment modality for end stage lung disease with over 3614 transplantations performed worldwide between July 2013 and June 2014[1]. In Australia alone, over 163 of lung transplantations were performed in 2014[2]. During the early 1960s when human lung transplantation was explored, multiple attempts at the procedure often failed rapidly due to rejection and issues with bronchial and vessel anastomoses. However, over years, this measure of treatment has achieved remarkable outcomes.
Given the inherent risks associated with lung transplantation, patients are carefully selected for their suitability for treatment. Improved outcomes have been associated with advancing surgical techniques, appropriate patient selection, cautious harvesting and preservation of organs, and improved immunosuppressive therapy[3]. Despite the pre-transplant strict selection criteria, complications are still frequent.
Postoperative complications can be categorised broadly into: Early complications; including but not limited to reperfusion oedema and acute rejection; and late complications including infections, anastomotic complications and chronic graft rejection. Understanding the timeline of post-operative complications is a key to making accurate diagnosis for early intervention.
Early complications of lung transplantation generally occur within few weeks post- operatively. These account for the significant proportion of mortality in patients who undergo lung transplantation.
Donor lung and recipient thoracic cage mismatch is a potential underlying cause of some of the early complications of lung transplantation. Therefore, meticulous attention to details in selecting appropriate donor lung is a crucial initial step in obviating the likelihood of complications relating to donor-recipient mismatch. These complications include pleural effusion and pneumothorax, which have been shown to develop especially if the donor lung is too small for the recipient thorax. These complications may, in certain cases require interventions such as thoracocentesis and antibiotic therapy. This contributes to a prolonged hospitalisation and increased overall cost of lung transplantation.
However, deliberately mismatching the donor lung and the recipient’s thorax may have some potential benefits. Moderately oversized donor lung has been shown to reduce the risk of early primary graft dysfunction[4]. However, in cases where the donor lung is too large in size, atelectasis and impaired ventilation may ensue[4].
Ischaemic-reperfusion injury, also known as primary graft dysfunction is a frequent complication following lung transplantation. It is one of the leading causes of early post-transplant morbidity and mortality. It is a severe acute lung injury syndrome that develops within the first 48-72 h post lung transplantation[5].
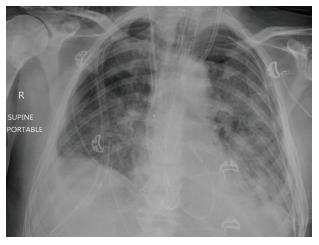
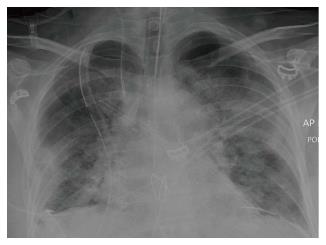
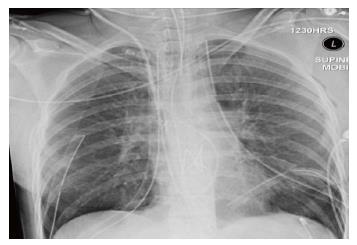
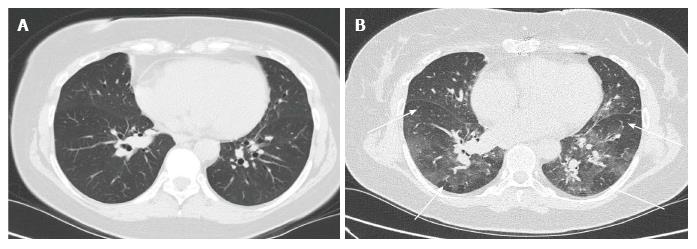
Reperfusion oedema has variable imaging features. On chest X-ray, it may present with hazy peri-hilar air-space opacity in milder cases, and dense peri-hilar consolidations with air bronchograms in more severe cases[6] (Figures 1 and 2).
High resolution computerised tomography (HRCT) will demonstrate the above features in greater details, even though these are not specific only to pulmonary oedema. Perihilar ground-glass opacities, peribronchovascular thickening and reticulations with predilection for the middle and lower lobes are elegantly demonstrable on HRCT[7,8].
Acute cellular rejection occurs generally within the first 2 wk post-lung transplantation. This complication is a potential cause of significant morbidity. Acute cellular rejection is a cell-mediated immune response to human leukocyte antigen (HLA) complex expressed in the donor lung. This immune response leads to perivascular lymphocytic infiltrate[9-11].
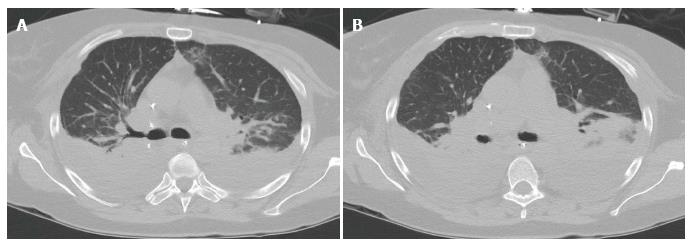
Imaging findings in the mild acute rejection may be very subtle and, hence, trans-bronchial lung biopsy is the gold standard for diagnosis in this setting. Normal findings on chest X-rays, therefore, does not exclude the diagnosis of acute rejection, especially in the mild form[10]. Radiological findings, demonstrable on HRCT, include lower lobe predominant peri-hilar ground glass opacities, peri-bronchial cuffing, interlobular septal thickening, and new or increasing pleural effusions[9,11] (Figures 3 and 4). Notably, the absence of ground glass opacities in HRCT within the first few weeks post lung transplantation virtually excludes the diagnosis of severe acute rejection[7].
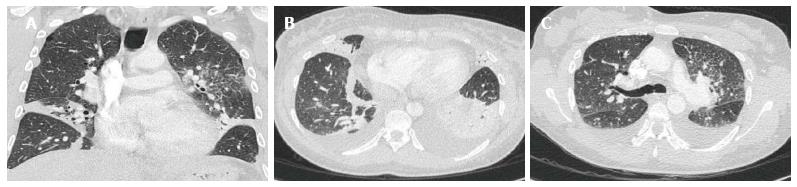
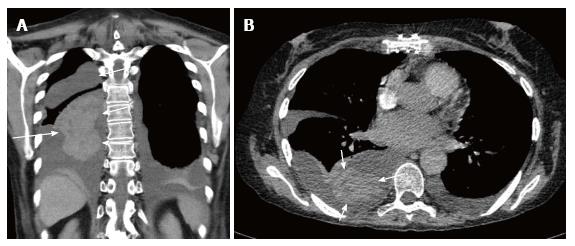
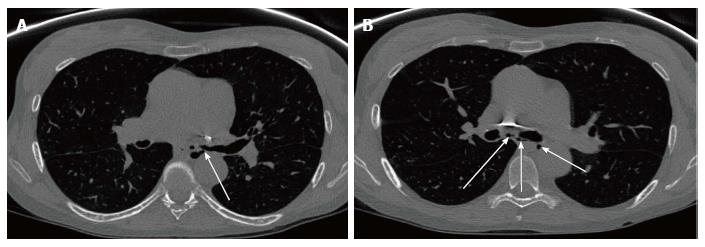
The spectrums of acute pleural complications include minor air leaks, haemothorax, pneumothorax, chylothorax and pleural effusions. Air leaks may occur transiently following lung transplantation or persist for quite a while. Pleural leaks (Figure 5) are considered transient if spontaneous resolution occurs within 7 d post-transplantation. Air leaks that are unresolved after 7 d post-transplantation are dubbed persistent and may signify more serious complications such as significant bronchial dehiscence or airway ischaemia. This may eventually lead to pneumomediastinum, persistent pneumothorax or subcutaneous emphysema[9].
Pleural effusion may persist for few months. At 3 mo, approximately 59% of patients may have some pleural effusions detectable on imaging study, especially on computerised tomography (CT) scan. However, majority of pleural effusion resolve completely by 12 mo (8%)[12]. Pleural thickening and calcification may manifest as a long-term complication. Figure 6 illustrates some of the more common pleural complications.
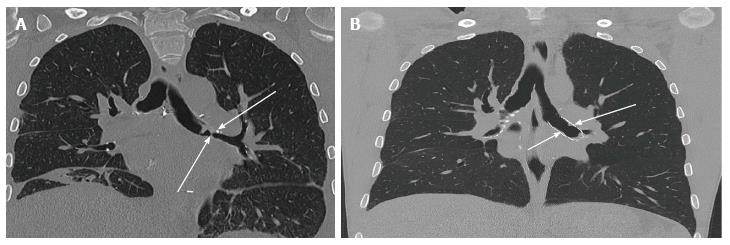
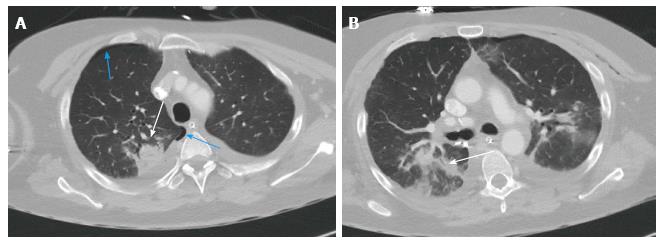
Bronchial dehiscence, bronchial stenosis, bronchomalacia and bronchopleural fistulas are some of the airway anastomotic complications that can occur. The most frequent of these complications is bronchial stenosis. There are two patterns of bronchial stenosis: Surgical site anastomotic stenosis and segmental non-anastomotic bronchial stenosis.
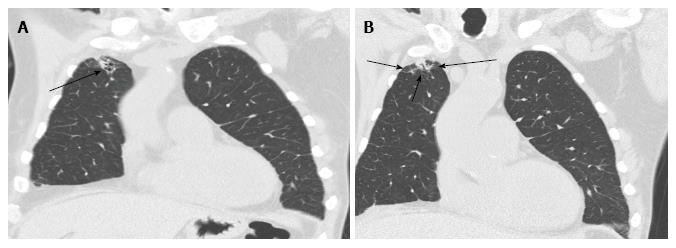
Bronchial stenosis is easily demonstrable in chest X-ray. Bronchial stenosis may be severe enough to cause the atelectasis of the affected lobe[13]. As demonstrated in Figures 7 and 8, helical CT with multiplanar reconstruction will demonstrate this and other associated features more elegantly and is said to have an accuracy of 94% for detecting bronchial stenosis[13].
Bronchial dehiscence results from ongoing mucosal necrosis of the donor bronchus secondary to disruption of the bronchial circulation[7]. Chest radiography is unreliable for the diagnosis of bronchial dehiscence due to the presence of peri-bronchial air that may obscure the major airways.
CT scan is more sensitive and readily able to identify the features of bronchial dehiscence including bronchial wall defects, fixed or dynamic bronchial narrowing, and peribronchial air around the anastomosis[13] ( Figure 9).
Bronchopleural fistula manifests as progressive increase in the intrapleural air, new or progressing hydropneumothorax and changes in the already present air-fluid levels. In severe cases, tension pneumothorax may occur with imaging demonstrating contralateral mediastinal shift, flattening of the ipsilateral diaphragm, ipsilateral widening of intercostal spaces and atelectasis of the contralateral lung.
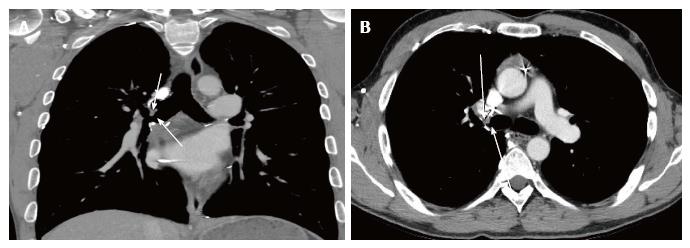
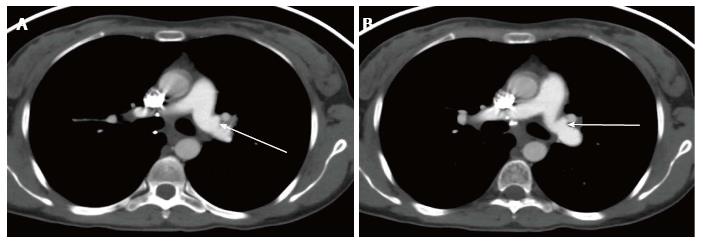
Complications involving the vasculature anastomotic sites post lung transplantation are much less frequent compared to airway anastomotic complications. Vascular complications include pulmonary artery stenosis, kinking of the pulmonary artery and pulmonary vein thrombosis. Peri-anastomotic pulmonary artery aneurysm is an unusual complication (Figure 10).
Pulmonary artery stenosis can occur early or late after lung transplantation and is generally a result of incongruent lengths of the donor and recipient segments, technical narrowing or twisting of the anastomosis[14].
CT angiogram is the acceptable imaging modality for investigating these complications. Narrowing or occlusion of the affected artery is readily demonstrable with CT angiogram. Diminished opacification of the corresponding pulmonary segment may indicate atelectasis or evolving pulmonary infarction[15].
Pulmonary infection after lung transplantation remains an important complication that is associated with high rates of morbidity and mortality. The incidence of infection is far more frequent in the lung transplant recipients than any other organ transplant recipients[9]. This is due to the higher level of immunosuppression and loss of local pulmonary host defences characterised by the reduction in lymphatic drainage, and reduced mucociliary clearance.
Bacterial pneumonia accounts for approximately 36% of pneumonias[16] occurring post lung transplantation. Staphylococcus aureus, pseudomonas aeruginosa and Enterobacteriaceae are the most common bacterial culprits.
Radiographic manifestation of bacterial pneumonia (Figures 11 and 12) may be nonspecific with the occurrence of patchy or confluent consolidation, ground-glass opacity, septal thickening and pleural effusions[16]. These features and the presence of tree-in-bud opacity on chest radiograph, in conjunction with the appropriate clinical picture, makes the radiographic diagnosis of pneumonia fairly obvious. Pleural effusion is nonspecific. It may be indicative of haemorrhage, rejection or empyema[17].
Late complications post lung transplantation can occur anytime from months to years. It is vitally important to have a high index of suspicion in recognising the signs of late complications as these largely contribute to the patients’ morbidity and mortality.
Chronic allograft rejection is one of the causes attributed to the increased rate of mortality and morbidity post lung transplantation, whether single lung transplantation or bilateral. Chronic allograft rejection is described clinically as bronchiolitis obliterans syndrome. Cryptogenic organising pneumonia may also be seen. Patho-physiologically, chronic rejection is typified by inflammatory and fibrotic processes. Eosinophilic hyaline fibrosis of the small airways leads to progressive concentric bronchiolar luminal narrowing and eventually bronchiolar occlusion[7].
Plain radiograph is of limited diagnostic value in chronic graft rejection. Non-specific features in plain radiograph that can suggest chronic rejection include pulmonary hyperinflation, decreased vascular markings, regional volume loss, subsegmental atelectasis, linear opacities and bronchiectasis[7].
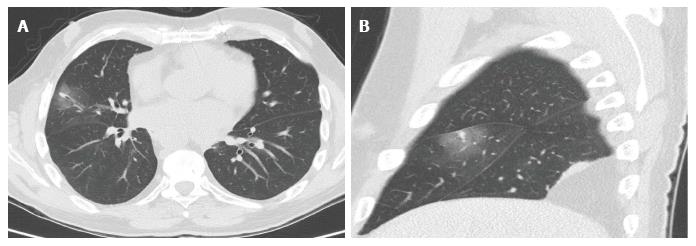
Chest CT is the imaging of choice for demonstrating the features of small airway and interstitial lung parenchymal changes that occur in chronic graft rejection (Figure 13). Some of these features, which are readily demonstrable on chest CT, include bronchial wall thickening, interlobular septal thickening, reticulo-nodular opacity, ground-glass opacity with mosaic attenuation, air trapping and peripherally predominant bronchiectasis[7,9,18].
Iatrogenic immunosuppression post lung transplantation is an important predisposing factor for infection with atypical organisms such as viruses, fungi and mycobacterium.
Viruses, particularly cytomegalovirus (CMV), are largely opportunistic infections and are a risk factor for the development of transplant rejection. Lung transplant recipients are particularly susceptible to CMV infection and the rate of infection in these patients can be as high as 50%[7]. Other viral culprits include parainfluenza virus, respiratory syncytia virus and adenovirus.
Features of viral chest infections are nonspecific. It is usually patchy with no particular lobar predilection. Nodular opacities, patchy consolidation, diffuse ground-glass opacity and bronchiolar thickening can be seen in viral chest infections. Adenoviral pneumonia imaging findings typically are more extensive compared to those caused by other viral infections[19].
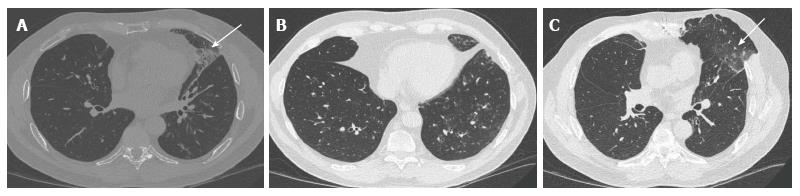
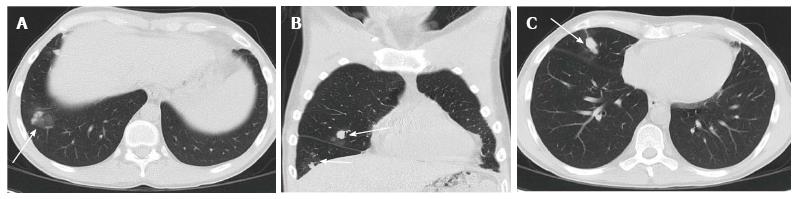
Fungal infection post lung transplantation is less frequent than bacterial and viral infections, however they are associated with high mortality rates. Aspergillus and Candida species are the most common causes of fungal infections in lung transplant recipients (Figure 14). Colonisation of the airways by aspergillus species is a common occurrence in lung transplant recipients, particularly those with underlying cystic fibrosis. Colonisation with these organisms increase the risk of developing invasive aspergillosis which could be fatal and may result in as high as 55% mortality in lung transplant recipients if not aggressively treated[20].
Fungal chest infections have various chest CT features including consolidation, lung nodules and cavitating nodules or masses. Ground glass opacity surrounding a lung nodule or mass (Figures 15 and 16) dubbed as a halo sign is highly suggestive, although not specific, of fungal infection in appropriate clinical settings[7].
Rate of tuberculosis infections usually vary by geographic location. However, tuberculosis infection has been shown to be significantly higher in transplant recipients compared to the general population irrespective of geographic location[21]. It presents commonly as a reactivation of the latent infection in the transplant recipients, but can also be acquired from unrecognised infected donor lung. Pulmonary tuberculosis is commonly seen radiologically as focal infiltrates or in a miliary pattern[22].
Thromboembolism (including pulmonary embolism and deep vein thrombosis) is a common complication that tends to occur within few months post-transplantation. It is important to recognise this since up to 27% of lung transplant recipients are prone to this complication[23]. This has been suggested to be due to the hypercoagulable state caused by the inflammatory response to the donor organ[11]. Potential thrombogenic surfaces, such as the pulmonary artery anastomotic site and central lines, also act as sources of venous thromboembolisms[23].
Lung transplant recipients are more susceptible to thromboembolic induced pulmonary infarcts due to a deficient dual blood supply (bronchial and pulmonary arterial supply) in the early post-operative period. Clues suggesting pulmonary thromboembolism on chest radiography include segmental oligaemia, pleural effusion, dilated central pulmonary arteries and cardiomegaly[7]. Wedge shaped sub-pleural opacity representing lung infarct is readily demonstrable on chest radiograph.
CT pulmonary angiogram is the gold standard for diagnosing pulmonary embolism. Filling defects with segmental partial or total occlusion in the central or segmental branches of the pulmonary arteries are the most reliable direct signs of thromboembolism[7,10]. Indirect features include consolidation in specific vascular territories, mosaic perfusion, atelectasis and pleural effusions[7,10]. Ventilation perfusion scans is a viable alternative in patients who have contraindications to CT pulmonary angiogram.
A diverse number of lymphoproliferative diseases may develop post lung transplantation. These are collectively termed post-transplantation lymphoproliferative disorders (PTLD) and occur in approximately 5% of lung transplant recipients[24]. Patho-physiologically, transplant recipients are predisposed to Epstein-Barr virus (EBV) which induces B-cell proliferative responses leading to PTLD, usually within a year after transplantation.
CT features of PTLD are variable, and may be seen as a single or multiple pulmonary nodules or masses with or without mediastinal, hilar and extra-thoracic adenopathy[25].
Primary lung carcinomas occurring in the lung allograft are rare[26]. This is attributable in part to the comprehensive screening process prior to transplantation aiming at excluding donors with underlying parenchymal lung disease and those with significant smoking history. The risk of developing primary pulmonary carcinoma in transplant recipients is therefore the same as the general population.
Nevertheless, in cases where primary lung allograft carcinomas arise, tumours tend to be more aggressive[26]. This is likely due to immunosuppression, and can be challenging radiologically to distinguish from an infectious process.
Despite lung transplantation being the only available therapy for end-stage lung disease, a number of diseases have been reported to recur in the lung allograft. Sarcoidosis has been demonstrated to have a high recurrence rate[27]. Lymphangioleiomyomatosis and diffuse panbronchiolitis and pulmonary alveolar proteinosis have also been reported to recur post lung transplantation. Radiological findings of these diseases, however, have slight morphological differences at recurrence compared with pre-transplantation[27].
Lung transplantation is essentially the only viable option for treating end stage lung disease. Despite this advanced procedure, it poses a diverse list of complications that are associated with morbidity and mortality for transplant recipients. Although radiological manifestations of post-lung transplant complications may be non-specific, understanding the main features of post-transplant complications over a time continuum is the key to improving patients’ survival. By recognising these radiological features, early treatment can be instituted.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cerwenka H, Li YZ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | International Society for Heart and Lung Transplantation. The registry of the international society for heart and lung transplantation: Thirty-second annual report. Available from: https://www.ishlt.org/downloadables/slides/2015/introduction.pptx. |
| 2. | ANZOD Committee. ANZOD Registry Annual Report 2015. Australia and New Zealand Organ Donation Registry. Available from: http://www.anzdata.org.au/anzod/ANZODReport/2015/2015ANZOD_annrpt.pdf. |
| 3. | O’Donovan PB. Imaging of complications of lung transplantation. Radiographics. 1993;13:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Eberlein M, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Shlobin OA, Shelhamer JH, Reed RM, Pearse DB, Orens JB. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Kundu S, Herman SJ, Winton TL. Reperfusion edema after lung transplantation: radiographic manifestations. Radiology. 1998;206:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Herman SJ, Rappaport DC, Weisbrod GL, Olscamp GC, Patterson GA, Cooper JD. Single-lung transplantation: imaging features. Radiology. 1989;170:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Krishnam MS, Suh RD, Tomasian A, Goldin JG, Lai C, Brown K, Batra P, Aberle DR. Postoperative complications of lung transplantation: radiologic findings along a time continuum. Radiographics. 2007;27:957-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Belmaati EO, Steffensen I, Jensen C, Kofoed KF, Mortensen J, Nielsen MB, Iversen M. Radiological patterns of primary graft dysfunction after lung transplantation evaluated by 64-multi-slice computed tomography: a descriptive study. Interact Cardiovasc Thorac Surg. 2012;14:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Diez Martinez P, Pakkal M, Prenovault J, Chevrier MC, Chalaoui J, Gorgos A, Ferraro P, Poirier C, Chartrand-Lefebvre C. Postoperative imaging after lung transplantation. Clin Imaging. 2013;37:617-623. [PubMed] [DOI] [Full Text] |
| 10. | Hochhegger B, Irion KL, Marchiori E, Bello R, Moreira J, Camargo JJ. Computed tomography findings of postoperative complications in lung transplantation. J Bras Pneumol. 2009;35:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Ahmad S, Shlobin OA, Nathan SD. Pulmonary complications of lung transplantation. Chest. 2011;139:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Ferrer J, Roldan J, Roman A, Bravo C, Monforte V, Pallissa E, Gic I, Sole J, Morell F. Acute and chronic pleural complications in lung transplantation. J Heart Lung Transplant. 2003;22:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009;6:79-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Anaya-Ayala JE, Loebe M, Davies MG. Endovascular management of early lung transplant-related anastomotic pulmonary artery stenosis. J Vasc Interv Radiol. 2015;26:878-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Madan R, Chansakul T, Goldberg HJ. Imaging in lung transplants: Checklist for the radiologist. Indian J Radiol Imaging. 2014;24:318-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Collins J, Müller NL, Kazerooni EA, Paciocco G. CT findings of pneumonia after lung transplantation. AJR Am J Roentgenol. 2000;175:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Judson MA, Handy JR, Sahn SA. Pleural effusions following lung transplantation. Time course, characteristics, and clinical implications. Chest. 1996;109:1190-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Bankier AA, Van Muylem A, Scillia P, De Maertelaer V, Estenne M, Gevenois PA. Air trapping in heart-lung transplant recipients: variability of anatomic distribution and extent at sequential expiratory thin-section CT. Radiology. 2003;229:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Matar LD, McAdams HP, Palmer SM, Howell DN, Henshaw NG, Davis RD, Tapson VF. Respiratory viral infections in lung transplant recipients: radiologic findings with clinical correlation. Radiology. 1999;213:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Singh N, Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J Heart Lung Transplant. 2003;22:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Morales P, Briones A, Torres JJ, Solé A, Pérez D, Pastor A. Pulmonary tuberculosis in lung and heart-lung transplantation: fifteen years of experience in a single center in Spain. Transplant Proc. 2005;37:4050-4055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27:1266-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 405] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Krivokuca I, van de Graaf EA, van Kessel DA, van den Bosch JM, Grutters JC, Kwakkel-van Erp JM. Pulmonary embolism and pulmonary infarction after lung transplantation. Clin Appl Thromb Hemost. 2011;17:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Kremer BE, Reshef R, Misleh JG, Christie JD, Ahya VN, Blumenthal NP, Kotloff RM, Hadjiliadis D, Stadtmauer EA, Schuster SJ. Post-transplant lymphoproliferative disorder after lung transplantation: a review of 35 cases. J Heart Lung Transplant. 2012;31:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Rappaport DC, Chamberlain DW, Shepherd FA, Hutcheon MA. Lymphoproliferative disorders after lung transplantation: imaging features. Radiology. 1998;206:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Grewal AS, Padera RF, Boukedes S, Divo M, Rosas IO, Camp PC, Fuhlbrigge A, Goldberg H, El-Chemaly S. Prevalence and outcome of lung cancer in lung transplant recipients. Respir Med. 2015;109:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Collins J, Hartman MJ, Warner TF, Müller NL, Kazerooni EA, McAdams HP, Slone RM, Parker LA. Frequency and CT findings of recurrent disease after lung transplantation. Radiology. 2001;219:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |