Copyright
©The Author(s) 2025.
World J Radiol. Jun 28, 2025; 17(6): 106682
Published online Jun 28, 2025. doi: 10.4329/wjr.v17.i6.106682
Published online Jun 28, 2025. doi: 10.4329/wjr.v17.i6.106682
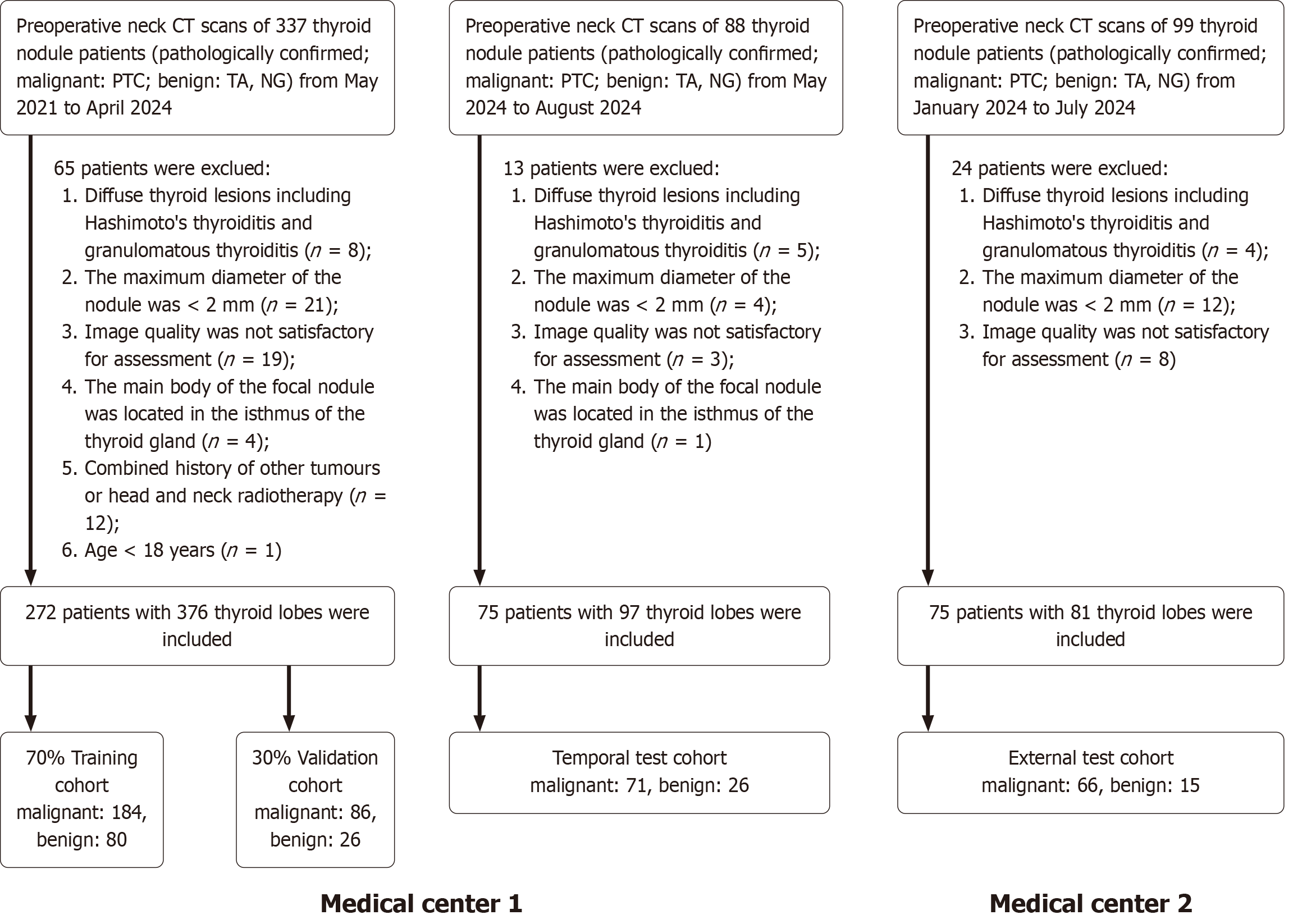
Figure 1 Study inclusion flowchart.
CT: Computed tomography; PTC: Papillary thyroid carcinoma; TA: Thyroid adenoma; NG: Nodular goiter.
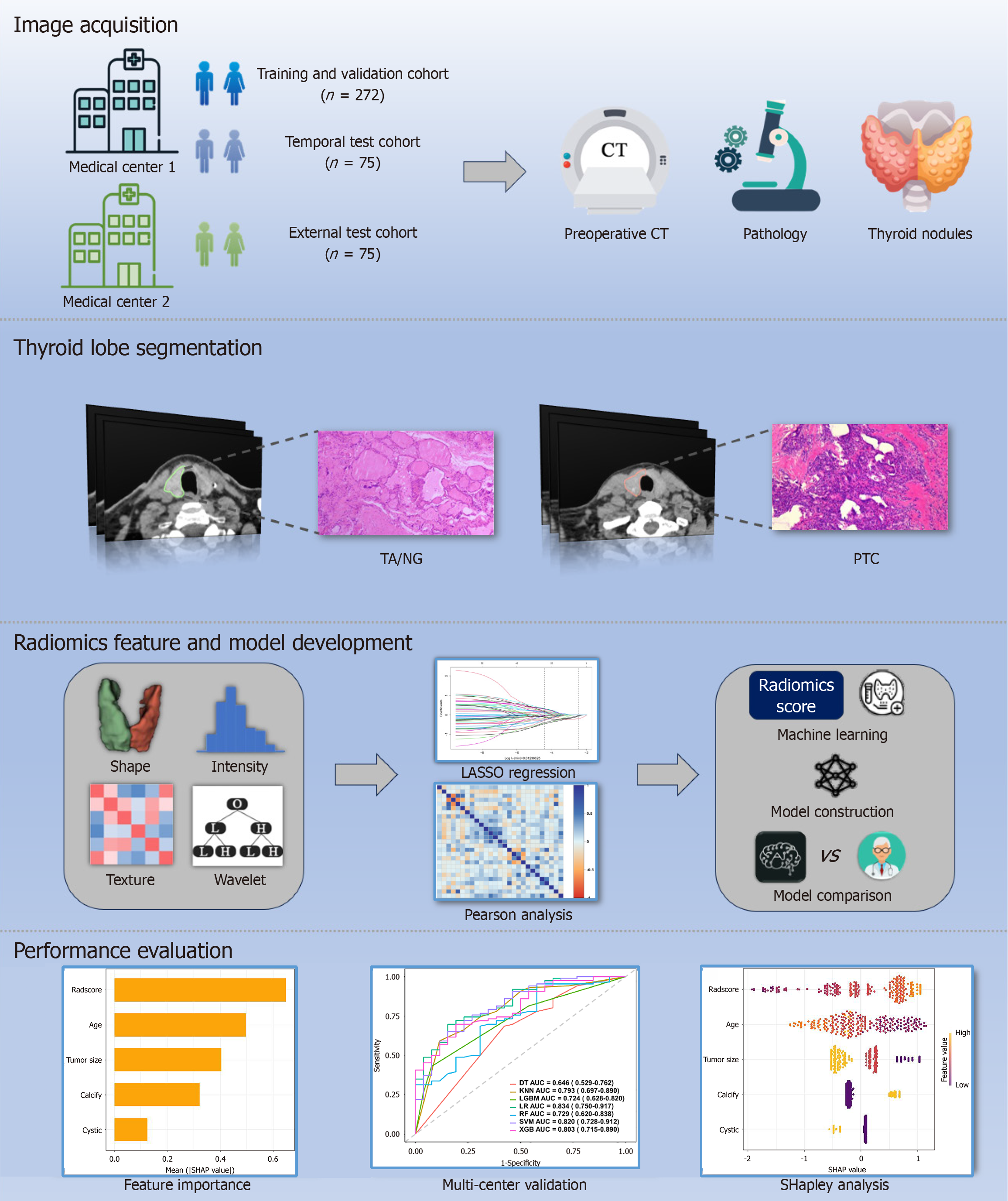
Figure 2 Workflow of model development.
CT: Computed tomography; PTC: Papillary thyroid carcinoma; TA: Thyroid adenoma; NG: Nodular goiter.
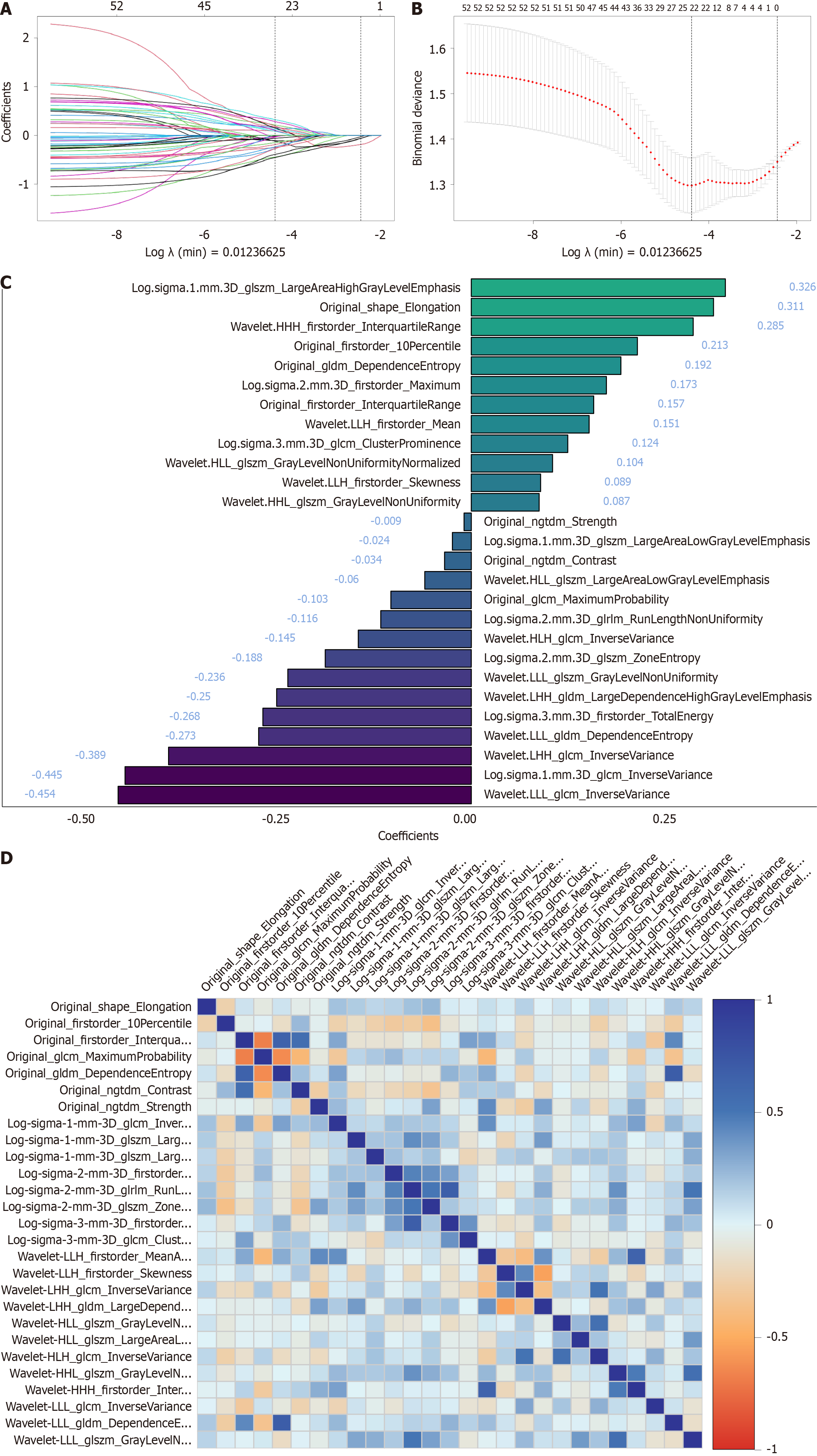
Figure 3 Least absolute shrinkage and selection operator feature selection and comparison.
A: Least absolute shrinkage and selection operator feature selection plot, where the horizontal axis represents the logarithm of the regularization parameter (λ), and the vertical axis denotes the corresponding feature weights. As λ increases, feature weights progressively decrease until exclusion at zero; B: 10-fold cross-validation curve, where the vertical axis represents the cross-validated mean squared error. The λ-value at the lowest point (λ min) is identified as the optimal regularization parameter, balancing model complexity and prediction error; C: 27 radiomic features and their corresponding weight coefficients retained after least absolute shrinkage and selection operator regression; D: Correlation heatmap of the 27 selected radiomic features, with darker colors indicating stronger correlations.
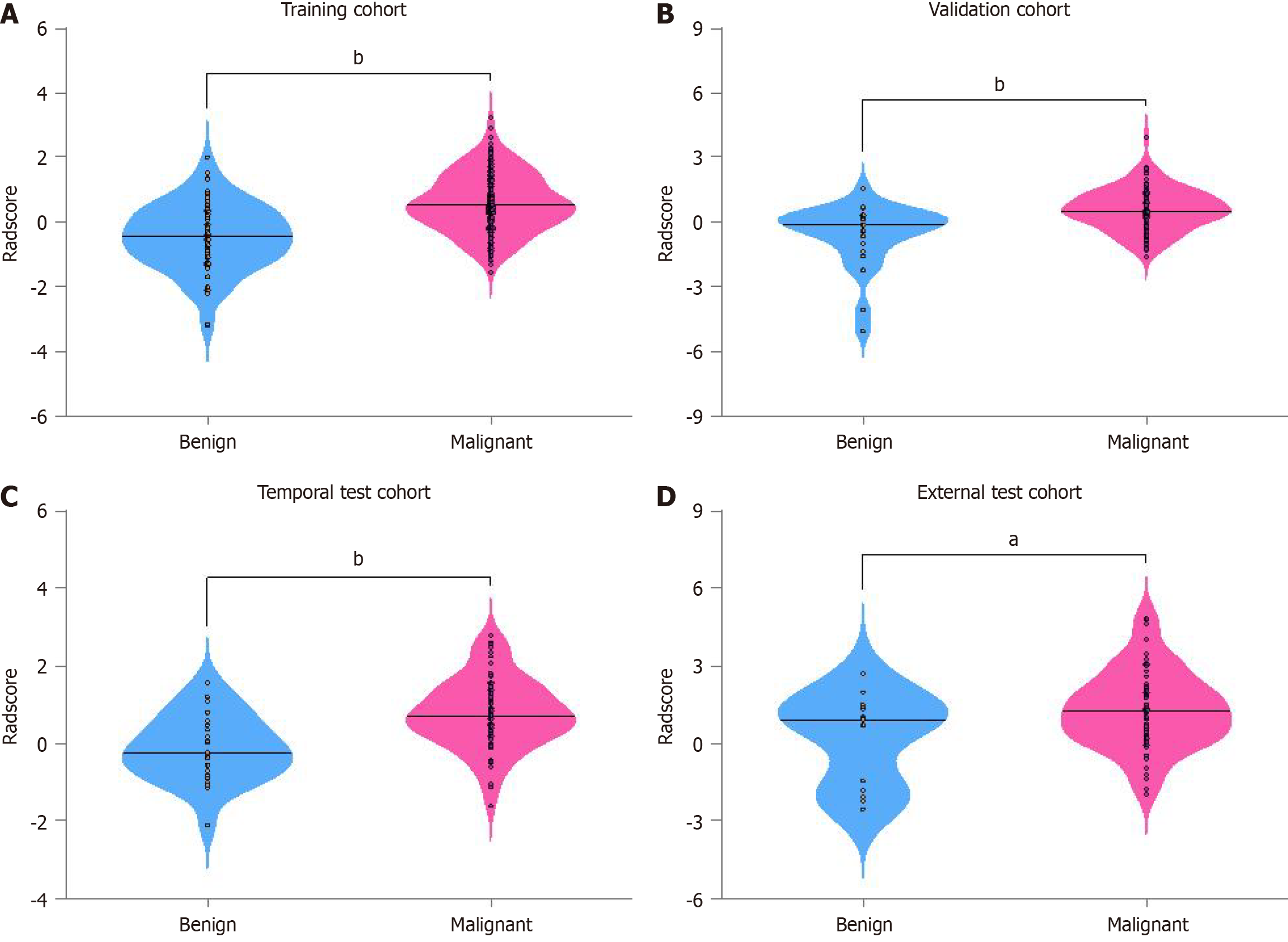
Figure 4 Radiomic score distribution across cohorts.
A: Comparison of radiomic score (Radscore) between benign and malignant lesions in the training; B: Comparison of Radscore between benign and malignant lesions in the validation; C: Comparison of Radscore between benign and malignant lesions in the temporal test; D: Comparison of Radscore between benign and malignant lesions in the external test cohorts. aP < 0.05; bP < 0.001.
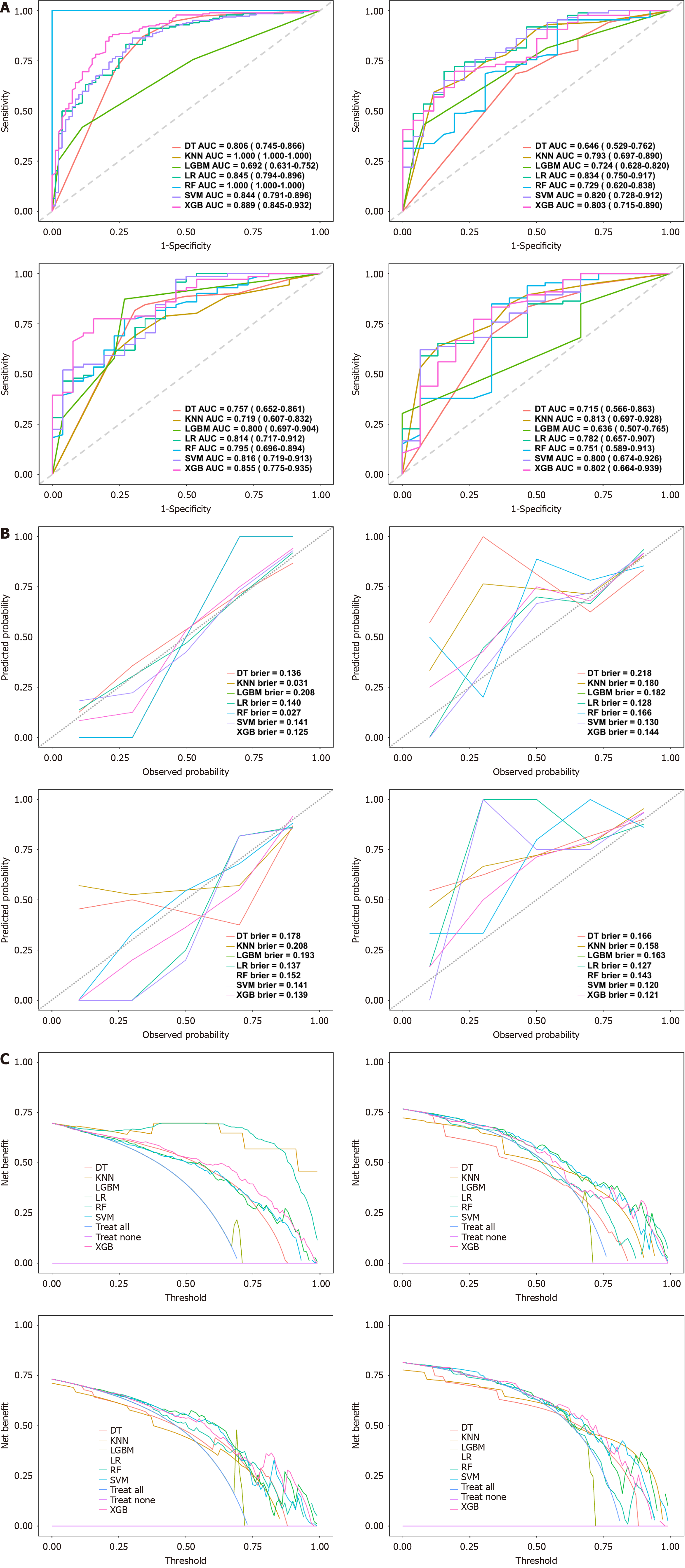
Figure 5 Performance evaluation of seven machine learning models.
A: Receiver operating characteristic curves of different models across the training, validation, temporal test, and external test cohorts (from left to right). The area under the receiver operating characteristic curve quantifies classification performance, with higher area under the receiver operating characteristic curve values indicating superior discrimination; B: Calibration curves for different models in the training, validation, temporal test, and external test cohorts (from left to right). These curves assess the agreement between predicted probabilities and actual outcomes, with lower Brier scores indicating better calibration, as reflected by proximity to the 45° diagonal line; C: Decision curve analysis in the training, validation, temporal test, and external test cohorts (from left to right), evaluating the clinical net benefit of different models across probability thresholds. Overall, the extreme gradient boosting model exhibits consistent and superior performance across multiple cohorts. DT: Decision trees; KNM: K-nearest neighbors; LR: Logistic regression; RF: Random forests; LGBM: Light gradient boosting machines; SVM: Support vector machines; XGB: Extreme gradient boosting; AUC: Area under the receiver operating characteristic curve.
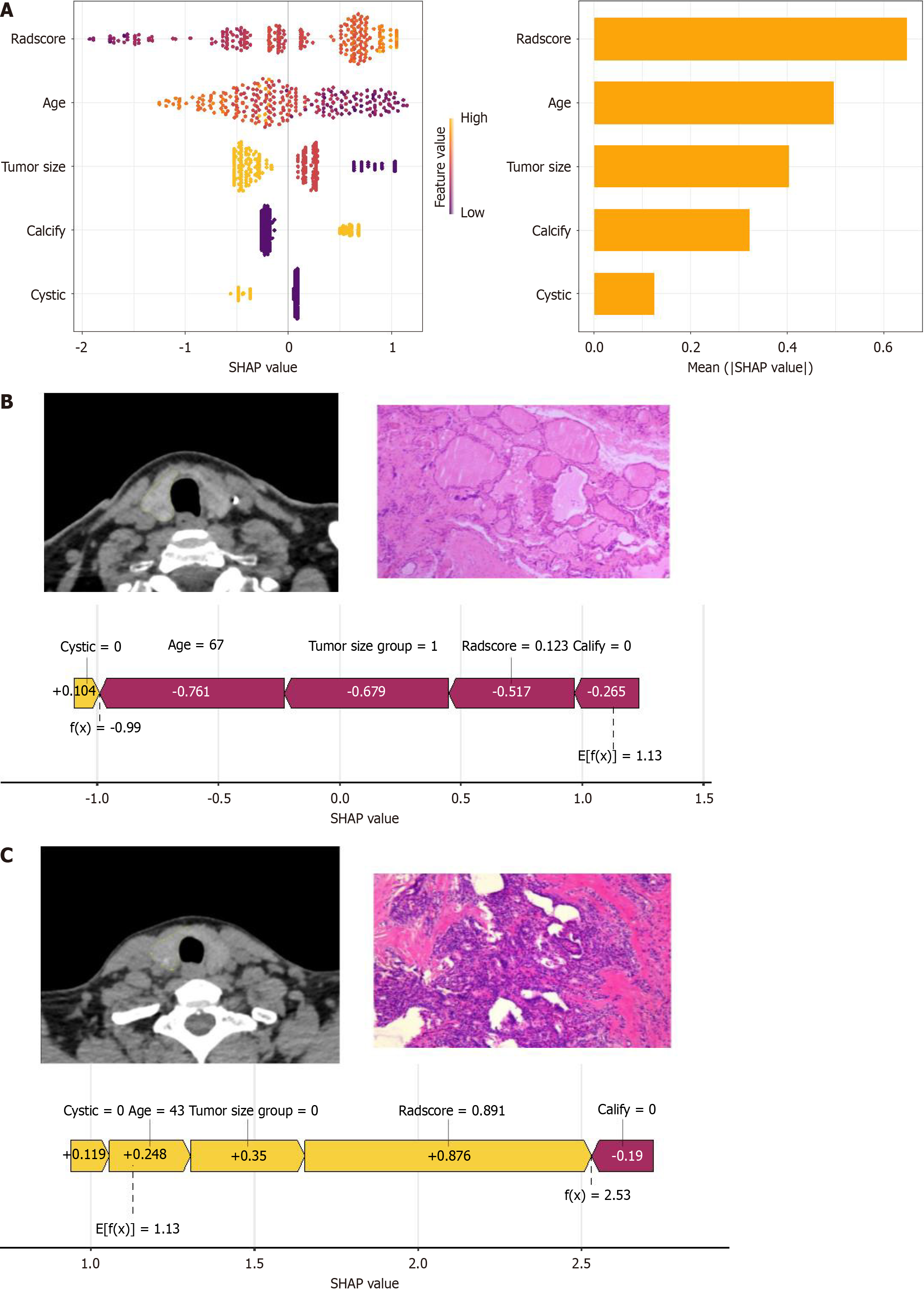
Figure 6 SHapley Additive exPlanations analysis and clinical application.
A: Honeycomb and bar plot ranking SHapley Additive exPlanations values by feature importance; B: Case illustration of a 67-year-old female with an 8 mm right thyroid lobe nodule, pathologically confirmed as nodular goiter; C: Case illustration of a 43-year-old female with a 4 mm right thyroid lobe nodule, pathologically confirmed as papillary thyroid carcinoma. SHAP: SHapley Additive exPlanations.
- Citation: Wang H, Wang X, Du YS, Wang Y, Bai ZJ, Wu D, Tang WL, Zeng HL, Tao J, He J. Non-contrast computed tomography radiomics model to predict benign and malignant thyroid nodules with lobe segmentation: A dual-center study. World J Radiol 2025; 17(6): 106682
- URL: https://www.wjgnet.com/1949-8470/full/v17/i6/106682.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i6.106682