Published online Aug 28, 2015. doi: 10.4329/wjr.v7.i8.212
Peer-review started: May 3, 2015
First decision: June 18, 2015
Revised: July 7, 2015
Accepted: July 29, 2015
Article in press: August 3, 2015
Published online: August 28, 2015
Processing time: 123 Days and 4.6 Hours
AIM: To develop a new type of calibrated, biodegradable, and imaging detectable microsphere and evaluated its embolization safety and efficacy on pig’s liver and spleen.
METHODS: Six kinds of pharmaceutical excipient were combined and atomized to form our microsphere. Twenty-four male Lanyu pigs weighing 25-30 kg were used. The arteries of spleen and liver were embolized with Gelfoam, Embosphere, or our microsphere. The serum biochemical tests, computed tomography (CT), liver perfusion scan, and tissue microscopy examination were done to evaluate the safety and efficacy of embolization.
RESULTS: Radiopaque microspheres with a size ranging from 300 to 400 μm were produced. Embolization of hepatic and splenic artery of pigs with our microsphere significantly reduced the blood flow of liver and resulted in splenic infarction. The follow-up CT imaging and the microscopic examination showed intraarterial degradation of Gelfoam and microsphere. The blood tests demonstrated insignificant changes with regards to liver and renal functions.
CONCLUSION: Our microspheres, with the unique characteristics, can be used for transcatheter arterial embolization with effects equivalent to or better than Gelfoam and Embosphere in pigs.
Core tip: Transcatheter arterial embolization (TAE) is the treatment of choice for intermediate stage hepatocellular carcinoma. Various embolization materials have been designed for this purpose. By using atomization technique and a mixture of pharmaceutical excipient, we developed a new type of calibrated, biodegradable, and imaging detectable microsphere. We proved that our microspheres, with the unique characteristics, can be used for TAE with effects equivalent to or better than Gelfoam and Embosphere in pigs.
- Citation: Liu YS, Lin XZ, Tsai HM, Tsai HW, Chen GC, Chen SF, Kang JW, Chou CM, Chen CY. Development of biodegradable radiopaque microsphere for arterial embolization-a pig study. World J Radiol 2015; 7(8): 212-219
- URL: https://www.wjgnet.com/1949-8470/full/v7/i8/212.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i8.212
Hepatocellular carcinoma (HCC), the most common primary liver cancer, is the sixth most commonly diagnosed malignancy worldwide[1]. It is also the third leading cause of cancer-related mortality[1]. Conventional transcatheter arterial chemoembolization (cTACE) stands for the treatment of choice for Barcelona Clinic Liver Cancer stage B HCC[2,3]. By introducing embolic agents through an angio-catheter into the blood vessel, transcatheter arterial embolization (TAE) occludes tumor feeding vessels and thereby results in tumor shrinkage[4,5]. By adding chemotoxic agent(s) to the embolic materials, the cTACE evolved into more a controlled delivery of chemotherapy in the form of drug-eluting bead transcatheter arterial chemoembolization (DEB-TACE)[6].
Commercially available embolic materials include metallic coils, oils (lipiodol), non-spherical particles (Gelfoam) and microspheres (Embosphere, DC Bead and Hepasphere)[7]. As a tumor may recanalize the occluded vessels or form new vessels, repeated TAE is required in order to control tumor growth and a biodegradable embolic material allowing for the re-catheterization of previously embolized vessels is therefore, ideally preferred. Gelfoam is the only commercially available biodegradable embolic material at this time; however, it is non-spherical which makes it unable to precisely control the level of embolization[8].
Calibrated microspheres allow the radiologist to choose the size of microspheres according to the size of the targeting vessels. The DEB-TACE using drug-loaded microspheres showed less systemic toxicity and drug-related side-effects as compared to the cTACE[9]. However, both the Hepasphere and the DC Bead are not biodegradable, and it is reported that the long-term presence of DC Bead microspheres containing a potentially harmful drug in the body elicits chronic inflammation and thus causes more tissue injury[10]. Furthermore, these microspheres including the Embosphere are not radiopaque and interventional radiologists can only estimate the devascularization through an angiography, but do not know the precise site of occlusion of the injected microspheres[11].
To develop a new type of spherical, biodegradable, imaging detectable, and drug-loadable embolic material is therefore crucial in order to improve the efficacy of tumor embolization treatment. A biodegradable excipient able to be formulated with chemotoxic agent(s) and radiopaque contrast with suitable consistency will be a candidate of material to construct a microsphere for drug delivery and vascular embolization. Atomizing technique which breaks up bulk liquids into droplets can be applied to produce particles of desired shape, size, and density. In this study, we constructed a biodegradable radiopaque microsphere by atomizing a mixture of pharmaceutical excipient and conducted arterial embolization study in pigs in an attempt to explore a new microsphere that fulfills the above requirements for arterial embolization of HCC.
The experiment was conducted after the approval of the ethical committee of the animal center of our university and in accordance with the guidelines set forth by the Agriculture Council of Taiwan on animal care. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) during experimentation. Twenty-four male Lanyu pigs weighing 25-30 kg were included in the study. Arterial embolization of the liver with concomitant partial embolization of the spleen was used to test our newly developed embolic microsphere. Two other commonly used embolic materials for cTACE-Gelfoam and Embosphere were used for comparison. To better understand the acute and midterm effect of the embolic materials on pigs while avoid the potential anesthesia effects on pigs, blood tests were only checked on the day before embolization, 1 and 25 d after the embolization. To observe the evolutional change of our microsphere, non-enhanced CT scans were performed on Day 4, 12, and 25 after the embolization. To estimate the blockade extent of liver blood flow by embolization materials, CT perfusion scans were performed on the pigs without embolization and immediately after embolization. All the animals were sacrificed 28 d after the embolization to examine the pathological changes in liver and/or spleen relating to embolization.
We combined several kinds of excipient from the handbook of pharmaceutical excipient to construct an excipient possessing suitable consistency for embolization. The excipient that we used included Lipiodol, Cetyl alcohol, Glycol monostearate, Stearyl acid, Polycaprolactone, and Cholesterol. All these materials are biodegradable and water insoluble. All these excipients were solid at room and body temperature, and become self-emulsifying oils at 65 °C. Such a characteristic allowed us to melt and atomize it to make it into microsphere. In brief, the atomization procedure included a pressure type atomization technique for mass production of microspheres and a high frequency resonated technique to produce microspheres with a specific range of size. The size of microspheres was further examined by using a scanning electron microscope. With an aim to embolize intrahepatic arteries, microspheres with sizes of 300 to 450 μm were selected for the following embolization experiment.
The animals were fasted overnight and given free access to water. They were premedicated with intramuscular injection of Atropine (Sintong, Taoyuan, Taiwan) 0.02 mg/kg, Xylazine (Bayer, Leverkusen, Germany) 0.1 mL/kg, and Zoletil 50 (Virbac, Carros, France) 10 mg/kg. Following endotracheal intubation, the animals were anesthetized by using Propofol 12-20 mg/kg per hour (Tongchou, Taipei, Taiwan) intravenous injection or Isoflurane (Baxter, Guayama, United States) 1%-3% 200 mL/kg per minute inhalation throughout the operation. All animals were subjected to celiac artery angiography before the embolization. The procedure was performed with a femoral approach by using the Seldinger technique. After placing a 4-F introducer sheath (Cordis, Roden, the Netherlands), a 2.7-F microcatheter catheter (Progreat, Terumo, Tokyo, Japan) was used to catheterize the hepatic proper artery for liver embolization and one of the branches of splenic artery for splenic embolization because complete embolization of spleen caused a significant morbidity and mortality. As many embolization materials were introduced as possible and the end point of the procedure was to obtain blood flow stasis of the selected hepatic and splenic arteries.
By using intramuscular injection of Xylazine (Bayer, Leverkusen, Germany) 0.1 mL/kg and Zoletil 50 (Virbac, Carros, France) 10 mg/kg to anesthetize pig, serum samples were obtained on the day before embolization and 1 and 25 d after the embolization. Serum levels of blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin were analyzed by using D and P modular analyzer (Roche, Mannheim, Germany).
Each pig was anesthetized when undergoing computed tomography (CT) scanning and liver perfusion study. CT scanning and perfusion study was performed by a 128-section multidetector CT scanner (Definition Flash, Siemens Medical Systems; Erlangen, Germany). A dynamic study of the selected area was performed in a single breath hold at the end of expiration at a static table position. A total of 50 mL of nonionic iodinated contrast medium was injected at a rate of 5 mL/s, through an 18-gauge intravenous cannula. The liver blood volume (mL/100 mL) and the time that the liver started to be enhanced by contrast (time to start, second) were used to estimate the immediate embolization effects on liver perfusion.
All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection. The transected liver and spleen harvested on the day of sacrifice were immediately fixed in a 10% formalin, sectioned, and stained with hematoxylin-eosin to investigate the changes of embolized arteries and peripheral tissues of both the spleen and liver.
The blood test results and the CT perfusion index between each group of pigs undergoing different treatments were analyzed by using one way ANOVA with LSD post-hoc test. A P value of < 0.05 was considered to be statistically significant.
As shown in Figure 1, microspheres with a size ranging from 300 to 400 μm were successfully produced. The size and shape were comparable to the current commercially used microsphere-Embosphere. Furthermore, due to radiopaque lipiodol being contained in our excipient mixture, our microsphere was different from the Embosphere in that it was radiopaque under fluoroscopy (Figure 2).
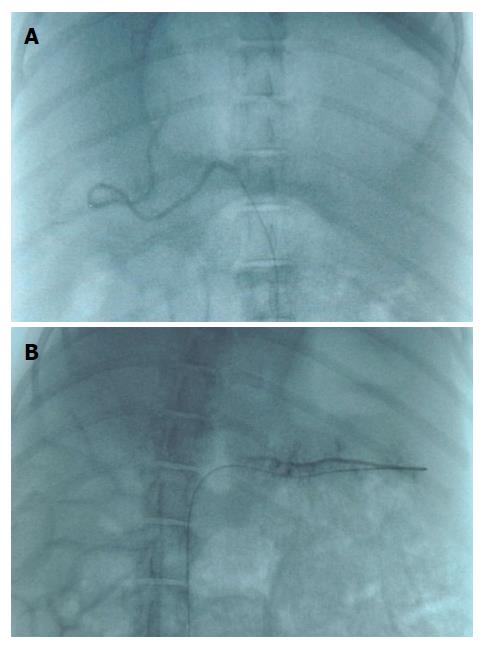
Figure 3A presents the angiography of the liver of pig. As there were no liver tumors, embolization materials were injected into the hepatic proper artery to embolize bilateral intrahepatic arteries. Besides the liver, we also embolized one of the branches of splenic artery to test the embolization effect on spleen (Figure 3B).
The blood tests confirmed the safety of the new microsphere based on our pig embolization experiment (Table 1). Similar to pigs embolized by Embosphere or Gelfoam, our microsphere embolization only caused mild increases in the serum levels of BUN, AST and ALT on the day after embolization and all returned to baseline at the end of experiment. Although the serum creatinine levels were higher in pigs receiving Gelfoam embolization on Day 25, the embolization did not cause any biochemical abnormality with regards to pig liver and the kidneys among groups embolized by different materials.
| Day 0 | Day 1 | Day 25 | |||||||
| Gelfoam | Embosphere | Microsphere | Gelfoam | Embosphere | Microsphere | Gelfoam | Embosphere | Microsphere | |
| (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 7) | (n = 8) | (n = 8) | |
| AST | 27.8 ± 15.3 | 24.4 ± 9.9 | 29.5 ± 12.3 | 56.4 ± 30.8a,c | 34.9 ± 16.7a | 103.0 ± 80.4b | 23.1 ± 10.5 | 30.1 ± 20.6 | 35.4 ± 10.8 |
| ALT | 48.3 ± 23.9 | 34.8 ± 12.1 | 44.4 ± 20.6 | 58.9 ± 20.1 | 46.4 ± 17.3 | 51.5 ± 26.7 | 34.3 ± 19.6a,c | 22.8 ± 12.6c | 40.8 ± 17.0c |
| T-BIL | 0.38 ± 0.15 | 0.40 ± 0.23 | 0.39 ± 0.15 | 0.29 ± 0.20 | 0.73 ± 1.00 | 0.68 ± 0.58 | 0.40 ± 0.14 | 0.39 ± 0.22 | 0.56 ± 0.34 |
| BUN | 12.1 ± 11.6 | 8.7 ± 6.4 | 8.5 ± 3.5 | 19.4 ± 9.4 | 18.3 ± 8.5 | 16.4 ± 9.2 | 8.7 ± 7.2 | 7.2 ± 4.1 | 10.5 ± 4.6 |
| Cr | 0.85 ± 0.19 | 0.75 ± 0.27 | 0.81 ± 0.21 | 0.84 ± 0.17 | 0.73 ± 0.28 | 0.86 ± 0.25 | 1.03 ± 0.26 | 0.86 ± 0.33 | 0.91 ± 0.18 |
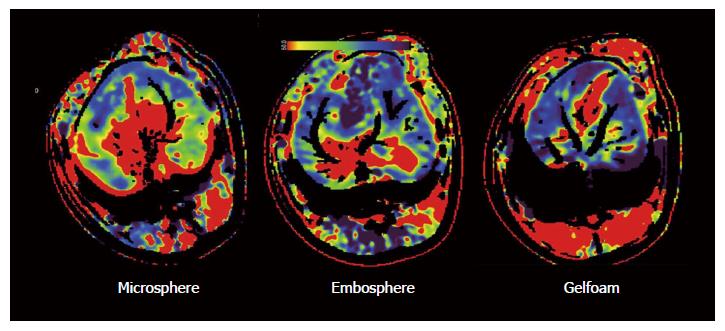
As shown in Table 2, the embolization effect of our microsphere was comparable to that of Embosphere and Gelfoam, in that all showed a significant reduction of perfused liver blood volume and a delayed contrast enhancement of the liver. The liver perfusion scan further demonstrated areas in the liver with a reduced blood flow after embolization (Figure 4).
| Treatment | Non-embolized | Embosphere | Microsphere | Gelfoam |
| (n = 3) | (n = 3) | (n = 2) | (n = 2) | |
| Blood volume1 | 12.75 ± 0.69 | 9.60 ± 1.48 | 9.42 ± 0.24 | 10.22 ± 1.24 |
| Mean decrease | 3.16 ± 0.82 | 3.33 ± 0.73 | 2.54 ± 0.82 | |
| P value | 0.008 | 0.004 | 0.021 | |
| Time to Start2 | 11.40 ± 1.57 | 15.44 ± 2.10 | 15.91 ± 0.39 | 15.44 ± 1.74 |
| Mean delay | 4.04 ± 1.33 | 4.51 ± 1.19 | 4.04 ± 1.33 | |
| P value | 0.023 | 0.009 | 0.023 |
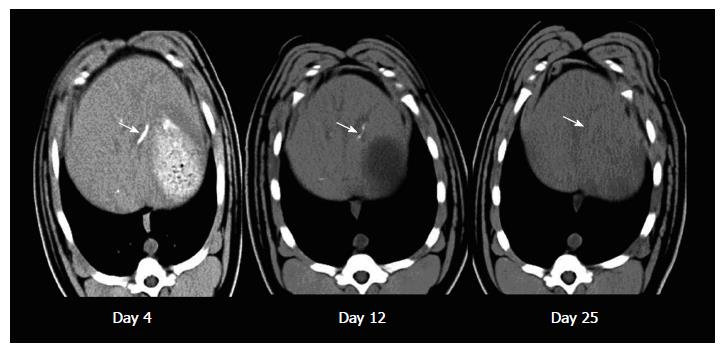
As shown in Figure 5, the CT imaging showed retention of lipiodol in the liver after embolization by using our microsphere which faded away gradually in the subsequent follow up imaging. In contrast, both Embosphere and Gelfoam are radiolucent and there was no hyper-intensity area found in the liver of pigs embolized with either one of them.
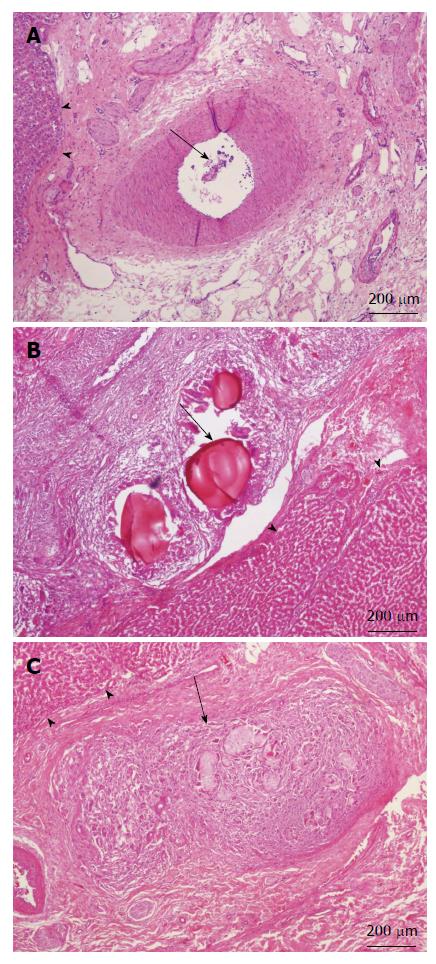
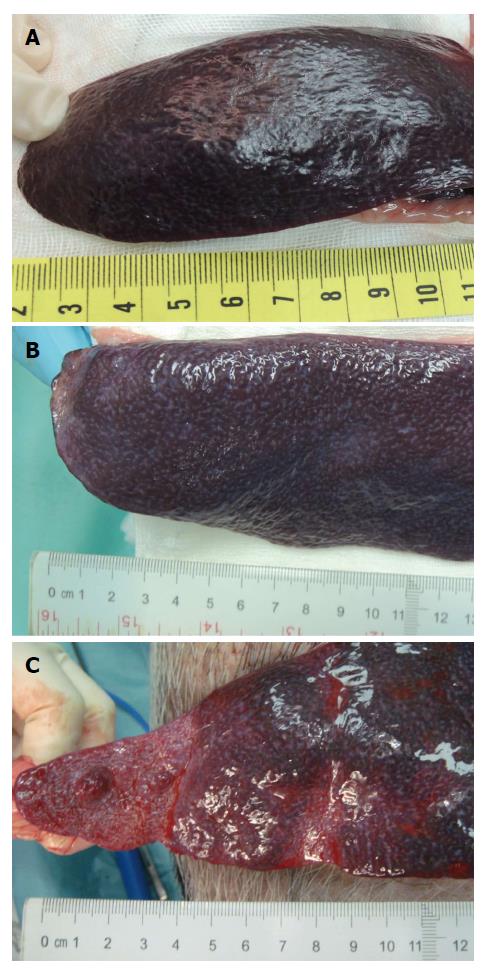
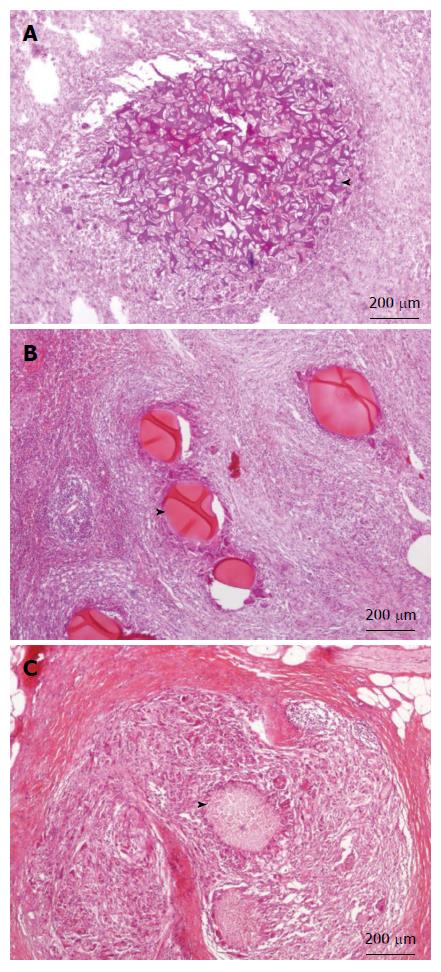
Although the ingredients we used for our microsphere were all pharmaceutical excipient, the possible liver toxicity caused by such mixture can be a concern and was checked at first. Microscopically, the liver lobules did not have a significant pathology change after embolization with any of the three embolization materials (Figure 6). Infarction with shrinkage of the embolized part of spleen was noted in the pigs that underwent Embosphere and our microsphere embolization (Figure 7A and B) but grossly normal, in pigs embolized with Gelfoam (Figure 7C). Upon microscopic examination, partial degradation of our microsphere and Gelfoam with peripheral leukocyte infiltration within and around the embolized splenic vessels was observed which was in contrast to the presence of intact Embosphere within the embolized vessels (Figure 8). The different severity of splenic infarction among gelfoam, embosphere, and our microsphere may be therefore, caused by the selection of arterial branch on TAE rather than the character of embolization materials per se.
In this study, we successfully manufactured a microsphere by atomizing mixture of pharmaceutical excipient. By using pig model, our microsphere was proven to be as safe and effective as currently used embolization materials-Embosphere and Gelfoam. Our microsphere was similar to Gelfoam in that it was biodegradable and Embosphere in that it was calibrated. Besides, our microsphere was radiopaque which can help radiologists to observe and monitor the entire embolization process.
Because the liver has dual blood supply coming from both portal vein and hepatic artery, arterial embolization by using commercial embolization materials or our microsphere did not cause any significant pathological or serum biochemical changes. The efficacy of embolization can only be investigated from the reduced blood flow of liver on CT perfusion imaging and the extent of splenic infarction after embolization of splenic artery. Based on these two findings, our microsphere was proven to be as effective as Embosphere and Gelfoam.
The size and the accurate caliber range of embolization microspheres is important to correctly deliver it to tumoral or peritumoral vessels. Drug-eluting or simple particles of 100-500 μm size are delivered into medium-sized vessels that irrigate tumor nodules with the aim of producing ischemia and finally exposing tumor cells to high concentrations of cytotoxic agents. Particles more than 500 μm occlude tumor feeding vessels and cause ischemia of both tumor and peritumoral liver[12]. By applying the atomizing technique, we were able to manufacture microspheres with a narrow size distribution as other calibrated materials such as DC-bead does using a microfluidics technique.
Drug eluting beads significantly reduced the peak plasma concentration of chemotoxic drug when compared with cTACE[13] and therefore, DEB-TACE has a lower frequency of adverse events than cTACE[14]. The mechanism of drug elution is attributed to an ionic exchange process between the hydrogel sulfonate or carboxyl counter ions of bead and anionic drug moieties[15,16]. Such a characteristic has limited the selection of chemotoxic drug to only drugs with anionic moieties. Excipient is a pharmacologically inactive substance and it can be formulated with the active gradient of a medication to give it a suitable consistency or form to a drug. Our microsphere was constructed by a mixture of excipient and thus has a greater potential to combine with various chemotoxic agents for cTACE.
Visualization of the microspheres during embolization would allow radiologists to investigate microsphere distribution within the tumor and liver and to evaluate as to whether distribution is homogeneous in the vasculature and whether the entire target tissue is embolized. All of this information regarding the distribution of the microsphere can be further correlated with the outcome of patients and would be extremely valuable to support the optimization of embolization protocols for a given type and size of tumor. Owning to the fact that lipiodol was included in our formulation of excipient, our microsphere therefore has an additional advantage over the currently used microsphere (i.e., Embosphere and DC-bead) in that it was visible under fluoroscopy.
Gelfoam is the only commercially available biodegradable embolic material at this time; however, it is not spherical and thus unable to accurately control the level of embolization. For temporary embolization such as repeated TAE designated for controlling tumor growth, a biodegradable embolic material clearly preferred. Our microsphere was manufactured by biodegradable excipient. As evidenced by the histology examination and serial follow up CT scan, our microsphere was proven to be biodegradable and was therefore, a more advantageous embolization material than Embosphere.
Although our microsphere has been proven to be useful for transcatheter arterial embolization in a pig model, the detailed physical properties such as rigidity to compression and in vivo deformation have not been studied. Deformation of microsphere in arteries and micro-catheters may lead to a more distal occlusion, and thus it is crucial when choosing a optimal sized microsphere to embolized targeted arteries[11]. In addition, we have not added chemotoxic agent to microsphere to evaluate the rate of drug eluting as it may complicate the evaluation of adverse effect of our microsphere if its safety has not been proved in advance. Studies regarding to these properties of our new microsphere are now ongoing.
In summary, our microspheres possess the characteristics of calibrated, radiopaque, and biodegradable and we proved their efficacy for TAE is equal to or better than Gelfoam and Embosphere.
Conventional transcatheter arterial chemoembolization (TACE) stands for the treatment of choice for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma (HCC). Drug-eluting bead transcatheter arterial chemoembolization controls the delivery of chemotoxic agent and reduces the side effects of chemotherapy. Biodegradable embolic material allows for the re-catheterization of embolized vessels and therefore, repetitive TACE. Calibrated microspheres allow the radiologist to choose the size of microspheres according to the size of the targeting vessels. Currently, there is no commercial microsphere that fulfills the above characteristics of an ideal embolization material.
The authors constructed a biodegradable radiopaque microsphere by atomizing a mixture of pharmaceutical excipient. The conducted arterial embolization study in pigs in an attempt to explore a new microsphere that fulfills the above requirements for arterial embolization of HCC.
Unlike DC-bead using a microfluidics technique to produce calibrated microsphere, the authors applied atomizing technique to manufacture microspheres with a narrow size distribution. The authors’ microsphere was constructed by a mixture of excipient and thus has a greater potential to combine with various chemotoxic agents than the currently developed drug-eluting beads which uses ionic exchange process between the bead and anionic drug moieties. As evidenced by the histology examination and serial follow up CT scan, the authors’ microsphere was proven to be biodegradable and was therefore, a more advantageous embolization material than Embosphere. By using pig model, their microsphere was proven to be as safe and effective as currently used embolization materials-Embosphere and Gelfoam.
Before applying the microsphere to patients with HCC, studies for the detailed physical properties such as rigidity to compression and in vivo deformation of microsphere and the rate of drug eluting from microsphere would be required. However, the study has demonstrated a brand new way and idea to produce embolization material for future arterial embolization.
Atomization is a technique which breaks up bulk liquids into droplets and has been applied to produce particles of desired shape, size, and density. Excipient is a pharmacologically inactive substance and it can be formulated with the active gradient of a medication to give it a suitable consistency or form to a drug.
This is a very well designed animal study. This biodegradable radiopaque microsphere has a high potential to develop into a commercial product used for transcatheter arterial embolization of HCC.
P- Reviewer: Kim HC, Lau WY, Morris DL S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 4. | Oerlemans C, Seevinck PR, van de Maat GH, Boulkhrif H, Bakker CJ, Hennink WE, Nijsen JF. Alginate-lanthanide microspheres for MRI-guided embolotherapy. Acta Biomater. 2013;9:4681-4687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Kang MJ, Park JM, Choi WS, Lee J, Kwak BK, Lee J. Highly spherical and deformable chitosan microspheres for arterial embolization. Chem Pharm Bull (Tokyo). 2010;58:288-292. [PubMed] |
| 6. | Salem R, Lewandowski RJ. Chemoembolization and radioembolization for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2013;11:604-611; quiz e43- e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Weng L, Rostamzadeh P, Nooryshokry N, Le HC, Golzarian J. In vitro and in vivo evaluation of biodegradable embolic microspheres with tunable anticancer drug release. Acta Biomater. 2013;9:6823-6833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Laurent A, Wassef M, Chapot R, Houdart E, Merland JJ. Location of vessel occlusion of calibrated tris-acryl gelatin microspheres for tumor and arteriovenous malformation embolization. J Vasc Interv Radiol. 2004;15:491-496. [PubMed] |
| 9. | Namur J, Citron SJ, Sellers MT, Dupuis MH, Wassef M, Manfait M, Laurent A. Embolization of hepatocellular carcinoma with drug-eluting beads: doxorubicin tissue concentration and distribution in patient liver explants. J Hepatol. 2011;55:1332-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 11. | Laurent A. Microspheres and nonspherical particles for embolization. Tech Vasc Interv Radiol. 2007;10:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | Gonzalez MV, Tang Y, Phillips GJ, Lloyd AW, Hall B, Stratford PW, Lewis AL. Doxorubicin eluting beads-2: methods for evaluating drug elution and in-vitro:in-vivo correlation. J Mater Sci Mater Med. 2008;19:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, Denys A, Lee C. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562-W570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Lencioni R, Crocetti L, Petruzzi P, Vignali C, Bozzi E, Della Pina C, Bargellini I, Cioni D, Oliveri F, De Simone P. Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: a pilot clinical study. J Hepatol. 2008;49:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Liu DM, Kos S, Buczkowski A, Kee S, Munk PL, Klass D, Wasan E. Optimization of doxorubicin loading for superabsorbent polymer microspheres: in vitro analysis. Cardiovasc Intervent Radiol. 2012;35:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |