Revised: October 22, 2013
Accepted: December 17, 2013
Published online: February 26, 2014
Processing time: 168 Days and 9 Hours
Vascular biology, endothelial and vascular smooth muscle and cardiac dysfunction play a primary role in the initiation and perpetuation of hypertension, cardiovascular disease and target organ damage. Nutrient-gene interactions and epigenetics are predominant factors in promoting beneficial or detrimental effects in cardiovascular health and hypertension. Macronutrients and micronutrients can prevent, control and treat hypertension through numerous mechanisms related to vascular biology. Oxidative stress, inflammation and autoimmune dysfunction initiate and propagate hypertension and cardiovascular disease. There is a role for the selected use of single and component nutraceutical supplements, vitamins, antioxidants and minerals in the treatment of hypertension based on scientifically controlled studies which complement optimal nutrition, coupled with other lifestyle modifications.
Core tip: Vascular biology and endothelial dysfunction play a primary roles in hypertension and subsequent cardiovascular disease. Micronutrients, macronutrients and optimal nutrition and nutritional supplements can prevent, control and treat hypertension through numerous mechanisms related to vascular biology. These treatments are complementary to drug therapy. Oxidative stress, inflammation and autoimmune dysfunction initiate and propagate hypertension and cardiovascular disease. There is a role for the selected use of single and component nutraceutical supplements, vitamins, antioxidants and minerals in the treatment of hypertension based on scientifically controlled studies which complement optimal nutrition, coupled with other lifestyle modifications.
- Citation: Houston M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J Cardiol 2014; 6(2): 38-66
- URL: https://www.wjgnet.com/1949-8462/full/v6/i2/38.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i2.38
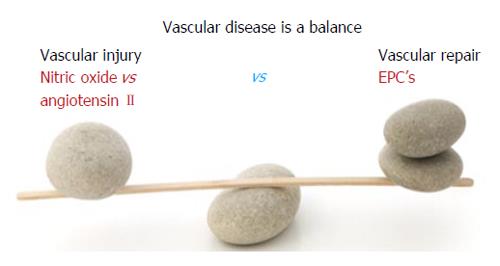
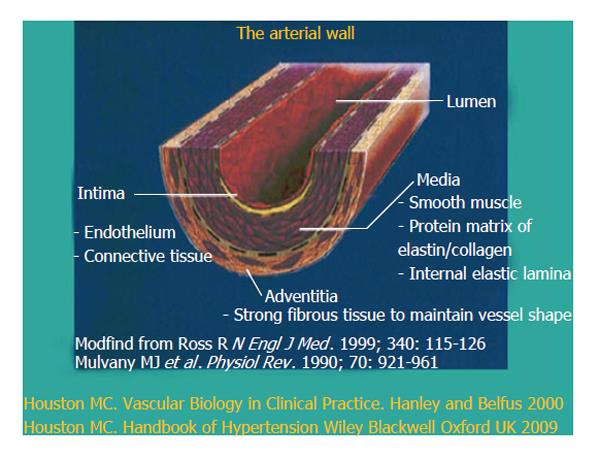
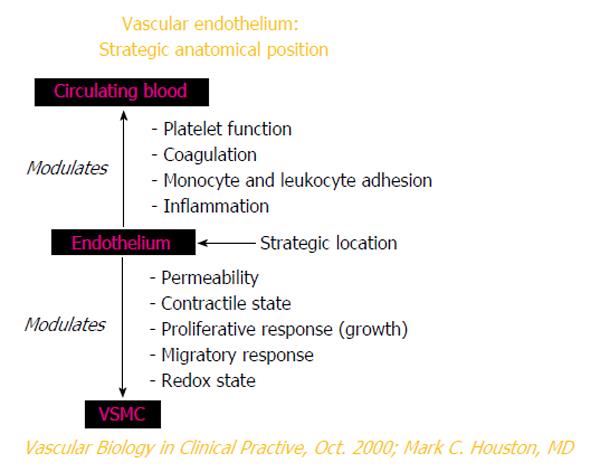
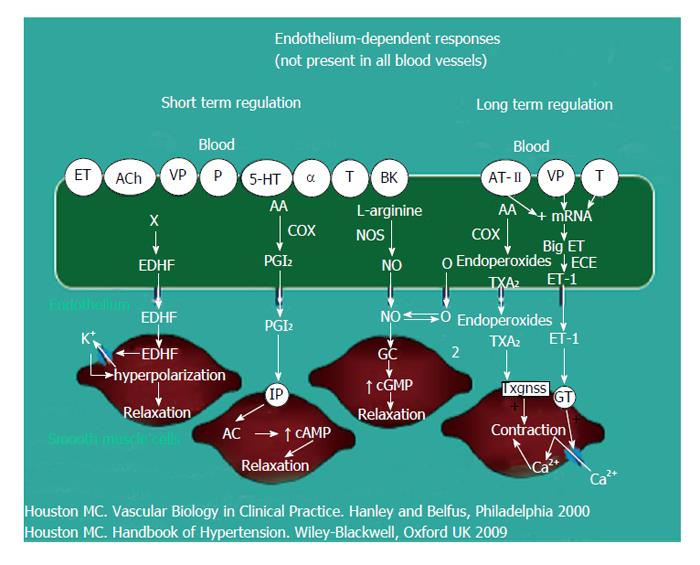
Vascular disease is a balance between vascular injury and repair (Figure 1). The endothelium is in a strategic location between the blood and the vascular smooth muscle and secretes various substances to maintain vascular homeostasis and health (Figures 2 and 3). Various insults that damage the endothelium, lead to endothelial dysfunction (ED) and may induce hypertension and other cardiovascular diseases. Hypertension may be a hemodynamic marker of injured endothelium and vascular smooth muscle related to finite responses of inflammation, oxidative stress and immune dysfunction of the arteries leading to ED, vascular and cardiac smooth muscle dysfunction, loss of arterial elasticity with reduced arterial compliance and increased systemic vascular resistance. Hypertension is a consequence of the interaction of genetics and environment. Macronutrients and micronutrients are crucial in the regulation of blood pressure (BP) and subsequent target organ damage (TOD). Nutrient-gene interactions, subsequent gene expression, epigenetics, oxidative stress, inflammation and autoimmune vascular dysfunction have positive or negative influences on vascular biology in humans. Endothelial activation with ED and vascular smooth muscle dysfunction (VSMD) initiate and perpetuate essential hypertension.
Macronutrient and micronutrient deficiencies are very common in the general population and may be even more common in patients with hypertension and cardiovascular disease due to genetics, environmental causes and prescription drug use. These deficiencies will have an enormous impact on present and future cardiovascular health outcomes such as hypertension, myocardial infarction (MI), stroke and renal disease. The diagnosis and treatment of these nutrient deficiencies will reduce BP and improve vascular health, ED, vascular biology and cardiovascular events.
Epidemiology underscores the etiologic role of diet and associated nutrient intake in hypertension. The transition from the Paleolithic diet to our modern diet has produced an epidemic of nutritionally-related diseases (Table 1). Hypertension, atherosclerosis, coronary heart disease (CHD), MI, congestive heart failure (CHF), cerebrovascular accidents (CVA), renal disease, type 2 diabetes mellitus (T2DM), metabolic syndrome (MS) and obesity are some of these diseases[1,2]. Table 1 contrasts intake of nutrients involved in BP regulation during the Paleolithic Era and modern time. Evolution from a pre-agricultural, hunter-gatherer milieu to an agricultural, refrigeration society has imposed an unnatural and unhealthful nutritional selection process. In sum, diet has changed more than our genetics can adapt.
| Nutrients and dietary characteristics | Paleolithic intake | Modern intake |
| Sodium | < 50 mmol/d (1.2 g) | 175 mmol/d (4 g) |
| Potassium | > 10000 meq/d (256 g) | 150 meq/d (6 g) |
| Sodium/potassium ratio | < 0.13/d | > 0.67/d |
| Protein | 37% | 20% |
| Carbohydrate | 41% | 40%-50% |
| Fat | 22% | 30%-40% |
| Polyunsaturated/saturated Fat ratio | 1.4 | 0.4 |
| Fiber | > 100 g/d | 9 g/d |
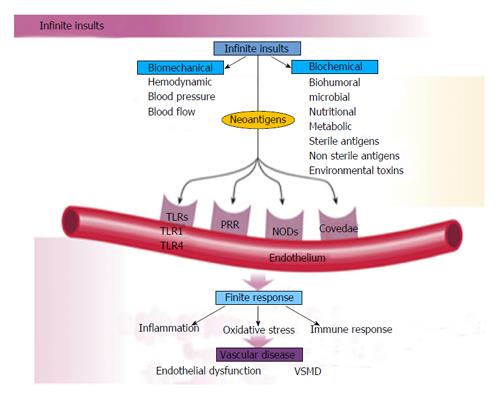
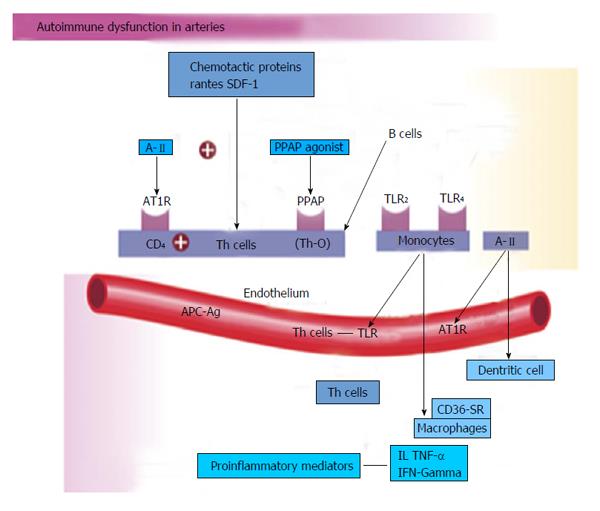
The human genetic makeup is 99.9% that of our Paleolithic ancestors, yet our nutritional, vitamin and mineral intakes are vastly different[3]. The macronutrient and micronutrient variations, oxidative stress from radical oxygen species (ROS) and radical nitrogen species (RNS) and inflammatory mediators such as cell adhesion molecules (CAMs), cytokines, signaling molecules and autoimmune vascular dysfunction of T cells and B cells, contribute to the higher incidence of hypertension and other cardiovascular diseases through complex nutrient-gene interactions, epigenetic and nutrient-caveolae interactions and nutrient reactions with pattern recognition receptors [toll like receptors (TLR) and nod like receptors] in the endothelium[4-9] (Figure 4). Reduction in nitric oxide bioavailability, increase in angiotensin II and endothelin coupled with endothelial activation initiate the vascular and cardiac dysfunction and hypertension. Poor nutrition, coupled with obesity and a sedentary lifestyle have resulted in an exponential increase in nutritionally-related diseases. In particular, the high Na+/K+ ratio of modern diets has contributed to hypertension, CVA, CHD, MI, CHF and renal disease[3,10] as have the relatively low intake of omega-3 PUFA, increase in omega-6 PUFA, saturated fat and trans fatty acids[11].
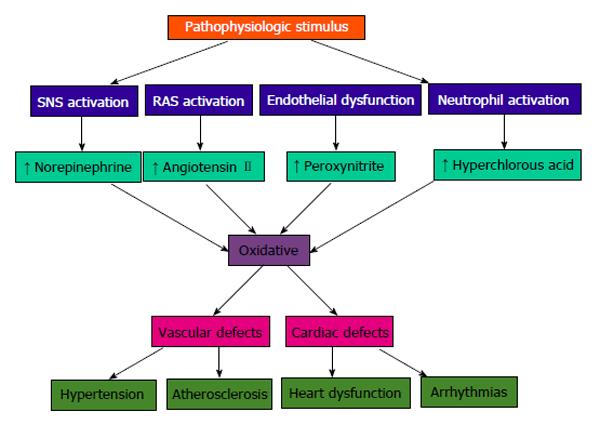
Vascular biology assumes a pivotal role in the initiation and perpetuation of hypertension and cardiovascular TOD[1]. Oxidative stress (ROS and RNS), inflammation (increased expression of redox-sensitive pro-inflammatory genes, CAMs and recruitment migration and infiltration of circulating cells) and autoimmune vascular dysfunction (T cells and B cells) are the primary pathophysiologic and functional mechanisms that induce vascular disease[1,12-14] (Figure 5). All three of these are closely inter-related and establish a deadly combination that leads to ED, vascular smooth muscle and cardiac dysfunction, hypertension, vascular disease, atherosclerosis and CVD. Hypertension is not a disease but is the correct and chronically dysregulated response with an exaggerated outcome of the infinite insults to the blood vessel with subsequent environmental-genetic expression patterns and downstream disturbances in which the vascular system is the innocent bystander. This becomes a maladaptive vascular response that was initially intended to provide vascular defense to the endothelial insults (Figure 6)[1,13-15]. Hypertension is a vasculopathy characterized by ED, structural remodeling, vascular inflammation, increased arterial stiffness, reduced distensibility and loss of elasticity[13]. These insults are biomechanical (BP, pulse pressure, blood flow, oscillatory flow, turbulence, augmentation, pulse wave velocity and reflected waves) and biohumoral or biochemical which includes all the non-mechanical causes such as metabolic, endocrine, nutritional, toxic, infectious and other etiologies[1] (Figure 4). In addition to the very well established connections for endocrine and nutritional causes of hypertension, toxins and infections also increase BP[16-20]. Various toxins such as polychlorinated biphenyls, mercury, lead, cadmium, arsenic and iron also increase BP and CVD[16,17]. Numerous microbial organisms have been implicated in hypertension and CHD[18-20]. All of these insults lead to impaired microvascular structure and function which manifests clinically as hypertension[12-14]. The level of BP may not give an accurate indication of the microvascular involvement and impairment in hypertension. Hypertensive patients have abnormal microvasculature in the form of inward eutrophic remodeling of the small resistance arteries leading to impaired vasodilatory capacity, increased vascular resistance, increased media to lumen ratio, decreased maximal organ perfusion and reduced flow reserve, especially in the heart with decreased coronary flow reserve[12-14]. Significant functional then structural microvascular impairment occurs even before the BP begins to rise in normotensive offspring of hypertensive parents evidenced by ED, impaired vasodilation, forearm vascular resistance, diastolic dysfunction, increased left ventricular mass index, increased septal and posterior wall thickness and left ventricular hypertrophy[12,15]. Thus, the cellular processes underlying the vascular perturbations constitute a vascular phenotype of hypertension that may be determined by early life programming and imprinting which is compounded by vascular aging[12-14].
Oxidative stress, with an imbalance between ROS and RNS and the anti-oxidant defense mechanisms, contributes to the etiology of hypertension in animals[10] and humans[11,12]. Radical oxygen species and RNS are generated by multiple cellular sources, including nicotinamide adenine dinucleotide phosphate hydrase (NADPH) oxidase, mitochondria, xanthine oxidase, uncoupled endothelium-derived nitric oxide (NO) synthase (U-eNOS), cyclo-oxygenase and lipo-oxygenase[11]. Superoxide anion is the predominant ROS species produced by these tissues, which neutralizes NO and also leads to downstream production of other ROS (Figure 3). Hypertensive patients have impaired endogenous and exogenous anti-oxidant defense mechanisms[21], an increased plasma oxidative stress and an exaggerated oxidative stress response to various stimuli[21,22]. Hypertensive subjects also have lower plasma ferric reducing ability of plasma, lower vitamin C levels and increased plasma 8-isoprostanes, which correlate with both systolic and diastolic BP. Various single-nucleotide polymorphisms (SNP’s) in genes that codify for anti-oxidant enzymes are directly related to hypertension[23]. These include NADPH oxidase, xanthine oxidase, superoxide dismutase 3 (SOD 3), catalase, glutathione peroxidase 1 (GPx 1) and thioredoxin. Antioxidant deficiency and excess free radical production have been implicated in human hypertension in numerous epidemiologic, observational and interventional studies (Table 2)[21,22,24]. Radical oxygen species directly damage endothelial cells, degrade NO, influence eicosanoid metabolism, oxidize LDL, lipids, proteins, carbohydrates, DNA and organic molecules, increase catecholamines, damage the genetic machinery, influence gene expression and transcription factors[1,21,22,25,26]. The inter-relations of neurohormonal systems, oxidative stress and cardiovascular disease are shown in Figures 6 and 7. The increased oxidative stress, inflammation and autoimmune vascular dysfunction in human hypertension results from a combination of increased generation of ROS and RNS, an exacerbated response to ROS and RNS and a decreased antioxidant reserve[24-29]. Increased oxidative stress in the rostral ventrolateral medulla (RVLM) enhances glutamatergic excitatory inputs and attenuates GABA-ergic inhibitory inputs to the RVLM which contributes to increased sympathetic nervous system (SNS) activity from the paraventricular nucleus[30]. Activation of the AT1R in the RVLM increases NADPH oxidase and increases oxidative stress and superoxide anion, increases SNS outflow causing an imbalance of SNS/PNS activity with elevation of BP, increased heart rate and alterations in heart rate variability and heart rate recovery time, which can be blocked by AT1R blockers[30,31].
| The cytotoxic reactive oxygen species and the natural defense mechanisms | |||
| Reactive oxygen apecies | Antioxidant defense mechanisms | ||
| Free radicals | Enzymatic scavengers | ||
| O2•- | Superoxide anion radical | SOD | Superoxide dismutase |
| OH• | Hydroxyl radical | 2O2•- + 2H+→ H2O2 + O2 | |
| ROO• | Lipid peroxide (peroxyl) | CAT | Catalase (peroxisomal-bound) |
| RO• | Alkoxyl | 2H2O2→ O2 + H2O | |
| RS• | Thiyl | GTP | Glutathione peroxidase |
| NO• | Nitric oxide | 2GSH + H2O2→ GSSG + 2H2O | |
| NO2• | Nitrogen dioxide | 2GSH + ROOH → GSSG + ROH + 2H2O | |
| ONOO- | Peroxynitrite | ||
| CCl3• | Trichloromethyl | Nonenzymatic scavengers | |
| Vitamin A | |||
| Non-radicals | Vitamin C (ascorbic acid) | ||
| H2O2 | Hydrogen peroxide | Vitamin E (α-tocopherol) | |
| HOCl | Hypochlorous acid | β-carotene | |
| ONOO- | Peroxynitrite | Cysteine | |
| 1O2 | Singlet oxygen | Coenzyme Q | |
| The superscripted bold dot indicates an unpaired electron and the negative charge indicates a gained electron. GSH, reduced glutathione; GSSG, oxidized glutathione; R, lipid chain. Singlet oxygen is an unstable molecule due to the two electrons present in its outer orbit spinning in opposite directions. | Uric acid | ||
| Flavonoids | |||
| Sulfh ydryl group | |||
| Thioether compounds | |||
The link between inflammation and hypertension has been suggested in both cross-sectional and longitudinal studies[32]. Increases in high sensitivity C-reactive protein (HS-CRP) as well as other inflammatory cytokines such as interleukin-1B, (IL-1B), IL-6, tumor necrosis alpha (TNF-α) and chronic leukocytosis occur in hypertension and hypertensive- related TOD, such as increased carotid IMT[33]. HS-CRP predicts future CV events[32,33]. Elevated HS-CRP is both a risk marker and risk factor for hypertension and CVD[34,35]. Increases in HS-CRP of over 3 μg/mL may increase BP in just a few days that is directly proportional to the increase in HS-CRP[34,35]. Nitric oxide and eNOS are inhibited by HS-CRP[34,35]. The AT2R, which normally counterbalances AT1R, is down-regulated by HS-CRP[34,35]. Angiotensin II (A-II) upregulates many of the cytokines, especially IL-6, CAMs and chemokines by activating nuclear factor Kappa B (NF-κB) leading to vasoconstriction. These events, along with the increases in oxidative stress and endothelin-1, elevate BP[32].
Innate and adaptive immune responses are linked to hypertension and hypertension-induced CVD through at least three mechanisms: cytokine production, central nervous system stimulation and renal damage. This includes salt-sensitive hypertension with increased renal inflammation as a result of T cell imbalance, dysregulation of CD4+ and CD8+ lymphocytes and chronic leukocytosis with increased neutrophils and reduced lymphocytes[36-38]. Leukocytosis, especially increased neutrophils and decreased lymphocyte count increase BP in Blacks by 6/2 mmHg in the highest vs the lowest tertile[38]. Macrophages and various T-cell subtypes regulate BP, invade the arterial wall, activate TLRs and induce autoimmune vascular damage[38,39]. Angiotensin II activates immune cells (T cells, macrophages and dendritic cells) and promotes cell infiltration into target organs[39]. CD4+ T lymphocytes express AT1R and PPAR gamma receptors, and release TNF-α, interferon and interleukins within the vascular wall when activated[39] (Figure 5). IL-17 produced by T cells may play a pivotal role in the genesis of hypertension caused by Angiotensin II[39]. Hypertensive patients have significantly higher TLR 4 mRNA in monocytes compared to normal[40]. Intensive reduction in BP to systolic BP (SBP) less than 130 mmHg vs SBP to only 140 mmHg lowers the TLR 4 more[40]. A-II activates the TLR expression leading to inflammation and activation of the innate immune system. When TLR 4 is activated there is downstream macrophage activation, migration, increase metalloproteinase 9, vascular remodeling, collagen accumulation in the artery, LVH and cardiac fibrosis[40]. The autonomic nervous system is critical in either increasing or decreasing immune dysfunction and inflammation[41]. Efferent cholinergic anti-inflammatory pathways via the vagal nerve innervate the spleen, nicotine acetylcholine receptor subunits and cytokine producing immune cells to influence vasoconstriction and BP[41]. Local CNS inflammation or ischemia may mediate vascular inflammation and hypertension[39].
Aldosterone is associated with increased adaptive immunity and autoimmune responses with CD4+ T cell activation and Th 17 polarization with increased IL 17, TGF-β and TNF-α which modulate over 30 inflammatory genes[42,43]. Increased serum aldosterone is an independent risk factor for CVD and CHD through non-hemodynamic effects as well as through increased BP[42,43]. Blockade of mineralocorticoid receptors in the heart, brain, blood vessels and immune cells reduces CV risk even with the persistence of hypertension[42,43].
Many of the natural compounds in food, certain nutraceutical supplements, vitamins, antioxidants or minerals function in a similar fashion to a specific class of antihypertensive drugs. Although the potency of these natural compounds may be less than the antihypertensive drug, when used in combination with other nutrients and nutraceutical supplements, the antihypertensive effect is additive or synergistic. Table 3 summarizes these natural compounds into the major antihypertensive drug classes such as diuretics, beta blockers, central alpha agonists, direct vasodilators, calcium channel blockers (CCB’s), angiotensin converting enzyme inhibitors (ACEI’s), angiotensin receptor blockers (ARB’s) and direct renin inhibitors (DRI).
| Antihypertensive therapeutic class (alphabetical listing) | Foods and ingredients listed by therapeutic class | Nutrients and other supplements listed by therapeutic class |
| Angiotensin converting enzyme inhibitors | Egg yolk Fish (specific): bonito, dried salted fish, fish sauce sardine muscle/protein tuna garlic gelatin hawthorne berry Milk products (specific): casein sour milk whey (hydrolyzed) sake sea vegetables (kelp) sea weed (wakame) wheat germ (hydrolyzed) zein (corn protein) | Melatonin omega-3 fatty acids pomegranate pycnogenol zinc |
| Angiotensin receptor blockers | Celery fiber garlic MUFA | Coenzyme Q10 gamma linolenic acid N-acetyl cysteine oleic acid resveratrol potassium taurine vitamin C vitamin B6 (pyridoxine) |
| Beta blockers | Hawthorne berry | |
| Calcium channel blockers | Celery garlic hawthorn berry MUFA | Alpha lipoic acid calcium magnesium N-acetyl cysteine oleic acid omega-3 fatty acids: eicosapentaenoic acid docosahexaenoic acid taurine vitamin B6 vitamin C vitamin E |
| Central alpha agonists (reduce sympathetic nervous system activity) | Celery fiber garlic protein | Coenzyme Q 10 gamma linolenic acid potassium restriction of sodium taurine vitamin C vitamin B6 zinc |
| Direct renin inhibitors | Vitamin D | |
| Direct vasodilators | Celery cooking oils with monounsaturated fats fiber garlic MUFA soy | Alpha linolenic acid arginine calcium flavonoids magnesium Omega-3 fatty acids potassium taurine vitamin C vitamin E |
| Diuretics | Celery hawthorn berry protein | Calcium coenzyme Q 10 fiber gamma linolenic acid l-carnitine magnesium potassium taurine vitamin B6 vitamin C Vitamin E: high gamma/delta tocopherols and tocotrienols. |
The Dietary Approaches to Stop Hypertension (DASH) I and II diets conclusively demonstrated significant reductions in BP in borderline and stage I hypertensive patients[44,45]. In DASH I untreated hypertensive subjects with SBP < 160 mmHg and DBP 80-95 mmHg were placed on one of three diets for 4 wk, control diet, fruit and vegetable diet (F + V) and combined diet that added F + V and low fat dairy[44]. DASH II added progressive sodium restriction in each group[45]. The control diet consisted of sodium at 3 g/d, potassium, magnesium and calcium at 25% of the US average, macronutrients at US average of 4 servings per day, a sodium/potassium ratio of 1.7 and fiber at 9 g/d. The F + V diet increased the potassium, magnesium and calcium to 75%, macronutrients to greater than the US average, a sodium potassium ratio of 0.7, 31 g of fiber and 8.5 servings of fruits and vegetables per day. The combined diet was similar to the F + V diet but added low fat dairy. At 2 wk the BP was decreased by 10.7/5.2 mmHg in the hypertensive patients in DASH I and 11.5/6.8 mmHg in the hypertensive patients in DASH II. These reductions persisted as long as the patients were on the diet. The DASH diet increases plasma renin activity (PRA) and serum aldosterone levels in response to the BP reductions[46,47]. The mean increase in PRA was 37 ng/mL per day[47]. There was an associated of response with the G46A polymorphism of beta 2 adrenergic receptor. The A allele of G46A had a greater BP reduction and blunted PRA and aldosterone. The arachidonic acid (AA) genotype had the best response and the GG genotype had no response. Adding an ARB, ACEI or DRI improved BP response to the DASH diet in the GG group due to blockade of the increase in PRA. A low sodium DASH diet decreases oxidative stress (urine F2-isoprostanes), improves vascular function (augmentation index) and lowers BP in salt sensitive subjects[48]. In addition, plasma nitrite increased and pulse wave velocity decreased at week two on the DASH diet[49].
The average sodium intake in the US is 5000 mg/d with some areas of the country consuming 15000-20000 mg/d[50]. However, the minimal requirement for sodium is probably about 500 mg/d[50]. Epidemiologic, observational and controlled clinical trials demonstrate that an increased sodium intake is associated with higher BP as well as increased risk for CVD, CVA, LVH, CHD, MI, renal insufficiency, proteinuria and over activity of the SNS[1,50]. A reduction in sodium intake in hypertensive patients, especially the salt sensitive patients, will significantly lower BP by 4-6/2-3 mmHg that is proportional to the degree of sodium restriction and may prevent or delay hypertension in high risk patients and reduce future CV events[51-53].
Salt sensitivity (≥ 10% increase in MAP with salt loading) occurs in about 51% of hypertensive patients and is a key factor in determining the cardiovascular, cerebrovascular, renal and BP responses to dietary salt intake[54]. Cardiovascular events are more common in the salt sensitive patients than in salt resistant ones, independent of BP[55]. An increased sodium intake has a direct positive correlation with BP and the risk of CVA and CHD[56]. The risk is independent of BP for CVA with a relative risk of 1.04 to 1.25 from the lowest to the highest quartile[56]. In addition, patients will convert to a nondipping BP pattern with increases in nocturnal BP as the sodium intake increases[56].
Increased sodium intake has a direct adverse effect on endothelial cells[57-61]. Sodium promotes cutaneous lymphangiogenesis, increases endothelial cell stiffness, reduces size, surface area, volume, cytoskeleton, deformability and pliability, reduces eNOS and NO production, increases asymmetric dimethyl arginine (ADMA), oxidative stress and TGF-β. All of these abnormal vascular responses are increased in the presence of aldosterone[57-61]. These changes occur independent of BP and may be partially counteract by dietary potassium[57-61]. The endothelial cells act as vascular salt sensors[62]. Endothelial cells are targets for aldosterone which activate epithelial sodium channels (ENaCs) and have a negative effects on release of NO and on endothelial function. The mechanical stiffness of the cell plasma membrane and the submembranous actin network (endothelial glcyocalyx) (“shell”) serve as a “firewall” to protect the endothelial cells and are regulated by serum sodium, potassium and aldosterone within the physiologic range[62]. Changes in shear-stress-dependent activity of the endothelial NO synthase located in the caveolae regulate the viscosity in this “shell”[62]. High plasma sodium gelates the shell of the endothelial cell, whereas the shell is fluidized by high potassium. These communications between extracellular ions and intracellular enzymes occur at the plasma membrane barrier, whereas 90% of the total cell mass remains uninvolved in these changes. Blockade of the ENaC with spironolactone (100%) or amiloride (84%) minimizes or stop many of these vascular endothelial responses and increase NO[58,63]. Nitric oxide release follows endothelial nanomechanics and not vice versa and membrane depolarization decreases vascular endothelial cell stiffness which improves flow mediated nitric-oxide dependent vasodilation[64,65]. In the presence of vascular inflammation and increased HS-CRP, the effects of aldosterone on the ENaC is enhanced further increasing vascular stiffness and BP[66]. High sodium intake also abolishes the AT2R-mediated vasodilation immediately with complete abolition of endothelial vasodilation (EDV) within 30 d[67]. Thus, it has become clear that increased dietary sodium has adverse effects on the vascular system, BP and CVD by altering the endothelial glycocalyx, which is a negatively charged biopolymer that lines the blood vessels and serves as a protective barrier against sodium overload, increased sodium permeability and sodium-induced TOD[68]. Certain SNP’s of salt inducible kinase I which alter Na+/K+ ATPase, determine sodium induced hypertension and LVH[69].
The sodium intake per day in hypertensive patients should be between 1500 to 2000 mg. Sodium restriction improves BP reduction in those on patients that are on pharmacologic treatment and the decrease in BP is additive with restriction of refined carbohydrates[70,71]. Reducing dietary sodium intake may reduce damage to the brain, heart, kidney and vasculature through mechanisms dependent on the small BP reduction as well as those independent of the decreased BP[72-75].
A balance of sodium with other nutrients, especially potassium, magnesium and calcium is important, not only in reducing and controlling BP, but also in decreasing cardiovascular and cerebrovascular events[3,72,73]. An increase in the sodium to potassium ratio is associated with significantly increased risk of CVD and all-cause mortality[72]. The Yanomamo Indians consume and excrete only 1 meq of sodium in 24 h and consume and excrete 152 meq of potassium in 24 h[73]. The Na+ to K+ ratio is 1/152 and is associated with elevated PRA, but BP does not increase with age. At age 50 the average BP in the Yanomamo is 100-108/64-69 mmHg[73].
The average U.S. dietary intake of potassium (K+) is 45 mmol/d with a potassium to sodium (K+/Na+) ratio of less than 1:2[10,74]. The recommended intake of K+ is 4700 mg/d (120 mmol) with a K+/Na+ ratio of about 4-5 to 1[10,74]. Numerous epidemiologic, observational and clinical trials have demonstrated a significant reduction in BP with increased dietary K+ intake in both normotensive and hypertensive patients[10,74,76]. The average BP reduction with a K+ supplementation of 60 to 120 mmol/d is 4.4/2.5 mmHg in hypertensive patients but may be as much as 8/4.1 mmHg with 120 mmol/d (4700 mg)[10,74,76,77]. In hypertensive patients, the linear dose-response relationship is 1.0 mmHg reduction in SBP and 0.52 mmHg reduction in diastolic BP per 0.6 g/d increase in dietary potassium intake that is independent of baseline dietary potassium ingestion[10]. The response depends on race (black > white), sodium, magnesium and calcium intake[10]. Those on a higher sodium intake have a greater reduction in BP with potassium[10]. Alteration of the K+/Na+ ratio to a higher level is important for both anti-hypertensive as well as cardiovascular and cerebrovascular effects[10,77]. High potassium intake reduces the incidence of cardiovascular (CHD, MI) and CVA independent of the BP reduction[10,74,76,77]. There are also reductions in CHF, LVH, diabetes mellitus and cardiac arrhythmias[10]. If the serum potassium is less than 4.0 meq/dL, there is an increased risk of CVD mortality, ventricular tachycardia, ventricular fibrillation and CHF[10]. Red blood cell potassium is a better indication of total body stores and CVD risk than is serum potassium[10]. Gu et al[77] found that potassium supplementation at 60 mmol of KCl per day for 12 wk significantly reduced SBP -5.0 mmHg (range -2.13 to -7.88 mmHg) (P < 0.001) in 150 Chinese men and women aged 35 to 64 years.
Potassium increases natriuresis, modulates baroreflex sensitivity, vasodilates, decreases the sensitivity to catecholamines and Angiotensin II, increases sodium potassium ATPase and DNA synthesis in the vascular smooth muscle cells and decreases SNS activity in cells with improved vascular function[10]. In addition, potassium increases bradykinin and urinary kallikrein, decreases NADPH oxidase, which lowers oxidative stress and inflammation, improves insulin sensitivity, decreases ADMA, reduces intracellular sodium and lowers production of TGF-β[10].
Each 1000 mg increase in potassium intake per day reduces all cause mortality by approximately 20%. Potassium intake of 4.7 g/d is estimated to decrease CVA by 8% to 15% and MI by 6%-11%[10]. Numerous SNP’s such as nuclear receptor subfamily 3 group C, angiotensin II type receptor and hydroxysteroid 11 beta dehydrogenase (HSD11B1 and B2) determine an individual’s response to dietary potassium intake[78]. Each 1000 mg decrease in sodium intake per day will decrease all cause mortality by 20%[10,73]. A recent analysis suggested a dose related response to CVA with urinary potassium excretion[79]. There was a RRR of CVA of 23% at 1.5-1.99 g, 27% at 2.0-2.49 g, 29% at 2.5-3 g and 32% over 3 g/d of potassium urinary excretion[79]. The recommended daily dietary intake for patients with hypertension is 4.7 to 5.0 g of potassium and less than 1500 mg of sodium[10]. Potassium in food or from supplementation should be reduced or used with caution in those patients with renal impairment or those on medications that increase renal potassium retention such as ACEI, ARB, DRI and serum aldosterone receptor antagonists[10].
A high dietary intake of magnesium of at least 500-1000 mg/d reduces BP in most of the reported epidemiologic, observational and clinical trials, but the results are less consistent than those seen with Na+ and K+[74,80]. In most epidemiologic studies, there is an inverse relationship between dietary magnesium intake and BP[74,80,81]. A study of 60 essential hypertensive subjects given magnesium supplements showed a significant reduction in BP over an eight week period documented by 24 h ambulatory BP, home and office blood BP[74,80,81]. The maximum reduction in clinical trials has been 5.6/2.8 mmHg but some studies have shown no change in BP[82]. The combination of high potassium and low sodium intake with increased magnesium intake had additive anti-hypertensive effects[82]. Magnesium also increases the effectiveness of all anti-hypertensive drug classe[82].
Magnesium competes with Na+ for binding sites on vascular smooth muscle and acts as a direct vasodilator, like a CCB. Magnesium increases prostaglandin E (PGE), regulates intracellular calcium, sodium, potassium and pH, increases nitric oxide, improves endothelial function, reduces oxLDL, reduces HS-CRP, TBxA2, A-II, and norepinephrine. Magnesium also improves insulin resistance, glucose and MS, binds in a necessary-cooperative manner with potassium, inducing EDV and BP reduction, reduces CVD and cardiac arrhythmias, decreases carotid IMT, lowers cholesterol, lowers cytokine production, inhibits nuclear factor Kb, reduces oxidative stress and inhibits platelet aggregation to reduce thrombosis[74,80-86].
Magnesium is an essential co-factor for the delta-6-desaturase enzyme that is the rate-limiting step for conversion of linoleic acid (LA) to gamma linolenic acid (GLA)[74,80,81,83-85] needed for synthesis of the vasodilator and platelet inhibitor PGE1. Altered TRPM7 channels, which are the transporter for magnesium occur in many hypertensive patients[83].
A meta-analysis of 241378 patients with 6477 strokes showed an inverse relationship of dietary magnesium to the incidence of ischemic stroke[84]. For each 100 mg of dietary magnesium intake, ischemic stroke was decreased by 8%. The proposed mechanism include inhibition of ischemia induced glutamate release, NMDA receptor blockade, CCB actions, mitochondrial calcium buffering, decrease in ATP depletion and vasodilation of the cerebral arteries[84]. A meta-analysis showed reductions in BP of 3-4/2-3 mmHg in 22 trials of 1173 patients[87].
Intracellular level of magnesium (RBC) is more indicative of total body stores and should be measured in conjunction with serum and urinary magnesium[83]. Magnesium may be supplemented in doses of 500 to 1000 mg/d. Magnesium formulations chelated to an amino acid may improve absorption and decrease the incidence of diarrhea[82]. Adding taurine at 1000 to 2000 mg/d will enhance the anti-hypertensive effects of magnesium[82]. Magnesium supplements should be avoided or used with caution in patients with known renal insufficiency or in those taking medications that induce magnesium retention[82].
Population studies show a link between hypertension and calcium[88], but clinical trials that administered calcium supplements to patients have shown inconsistent effects on BP[88]. The heterogeneous responses to calcium supplementation have been explained by Resnick[89]. This is the “ionic hypothesis”[89] of hypertension, cardiovascular disease and associated metabolic, functional and structural disorders. Calcium supplementation is not recommended at this time as an effective means to reduce BP.
Low serum zinc levels in observational studies correlate with hypertension as well as CHD, type II DM, hyperlipidemia, elevated lipoprotein a [Lp(a)], increased 2 h postprandial plasma insulin levels and insulin resistance[90,91]. Zinc is transported into cardiac and vascular muscle and other tissues by metallothionein[92]. Genetic deficiencies of metallothionein with intramuscular zinc deficiencies may lead to increased oxidative stress, mitochondrial dysfunction, cardiomyocyte dysfunction and apoptosis with subsequent myocardial fibrosis, abnormal cardiac remodeling, heart disease, heart failure, or hypertension[92]. Intracellular calcium increases oxidative stress which is reduced by zinc[92].
Bergomi et al[93] evaluated Zinc (Zn++) status in 60 hypertensive subjects compared to 60 normotensive control subjects. An inverse correlation of BP and serum Zn++ was observed. The BP was also inversely correlated to a Zn++ dependent enzyme-lysyl oxidase activity. Zn++ inhibits gene expression and transcription through NF-κB and activated protein-1 and is an important cofactor for SOD[90,92]. These effects plus those on insulin resistance, membrane ion exchange, RAAS and SNS effects may account for Zn++ antihypertensive effects[90,92]. Zinc intake should be 50 mg/d[1].
Observational and epidemiologic studies demonstrate a consistent association between a high protein intake and a reduction in BP and incident BP[94,95]. The protein source is an important factor in the BP effect; animal protein being less effective than non-animal or plant protein, especially almonds[94-97]. In the Inter-Salt Study of over 10000 subjects, those with a dietary protein intake 30% above the mean had a lower BP by 3.0/2.5 mmHg compared to those that were 30% below the mean (81 vs 44 g/d)[94]. However, lean or wild animal protein with less saturated fat and more essential omega-3 fatty acids may reduce BP, lipids and CHD risk[94,97]. A meta-analysis confirmed these findings and also suggested that hypertensive patients and the elderly have the greatest BP reduction with protein intake[95]. Another meta-analysis of 40 trials with 3277 patients found reductions in BP of 1.76/1.15 mmHg compared to carbohydrate intake (P < 0.001)[98]. Both vegetable and animal protein significantly and equally reduced BP at 2.27/1.26 mmHg and 2.54/0.95 mmHg respectively[98]. Increased dietary protein intake is inversely associated with risk for stroke in women with hypertension[99]. A randomized cross-over study in 352 adults with pre-hypertension and stage I hypertension found a significant reduction in SBP of 2.0 mmHg with soy protein and 2.3 mmHg with milk protein compared to a high glycemic index diet over each of the 8 wk treatment periods[100]. There was a non-significant reduction in DBP. Another RDB parallel study over 4 wk of 94 subjects with prehypertension and stage I hypertension found significant reductions on office BP of 4.9/2.7 mmHg in those given a combination of 25% protein intake vs the control group given 15% protein in an isocaloric manner[101]. The protein consisted of 20% pea, 20% soy, 30% egg and 30% milk-protein isolate[101]. The daily recommended intake of protein from all sources is 1.0 to 1.5 g/kg body weight, varying with exercise level, age, renal function and other factors[1,70,71].
Fermented milk supplemented with whey protein concentrate significantly reduces BP in human studies[102-106]. Administration of 20 g/d of hydrolyzed whey protein supplement rich in bioactive peptides significantly reduced BP over 6 wk by 8.0 ± 3.2 mmHg in SBP and 5.5 ± 2.1 mm in diastolic BP[103]. Milk peptides which contain both caseins and whey proteins are a rich source of ACEI peptides. Val-Pro-Pro and Ile-Pro-Pro given at 5 to 60 mg/d have variable reductions in BP with an average decrease in pooled studies of about 1.28-4.8/0.59-2.2 mmHg[71,100,104-107]. However several recent meta-analysis did not show significant reductions in BP in humans[106,108]. Powdered fermented milk with Lactobacillus helveticus given at 12 g/d significantly lowered BP by 11.2/6.5 mmHg in 4 wk in one study[104]. Milk peptides are beneficial in treating MS[109]. A dose response study showed insignificant reductions in BP[110]. The clinical response is attributed to fermented milk’s active peptides which inhibit ACE.
Pins et al[111] administered 20 g of hydrolyzed whey protein to 56 hypertensive subjects and noted a BP reduction of 11/7 mmHg compared to controls at one week that was sustained throughout the study. Whey protein is effective in improving lipids, insulin resistance, glucose, arterial stiffness and BP[112]. These data indicate that the whey protein must be hydrolyzed in order to exhibit an antihypertensive effect, and the maximum BP response is dose dependent.
Bovine casein-derived peptides and whey protein-derived peptides exhibit ACEI activity[102-111]. These components include B-caseins, B-lg fractions, B2-microglobulin and serum albumin[102-104,111]. The enzymatic hydrolysis of whey protein isolates releases ACEI peptides.
Marine collagen peptides (MCPs) from deep sea fish have anti-hypertensive activity[113-115]. A double-blind placebo controlled trial in 100 hypertensive subjects with diabetes who received MCPs twice a day for 3 mo had significant reductions in DBP and mean arterial pressure[113]. Bonito protein (Sarda Orientalis), from the tuna and mackerel family has natural ACEI inhibitory peptides and reduces BP 10.2/7 mmHg at 1.5 g/d[114,116].
Sardine muscle protein, which contains Valyl-Tyrosine (VAL-TYR), significantly lowers BP in hypertensive subjects[117]. Kawasaki et al[117] treated 29 hypertensive subjects with 3 mg of VAL-TYR sardine muscle concentrated extract for four wk and lowered BP 9.7/5.3 mmHg (P < 0.05). Levels of A-I increased as serum A-II and aldosterone decreased indicating that VAL-TYR is a natural ACEI. A similar study with a vegetable drink with sardine protein hydrolysates significantly lowered BP by 8/5 mmHg in 13 wk[118].
Soy protein lowers BP in hypertensive patients in most studies[100,119-127]. Soy protein intake was significantly and inversely associated with both SBP and DBP in 45694 Chinese women consuming 25 g/d or more of soy protein over 3 years and the association increased with age[119]. The SBP reduction was 1.9 to 4.9 mm lower and the DBP 0.9 to 2.2 mmHg lower[119]. However, randomized clinical trials and meta-analysis have shown mixed results on BP with no change in BP to reductions of 7% to 10 % for SBP and DBP[121-125]. The recent meta-analysis of 27 trials found a significant reduction in BP of 2.21/1.44 mmHg[120]. Some studies suggest improvement in endothelial function, improved arterial compliance, reduction in HS-CRP and inflammation, ACEI activity, reduction in sympathetic tone, diuretic action and reduction in both oxidative stress and aldosterone levels[125-127]. Fermented soy at about 25 g/d is recommended.
In addition to ACEI effects, protein intake may also alter catecholamine responses and induce a natriuretic effect[117,118]. Low protein intake coupled with low omega 3 fatty acid intake may contribute to hypertension in animal models[128]. The optimal protein intake, depending on level of activity, renal function, stress and other factors, is about 1.0 to 1.5 g/kg per day[1].
L-arginine: L-arginine and endogenous methylarginines are the primary precursors for the production of NO, which has numerous beneficial cardiovascular effects, mediated through conversion of L-arginine to NO by eNOS. Patients with hypertension, hyperlipidemia, diabetes mellitus and atherosclerosis have increased levels of HSCRP and inflammation, increased microalbumin, low levels of apelin (stimulates NO in the endothelium), increased levels of arginase (breaks down arginine) and elevated serum levels of ADMA, which inactivates NO[129-133].
Under normal physiological conditions, intracellular arginine levels far exceed the Km [Michaelis Menton constant(MMC)] of eNOS which is less than 5 μmol[134]. However, endogenous NO formation is dependent on extracellular arginine concentration[134]. The intracellular concentrations of L-arginine are 0.1-3.8 mmol/L in endothelial cells while the plasma concentration of arginine is 80-120 μmol/L which is about 20-25 times greater than the MMC[135,136]. Despite this, cellular NO formation depends on exogenous L-arginine and this is the arginine paradox. Renal arginine regulates BP and blocks the formation of endothelin, reduces renal sodium reabsorption and is a potent antioxidant[134]. The NO production in endothelial cells is closely coupled to cellular arginine uptake indicating that arginine transport mechanisms play a major role in the regulation of NO-dependent function. Exogenous arginine can increase renal vascular and tubular NO bioavailability and influence renal perfusion, function and BP[132]. Molecular eNOS uncoupling may occur in the absence of tetrahydrobiopterin which stabilizes eNOS, which leads to production of ROS[135].
Human studies in hypertensive and normotensive subjects of parenteral and oral administrations of L-arginine demonstrate an antihypertensive effect as well as improvement in coronary artery blood flow and peripheral blood flow in PAD[129,136-140]. The BP decreased by 6.2/6.8 mmHg on 10 g/d of L-arginine when provided as a supplement or though natural foods to a group of hypertensive subjects[136]. Arginine produces a statistically and biologically significant decrease in BP and improved metabolic effect in normotensive and hypertensive humans that is similar in magnitude to that seen in the DASH-I diet[136]. Arginine given at 4 g/d also significantly lowered BP in women with gestational hypertension without proteinuria, reduced the need for anti-hypertensive therapy, decreased maternal and neonatal complications and prolonged the pregnancy[137,138]. The combination of arginine (1200 mg/d) and N-acetyl cysteine (NAC) (600 mg bid) administered over 6 mo to hypertensive patients with type 2 diabetes, lowered SBP and DBP (P < 0.05), increased HDL-C, decreased LDL-C and oxLDL, reduced HSCRP, ICAM, VCAM, PAI-I, fibrinogen and IMT[139]. A study of 54 hypertensive subjects given arginine 4 g three times per day for four weeks had significant reductions in 24 h ABM[140]. A meta-analysis of 11 trials with 383 subjects administered arginine 4-24 g/d found average reduction in BP of 5.39/2.66 mmHg (P < 0.001) in 4 wk[141]. Although these doses of L-arginine appear to be safe, no long term studies in humans have been published at this time and there are concerns of a pro-oxidative effect or even an increase in mortality in patients who may have severely dysfunctional endothelium, advanced atherosclerosis, CHD, ACS or MI[142]. In addition to the arginine-NO path, there exists an nitrate/nitrite pathway that is related to dietary nitrates from vegetables, beetroot juice and the DASH diet that are converted to nitrites by symbiotic, salivary, GI and oral bacteria[143]. Administration of beetroot juice or extract at 500 mg/d will increase nitrites and lower BP, improve endothelial function, increase cerebral, coronary and peripheral blood flow[143].
L-carnitine and acetyl -L-carnitine: L-carnitine is a nitrogenous constituent of muscle primarily involved in the oxidation of fatty acids in mammals. Animal studies indicate that carnitine has both systemic anti-hypertensive effects as well as anti-oxidant effects in the heart by upregulation of eNOS and PPAR gamma, inhibition of RAAS, modulation of NF-κB and down regulation of NOX2, NOX4, TGF-β and CTGF that reduces cardiac fibrosis[144,145]. Endothelial function, NO and oxidative defense are improved while oxidative stress and BP are reduced[144-147].
Human studies on the effects of L-carnitine and acetyl-L-carnitine are limited, with minimal to no change in BP[148-153]. In patients with MS, acetyl-L-carnitine at one gram bid over 8 wk, improved dysglycemia and reduced SBP by 7-9 mmHg, but diastolic BP was significantly decreased only in those with higher glucose[151]. Low carnitine levels are associated with a nondipping BP pattern in Type 2 DM[153]. Carnitine has antioxidant and anti-inflammatory effects and may be useful in the treatment of essential hypertension, type II DM with hypertension, hyperlipidemia, cardiac arrhythmias, CHF and cardiac ischemic syndromes[1,149,150,153]. Doses of 2-3 g twice per day are recommended.
Taurine: Taurine is a sulfonic beta-amino acid that is considered a conditionally-essential amino acid, which is not utilized in protein synthesis, but is found free or in simple peptides with its highest concentration in the brain, retina and myocardium[154]. In cardiomyocytes, it represents about 50% of the free amino acids and has a role of an osmoregulator, inotropic factor and anti-hypertensive agent[155].
Human studies have noted that essential hypertensive subjects have reduced urinary taurine as well as other sulfur amino acids[1,154,155]. Taurine lowers BP, SVR and HR, decreases arrhythmias, CHF symptoms and SNS activity, increases urinary sodium and water excretion, increases atrial natriuretic factor, improves insulin resistance, increases NO and improves endothelial function. Taurine also decreases A-II, PRA, aldosterone, SNS activity, plasma norepinephrine, plasma and urinary epinephrine, lowers homocysteine, improves insulin sensitivity, kinins and acetyl choline responsiveness, decreases intracellular calcium and sodium, lowers response to beta receptors and has antioxidant, anti-atherosclerotic and anti-inflammatory activities, decreases IMT and arterial stiffness and may protect from risk of CHD[1,154-160]. A lower urinary taurine is associated with increased risk of hypertension and CVD[160,161]. A study of 31 Japanese males with essential hypertension placed on an exercise program for 10 wk showed a 26% increase in taurine levels and a 287% increase in cysteine levels. The BP reduction of 14.8/6.6 mmHg was proportional to increases in serum taurine and reductions in plasma norepinephrine[162]. Fujita et al[155] demonstrated a reduction in BP of 9/4.1 mmHg (P < 0.05) in 19 hypertension subjects given 6 g of taurine for 7 d. Taurine has numerous beneficial effects on the cardiovascular system and BP[156]. The recommended dose of taurine is 2 to 3 g/d at which no adverse effects are noted, but higher doses up to 6 g/d may be needed to reduce BP significantly[1,70,71,154-162].
The omega-3 fatty acids found in cold water fish, fish oils, flax, flax seed, flax oil and nuts lower BP in observational, epidemiologic and in prospective clinical trials[163-173]. The findings are strengthened by a dose-related response in hypertension as well as a relationship to the specific concomitant diseases associated with hypertension[163-173].
Studies indicate that DHA at 2 g/d reduces BP and heart rate[163,173]. The average reduction in BP is 8/5 mmHg and heart rate falls about 6 beats/min usually in about 6 wk[1,70,71,91-175]. Fish oil at 4-9 g/d or combination of DHA and EPA at 3-5 g/d will also reduce BP[1,168-173]. However, formation of EPA and ultimately DHA from ALA is decreased in the presence of high LA (the essential omega-6 fatty acid), saturated fats, trans fatty acids, alcohol, several nutrient deficiencies (magnesium, vitamin B6) and aging, all of which inhibit the desaturase enzymes[163]. Eating cold water fish three times per week may be as effective as high dose fish oil in reducing BP in hypertensive patients, and the protein in the fish may also have antihypertensive effects[1,163]. In patients with chronic kidney disease 4 g of omega 3 fatty acids reduced BP measured with 24 h ABM over 8 wk by 3.3/2.9 mmHg compared to placebo (P < 0.0001)[167].
The ideal ratio of omega-6 FA to omega-3 FA is between 1:1 to 1:4 with a polyunsaturated to saturated fat ratio greater than 1.5 to 2:0[2]. Omega 3 fatty acids increase eNOS and nitric oxide, improve endothelial function, improve insulin sensitivity, reduce calcium influx, suppress ACE activity and improve parasympathetic tone[1,163-171]. The omega-6 FA family includes LA, GLA, dihomo-GLA and AA which do not usually lower BP significantly, but may prevent increases in BP induced by saturated fats[176]. GLA may block stress-induced hypertension by increasing PGE1 and PGI2, reducing aldosterone levels, reducing adrenal AT1R density and affinity[175].
The omega-3 FA have a multitude of other cardiovascular consequences which modulates BP such as increases in eNOS and nitric oxide, improvement in ED, reduction in plasma nor-epinephrine and increase in paraSNS tone, suppression of ACE activity and improvement in insulin resistance[176]. The recommended daily dose is 3000 to 5000 mg/d of combined DHA and EPA in a ratio of 3 parts EPA to 2 parts DHA and about 50% of this dose as GLA combined with gamma/delta tocopherol at 100 mg per gram of DHA and EPA to get the omega 3 index to 8% or higher to reduce BP and provide optimal cardioprotection[177]. DHA is more effective than EPA for reducing BP and should be given at 2 g/d if administered alone[163,173].
Olive oil is rich in the omega-9 monounsaturated fat (MUFA) oleic acid, which has been associated with BP and lipid reduction in Mediterranean and other diets[178-180]. Olive oil and MUFAs have shown consistent reductions in BP in most clinical studies in humans[178-190]. In one study, the SBP fell 8 mmHg (P≤ 0.05) and the DBP fell 6 mmHg (P≤ 0.01) in both clinic and 24 h ambulatory BP monitoring in the MUFA treated subjects compared to the PUFA treated subjects[178]. In addition, the need for antihypertensive medications was reduced by 48% in the MUFA group vs 4% in the omega-6 PUFA group (P < 0.005). Extra virgin olive oil (EVOO) was more effective than sunflower oil in lowering SBP in a group of 31 elderly hypertensive patients in a double blind randomized crossover study[187]. The SBP was 136 mmHg in the EVOO treated subjects vs 150 mmHg in the sunflower treated group (P < 0.01). Olive oil also reduces BP in hypertensive diabetic subjects[188]. It is the high oleic acid content in olive oil that reduces BP[180]. In stage I hypertensive patients, oleuropein-olive leaf (Olea Eurpoaea) extract 500 mg bid for 8 wk reduced BP 11.5/4.8 mmHg which was similar to captopril 25 mg bid[189]. Olea Eupopea L aqueous extract administered to 12 patients with hypertension at 400 mg qid for 3 mo significantly reduced BP (P < 0.001)[181]. Olive oil intake in the EPIC study of 20343 subjects was inversely associated with both systolic and diastolic BP[182]. In the SUN study of 6863 subjects, BP was inversely associated with olive oil consumption, but only in men[183]. In a study of 40 hypertensive monozygotic twins, olive leaf extract demonstrated a dose response reduction in BP at doses of 500 to 1000 mg/d in 8 wk compared to placebo[184]. The low dose groups decreased BP 3/1 mmHg and the high dose 11/4 mmHg[184]. A double blind, randomized, crossover dietary intervention study over 4 mo using polyphenol rich olive oil 30 mg/d decreased BP in the study group by 7.91/6.65 mmHg and improved endothelial function[185]. The ADMA levels, oxLDL and HS-CRP were reduced in the olive oil group. Plasma nitrites and nitrates increased and hyperemic area after ischemia improved in the treated group. Olive oil inhibits the AT1R receptor, exerts L-type calcium channel antagonist effects and improves wave reflections and augmentation index[191-193].
EVOO is also contains lipid-soluble phytonutrients such as polyphenols. Approximately 5 mg of phenols are found in 10 g of EVOO[178,186]. About 4 tablespoons of EVOO is equal to 40 g of EVOO which is the amount required to get significant reductions in BP.
The clinical trials with various types of fiber to reduce BP have been inconsistent[194,195]. Soluble fiber, guar gum, guava, psyllium and oat bran may reduce BP and reduce the need for antihypertensive medications in hypertensive subjects, diabetic subjects and hypertensive-diabetic subjects[1,70,71,194,195]. The average reduction in BP is about 7.5/5.5 mmHg on 40 to 50 g/d of a mixed fiber. There is improvement in insulin sensitivity, endothelial function, reduction in SNS activity and increase in renal sodium loss[1,70,71,194].
Vitamin C is a potent water-soluble electron-donor. At physiologic levels it is an antioxidant although at supraphysiologic doses such as those achieved with intravenous vitamin C it donates electrons to different enzymes which results in pro-oxidative effects. At physiologic doses vitamin C recycles vitamin E, improves ED and produces a diuresis[196]. Dietary intake of vitamin C and plasma ascorbate concentration in humans is inversely correlated to SBP, DBP and heart rate[196-210].
An evaluation of published clinical trials indicate that vitamin C dosing at 250 mg twice daily will significantly lower SBP 5-7 mmHg and diastolic BP 2-4 mmHg over 8 wk[196-210]. Vitamin C will induce a sodium water diuresis, improve arterial compliance, improve endothelial function, increase nitric oxide and PGI2, decrease adrenal steroid production, improve sympathovagal balance, increase RBC Na/K ATPase, increase SOD, improve aortic elasticity and compliance, improve flow mediated vasodilation, decrease pulse wave velocity and augmentation index, increase cyclic GMP, activate potassium channels, reduce cytosolic calcium and reduce serum aldehydes[208]. Vitamin C prevents ED induced by an oral glucose load. Vitamin C enhances the efficacy of amlodipine, decreases the binding affinity of the AT 1 receptor for angiotensin II by disrupting the ATR1 disulfide bridges and enhances the anti-hypertensive effects of medications in the elderly with refractory hypertension[1,70,71,200-205]. In elderly patients with refractory hypertension already on maximum pharmacologic therapy, 600 mg of vitamin C daily lowered the BP by 20/16 mmHg[205]. The lower the initial ascorbate serum level, the better is the BP response. A serum level of 100 μmol/L is recommended[1,70,71]. The SBP and 24 ABM show the most significant reductions with chronic oral administration of Vitamin C[200-205]. Block et al[206] in an elegant depletion-repletion study of vitamin C demonstrated an inverse correlation of plasma ascorbate levels, SBP and DBP. In a meta-analysis of thirteen clinical trials with 284 patients, vitamin C at 500 mg/d over 6 wk reduced SBP 3.9 mmHg and DBP 2.1 mmHg[207]. Hypertensive subjects were found to have significantly lower plasma ascorbate levels compared to normotensive subjects (40 μmol/L vs 57 μmol/L respectively)[211], and plasma ascorbate is inversely correlated with BP even in healthy, normotensive individuals[206].
Most studies have not shown reductions in BP with most forms of tocopherols or tocotrienols[1,70,71]. Patients with T2DM and controlled hypertension (130/76 mmHg) on prescription medications with an average BP of 136/76 mmHg were administered mixed tocopherols containing 60% gamma, 25% delta and 15% alpha tocopherols[212]. The BP actually increased by 6.8/3.6 mmHg in the study patients (P < 0.0001) but was less compared to the increase with alpha tocopherol of 7/5.3 mmHg (P < 0.0001). This may be a reflection of drug interactions with tocopherols via cytochrome P 450 (3A4 and 4F2) and reduction in the serum levels of the pharmacologic treatments that were simultaneously being given[212]. Gamma tocopherol may have natriuretic effects by inhibition of the 70pS potassium channel in the thick ascending limb of the loop of Henle and lower BP[213]. Both alpha and gamma tocopherol improve insulin sensitivity and enhance adiponectin expression via PPAR gamma dependent processes, which have the potential to lower BP and serum glucose[214]. If vitamin E has an antihypertensive effect, it is probably small and may be limited to untreated hypertensive patients or those with known vascular disease or other concomitant problems such as diabetes or hyperlipidemia.
Vitamin D3 may have an independent and direct role in the regulation of BP and insulin metabolism[215-225]. Vitamin D influences BP by its effects on calcium-phosphate metabolism, RAA system, immune system, control of endocrine glands and ED[216]. If the Vitamin D level is below 30 ng/mL the circulating PRA levels are higher which increases angiotensin II, increases BP and blunts plasma renal blood flow[221]. The lower the level of Vitamin D, the greater the risk of hypertension, with the lowest quartile of serum Vitamin D having a 52% incidence of hypertension and the highest quartile having a 20% incidence[221]. Vitamin D3 markedly suppresses renin transcription by a VDR-mediated mechanism via the JGA apparatus. Its role in electrolytes, volume and BP homeostasis indicates that Vitamin D3 is important in amelioration of hypertension. Vitamin D lower ADMA, suppresses pro-inflammatory cytokines such as TNF-α, increases nitric oxide, improves endothelial function and arterial elasticity, decreases vascular smooth muscle hypertrophy, regulates electrolytes and blood volume, increases insulin sensitivity, reduces free fatty acid concentration, regulates the expression of the natriuretic peptide receptor and lowers HS-CRP[217-219,221].
The hypotensive effect of vitamin D was inversely related to the pretreatment serum levels of 1,25(OH)2D3 and additive to antihypertensive medications. Pfeifer et al[225] showed that short-term supplementation with vitamin D3 and calcium is more effective in reducing SBP than calcium alone. In a group of 148 women with low 25(OH)2D3 levels, the administration of 1200 mg calcium plus 800 IU of vitamin D3 reduced SBP 9.3% more (P < 0.02) compared to 1200 mg of calcium alone. The HR fell 5.4% (P = 0.02), but DBP was not changed. The range in BP reduction was 3.6/3.1 to 13.1/7.2 mmHg. The reduction in BP is related to the pretreatment level of vitamin D3, the dose of vitamin D3 and serum level of vitamin D3, but BP is reduced only in hypertensive patients. Although vitamin D deficiency is associated with hypertension in observational studies, randomized clinical trials and their meta-analysis have yielded inconclusive results[223]. In addition, vitamin D receptor gene polymorphisms may effect the risk of hypertension in men[224]. A 25 hydroxyvitamin D level of 60 ng/mL is recommended.
Low serum vitamin B6 (pyridoxine) levels are associated with hypertension in humans[226]. One human study by Aybak et al[227] proved that high dose vitamin B6 at 5 mg/kg per day for 4 wk significantly lowered BP by 14/10 mmHg. Pyridoxine (vitamin B6) is a cofactor in neurotransmitter and hormone synthesis in the central nervous system(norepinephrine, epinephrine, serotonin, GABA and kynurenine), increases cysteine synthesis to neutralize aldehydes, enhances the production of glutathione, blocks calcium channels, improves insulin resistance, decreases central sympathetic tone and reduces end organ responsiveness to glucocorticoids and mineralocorticoids[1,70,71,228,229]. Vitamin B6 is reduced with chronic diuretic therapy and heme pyrollactams. Vitamin B6 thus has similar action to central alpha agonists, diuretics and CCB’s. The recommended dose is 200 mg/d orally.
Over 4000 naturally occurring flavonoids have been identified in such diverse substances as fruits, vegetables, red wine, tea, soy and licorice[230]. Flavonoids (flavonols, flavones and isoflavones) are potent free radical scavengers that inhibit lipid peroxidation, prevent atherosclerosis, promote vascular relaxation and have antihypertensive properties[230]. In addition, they reduce stroke and provide cardioprotective effects that reduce CHD morbidity and mortality[231].
Resveratrol is a potent antioxidant and antihypertensive found in the skin of red grapes and in red wine. Resveratrol administration to humans reduces augmentation index, improves arterial compliance and lowers central arterial pressure when administered as 250 mL of either regular or dealcoholized red wine[232]. There was a significant reduction in the aortic augmentation index of 6.1% with the dealcoholized red wine and 10.5% with regular red wine. The central arterial pressure was significantly reduced by dealcoholized red wine at 7.4 mmHg and 5.4 mmHg by regular red wine. Resveratrol increases flow mediated vasodilation in a dose related manner, improves ED, prevents uncoupling of eNOS, increases adiponectin, lowers HS-CRP and blocks the effects of angiotensin II[233-236]. The recommended dose is 250 mg/d of trans resveratrol[234].
Lycopene is a fat-soluble phytonutrient in the carotenoid family. Dietary sources include tomatoes, guava, pink grapefruit, watermelon, apricots and papaya in high concentrations[237-241]. Lycopene produces a significant reduction in BP, serum lipids and oxidative stress markers[237-241]. Paran et al[241] evaluated 30 subjects with Grade I hypertension, age 40-65, taking no antihypertensive or anti-lipid medications treated with a tomato lycopene extract (10 mg lycopene) for eight weeks. The SBP was reduced from 144 to 135 mmHg (9 mmHg reduction, P < 0.01) and DBP fell from 91 to 84 mmHg (7 mmHg reduction, P < 0.01). Another study of 35 subjects with Grade I hypertension showed similar results on SBP, but not DBP[237]. Englehard gave a tomato extract to 31 hypertensive subjects over 12 wk demonstrating a significant BP reduction of 10/4 mmHg[238]. Patients on various anti-hypertensive agents including ACEI, CCB and diuretics had a significant BP reduction of 5.4/3 mmHg over 6 wk when administered a standardized tomato extract[239]. Other studies have not shown changes in BP with lycopene[240]. Lycopene and tomato extract improve ED and reduce plasma total oxidative stress[242]. The recommended daily intake of lycopene is 10-20 mg in food or supplement form.
Pycnogenol, a bark extract from the French maritime pine, at doses of 200 mg/d resulted in a significant reduction in SBP from 139.9 mmHg to 132.7 mmHg (P < 0.05) in eleven patients with mild hypertension over eight weeks in a double-blind randomized placebo crossover trial. Diastolic BP fell from 93.8 mmHg to 92.0 mmHg. Pycnogenol acts as a natural ACEI, protects cell membranes from oxidative stress, increases NO and improves endothelial function, reduces serum thromboxane concentrations, decreases myelo-peroxidase activity, improves renal cortical blood flow, reduces urinary albumin excretion and decreases HS-CRP[243-247]. Other studies have shown reductions in BP and a decreased need for ACEI and CCB, reductions in endothelin-1, HgA1C, fasting glucose, LDL-C and myeloperoxidase[244,245,247].
Clinical trials utilizing the correct dose, type of garlic and well absorbed long acting preparations have shown consistent reductions in BP in hypertensive patients with an average reduction in BP of 8.4/7.3 mmHg[248,249]. Not all garlic preparations are processed similarly and are not comparable in antihypertensive potency[1]. In addition, cultivated garlic (allium sativum), wild uncultivated garlic or bear garlic (allium urisinum) as well as the effects of aged, fresh and long acting garlic preparations differ[1,70,71,248,249]. Garlic is also effective in reducing BP in patients with uncontrolled hypertension already on anti-hypertensive medication[249,250]. A garlic homogenate-based supplement was administered to 34 prehypertensive and stage I hypertensive patients at 300 mg/d over 12 wk with a reduction in BP of 6.6-7.5/4.6-5.2 mmHg[249]. Aged garlic at doses of 240 to 960 mg/d given to 79 hypertensive subjects over 12 wk significantly lowered SBP 11.8 ± 5.4 mmHg in the high dose garlic group[249]. A time released garlic may reduce BP better than the shorter acting garlic[249]. A Cochrane Database review indicated a net reduction in BP of 10-12/6-9 mmHg in all clinical trials with garlic[249]. In a double-blind parallel randomized placebo-controlled trial of 50 patients, 900 mg of aged garlic extract with 2.4 mg of S-allylcysteine was administered daily for 12 wk and reduced SBP 10.2 mmHg (P = 0.03) more than the control group[250].
Approximately 10000 mcg of allicin (one of the active ingredients in garlic) per day, the amount contained in four cloves of garlic (5 g) is required to achieve a significant BP lowering effect[1,70,71,249,250]. Garlic has ACEI activity, calcium channel blocking activity, reduces catecholamine sensitivity, improves arterial compliance, increases bradykinin and nitric oxide and contains adenosine, magnesium, flavonoids, sulfur, allicin, phosphorous and ajoenes that reduce BP[1,70,71].
Wakame seaweed (Undaria pinnatifida) is the most popular, edible seaweed in Japan[251]. In humans, 3.3 g of dried Wakame for four wk significantly reduced both the SBP 14 ± 3 mmHg and the DBP 5 ± 2 mmHg (P < 0.01)[252]. In a study of 62 middle-aged, male subjects with mild hypertension given a potassium-loaded, ion-exchanging, sodium-adsorbing, potassium-releasing seaweed preparation, significant BP reductions occurred at four weeks on 12 and 24 g/d of the seaweed preparation (P < 0.01)[253]. The MAP fell 11.2 mmHg (P < 0.001) in the sodium-sensitive subjects and 5.7 mmHg (P < 0.05) in the sodium-insensitive subjects, which correlated with PRA.
Seaweed and sea vegetables contain most all of the seawater’s 77I minerals and rare earth elements, fiber and alginate in a colloidal form[251-253]. The primary effect of Wakame appears to be through its ACEI activity from at least four parent tetrapeptides and possibly their dipeptide and tripeptide metabolites, especially those containing the amino acid sequence Val-Tyr, Ile-Tyr, Phe-Tyr and Ile-Try in some combination[251,254,255]. Its long-term use in Japan has demonstrated its safety. Other varieties of seaweed may reduce BP by reducing intestinal sodium absorption and increasing intestinal potassium absorption[253].
Sesame has been shown to reduce BP in a several small randomized, placebo controlled human studies over 30-60 d[256-264]. Sesame lowers BP alone[257-261] or in combination with nifedipine[256,260] diuretics and beta blockers[257,261]. In a group of 13 mild hypertensive subjects, 60 mg of sesamin for 4 wk lowered SBP 3.5 mmHg (P < 0.044) and DBP 1.9 mmHg (P < 0.045)[258]. Black sesame meal at 2.52 g/d over 4 wk in 15 subjects reduced SBP by 8.3 mmHg (P < 0.05) but there was a non-significant reduction in DBP of 4.2 mmHg[259]. Sesame oil at 35 g/d significantly lowered central BP within 1 h and also maintained BP reduction chronically in 30 hypertensive subjects, reduced heart rate, reduced arterial stiffness, decreased augmentation index and pulse wave velocity, decreased HSCRP, improved NO, decreased endothelin-I and improved antioxidant capacity[264]. In addition sesame lowers serum glucose, HgbAIC and LDL-C, increases HDL, reduces oxidative stress markers and increases glutathione, SOD, GPx, CAT, vitamins C, E and A[256,257,258-261]. The active ingredients are natural ACEI’s such as sesamin, sesamolin, sesaminol glucosides, furoufuran lignans which also suppressors of NF-κB[262,263]. All of these effects lower inflammation and oxidative stress, improve oxidative defense and reduce BP[262,263].
Green tea, black tea and extracts of active components in both have demonstrated reduction in BP in humans[265-271]. In a double blind placebo controlled trial of 379 hypertensive subjects given green tea extract 370 mg/d for 3 mo, BP was reduced significantly at 4/4 mmHg with simultaneous decrease in HS CRP, TNF-α, glucose and insulin levels[268].
Dark chocolate (100 g) and cocoa with a high content of polyphenols (30 mg or more) have been shown to significantly reduce BP in humans[272-283]. A meta-analysis of 173 hypertensive subjects given cocoa for a mean duration of 2 wk had a significant reduction in BP 4.7/2.8 mmHg (P = 0.002 for SBP and 0.006 for DBP)[276]. Fifteen subjects given 100 g of dark chocolate with 500 mg of poly-phenols for 15 d had a 6.4 mmHg reduction in SBP (P < 0.05) with a non significant change in DBP[273]. Cocoa at 30 mg of poly-phenols reduced BP in pre-hypertensive and stage I hypertensive patients by 2.9/1.9 mmHg at 18 wk (P < 0.001)[274]. Two more recent meta-analysis of 13 trials and 10 trials with 297 patients found a significant reduction in BP of 3.2/2.0 mmHg and 4.5/3.2 mmHg respectively[276,279]. The BP reduction is the greatest in those with the highest baseline BP and those with at least 50%-70% cocoa at doses of 6 to 100 g/d[280,282]. Cocoa may also improve insulin resistance and endothelial function[276,279,281].
Polyphenols, chlorogenic acids (CGAs), the ferulic acid metabolite of CGAs and di-hydro-caffeic acids decrease BP in a dose dependent manner, increase eNOS and improve endothelial function in humans[284-286]. CGAs in green coffee been extract at doses of 140 mg/d significantly reduced SBP and DBP in 28 subjects in a placebo- controlled randomized clinical trial. A study of 122 male subjects demonstrated a dose response in SBP and DBP with doses of CGA from 46 mg/d to 185 mg/d. The group that received the 185 mg dose had a significant reduction in BP of 5.6/3.9 mmHg (P < 0.01) over 28 d. Hydroxyhydroquinone is another component of coffee beans which reduces the efficacy of CGAs in a dose-dependent manner which partially explains the conflicting results of coffee ingestion on BP[284,286]. Furthermore, there is genetic variation in the enzyme responsible for the metabolism of caffeine modifies the association between coffee intake, amount of coffee ingested and the risk of hypertension, heart rate, MI, arterial stiffness, arterial wave reflections and urinary catecholamine levels[287]. Fifty-nine percent of the population has the IF/IA allele of the CYP1A2 genotype which confers slow metabolism of caffeine. Heavy coffee drinkers who are slow metabolizers had a 3.00 HR for developing hypertension. In contrast, fast metabolizers with the IA/IA allele have a 0.36 HR for incident hypertension[288].
Melatonin demonstrates significant anti-hypertensive effects in humans in a numerous double-blind randomized placebo controlled clinical trials at 3-5 mg/d[289-299]. The average reduction in BP is 6/3 mmHg. Melatonin stimulates GABA receptors in the CNS and vascular melatonin receptors, inhibits plasma A II levels, improves endothelial function, increases NO, vasodilates, improves nocturnal dipping, lowers cortisol and is additive with ARBs. Beta blockers reduce melatonin secretion[300].
Hesperidin significantly lowered DBP 3-4 mmHg (P < 0.02) and improved microvascular endothelial reactivity in 24 obese hypertensive male subjects in a randomized, controlled crossover study over 4 wk for each of three treatment groups consuming 500 mL of orange juice, hesperidin or placebo[301].
Pomegranate juice is rich in tannins and has numerous other properties that improve vascular health and reduces the SBP by 5%-12%[302,303]. A study of 51 healthy subjects given 330 mg/d of pomegranate juice had reduction in BP of 3.14/2.33 mmHg (P < 0.001)[303]. Pomegranate juice also suppresses the postprandial increase in SBP following a high-fat meal[303]. Pomegranate juice reduces serum ACE activity by 36%, and has anti-atherogenic, antioxidant and anti-inflammatory effects[302,303]. Pomegranate juice at 50 mL/d reduced carotid IMT by 30% over one year, increased PON 83%, decreased oxLDL by 59%-90%, decreased antibodies to oxLDL by 19%, increased total antioxidant status by 130 %, reduced TGF-β, increased catalase, SOD and GPx, increased eNOS and NO and improved endothelial function[304,305]. Pomegranate juice works like an ACEI.
Grape seed extract (GSE) was administered to subjects in nine randomized trials, meta-analysis of 390 subjects and demonstrated a significant reduction in SBP of 1.54 mmHg (P < 0.02)[304,305]. Significant reduction in BP of 11/8 mmHg (P < 0.05) were seen in another dose response study with 150 to 300 mg/d of GSE over 4 wk[306]. GSE has high phenolic content which activates the PI3K/Akt signaling pathway that phosphorylates eNOS and increases NO[306,307].
Coenzyme Q10 has consistent and significant antihypertensive effects in patients with essential hypertension[1,308-317]. The literature is summarized below: (1) Compared to normotensive patients, essential hypertensive patients have a higher incidence (6 fold) of coenzyme Q10 deficiency documented by serum levels[1]; (2) Doses of 120 to 225 mg/d of coenzyme Q10, depending on the delivery method or the concomitant ingestion with a fatty meal, are necessary to achieve a therapeutic level of 3 ug/mL[1,313,314]. This dose is usually 3-5 mg/kg per day of coenzyme Q10. Oral dosing levels may become lower with nanoparticle and emulsion delivery systems intended to facilitate absorption[315]. Adverse effects have not been characterized in the literature; (3) Patients with the lowest coenzyme Q10 serum levels may have the best antihypertensive response to supplementation; (4) The average reduction in BP is about 15/10 mmHg and heart rate falls 5 beats/min based on reported studies and meta-analysis; (5) The antihypertensive effect takes time to reach its peak level at 4 wk. Then the BP remains stable during long term treatment. The antihypertensive effect is gone within two weeks after discontinuation of coenzyme Q10. The reduction in BP and SVR are correlated with the pretreatment and post treatment serum levels of coenzyme Q10. About 50% of patients respond to oral coenzyme Q10 supplementation for BP[309]; (6) Approximately 50% of patients on antihypertensive drugs may be able to stop between one and three agents. Both total dose and frequency of administration may be reduced. (7) Patients administered coenzyme Q10 with enalapril improved the 24 h ABM better than with enalapril monotherapy and also normalized endothelial function[310]; and (8) Coenzyme Q10 is a lipid phase antioxidant and free radical scavenger, increases eNOS and NO, reduces inflammation and NF-κB and improves endothelial function and vascular elasticity[1,311,312].
Other favorable effects on cardiovascular risk factors include improvement in the serum lipid profile and carbohydrate metabolism with reduced glucose and improved insulin sensitivity, reduced oxidative stress, reduced heart rate, improved myocardial LV function and oxygen delivery and decreased catecholamine levels[1,311,312].
Alpha lipoic acid (ALA) is known as thioctic acid in Europe where it is a prescription medication. It is a sulfur-containing compound with antioxidant activity both in water and lipid phases[1,70,71]. Its use is well-established in the treatment of certain forms of liver disease and in the delay of onset of peripheral neuropathy in patients with diabetes. Recent research has evaluated its potential role in the treatment of hypertension, especially as part of the MS[318-321]. In a double-blind cross over study of 36 patients over 8 wk with CHD and hypertension, 200 mg of lipoic acid with 500 mg of acetyl-L-carnitine significantly reduced BP 7/3 mmHg and increased brachial artery diameter[320]. The QUALITY study of 40 patients with DM and stage I hypertension showed significant improvements in BP, urinary albumin excretion, FMD and insulin sensitivity over 8 wk with a combination of Quinapril (40 mg/d) and lipoic acid (600 mg/d) that was greater than either alone[320]. Lipoic acid increases levels of glutathione, cysteine, vitamin C and vitamin E, inhibits NF-κB, reduces endothelin-1, tissue factor and VCAM-1, increases cAMP, downregulates CD4 immune expression on mononuclear cells, reduces oxidative stress, inflammation, reduces atherosclerosis in animal models, decreases serum aldehydes and closes calcium channels which improves vasodilation, increases NO and nitrosothiols, improves endothelial function and lowers BP[1,318-321]. Lipoic acid normalizes membrane calcium channels by providing sulfhydryl groups, decreasing cytosolic free calcium and lowers SVR. In addition, lipoic acid improves insulin sensitivity which lowers glucose and advanced glycosylation end products which improves BP control and lowers serum triglycerides. Morcos et al[321], showed stabilization of urinary albumin excretion in DM subjects given 600 mg of ALA compared to placebo for 18 mo (P < 0.05).
The recommended dose is 100 to 200 mg/d of R-lipoic acid with biotin 2-4 mg/d to prevent biotin depletion with long term use of lipoic acid. R-lipoic acid is preferred to the L isomer because of its preferred use by the mitochondria[1,32,71].
NAC: NAC and L arginine (ARG) in combination reduce endothelial activation and BP in hypertensive patients with type 2 DM[141]. Over 6 mo 24 subjects given placebo or NAC with ARG, significantly reduced both systolic and diastolic BP (P = 0.05)[141]. In addition, ox LDL, HSCRP, ICAM, VCAM, fibrinogen and PAI-1 were decreased while HDL, NO and endothelial postischemic vasodilation increased[141]. NAC increases NO via IL-1b and increases iNOS MRNA, increases glutathione by increasing cysteine levels, reduces the affinity for the AT1 receptor by disrupting disulfide groups, blocks the L type calcium channel, lowers homocysteine, and improves IMT[141,322-324]. The recommended dose is 500 to 1000 mg bid.
Hawthorne extract has been used for centuries for the treatment of hypertension, CHF and other cardiovascular diseases[325-329]. A recent four-period crossover design, dose response study in 21 subjects with prehypertension or mild hypertension over 3½ d, did not show changes in FMD or BP on standardized extract with 50 mg of oligomeric procyanidin per 250 mg extract with 1000 mg, 1500 or 2500 mg of the extract[325]. Hawthorne showed non inferiority of ACEI and diuretics in the treatment of 102 patients with NYHC II CHF over 8 wk[327]. Patients with hypertension and type 2 DM on medications for BP and DM were randomized to 1200 mg of hawthorne extract for 16 wk showed significant reductions in DBP of 2.6 mmHg (P = 0.035)[328]. Thirty six mildly hypertensive patients were administered 500 mg of hawthorne extract for 10 wk and showed a non significant trend in DBP reduction (P = 0.081) compared to placebo[329]. Hawthorne acts like an ACEI, BB, CCB and diuretic. More studies are needed to determine the efficacy, long term effects and dose of hawthorne for the treatment of hypertension.
Quercetin is an antioxidant flavonol found in apples, berries and onions that reduces BP in hypertensive individuals[330,331] but the hypotensive effects do not appear to be mediated by changes in HSCRP, TNF-α, ACE activity, ET-1, NO, vascular reactivity or FMD[330]. Quercetin is metabolized by CYP 3A4. Quercetin was administered to 12 hypertensive men at an oral dose of 1095 mg with reduction in mean BP by 5 mmHg, SBP by 7 mmHg and DBP by 3 mmHg[330]. The maximal plasma level at 10 h was 2.3 ± 1.8 μmol/L, with return to baseline levels at 17 h. Forty one pre-hypertensive and stage I hypertensive subjects were enrolled in a randomized, double-blind, placebo-controlled, crossover study with 730 mg of quercetin per day vs placebo[331]. In the stage I hypertensive patients, the BP was reduced by 7/5 mmHg (P < 0.05) but there were no changes in oxidative stress markers[331]. Quercetin administered to 93 overweight or obese subjects at 150 mg/d (plasma levels of 269 nmol/L) over 6 wk lowered SBP 2.9 mmHg in the hypertensive group and up to 3.7 mmHg in SBP in the patients 25-50 years of age[332]. The recommended dose of quercetin is 500 mg bid.
Several of the strategic combinations of nutraceutical supplements together or with anti-hypertensive drugs, have been shown to lower BP more than the medication alone: (1) Sesame with beta blockers, diuretics and nifedipine; (2) Pycnogenol with ACEI and CCB; (3) Lycopene with ACEI, CCB and diuretics; (4) ALA with ACEI or acetyl -L Carnitine; (5) Vitamin C with CCB’s; (6) NAC with arginine; (7) Garlic with ACEI, diuretics and beta blockers; (8) Coenzyme Q10 with ACEI and CCB; (9) Taurine with magnesium; (10) Potassium with all anti-hypertensive agents; and (11) Magnesium with all anti-hypertensive agents.
Many anti-hypertensive drugs may cause nutrient depletions that can actually interfere with their anti-hypertensive action or cause other metabolic adverse effects manifest through the lab or with clinical symptoms[333]. Diuretics decrease potassium, magnesium, phosphorous, sodium, chloride, folate, vitamin B6, zinc, iodine and coenzyme Q10; increase homocysteine, calcium and creatinine; and elevate serum glucose by inducing insulin resistance. Beta blockers reduce coenzyme Q10. ACEI and ARB’s reduce zinc.
Vascular biology such as endothelial and VSMD plays a primary role in the initiation and perpetuation of hypertension, CVD and TOD. Nutrient-gene interactions and epigenetics are a predominant factor in promoting beneficial or detrimental effects in cardiovascular health and hypertension. Food and nutrients can prevent, control and treat hypertension through numerous vascular biology mechanisms. Oxidative stress, inflammation and autoimmune dysfunction initiate and propagate hypertension and cardiovascular disease. There is a role for the selected use of single and component nutraceutical supplements vitamins, antioxidants and minerals in the treatment of hypertension based on scientifically controlled studies as a complement to optimal nutritional, dietary intake from food and other lifestyle modifications[333]. A clinical approach which incorporates diet, foods, nutrients, exercise, weight reduction, smoking cessation, alcohol and caffeine restriction, and other lifestyle strategies can be systematically and successfully incorporated into clinical practice (Table 4).
| Intervention category | Therapeutic intervention | Daily intake |
| Diet characteristics | DASH I, DASH II-Na+ or premier diet | Diet type |
| Sodium restriction | 1500 mg | |
| Potassium | 5000 mg | |
| Potassium/sodium ratio | > 3:1 | |
| Magnesium | 1000 mg | |
| Zinc | 50 mg | |
| Macronutrients | Protein total intake from non-animal sources, organic lean or wild animal protein, or coldwater fish | 30% of total calories, which 1.5-1.8 g/kg body weight |
| Whey protein | 30 g | |
| Soy protein (fermented sources are preferred) | 30 g | |
| Sardine muscle concentrate extract | 3 g | |
| Milk peptides | 30-60 mg | |
| Fat | 30% of total calories | |
| Omega-3 fatty acids | 2-3 g | |
| Omega-6 fatty acids | 1 g | |
| Omega-9 fatty acids | 2-4 tablespoons of olive or nut oil or 10-20 olives | |
| Saturated fatty acids from wild game, bison, or other lean meat | < 10% total calories | |
| Polyunsaturated to saturated fat ratio | < 2.0 | |
| Omega 3 to omega 6 ratio | 1.1-1.2 | |
| Synthetic trans fatty acids | None (completely remove from diet) | |
| Nuts in variety | Ad libidum | |
| Carbohydrates as primarily complex carbohydrates and fiber | 40% of total calories | |
| Oatmeal or | 60 g | |
| Oatbran or | 40 g | |
| Beta-glucan or | 3 g | |
| Psyllium | 7 g | |
| Specific foods | Garlic as fresh cloves or aged kyolic garlic | 4 fresh cloves (4 g) or 600 mg aged garlic taken twice daily |
| Sea vegetables, specifically dried wakame | 3.0-3.5 g | |
| Lycopene as tomato products, guava, watermelon, apricots, pink grapefruit, papaya or supplements | 10-20 mg | |
| Dark chocolate | 100 g | |
| Pomegranate juice or seeds | 8 ounces or one cup | |
| Sesame | 60 mg sesamin or 2.5 g sesame meal | |
| Exercise | Aerobic | 20 min daily at 4200 kJ/wk |
| Resistance | 40 min/d | |
| Weight reduction | Body mass index < 25 | Lose 1-2 pounds per week and |
| Waist circumference: | increasing the proportion of lean muscle | |
| < 35 inches for women | ||
| < 40 inches for men | ||
| Total body fat: | ||
| < 22% for women | ||
| < 16% for men | ||
| Other lifestyle recommendations | Alcohol restriction: Among the choice of alcohol red wine is preferred due to its vasoactive phytonutrients | < 20 g/d |
| Wine < 10 ounces | ||
| Beer < 24 ounces | ||
| Liquor < 2 ounces | ||
| Caffeine restriction or elimination depending on CYP 450 type | < 100 mg/d | |
| Tobacco and smoking | Stop | |
| Medical considerations | Medications which may increase blood pressure. | Minimize use when possible, such as by using disease-specific nutritional interventions |
| Supplemental foods and nutrients | Alpha lipoic acid with biotin | 100-200 mg twice daily |
| Amino acids: | ||
| Arginine | 5 g twice daily | |
| Carnitine | 1 to 2 g twice daily | |
| Taurine | 1 to 3 g twice daily | |
| Chlorogenic acids | 150-200 mg | |
| Coenzyme Q10 | 100mg once to twice daily | |
| Grape seed extract | 300 mg | |
| Hawthorne extract | 500 mg twice a day | |
| Melatonin | 2.5 mg | |
| N-acetyl cysteine | 500 mg twice a day | |
| Olive leaf extract (oleuropein) | 500 mg twice a day | |
| Pycnogenol | 200 mg | |
| Quercetin | 500 mg twice a day | |
| Resveratrol (trans) | 250 mg | |
| Vitamin B6 | 100 mg once to twice daily | |
| Vitamin C | 250-500 mg twice daily | |
| Vitamin D3 | Dose to raise 25-hydroxyvitamin | |
| D serum level to 60 ng/mL | ||
| Vitamin E as mixed tocopherols | 400 IU |
Vascular biology, endothelial and vascular smooth muscle and cardiac dysfunction play a primary role in the initiation and perpetuation of hypertension, cardiovascular disease and TOD. Nutrient-gene interactions and epigenetics are predominant factors in promoting beneficial or detrimental effects in cardiovascular health and hypertension. Macronutrients and micronutrients can prevent, control and treat hypertension through numerous mechanisms related to vascular biology.
Oxidative stress, inflammation and autoimmune dysfunction initiate and propagate hypertension and cardiovascular disease. There is a role for the selected use of single and component nutraceutical supplements, vitamins, antioxidants and minerals in the treatment of hypertension based on scientifically controlled studies which complement optimal nutrition, coupled with other lifestyle modifications.
P- Reviewer: Tsapenko MV S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Houston MC. Treatment of hypertension with nutraceuticals, vitamins, antioxidants and minerals. Expert Rev Cardiovasc Ther. 2007;5:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Eaton SB, Eaton SB, Konner MJ. Paleolithic nutrition revisited: a twelve-year retrospective on its nature and implications. Eur J Clin Nutr. 1997;51:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 164] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Houston MC, Harper KJ. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J Clin Hypertens (Greenwich). 2008;10:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Layne J, Majkova Z, Smart EJ, Toborek M, Hennig B. Caveolae: a regulatory platform for nutritional modulation of inflammatory diseases. J Nutr Biochem. 2011;22:807-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Dandona P, Ghanim H, Chaudhuri A, Dhindsa S, Kim SS. Macronutrient intake induces oxidative and inflammatory stress: potential relevance to atherosclerosis and insulin resistance. Exp Mol Med. 2010;42:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Berdanier CD. Nutrient-gene interactions. Present Knowledge in Nutrition, 7th Ed. Washington. DC: ILSI Press 1996; 574-580. |
| 7. | Talmud PJ, Waterworth DM. In-vivo and in-vitro nutrient-gene interactions. Curr Opin Lipidol. 2000;11:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Lundberg AM, Yan ZQ. Innate immune recognition receptors and damage-associated molecular patterns in plaque inflammation. Curr Opin Lipidol. 2011;22:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Zhao L, Lee JY, Hwang DH. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr Rev. 2011;69:310-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 10. | Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep. 2011;13:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Broadhurst CL. Balanced intakes of natural triglycerides for optimum nutrition: an evolutionary and phytochemical perspective. Med Hypotheses. 1997;49:247-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Eftekhari A, Mathiassen ON, Buus NH, Gotzsche O, Mulvany MJ, Christensen KL. Disproportionally impaired microvascular structure in essential hypertension. J Hypertens. 2011;29:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Touyz RM. New insights into mechanisms of hypertension. Curr Opin Nephrol Hypertens. 2012;21:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Xing T, Wang F, Li J, Wang N. Hypertension: an immunologic disease. J Hypertens. 2012;30:2440-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Giannattasio C, Cattaneo BM, Mangoni AA, Carugo S, Stella ML, Failla M, Trazzi S, Sega R, Grassi G, Mancia G. Cardiac and vascular structural changes in normotensive subjects with parental hypertension. J Hypertens. 1995;13:259-264. [PubMed] |
| 16. | Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J Hypertens. 2010;28:2053-2060. [PubMed] |
| 17. | Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich). 2011;13:621-627. [PubMed] |
| 18. | Al-Ghamdi A. Role of herpes simplex virus-1, cytomegalovirus and Epstein-Barr virus in atherosclerosis. Pak J Pharm Sci. 2012;25:89-97. [PubMed] |
| 19. | Kotronias D, Kapranos N. Herpes simplex virus as a determinant risk factor for coronary artery atherosclerosis and myocardial infarction. In Vivo. 2005;19:351-357. [PubMed] |
| 20. | Grahame-Clarke C, Chan NN, Andrew D, Ridgway GL, Betteridge DJ, Emery V, Colhoun HM, Vallance P. Human cytomegalovirus seropositivity is associated with impaired vascular function. Circulation. 2003;108:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Nayak DU, Karmen C, Frishman WH, Vakili BA. Antioxidant vitamins and enzymatic and synthetic oxygen-derived free radical scavengers in the prevention and treatment of cardiovascular disease. Heart Dis. 2001;3:28-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovasc Ther. 2010;28:e20-e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Kitiyakara C, Wilcox CS. Antioxidants for hypertension. Curr Opin Nephrol Hypertens. 1998;7:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, Corrocher R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens. 1998;16:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Tse WY, Maxwell SR, Thomason H, Blann A, Thorpe GH, Waite M, Holder R. Antioxidant status in controlled and uncontrolled hypertension and its relationship to endothelial damage. J Hum Hypertens. 1994;8:843-849. [PubMed] |
| 26. | Mansego ML, Solar Gde M, Alonso MP, Martínez F, Sáez GT, Escudero JC, Redón J, Chaves FJ. Polymorphisms of antioxidant enzymes, blood pressure and risk of hypertension. J Hypertens. 2011;29:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Galley HF, Thornton J, Howdle PD, Walker BE, Webster NR. Combination oral antioxidant supplementation reduces blood pressure. Clin Sci (Lond). 1997;92:361-365. [PubMed] |
| 28. | Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 969] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 29. | Saez G, Tormos MC, Giner V, Lorano JV, Chaves FJ, Armengod ME, Redon J. P-653: Oxidative stress and enzymatic antioxidant mechanisms in essential hypertension. Am J Hypertens. 2001;14:248A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Nishihara M, Hirooka Y, Matsukawa R, Kishi T, Sunagawa K. Oxidative stress in the rostral ventrolateral medulla modulates excitatory and inhibitory inputs in spontaneously hypertensive rats. J Hypertens. 2012;30:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Konno S, Hirooka Y, Kishi T, Sunagawa K. Sympathoinhibitory effects of telmisartan through the reduction of oxidative stress in the rostral ventrolateral medulla of obesity-induced hypertensive rats. J Hypertens. 2012;30:1992-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Ghanem FA, Movahed A. Inflammation in high blood pressure: a clinician perspective. J Am Soc Hypertens. 2007;1:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Amer MS, Elawam AE, Khater MS, Omar OH, Mabrouk RA, Taha HM. Association of high-sensitivity C-reactive protein with carotid artery intima-media thickness in hypertensive older adults. J Am Soc Hypertens. 2011;5:395-400. [PubMed] |
| 34. | Vongpatanasin W, Thomas GD, Schwartz R, Cassis LA, Osborne-Lawrence S, Hahner L, Gibson LL, Black S, Samols D, Shaul PW. C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation. 2007;115:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Razzouk L, Muntner P, Bansilal S, Kini AS, Aneja A, Mozes J, Ivan O, Jakkula M, Sharma S, Farkouh ME. C-reactive protein predicts long-term mortality independently of low-density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention. Am Heart J. 2009;158:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Kvakan H, Luft FC, Muller DN. Role of the immune system in hypertensive target organ damage. Trends Cardiovasc Med. 2009;19:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Rodríguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2012;39:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Tian N, Penman AD, Mawson AR, Manning RD, Flessner MF. Association between circulating specific leukocyte types and blood pressure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Hypertens. 2010;4:272-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Muller DN, Kvakan H, Luft FC. Immune-related effects in hypertension and target-organ damage. Curr Opin Nephrol Hypertens. 2011;20:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Marketou ME, Kontaraki JE, Zacharis EA, Kochiadakis GE, Giaouzaki A, Chlouverakis G, Vardas PE. TLR2 and TLR4 gene expression in peripheral monocytes in nondiabetic hypertensive patients: the effect of intensive blood pressure-lowering. J Clin Hypertens (Greenwich). 2012;14:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Luft FC. Neural regulation of the immune system modulates hypertension-induced target-organ damage. J Am Soc Hypertens. 2012;6:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Herrada AA, Campino C, Amador CA, Michea LF, Fardella CE, Kalergis AM. Aldosterone as a modulator of immunity: implications in the organ damage. J Hypertens. 2011;29:1684-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Colussi G, Catena C, Sechi LA. Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J Hypertens. 2013;31:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117-1124. [PubMed] |
| 45. | Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3-10. [PubMed] |
| 46. | Sun B, Williams JS, Svetkey LP, Kolatkar NS, Conlin PR. Beta2-adrenergic receptor genotype affects the renin-angiotensin-aldosterone system response to the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am J Clin Nutr. 2010;92:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Chen Q, Turban S, Miller ER, Appel LJ. The effects of dietary patterns on plasma renin activity: results from the Dietary Approaches to Stop Hypertension trial. J Hum Hypertens. 2012;26:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Al-Solaiman Y, Jesri A, Zhao Y, Morrow JD, Egan BM. Low-Sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J Hum Hypertens. 2009;23:826-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Lin PH, Allen JD, Li YJ, Yu M, Lien LF, Svetkey LP. Blood Pressure-Lowering Mechanisms of the DASH Dietary Pattern. J Nutr Metab. 2012;2012:472396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Kotchen TA, McCarron DA. Dietary electrolytes and blood pressure: a statement for healthcare professionals from the American Heart Association Nutrition Committee. Circulation. 1998;98:613-617. [PubMed] |
| 51. | Cutler JA, Follmann D, Allender PS. Randomized trials of sodium reduction: an overview. Am J Clin Nutr. 1997;65:643S-651S. [PubMed] |
| 52. | Svetkey LP, Sacks FM, Obarzanek E, Vollmer WM, Appel LJ, Lin PH, Karanja NM, Harsha DW, Bray GA, Aickin M. The DASH Diet, Sodium Intake and Blood Pressure Trial (DASH-sodium): rationale and design. DASH-Sodium Collaborative Research Group. J Am Diet Assoc. 1999;99:S96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Kawada T, Suzuki S. Attention of salt awareness to prevent hypertension in the young. J Clin Hypertens (Greenwich). 2011;13:933-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 54. | Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 572] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 55. | Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734-1737. [PubMed] |
| 56. | Tomonari T, Fukuda M, Miura T, Mizuno M, Wakamatsu TY, Ichikawa T, Miyagi S, Shirasawa Y, Ito A, Yoshida A. Is salt intake an independent risk factor of stroke mortality Demographic analysis by regions in Japan. J Am Soc Hypertens. 2011;5:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Kanbay M, Chen Y, Solak Y, Sanders PW. Mechanisms and consequences of salt sensitivity and dietary salt intake. Curr Opin Nephrol Hypertens. 2011;20:37-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Dubach JM, Das S, Rosenzweig A, Clark HA. Visualizing sodium dynamics in isolated cardiomyocytes using fluorescent nanosensors. Proc Natl Acad Sci USA. 2009;106:16145-16150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmüller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci USA. 2009;106:2829-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 60. | Oberleithner H, Riethmüller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA. 2007;104:16281-16286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 381] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 61. | Fels J, Oberleithner H, Kusche-Vihrog K. Ménage à trois: aldosterone, sodium and nitric oxide in vascular endothelium. Biochim Biophys Acta. 2010;1802:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Oberleithner H, Kusche-Vihrog K, Schillers H. Endothelial cells as vascular salt sensors. Kidney Int. 2010;77:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Kusche-Vihrog K, Callies C, Fels J, Oberleithner H. The epithelial sodium channel (ENaC): Mediator of the aldosterone response in the vascular endothelium. Steroids. 2010;75:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Fels J, Callies C, Kusche-Vihrog K, Oberleithner H. Nitric oxide release follows endothelial nanomechanics and not vice versa. Pflugers Arch. 2010;460:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Callies C, Fels J, Liashkovich I, Kliche K, Jeggle P, Kusche-Vihrog K, Oberleithner H. Membrane potential depolarization decreases the stiffness of vascular endothelial cells. J Cell Sci. 2011;124:1936-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Kusche-Vihrog K, Urbanova K, Blanqué A, Wilhelmi M, Schillers H, Kliche K, Pavenstädt H, Brand E, Oberleithner H. C-reactive protein makes human endothelium stiff and tight. Hypertension. 2011;57:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Foulquier S, Dupuis F, Perrin-Sarrado C, Maguin Gaté K, Merhi-Soussi F, Liminana P, Kwan YW, Capdeville-Atkinson C, Lartaud I, Atkinson J. High salt intake abolishes AT(2)-mediated vasodilation of pial arterioles in rats. J Hypertens. 2011;29:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Kusche-Vihrog K, Oberleithner H. An emerging concept of vascular salt sensitivity. F1000 Biol Rep. 2012;4:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Popov S, Silveira A, Wågsäter D, Takemori H, Oguro R, Matsumoto S, Sugimoto K, Kamide K, Hirose T, Satoh M. Salt-inducible kinase 1 influences Na(+),K(+)-ATPase activity in vascular smooth muscle cells and associates with variations in blood pressure. J Hypertens. 2011;29:2395-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Houston MC. Nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Prog Cardiovasc Dis. 2005;47:396-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Houston MC. Nutrition and nutraceutical supplements in the treatment of hypertension. Expert Rev Cardiovasc Ther. 2010;8:821-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Messerli FH, Schmieder RE, Weir MR. Salt. A perpetrator of hypertensive target organ disease. Arch Intern Med. 1997;157:2449-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 306] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 357] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens. 2011;29:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Whelton PK, He J. Potassium in preventing and treating high blood pressure. Semin Nephrol. 1999;19:494-499. [PubMed] |
| 77. | Gu D, He J, Wu X, Duan X, Whelton PK. Effect of potassium supplementation on blood pressure in Chinese: a randomized, placebo-controlled trial. J Hypertens. 2001;19:1325-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | He J, Gu D, Kelly TN, Hixson JE, Rao DC, Jaquish CE, Chen J, Zhao Q, Gu C, Huang J. Genetic variants in the renin-angiotensin-aldosterone system and blood pressure responses to potassium intake. J Hypertens. 2011;29:1719-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 354] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 80. | Widman L, Wester PO, Stegmayr BK, Wirell M. The dose-dependent reduction in blood pressure through administration of magnesium. A double blind placebo controlled cross-over study. Am J Hypertens. 1993;6:41-45. [PubMed] |
| 81. | Laurant P, Touyz RM. Physiological and pathophysiological role of magnesium in the cardiovascular system: implications in hypertension. J Hypertens. 2000;18:1177-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Houston M. The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens (Greenwich). 2011;13:843-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 83. | Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated. Nutr Rev. 2012;70:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 84. | Song Y, Liu S. Magnesium for cardiovascular health: time for intervention. Am J Clin Nutr. 2012;95:269-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Kupetsky-Rincon EA, Uitto J. Magnesium: novel applications in cardiovascular disease--a review of the literature. Ann Nutr Metab. 2012;61:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Cunha AR, Umbelino B, Correia ML, Neves MF. Magnesium and vascular changes in hypertension. Int J Hypertens. 2012;2012:754250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr. 2012;66:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 88. | McCarron DA. Role of adequate dietary calcium intake in the prevention and management of salt-sensitive hypertension. Am J Clin Nutr. 1997;65:712S-716S. [PubMed] |
| 89. | Resnick LM. Calcium metabolism in hypertension and allied metabolic disorders. Diabetes Care. 1991;14:505-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | García Zozaya JL, Padilla Viloria M. [Alterations of calcium, magnesium, and zinc in essential hypertension: their relation to the renin-angiotensin-aldosterone system]. Invest Clin. 1997;38 Suppl 2:27-40. [PubMed] |
| 91. | Carpenter WE, Lam D, Toney GM, Weintraub NL, Qin Z. Zinc, copper, and blood pressure: Human population studies. Med Sci Monit. 2013;19:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Shahbaz AU, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC, McGee JE, Weber KT. Fibrosis in hypertensive heart disease: molecular pathways and cardioprotective strategies. J Hypertens. 2010;28 Suppl 1:S25-S32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Bergomi M, Rovesti S, Vinceti M, Vivoli R, Caselgrandi E, Vivoli G. Zinc and copper status and blood pressure. J Trace Elem Med Biol. 1997;11:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Stamler J, Elliott P, Kesteloot H, Nichols R, Claeys G, Dyer AR, Stamler R. Inverse relation of dietary protein markers with blood pressure. Findings for 10,020 men and women in the INTERSALT Study. INTERSALT Cooperative Research Group. INTERnational study of SALT and blood pressure. Circulation. 1996;94:1629-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G, van ‘t Veer P, Geleijnse JM. Dietary protein and blood pressure: a systematic review. PLoS One. 2010;5:e12102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 96. | Jenkins DJ, Kendall CW, Faulkner DA, Kemp T, Marchie A, Nguyen TH, Wong JM, de Souza R, Emam A, Vidgen E. Long-term effects of a plant-based dietary portfolio of cholesterol-lowering foods on blood pressure. Eur J Clin Nutr. 2008;62:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Elliott P, Dennis B, Dyer AR. Relation of dietary protein (total, vegetable, animal) to blood pressure: INTERMAP epidemiologic study. Presented at the 18th Scientific Meeting of the International Society of Hypertension. Chicago: IL; 20-24. [PubMed] |
| 98. | Rebholz CM, Friedman EE, Powers LJ, Arroyave WD, He J, Kelly TN. Dietary protein intake and blood pressure: a meta-analysis of randomized controlled trials. Am J Epidemiol. 2012;176 Suppl 7:S27-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 99. | Larsson SC, Virtamo J, Wolk A. Dietary protein intake and risk of stroke in women. Atherosclerosis. 2012;224:247-251. [PubMed] |
| 100. | He J, Wofford MR, Reynolds K, Chen J, Chen CS, Myers L, Minor DL, Elmer PJ, Jones DW, Whelton PK. Effect of dietary protein supplementation on blood pressure: a randomized, controlled trial. Circulation. 2011;124:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Teunissen-Beekman KF, Dopheide J, Geleijnse JM, Bakker SJ, Brink EJ, de Leeuw PW, van Baak MA. Protein supplementation lowers blood pressure in overweight adults: effect of dietary proteins on blood pressure (PROPRES), a randomized trial. Am J Clin Nutr. 2012;95:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | FitzGerald RJ, Murray BA, Walsh DJ. Hypotensive peptides from milk proteins. J Nutr. 2004;134:980S-988S. [PubMed] |
| 103. | Pins JJ, Keenan JM. Effects of whey peptides on cardiovascular disease risk factors. J Clin Hypertens (Greenwich). 2006;8:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 104. | Aihara K, Kajimoto O, Hirata H, Takahashi R, Nakamura Y. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr. 2005;24:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 105. | Germino FW, Neutel J, Nonaka M, Hendler SS. The impact of lactotripeptides on blood pressure response in stage 1 and stage 2 hypertensives. J Clin Hypertens (Greenwich). 2010;12:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Geleijnse JM, Engberink MF. Lactopeptides and human blood pressure. Curr Opin Lipidol. 2010;21:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Cicero AF, Aubin F, Azais-Braesco V, Borghi C. Do the lactotripeptides isoleucine-proline-proline and valine-proline-proline reduce systolic blood pressure in European subjects A meta-analysis of randomized controlled trials. Am J Hypertens. 2013;26:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 108. | Usinger L, Reimer C, Ibsen H. Fermented milk for hypertension. Cochrane Database Syst Rev. 2012;4:CD008118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 109. | Ricci-Cabello I, Herrera MO, Artacho R. Possible role of milk-derived bioactive peptides in the treatment and prevention of metabolic syndrome. Nutr Rev. 2012;70:241-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 110. | Jauhiainen T, Niittynen L, Orešič M, Järvenpää S, Hiltunen TP, Rönnback M, Vapaatalo H, Korpela R. Effects of long-term intake of lactotripeptides on cardiovascular risk factors in hypertensive subjects. Eur J Clin Nutr. 2012;66:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Pins JJ, Keenan JM. The antihypertensive effects of a hydrolyzed whey protein isolate supplement. Cardiovasc Drugs Ther. 2002;16:68. |
| 112. | Pal S, Radavelli-Bagatini S. The effects of whey protein on cardiometabolic risk factors. Obes Rev. 2013;14:324-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 113. | Zhu CF, Li GZ, Peng HB, Zhang F, Chen Y, Li Y. Therapeutic effects of marine collagen peptides on Chinese patients with type 2 diabetes mellitus and primary hypertension. Am J Med Sci. 2010;340:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | De Leo F, Panarese S, Gallerani R, Ceci LR. Angiotensin converting enzyme (ACE) inhibitory peptides: production and implementation of functional food. Curr Pharm Des. 2009;15:3622-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 115. | Lordan S, Ross RP, Stanton C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar Drugs. 2011;9:1056-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 508] [Cited by in RCA: 347] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 116. | Fujita H, Yoshikawa M. LKPNM: a prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology. 1999;44:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 117. | Kawasaki T, Seki E, Osajima K, Yoshida M, Asada K, Matsui T, Osajima Y. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J Hum Hypertens. 2000;14:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 118. | Kawasaki T, Jun CJ, Fukushima Y, Kegai K, Seki E, Osajima K, Itoh K, Matsui T, Matsumoto K. [Antihypertensive effect and safety evaluation of vegetable drink with peptides derived from sardine protein hydrolysates on mild hypertensive, high-normal and normal blood pressure subjects]. Fukuoka Igaku Zasshi. 2002;93:208-218. [PubMed] |
| 119. | Yang G, Shu XO, Jin F, Zhang X, Li HL, Li Q, Gao YT, Zheng W. Longitudinal study of soy food intake and blood pressure among middle-aged and elderly Chinese women. Am J Clin Nutr. 2005;81:1012-1017. [PubMed] |
| 120. | Dong JY, Tong X, Wu ZW, Xun PC, He K, Qin LQ. Effect of soya protein on blood pressure: a meta-analysis of randomised controlled trials. Br J Nutr. 2011;106:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 121. | Teede HJ, Giannopoulos D, Dalais FS, Hodgson J, McGrath BP. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J Am Coll Nutr. 2006;25:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 122. | Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007;167:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 123. | Rosero Arenas MA, Rosero Arenas E, Portaceli Armiñana MA, García García MA. [Usefulness of phyto-oestrogens in reduction of blood pressure. Systematic review and meta-analysis]. Aten Primaria. 2008;40:177-186. [PubMed] |
| 124. | Nasca MM, Zhou JR, Welty FK. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am J Cardiol. 2008;102:84-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 125. | He J, Gu D, Wu X, Chen J, Duan X, Chen J, Whelton PK. Effect of soybean protein on blood pressure: a randomized, controlled trial. Ann Intern Med. 2005;143:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 126. | Hasler CM, Kundrat S, Wool D. Functional foods and cardiovascular disease. Curr Atheroscler Rep. 2000;2:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Tikkanen MJ, Adlercreutz H. Dietary soy-derived isoflavone phytoestrogens. Could they have a role in coronary heart disease prevention. Biochem Pharmacol. 2000;60:1-5. [PubMed] |
| 128. | Begg DP, Sinclair AJ, Stahl LA, Garg ML, Jois M, Weisinger RS. Dietary protein level interacts with omega-3 polyunsaturated fatty acid deficiency to induce hypertension. Am J Hypertens. 2010;23:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20 Suppl 12:S60-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 316] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 130. | Sonmez A, Celebi G, Erdem G, Tapan S, Genc H, Tasci I, Ercin CN, Dogru T, Kilic S, Uckaya G. Plasma apelin and ADMA Levels in patients with essential hypertension. Clin Exp Hypertens. 2010;32:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 131. | Michell DL, Andrews KL, Chin-Dusting JP. Endothelial dysfunction in hypertension: the role of arginase. Front Biosci (Schol Ed). 2011;3:946-960. [PubMed] |
| 132. | Rajapakse NW, Mattson DL. Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol. 2009;36:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 133. | Tsioufis C, Dimitriadis K, Andrikou E, Thomopoulos C, Tsiachris D, Stefanadi E, Mihas C, Miliou A, Papademetriou V, Stefanadis C. ADMA, C-reactive protein, and albuminuria in untreated essential hypertension: a cross-sectional study. Am J Kidney Dis. 2010;55:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 134. | Rajapakse NW, Mattson DL. Role of cellular L-arginine uptake and nitric oxide production on renal blood flow and arterial pressure regulation. Curr Opin Nephrol Hypertens. 2013;22:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 135. | Ruiz-Hurtado G, Delgado C. Nitric oxide pathway in hypertrophied heart: new therapeutic uses of nitric oxide donors. J Hypertens. 2010;28 Suppl 1:S56-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 136. | Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am J Hypertens. 2000;13:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 137. | Facchinetti F, Saade GR, Neri I, Pizzi C, Longo M, Volpe A. L-arginine supplementation in patients with gestational hypertension: a pilot study. Hypertens Pregnancy. 2007;26:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 138. | Neri I, Monari F, Sgarbi L, Berardi A, Masellis G, Facchinetti F. L-arginine supplementation in women with chronic hypertension: impact on blood pressure and maternal and neonatal complications. J Matern Fetal Neonatal Med. 2010;23:1456-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 139. | Martina V, Masha A, Gigliardi VR, Brocato L, Manzato E, Berchio A, Massarenti P, Settanni F, Della Casa L, Bergamini S. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care. 2008;31:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 140. | Ast J, Jablecka A, Bogdanski P, Smolarek I, Krauss H, Chmara E. Evaluation of the antihypertensive effect of L-arginine supplementation in patients with mild hypertension assessed with ambulatory blood pressure monitoring. Med Sci Monit. 2010;16:CR266-CR271. [PubMed] |
| 141. | Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 142. | Miller GD, Marsh AP, Dove RW, Beavers D, Presley T, Helms C, Bechtold E, King SB, Kim-Shapiro D. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutr Res. 2012;32:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 143. | Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 144. | Miguel-Carrasco JL, Monserrat MT, Mate A, Vázquez CM. Comparative effects of captopril and l-carnitine on blood pressure and antioxidant enzyme gene expression in the heart of spontaneously hypertensive rats. Eur J Pharmacol. 2010;632:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 145. | Zambrano S, Blanca AJ, Ruiz-Armenta MV, Miguel-Carrasco JL, Arévalo M, Vázquez MJ, Mate A, Vázquez CM. L-Carnitine protects against arterial hypertension-related cardiac fibrosis through modulation of PPAR-γ expression. Biochem Pharmacol. 2013;85:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 146. | Vilskersts R, Kuka J, Svalbe B, Cirule H, Liepinsh E, Grinberga S, Kalvinsh I, Dambrova M. Administration of L-carnitine and mildronate improves endothelial function and decreases mortality in hypertensive Dahl rats. Pharmacol Rep. 2011;63:752-762. [PubMed] |
| 147. | Mate A, Miguel-Carrasco JL, Monserrat MT, Vázquez CM. Systemic antioxidant properties of L-carnitine in two different models of arterial hypertension. J Physiol Biochem. 2010;66:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 148. | Digiesi V, Cantini F, Bisi G, Guarino G, Brodbeck B. L-carnitine adjuvant therapy in essential hypertension. Clin Ter. 1994;144:391-395. [PubMed] |
| 149. | Ghidini O, Azzurro M, Vita G, Sartori G. Evaluation of the therapeutic efficacy of L-carnitine in congestive heart failure. Int J Clin Pharmacol Ther Toxicol. 1988;26:217-220. [PubMed] |
| 150. | Digiesi V, Palchetti R, Cantini F. [The benefits of L-carnitine therapy in essential arterial hypertension with diabetes mellitus type II]. Minerva Med. 1989;80:227-231. [PubMed] |
| 151. | Ruggenenti P, Cattaneo D, Loriga G, Ledda F, Motterlini N, Gherardi G, Orisio S, Remuzzi G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: effects of acetyl-L-carnitine therapy. Hypertension. 2009;54:567-574. [PubMed] |
| 152. | Mate A, Miguel-Carrasco JL, Vázquez CM. The therapeutic prospects of using L-carnitine to manage hypertension-related organ damage. Drug Discov Today. 2010;15:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Korkmaz S, Yıldız G, Kılıçlı F, Yılmaz A, Aydın H, Içağasıoğlu S, Candan F. [Low L-carnitine levels: can it be a cause of nocturnal blood pressure changes in patients with type 2 diabetes mellitus]. Anadolu Kardiyol Derg. 2011;11:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 154. | Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101-163. [PubMed] |
| 155. | Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation. 1987;75:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 156. | Huxtable RJ, Sebring LA. Cardiovascular actions of taurine. Prog Clin Biol Res. 1983;125:5-37. [PubMed] |
| 157. | Feng Y, Li J, Yang J, Yang Q, Lv Q, Gao Y, Hu J. Synergistic effects of taurine and L-arginine on attenuating insulin resistance hypertension. Adv Exp Med Biol. 2013;775:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 158. | Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Pearte C, Costa M, Chen Y. Serum taurine and risk of coronary heart disease: a prospective, nested case-control study. Eur J Nutr. 2013;52:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 159. | Abebe W, Mozaffari MS. Role of taurine in the vasculature: an overview of experimental and human studies. Am J Cardiovasc Dis. 2011;1:293-311. [PubMed] |
| 160. | Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17 Suppl 1:S6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 161. | Yamori Y, Taguchi T, Mori H, Mori M. Low cardiovascular risks in the middle aged males and females excreting greater 24-hour urinary taurine and magnesium in 41 WHO-CARDIAC study populations in the world. J Biomed Sci. 2010;17 Suppl 1:S21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 162. | Tanabe Y, Urata H, Kiyonaga A, Ikeda M, Tanaka H, Shindo M, Arakawa K. Changes in serum concentrations of taurine and other amino acids in clinical antihypertensive exercise therapy. Clin Exp Hypertens A. 1989;11:149-165. [PubMed] |
| 163. | Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 268] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 164. | Bønaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromsø study. N Engl J Med. 1990;322:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 277] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 165. | Mori TA, Burke V, Puddey I, Irish A, Cowpland CA, Beilin L, Dogra G, Watts GF. The effects of [omega]3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: a randomized controlled trial. J Hypertens. 2009;27:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 166. | Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ, Carnethon MR, Daviglus ML, He K, Moag-Stahlberg A, Rodriguez BL. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension. 2007;50:313-319. [PubMed] |
| 167. | Mori TA. Omega-3 fatty acids and hypertension in humans. Clin Exp Pharmacol Physiol. 2006;33:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 168. | Noreen EE, Brandauer J. The effects of supplemental fish oil on blood pressure and morning cortisol in normotensive adults: a pilot study. J Complement Integr Med. 2012;9:1553-3840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 169. | Bhise A, Krishnan PV, Aggarwal R, Gaiha M, Bhattacharjee J. Effect of low-dose omega-3 fatty acids substitution on blood pressure, hyperinsulinemia and dyslipidemia in Indians with essential hypertension: A pilot study. Indian J Clin Biochem. 2005;20:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 170. | Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Br J Nutr. 2012;107 Suppl 2:S195-S200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 171. | Huang T, Shou T, Cai N, Wahlqvist ML, Li D. Associations of plasma n-3 polyunsaturated fatty acids with blood pressure and cardiovascular risk factors among Chinese. Int J Food Sci Nutr. 2012;63:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 172. | Sagara M, Njelekela M, Teramoto T, Taguchi T, Mori M, Armitage L, Birt N, Birt C, Yamori Y. Effects of docosahexaenoic Acid supplementation on blood pressure, heart rate, and serum lipids in Scottish men with hypertension and hypercholesterolemia. Int J Hypertens. 2011;2011:809198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 173. | Passfall J, Philipp T, Woermann F, Quass P, Thiede M, Haller H. Different effects of eicosapentaenoic acid and olive oil on blood pressure, intracellular free platelet calcium, and plasma lipids in patients with essential hypertension. Clin Investig. 1993;71:628-633. [PubMed] |
| 174. | Liu JC, Conklin SM, Manuck SB, Yao JK, Muldoon MF. Long-chain omega-3 fatty acids and blood pressure. Am J Hypertens. 2011;24:1121-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 175. | Engler MM, Schambelan M, Engler MB, Ball DL, Goodfriend TL. Effects of dietary gamma-linolenic acid on blood pressure and adrenal angiotensin receptors in hypertensive rats. Proc Soc Exp Biol Med. 1998;218:234-237. [PubMed] |
| 176. | Chin JP. Marine oils and cardiovascular reactivity. Prostaglandins Leukot Essent Fatty Acids. 1994;50:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 177. | Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 178. | Ferrara LA, Raimondi AS, d’Episcopo L, Guida L, Dello Russo A, Marotta T. Olive oil and reduced need for antihypertensive medications. Arch Intern Med. 2000;160:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 179. | Alonso A, Ruiz-Gutierrez V, Martínez-González MA. Monounsaturated fatty acids, olive oil and blood pressure: epidemiological, clinical and experimental evidence. Public Health Nutr. 2006;9:251-257. [PubMed] |
| 180. | Terés S, Barceló-Coblijn G, Benet M, Alvarez R, Bressani R, Halver JE, Escribá PV. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc Natl Acad Sci USA. 2008;105:13811-13816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 181. | Cherif S, Rahal N, Haouala M, Hizaoui B, Dargouth F, Gueddiche M, Kallel Z, Balansard G, Boukef K. [A clinical trial of a titrated Olea extract in the treatment of essential arterial hypertension]. J Pharm Belg. 1996;51:69-71. [PubMed] |
| 182. | Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2004;80:1012-1018. [PubMed] |
| 183. | Alonso A, Martínez-González MA. Olive oil consumption and reduced incidence of hypertension: the SUN study. Lipids. 2004;39:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 184. | Perrinjaquet-Moccetti T, Busjahn A, Schmidlin C, Schmidt A, Bradl B, Aydogan C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytother Res. 2008;22:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 185. | Moreno-Luna R, Muñoz-Hernandez R, Miranda ML, Costa AF, Jimenez-Jimenez L, Vallejo-Vaz AJ, Muriana FJ, Villar J, Stiefel P. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am J Hypertens. 2012;25:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 186. | Thomsen C, Rasmussen OW, Hansen KW, Vesterlund M, Hermansen K. Comparison of the effects on the diurnal blood pressure, glucose, and lipid levels of a diet rich in monounsaturated fatty acids with a diet rich in polyunsaturated fatty acids in type 2 diabetic subjects. Diabet Med. 1995;12:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 187. | Perona JS, Cañizares J, Montero E, Sánchez-Domínguez JM, Catalá A, Ruiz-Gutiérrez V. Virgin olive oil reduces blood pressure in hypertensive elderly subjects. Clin Nutr. 2004;23:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 188. | Perona JS, Montero E, Sánchez-Domínguez JM, Cañizares J, Garcia M, Ruiz-Gutiérrez V. Evaluation of the effect of dietary virgin olive oil on blood pressure and lipid composition of serum and low-density lipoprotein in elderly type 2 diabetic subjects. J Agric Food Chem. 2009;57:11427-11433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 189. | Susalit E, Agus N, Effendi I, Tjandrawinata RR, Nofiarny D, Perrinjaquet-Moccetti T, Verbruggen M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 190. | López-Miranda J, Pérez-Jiménez F, Ros E, De Caterina R, Badimón L, Covas MI, Escrich E, Ordovás JM, Soriguer F, Abiá R. Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis. 2010;20:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 347] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 191. | Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res. 2010;107:540-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 192. | Scheffler A, Rauwald HW, Kampa B, Mann U, Mohr FW, Dhein S. Olea europaea leaf extract exerts L-type Ca(2+) channel antagonistic effects. J Ethnopharmacol. 2008;120:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 193. | Papamichael CM, Karatzi KN, Papaioannou TG, Karatzis EN, Katsichti P, Sideris V, Zakopoulos N, Zampelas A, Lekakis JP. Acute combined effects of olive oil and wine on pressure wave reflections: another beneficial influence of the Mediterranean diet antioxidants. J Hypertens. 2008;26:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 194. | He J, Whelton PK. Effect of dietary fiber and protein intake on blood pressure: a review of epidemiologic evidence. Clin Exp Hypertens. 1999;21:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 195. | Pruijm M, Wuerzer G, Forni V, Bochud M, Pechère-Bertschi A, Burnier M. [Nutrition and hypertension: more than table salt]. Rev Med Suisse. 2010;6:1715-1716, 1718-1720. [PubMed] |
| 196. | Sherman DL, Keaney JF, Biegelsen ES, Duffy SJ, Coffman JD, Vita JA. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension. 2000;35:936-941. [PubMed] |
| 197. | Ness AR, Khaw KT, Bingham S, Day NE. Vitamin C status and blood pressure. J Hypertens. 1996;14:503-508. [PubMed] |
| 198. | Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Vita JA. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 199. | Enstrom JE, Kanim LE, Klein MA. Vitamin C intake and mortality among a sample of the United States population. Epidemiology. 1992;3:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 299] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 200. | Block G, Jensen CD, Norkus EP, Hudes M, Crawford PB. Vitamin C in plasma is inversely related to blood pressure and change in blood pressure during the previous year in young Black and White women. Nutr J. 2008;7:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 201. | Hatzitolios A, Iliadis F, Katsiki N, Baltatzi M. Is the anti-hypertensive effect of dietary supplements via aldehydes reduction evidence based A systematic review. Clin Exp Hypertens. 2008;30:628-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 202. | Mahajan AS, Babbar R, Kansal N, Agarwal SK, Ray PC. Antihypertensive and antioxidant action of amlodipine and vitamin C in patients of essential hypertension. J Clin Biochem Nutr. 2007;40:141-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 203. | Leclerc PC, Proulx CD, Arguin G, Bélanger S, Gobeil F, Escher E, Leduc R, Guillemette G. Ascorbic acid decreases the binding affinity of the AT1 receptor for angiotensin II. Am J Hypertens. 2008;21:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 204. | Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, Salvetti A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 205. | Sato K, Dohi Y, Kojima M, Miyagawa K, Takase H, Katada E, Suzuki S. Effects of ascorbic acid on ambulatory blood pressure in elderly patients with refractory hypertension. Arzneimittelforschung. 2006;56:535-540. [PubMed] |
| 206. | Block G, Mangels AR, Norkus EP, Patterson BH, Levander OA, Taylor PR. Ascorbic acid status and subsequent diastolic and systolic blood pressure. Hypertension. 2001;37:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 207. | McRae MP. Is vitamin C an effective antihypertensive supplement A review and analysis of the literature. J Chiropr Med. 2006;5:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 208. | Simon JA. Vitamin C and cardiovascular disease: a review. J Am Coll Nutr. 1992;11:107-125. [PubMed] |
| 209. | Ness AR, Chee D, Elliott P. Vitamin C and blood pressure--an overview. J Hum Hypertens. 1997;11:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 210. | Trout DL. Vitamin C and cardiovascular risk factors. Am J Clin Nutr. 1991;53:322S-325S. [PubMed] |
| 211. | Fulwood R, Johnson CL, Bryner JD, National Center for Health Statistics . Hematological and Nutritional Biochemistry Reference Data for Persons 6 Months-74 Years of Age: United States, 1976-1980. Washington: DC; US Public Health Service, 1982 Vital and Health Statistics series 11, No. 232, DHHS publication No. (PHS) 83-1682; . |
| 212. | Ward NC, Wu JH, Clarke MW, Puddey IB, Burke V, Croft KD, Hodgson JM. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J Hypertens. 2007;25:227-234. [PubMed] |
| 213. | Murray ED, Wechter WJ, Kantoci D, Wang WH, Pham T, Quiggle DD, Gibson KM, Leipold D, Anner BM. Endogenous natriuretic factors 7: biospecificity of a natriuretic gamma-tocopherol metabolite LLU-alpha. J Pharmacol Exp Ther. 1997;282:657-662. [PubMed] |
| 214. | Gray B, Swick J, Ronnenberg AG. Vitamin E and adiponectin: proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr Rev. 2011;69:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 215. | Lind L, Hänni A, Lithell H, Hvarfner A, Sörensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995;8:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 216. | Bednarski R, Donderski R, Manitius J. [Role of vitamin D3 in arterial blood pressure control]. Pol Merkur Lekarski. 2007;23:307-310. [PubMed] |
| 217. | Ngo DT, Sverdlov AL, McNeil JJ, Horowitz JD. Does vitamin D modulate asymmetric dimethylarginine and C-reactive protein concentrations. Am J Med. 2010;123:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 218. | Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 579] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 219. | Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 486] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 220. | Motiwala SR, Wang TJ. Vitamin D and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 221. | Bhandari SK, Pashayan S, Liu IL, Rasgon SA, Kujubu DA, Tom TY, Sim JJ. 25-hydroxyvitamin D levels and hypertension rates. J Clin Hypertens (Greenwich). 2011;13:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 222. | Kienreich K, Tomaschitz A, Verheyen N, Pieber TR, Pilz S. Vitamin D and arterial hypertension: treat the deficiency! Am J Hypertens. 2013;26:158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 223. | Tamez H, Kalim S, Thadhani RI. Does vitamin D modulate blood pressure. Curr Opin Nephrol Hypertens. 2013;22:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 224. | Wang L, Ma J, Manson JE, Buring JE, Gaziano JM, Sesso HD. A prospective study of plasma vitamin D metabolites, vitamin D receptor gene polymorphisms, and risk of hypertension in men. Eur J Nutr. 2013;52:1771-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 225. | Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 226. | Keniston R, Enriquez J Sr. Relationship between blood pressure and Plasma Vitamin B6 Levels in Healthy Middle-Aged Adults. Ann N Y Acad Sci. 1990;585:499-501. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 227. | Aybak M, Sermet A, Ayyildiz MO, Karakilçik AZ. Effect of oral pyridoxine hydrochloride supplementation on arterial blood pressure in patients with essential hypertension. Arzneimittelforschung. 1995;45:1271-1273. [PubMed] |
| 228. | Paulose CS, Dakshinamurti K, Packer S, Stephens NL. Sympathetic stimulation and hypertension in the pyridoxine-deficient adult rat. Hypertension. 1988;11:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 229. | Dakshinamurti K, Lal KJ, Ganguly PK. Hypertension, calcium channel and pyridoxine (vitamin B6). Mol Cell Biochem. 1998;188:137-148. [PubMed] |
| 230. | Moline J, Bukharovich IF, Wolff MS, Phillips R. Dietary flavonoids and hypertension: is there a link. Med Hypotheses. 2000;55:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 231. | Knekt P, Reunanen A, Järvinen R, Seppänen R, Heliövaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol. 1994;139:1180-1189. [PubMed] |
| 232. | Karatzi KN, Papamichael CM, Karatzis EN, Papaioannou TG, Aznaouridis KA, Katsichti PP, Stamatelopoulos KS, Zampelas A, Lekakis JP, Mavrikakis ME. Red wine acutely induces favorable effects on wave reflections and central pressures in coronary artery disease patients. Am J Hypertens. 2005;18:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 233. | Biala A, Tauriainen E, Siltanen A, Shi J, Merasto S, Louhelainen M, Martonen E, Finckenberg P, Muller DN, Mervaala E. Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press. 2010;19:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 234. | Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 235. | Bhatt SR, Lokhandwala MF, Banday AA. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol. 2011;667:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 236. | Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 237. | Paran E, Engelhard YN. Effect of lycopene, an oral natural antioxidant on blood pressure. J Hypertens. 2001;19:S74. Abstract P 1.204. |
| 238. | Engelhard YN, Gazer B, Paran E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: a double-blind, placebo-controlled pilot study. Am Heart J. 2006;151:100. [PubMed] |
| 239. | Paran E, Novack V, Engelhard YN, Hazan-Halevy I. The effects of natural antioxidants from tomato extract in treated but uncontrolled hypertensive patients. Cardiovasc Drugs Ther. 2009;23:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 240. | Ried K, Frank OR, Stocks NP. Dark chocolate or tomato extract for prehypertension: a randomised controlled trial. BMC Complement Altern Med. 2009;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 241. | Paran E, Engelhard Y. P-333: Effect of tomato’s lycopene on blood pressure, serum lipoproteins, plasma homocysteine and oxidative stress markers in grade I hypertensive patients. Am J Hypertens. 2001;14:141A. Abstract P-333. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 242. | Xaplanteris P, Vlachopoulos C, Pietri P, Terentes-Printzios D, Kardara D, Alexopoulos N, Aznaouridis K, Miliou A, Stefanadis C. Tomato paste supplementation improves endothelial dynamics and reduces plasma total oxidative status in healthy subjects. Nutr Res. 2012;32:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 243. | Hosseini S, Lee J, Sepulveda RT, Rohdewaldc P, Watson RR. A randomized, double-blind, placebo-controlled, prospective 16 week crossover study to determine the role of pycnogenol in modifying blood pressure in mildly hypertensive patients. Nutr Res. 2001;21:1251-1260. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 244. | Zibadi S, Rohdewald PJ, Park D, Watson RR. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr Res. 2008;28:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 245. | Liu X, Wei J, Tan F, Zhou S, Würthwein G, Rohdewald P. Pycnogenol, French maritime pine bark extract, improves endothelial function of hypertensive patients. Life Sci. 2004;74:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 246. | van der Zwan LP, Scheffer PG, Teerlink T. Reduction of myeloperoxidase activity by melatonin and pycnogenol may contribute to their blood pressure lowering effect. Hypertension. 2010;56:e34; author reply e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 247. | Cesarone MR, Belcaro G, Stuard S, Schönlau F, Di Renzo A, Grossi MG, Dugall M, Cornelli U, Cacchio M, Gizzi G. Kidney flow and function in hypertension: protective effects of pycnogenol in hypertensive participants--a controlled study. J Cardiovasc Pharmacol Ther. 2010;15:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 248. | Simons S, Wollersheim H, Thien T. A systematic review on the influence of trial quality on the effect of garlic on blood pressure. Neth J Med. 2009;67:212-219. [PubMed] |
| 249. | Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: a meta-analysis. Ann Pharmacother. 2008;42:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 250. | Ried K, Frank OR, Stocks NP. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: a randomised controlled trial. Maturitas. 2010;67:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 251. | Suetsuna K, Nakano T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J Nutr Biochem. 2000;11:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 252. | Nakano T, Hidaka H, Uchida J, Nakajima K, Hata Y. Hypotensive effects of wakame. J Jpn Soc Clin Nutr. 1998;20:92. |
| 253. | Krotkiewski M, Aurell M, Holm G, Grimby G, Szczepanik J. Effects of a sodium-potassium ion-exchanging seaweed preparation in mild hypertension. Am J Hypertens. 1991;4:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 254. | Sato M, Oba T, Yamaguchi T, Nakano T, Kahara T, Funayama K, Kobayashi A, Nakano T. Antihypertensive effects of hydrolysates of wakame (Undaria pinnatifida) and their angiotensin-I-converting enzyme inhibitory activity. Ann Nutr Metab. 2002;46:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 255. | Sato M, Hosokawa T, Yamaguchi T, Nakano T, Muramoto K, Kahara T, Funayama K, Kobayashi A, Nakano T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J Agric Food Chem. 2002;50:6245-6252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 256. | Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta. 2005;355:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 257. | Sankar D, Rao MR, Sambandam G, Pugalendi KV. Effect of sesame oil on diuretics or Beta-blockers in the modulation of blood pressure, anthropometry, lipid profile, and redox status. Yale J Biol Med. 2006;79:19-26. [PubMed] |
| 258. | Miyawaki T, Aono H, Toyoda-Ono Y, Maeda H, Kiso Y, Moriyama K. Antihypertensive effects of sesamin in humans. J Nutr Sci Vitaminol (Tokyo). 2009;55:87-91. [PubMed] |
| 259. | Wichitsranoi J, Weerapreeyakul N, Boonsiri P, Settasatian C, Settasatian N, Komanasin N, Sirijaichingkul S, Teerajetgul Y, Rangkadilok N, Leelayuwat N. Antihypertensive and antioxidant effects of dietary black sesame meal in pre-hypertensive humans. Nutr J. 2011;10:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 260. | Sudhakar B, Kalaiarasi P, Al-Numair KS, Chandramohan G, Rao RK, Pugalendi KV. Effect of combination of edible oils on blood pressure, lipid profile, lipid peroxidative markers, antioxidant status, and electrolytes in patients with hypertension on nifedipine treatment. Saudi Med J. 2011;32:379-385. [PubMed] |
| 261. | Sankar D, Rao MR, Sambandam G, Pugalendi KV. A pilot study of open label sesame oil in hypertensive diabetics. J Med Food. 2006;9:408-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 262. | Harikumar KB, Sung B, Tharakan ST, Pandey MK, Joy B, Guha S, Krishnan S, Aggarwal BB. Sesamin manifests chemopreventive effects through the suppression of NF-kappa B-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol Cancer Res. 2010;8:751-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 263. | Nakano D, Ogura K, Miyakoshi M, Ishii F, Kawanishi H, Kurumazuka D, Kwak CJ, Ikemura K, Takaoka M, Moriguchi S. Antihypertensive effect of angiotensin I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2006;70:1118-1126. [PubMed] |
| 264. | Karatzi K, Stamatelopoulos K, Lykka M, Mantzouratou P, Skalidi S, Manios E, Georgiopoulos G, Zakopoulos N, Papamichael C, Sidossis LS. Acute and long-term hemodynamic effects of sesame oil consumption in hypertensive men. J Clin Hypertens (Greenwich). 2012;14:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 265. | Hodgson JM, Puddey IB, Burke V, Beilin LJ, Jordan N. Effects on blood pressure of drinking green and black tea. J Hypertens. 1999;17:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 266. | Kurita I, Maeda-Yamamoto M, Tachibana H, Kamei M. Antihypertensive effect of Benifuuki tea containing O-methylated EGCG. J Agric Food Chem. 2010;58:1903-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 267. | McKay DL, Chen CY, Saltzman E, Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr. 2010;140:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 268. | Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 269. | Hodgson JM, Woodman RJ, Puddey IB, Mulder T, Fuchs D, Croft KD. Short-term effects of polyphenol-rich black tea on blood pressure in men and women. Food Funct. 2013;4:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 270. | Medina-Remón A, Estruch R, Tresserra-Rimbau A, Vallverdú-Queralt A, Lamuela-Raventos RM. The effect of polyphenol consumption on blood pressure. Mini Rev Med Chem. 2013;13:1137-1149. [PubMed] |
| 271. | Jiménez R, Duarte J, Perez-Vizcaino F. Epicatechin: endothelial function and blood pressure. J Agric Food Chem. 2012;60:8823-8830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 272. | Taubert D, Roesen R, Schömig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 273. | Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614. [PubMed] |
| 274. | Taubert D, Roesen R, Lehmann C, Jung N, Schömig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 322] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 275. | Cohen DL, Townsend RR. Cocoa ingestion and hypertension-another cup please. J Clin Hypertens (Greenwich). 2007;9:647-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 276. | Ried K, Sullivan T, Fakler P, Frank OR, Stocks NP. Does chocolate reduce blood pressure A meta-analysis. BMC Med. 2010;8:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 277. | Egan BM, Laken MA, Donovan JL, Woolson RF. Does dark chocolate have a role in the prevention and management of hypertension: commentary on the evidence. Hypertension. 2010;55:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 278. | Desch S, Kobler D, Schmidt J, Sonnabend M, Adams V, Sareban M, Eitel I, Blüher M, Schuler G, Thiele H. Low vs. higher-dose dark chocolate and blood pressure in cardiovascular high-risk patients. Am J Hypertens. 2010;23:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 279. | Desch S, Schmidt J, Kobler D, Sonnabend M, Eitel I, Sareban M, Rahimi K, Schuler G, Thiele H. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 280. | Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671-1676. [PubMed] |
| 281. | Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 282. | Ellinger S, Reusch A, Stehle P, Helfrich HP. Epicatechin ingested via cocoa products reduces blood pressure in humans: a nonlinear regression model with a Bayesian approach. Am J Clin Nutr. 2012;95:1365-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 283. | Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 445] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 284. | Yamaguchi T, Chikama A, Mori K, Watanabe T, Shioya Y, Katsuragi Y, Tokimitsu I. Hydroxyhydroquinone-free coffee: a double-blind, randomized controlled dose-response study of blood pressure. Nutr Metab Cardiovasc Dis. 2008;18:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 285. | Chen ZY, Peng C, Jiao R, Wong YM, Yang N, Huang Y. Anti-hypertensive nutraceuticals and functional foods. J Agric Food Chem. 2009;57:4485-4499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 286. | Ochiai R, Chikama A, Kataoka K, Tokimitsu I, Maekawa Y, Ohishi M, Rakugi H, Mikami H. Effects of hydroxyhydroquinone-reduced coffee on vasoreactivity and blood pressure. Hypertens Res. 2009;32:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 287. | Kozuma K, Tsuchiya S, Kohori J, Hase T, Tokimitsu I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens Res. 2005;28:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 288. | Palatini P, Ceolotto G, Ragazzo F, Dorigatti F, Saladini F, Papparella I, Mos L, Zanata G, Santonastaso M. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J Hypertens. 2009;27:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 289. | Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 314] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 290. | Cavallo A, Daniels SR, Dolan LM, Khoury JC, Bean JA. Blood pressure response to melatonin in type 1 diabetes. Pediatr Diabetes. 2004;5:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 291. | Cavallo A, Daniels SR, Dolan LM, Bean JA, Khoury JC. Blood pressure-lowering effect of melatonin in type 1 diabetes. J Pineal Res. 2004;36:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 292. | Cagnacci A, Cannoletta M, Renzi A, Baldassari F, Arangino S, Volpe A. Prolonged melatonin administration decreases nocturnal blood pressure in women. Am J Hypertens. 2005;18:1614-1618. [PubMed] |
| 293. | Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, Kamari Y, Shen-Orr Z, Zisapel N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med. 2006;119:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 294. | Rechciński T, Kurpesa M, Trzos E, Krzeminska-Pakuła M. [The influence of melatonin supplementation on circadian pattern of blood pressure in patients with coronary artery disease--preliminary report]. Pol Arch Med Wewn. 2006;115:520-528. [PubMed] |
| 295. | Merkur’eva GA, Ryzhak GA. [Effect of the pineal gland peptide preparation on the diurnal profile of arterial pressure in middle-aged and elderly women with ischemic heart disease and arterial hypertension]. Adv Gerontol. 2008;21:132-142. [PubMed] |
| 296. | Zaslavskaia RM, Shcherban’ EA, Logvinenko SI. [Melatonin in combined therapy of patients with stable angina and arterial hypertension]. Klin Med (Mosk). 2008;86:64-67. [PubMed] |
| 297. | Zamotaev IuN, Enikeev AKh, Kolomoets NM. [The use of melaxen in combined therapy of arterial hypertension in subjects occupied in assembly line production]. Klin Med (Mosk). 2009;87:46-49. [PubMed] |
| 298. | Rechciński T, Trzos E, Wierzbowska-Drabik K, Krzemińska-Pakuła M, Kurpesa M. Melatonin for nondippers with coronary artery disease: assessment of blood pressure profile and heart rate variability. Hypertens Res. 2010;33:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 299. | Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011;50:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 300. | De Leersnyder H, de Blois MC, Vekemans M, Sidi D, Villain E, Kindermans C, Munnich A. beta(1)-adrenergic antagonists improve sleep and behavioural disturbances in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2001;38:586-590. [PubMed] |
| 301. | Morand C, Dubray C, Milenkovic D, Lioger D, Martin JF, Scalbert A, Mazur A. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr. 2011;93:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 302. | Basu A, Penugonda K. Pomegranate juice: a heart-healthy fruit juice. Nutr Rev. 2009;67:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 303. | Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L, Volkova N, Presser D, Attias J, Liker H. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 367] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 304. | Aviram M, Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis. 2001;158:195-198. [PubMed] |
| 305. | Feringa HH, Laskey DA, Dickson JE, Coleman CI. The effect of grape seed extract on cardiovascular risk markers: a meta-analysis of randomized controlled trials. J Am Diet Assoc. 2011;111:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 306. | Sivaprakasapillai B, Edirisinghe I, Randolph J, Steinberg F, Kappagoda T. Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metabolism. 2009;58:1743-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 307. | Edirisinghe I, Burton-Freeman B, Tissa Kappagoda C. Mechanism of the endothelium-dependent relaxation evoked by a grape seed extract. Clin Sci (Lond). 2008;114:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 308. | Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong JY, Watts GF. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens. 2007;21:297-306. [PubMed] |
| 309. | Burke BE, Neuenschwander R, Olson RD. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South Med J. 2001;94:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 310. | Mikhin VP, Kharchenko AV, Rosliakova EA, Cherniatina MA. [Application of coenzyme Q(10) in combination therapy of arterial hypertension]. Kardiologiia. 2011;51:26-31. [PubMed] |
| 311. | Tsai KL, Huang YH, Kao CL, Yang DM, Lee HC, Chou HY, Chen YC, Chiou GY, Chen LH, Yang YP. A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. J Nutr Biochem. 2012;23:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 312. | Sohet FM, Delzenne NM. Is there a place for coenzyme Q in the management of metabolic disorders associated with obesity. Nutr Rev. 2012;70:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 313. | Digiesi V, Cantini F, Oradei A, Bisi G, Guarino GC, Brocchi A, Bellandi F, Mancini M, Littarru GP. Coenzyme Q10 in essential hypertension. Mol Aspects Med. 1994;15 Suppl:s257-s263. [PubMed] |
| 314. | Langsjoen P, Langsjoen P, Willis R, Folkers K. Treatment of essential hypertension with coenzyme Q10. Mol Aspects Med. 1994;15 Suppl:S265-S272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 315. | Ankola DD, Viswanad B, Bhardwaj V, Ramarao P, Kumar MN. Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy. Eur J Pharm Biopharm. 2007;67:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 316. | Trimarco V, Cimmino CS, Santoro M, Pagnano G, Manzi MV, Piglia A, Giudice CA, De Luca N, Izzo R. Nutraceuticals for blood pressure control in patients with high-normal or grade 1 hypertension. High Blood Press Cardiovasc Prev. 2012;19:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 317. | Young JM, Florkowski CM, Molyneux SL, McEwan RG, Frampton CM, Nicholls MG, Scott RS, George PM. A randomized, double-blind, placebo-controlled crossover study of coenzyme Q10 therapy in hypertensive patients with the metabolic syndrome. Am J Hypertens. 2012;25:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 318. | McMackin CJ, Widlansky ME, Hamburg NM, Huang AL, Weller S, Holbrook M, Gokce N, Hagen TM, Keaney JF, Vita JA. Effect of combined treatment with alpha-Lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich). 2007;9:249-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 319. | Salinthone S, Schillace RV, Tsang C, Regan JW, Bourdette DN, Carr DW. Lipoic acid stimulates cAMP production via G protein-coupled receptor-dependent and -independent mechanisms. J Nutr Biochem. 2011;22:681-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 320. | Rahman ST, Merchant N, Haque T, Wahi J, Bhaheetharan S, Ferdinand KC, Khan BV. The impact of lipoic acid on endothelial function and proteinuria in quinapril-treated diabetic patients with stage I hypertension: results from the QUALITY study. J Cardiovasc Pharmacol Ther. 2012;17:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 321. | Morcos M, Borcea V, Isermann B, Gehrke S, Ehret T, Henkels M, Schiekofer S, Hofmann M, Amiral J, Tritschler H. Effect of alpha-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: an exploratory study. Diabetes Res Clin Pract. 2001;52:175-183. [PubMed] |
| 322. | Jiang B, Haverty M, Brecher P. N-acetyl-L-cysteine enhances interleukin-1beta-induced nitric oxide synthase expression. Hypertension. 1999;34:574-579. [PubMed] |
| 323. | Vasdev S, Singal P, Gill V. The antihypertensive effect of cysteine. Int J Angiol. 2009;18:7-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 324. | Meister A, Anderson ME, Hwang O. Intracellular cysteine and glutathione delivery systems. J Am Coll Nutr. 1986;5:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 325. | Asher GN, Viera AJ, Weaver MA, Dominik R, Caughey M, Hinderliter AL. Effect of hawthorn standardized extract on flow mediated dilation in prehypertensive and mildly hypertensive adults: a randomized, controlled cross-over trial. BMC Complement Altern Med. 2012;12:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 326. | Koçyildiz ZC, Birman H, Olgaç V, Akgün-Dar K, Melikoğlu G, Meriçli AH. Crataegus tanacetifolia leaf extract prevents L-NAME-induced hypertension in rats: a morphological study. Phytother Res. 2006;20:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 327. | Schröder D, Weiser M, Klein P. Efficacy of a homeopathic Crataegus preparation compared with usual therapy for mild (NYHA II) cardiac insufficiency: results of an observational cohort study. Eur J Heart Fail. 2003;5:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 328. | Walker AF, Marakis G, Simpson E, Hope JL, Robinson PA, Hassanein M, Simpson HC. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: a randomised controlled trial. Br J Gen Pract. 2006;56:437-443. [PubMed] |
| 329. | Walker AF, Marakis G, Morris AP, Robinson PA. Promising hypotensive effect of hawthorn extract: a randomized double-blind pilot study of mild, essential hypertension. Phytother Res. 2002;16:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 330. | Larson A, Witman MA, Guo Y, Ives S, Richardson RS, Bruno RS, Jalili T, Symons JD. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: nitric oxide. Nutr Res. 2012;32:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 331. | Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137:2405-2411. [PubMed] |
| 332. | Egert S, Bosy-Westphal A, Seiberl J, Kürbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 386] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 333. | Trovato A, Nuhlicek DN, Midtling JE. Drug-nutrient interactions. Am Fam Physician. 1991;44:1651-1658. [PubMed] |