Published online Aug 26, 2025. doi: 10.4330/wjc.v17.i8.110163
Revised: June 12, 2025
Accepted: July 18, 2025
Published online: August 26, 2025
Processing time: 83 Days and 6.4 Hours
Cardiovascular disease remains the leading global cause of mortality, projected to increase by 73.4% from 2025 to 2050 despite declining age-standardized rates. Contemporary interventions, such as percutaneous coronary intervention and statins, reduce major adverse cardiovascular events (MACE) by 25%-30%, yet a 20% five-year MACE risk persists in high-risk cohorts. These approaches, historically focused on luminal stenosis, fail to address systemic atherogenesis drivers like endothelial dysfunction and inflammation. Specifically, dietary linoleic acid restriction (< 5 g/day) reduces oxidized low-density lipoprotein by approximately 15% by limiting peroxidation-prone bisallylic bonds, mitigating arterial inflammation, a key atherogenic trigger. Enhanced external counterpulsation, through pulsatile shear stress, enhances nitric oxide-mediated coronary perfusion, alle
Core Tip: Cardiovascular disease care still leaves a 20% five-year major adverse cardiovascular events risk. This review outlines an integrative, root-cause strategy combining dietary linoleic acid restriction (< 5 g/day) to lower oxidized low-density lipoprotein, non-invasive enhanced external counterpulsation to boost nitric-oxide-mediated perfusion, and plaque-targeted nanoliposomal chelation to reverse calcification. These scalable, affordable interventions collectively target oxidative stress, endothelial inflammation and plaque instability, offering a realistic roadmap to transform coronary events from commonplace to rare and potentially reshape future cardiovascular disease management.
- Citation: Mercola J. Integrative cardiovascular disease therapy: Linoleic acid restriction, enhanced external counterpulsation, and emerging nanotherapies. World J Cardiol 2025; 17(8): 110163
- URL: https://www.wjgnet.com/1949-8462/full/v17/i8/110163.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i8.110163
Cardiovascular disease (CVD) is projected to persist as the leading cause of global mortality, with an estimated 23.6 million deaths annually by 2030[1]. Over recent decades, substantial progress in acute care has improved patient out
Even with optimal medical therapy (OMT), the five-year risk of major adverse cardiovascular events (MACE) persists at levels as high as 20% in high-risk cohorts[7]. Furthermore, mechanical interventions such as PCI and CABG, though effective in relieving acute symptoms and enhancing survival in specific contexts, fail to arrest the systemic progression of atherosclerosis or prevent the formation of new lesions[8]. A meta-analysis of randomized trials has demonstrated that, in stable coronary artery disease (CAD), PCI does not significantly reduce mortality compared to medical therapy alone, underscoring the limitations of a lesion-centric approach[9].
As depicted in Figure 1, this persistent morbidity and mortality highlight the urgent need for a paradigm shift in cardiovascular medicine, from an emphasis on ameliorating acute manifestations and localized vascular obstructions to strategies that address the fundamental drivers of atherogenesis and restore physiologic vascular function[10]. Despite advancements in acute care, current CVD interventions such as PCI and statins primarily target symptomatic manifestations like luminal stenosis and elevated low-density lipoprotein cholesterol (LDL-C)[11,12], leaving a 20% five-year residual risk of MACE in high-risk patients[13,14] due to unaddressed oxidative stress[15], endothelial dysfunction[16], and plaque instability[17]. This gap underscores the necessity of an integrative model that synergistically targets these root causes to not only halt disease progression but also reverse existing atherosclerosis, offering a more comprehensive solution where traditional approaches fall short[18-20]. Moreover, while CABG offers superior long-term angina relief compared to PCI, it carries a higher peri-operative stroke risk, a nuance important to patient-specific decision-making. Historically, therapeutic efforts have prioritized downstream consequences of atherosclerosis, such as luminal narrowing, over the upstream pathophysiologic processes that initiate and perpetuate plaque formation[21].
In this context, emerging evidence suggests that while mechanical revascularization and cholesterol-lowering therapies remain essential, they are insufficient to halt disease progression comprehensively[22]. Consequently, a more integrative framework is warranted, one that synergizes metabolic optimization, circulatory enhancement, and plaque modification to not only prevent further atherosclerosis but also potentially reverse existing disease[23]. Additionally, dietary interventions targeting lipid profiles, such as reducing linoleic acid (LA) intake, which may exacerbate inflammation[24], and increasing odd-chain fatty acids, linked to a 20% reduction in coronary heart disease risk[25], offer potential to modulate atherogenic pathways at a molecular level.
Furthermore, advanced chelation techniques, which aim to extract heavy metals and stabilize atherosclerotic plaques, have demonstrated preliminary reductions in cardiovascular events[26], though these findings await confirmation through larger prospective studies[27]. These innovative approaches, when combined, represent a multifaceted strategy to address the complex etiology of atherosclerosis beyond the scope of traditional interventions[28]. Importantly, the efficacy of these novel therapies is amplified when integrated with established preventive measures, including smoking cessation[29], and regular physical activity[30]. The adoption of this integrative paradigm into clinical practice could not only enhance patient outcomes[31] but also mitigate the economic toll of CVD, projected to surpass 1 trillion dollars annually in the United States by 2035[32]. By aligning these strategies, clinicians can target the multifactorial nature of atherosclerosis, from endothelial dysfunction and inflammation to plaque instability and circulatory insufficiency[33].
This paper seeks to delineate a comprehensive, evidence-based roadmap that leverages both conventional and cutting-edge therapies to confront the pervasive challenge of CVD[34]. By redirecting focus from symptomatic relief to the eradication of underlying causes, we envision a future where the incidence of coronary events is substantially di
Revascularization strategies, including PCI with stent implantation and coronary CABG, have long served as cornerstones of CAD management, particularly in acute coronary syndromes. These interventions aim to restore luminal patency in stenotic coronary arteries, thereby alleviating ischemia and improving quality of life[39]. However, in stable ischemic heart disease, their efficacy in reducing mortality or MI is less pronounced, and their procedural limitations underscore the need for therapies targeting the systemic atherogenic milieu[40]. This narrative examines the constraints of PCI and CABG, emphasizing their inability to address the underlying pathophysiology of atherosclerosis and high
Historically, PCI and CABG were presumed to confer survival benefits across the spectrum of CAD[42]. However, the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches, published in 2020, challenged this assumption[43]. In 5179 patients with stable CAD and moderate-to-severe ischemia, an initial invasive strategy of PCI or CABG, compared with OMT, yielded no significant reduction in the composite endpoint of cardiova
Despite its utility, PCI is not without drawbacks. Stent thrombosis, occurring in approximately 1% of patients within the first year post-implantation[46], and in-stent restenosis, affecting 5%-10% of patients with drug-eluting stents[47], often necessitate repeat procedures. These complications stem from endothelial injury during stent deployment, which triggers neointimal hyperplasia or thrombus formation[48]. Furthermore, PCI targets focal stenoses but does not mitigate the diffuse atherogenic process[49]. Consequently, untreated segments of the coronary vasculature remain susceptible to plaque progression, with studies indicating a 20%-30% incidence of new significant lesions within five years post-PCI[50]. This limitation highlights the reactive nature of stenting, addressing only the immediate mechanical obstruction without altering the broader disease trajectory.
In contrast, CABG offers more durable coronary flow, particularly in patients with multivessel disease or left main coronary artery stenosis[51]. By bypassing occluded segments with autologous grafts, CABG achieves complete revascularization in approximately 90% of cases, compared with 60%-70% for PCI. However, this benefit comes at a cost. CABG carries a 1%-2% perioperative mortality rate[52], a 5% incidence of wound infections[53], and a 10%-20% risk of postope
Both PCI and CABG, while effective in restoring flow, fail to address the underlying biology of atherosclerosis. Atherosclerotic plaques form and destabilize through a complex interplay of endothelial injury, lipid accumulation, and inflammatory cell infiltration, mediated by cytokines such as interleukin-6 (IL-6) and C-reactive protein, which are elevated in 30%-40% of CAD patients[57]. Neither procedure modulates these pathways, nor do they prevent plaque rupture in non-target vessels, which accounts for 50% of recurrent ischemic events[58]. This gap underscores the need for therapies that stabilize plaques and enhance endothelial health across the vascular tree. For instance, high-dose statins, which reduce LDL-C by 40%-50%, have demonstrated a 25% reduction in MACE by stabilizing plaque morphology and attenuating inflammation[59].
Current knowledge gaps persist, particularly regarding the optimal integration of revascularization with emerging therapies targeting vascular biology, such as proprotein convertase subtilisin/kexin type 9 inhibitors or anti-inflammatory agents like canakinumab[60]. These limitations emphasize the need for a paradigm shift in CAD management[61]. Future research should prioritize strategies that address the systemic nature of atherosclerosis, potentially combining revascularization with therapies that modulate endothelial function, reduce plaque vulnerability, and mitigate inflammatory cascades[62]. Such an approach promises to reduce the burden of CAD more effectively, moving beyond the mechanical restoration of flow to the prevention of disease progression across the coronary vasculature[63].
Statins, or 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, have been a cornerstone of CVD management since their introduction in the late 1980s[64]. By inhibiting hepatic cholesterol synthesis, these agents effectively reduce LDL-C concentrations, typically by 30%-50%[65], and decrease the incidence of MACE, such as MI and stroke, by approximately 25%-35% in high-risk populations[66]. Despite this efficacy, statins fall short of eliminating cardiovascular risk entirely. A significant proportion of patients on optimal statin therapy continue to experience MACE, a phenomenon termed residual atherosclerotic cardiovascular risk[67]. This persistent vulnerability highlights the limitations of a pharmacotherapeutic strategy centered predominantly on LDL-C reduction and underscores the multifactorial etiology of atherosclerosis.
Clinical evidence substantiates the presence of residual risk[12]. In the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin, rosuvastatin reduced MACE by 44% in individuals with baseline LDL-C below 130 mg/dL but elevated high-sensitivity C-reactive protein (hsCRP) levels exceeding 2 mg/L. Nevertheless, the event rate in the treatment arm remained 0.77 per 100 person-years, indicating that nearly 1% of treated patients annually still faced cardiovascular events[68]. Similarly, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk trial demonstrated that adding evolocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, to statin therapy lowered LDL-C to a median of 30 mg/dL and reduced MACE by 15%[69]. Yet, the annualized event rate persisted at 3.4%, reinforcing that even profound LDL-C reduction does not abolish risk. These data reveal that while statins address a primary driver of atherosclerosis, they do not fully mitigate the underlying disease process[70].
Atherosclerosis is a complex, multifactorial condition influenced by factors beyond circulating LDL-C[71]. Arterial inflammation, driven by immune mediators such as IL-6 and tumor necrosis factor-alpha, persists in many patients despite lipid optimization[72]. Oxidized low-density lipoprotein (oxLDL) particles, which are highly atherogenic, evade complete neutralization by statins[73]. Lipoprotein(a) [Lp(a)], an independent genetic risk factor present in elevated levels (> 50 mg/dL) in approximately 20% of CVD patients, remains largely unaffected by 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibition[74]. Furthermore, insulin resistance, a hallmark of metabolic syndrome prevalent in 35% of adults with CVD, exacerbates endothelial dysfunction and atherogenesis[75]. The Canakinumab Anti-inflammatory Thrombosis Outcome Study illustrated this broader pathology: Canakinumab, targeting IL-1β, reduced MACE by 15% in statin-treated patients with hsCRP above 2 mg/L, independent of LDL-C changes[76]. This suggests that inflammation-targeted therapies can address residual risk where statins fall short[77].
In addition to incomplete risk attenuation, statins present tolerability challenges that limit their universal application[78]. Approximately 10%-15% of users report myalgia or muscle weakness, with 1%-2% discontinuing therapy due to these symptoms[79]. More rarely, severe adverse effects such as rhabdomyolysis (incidence < 0.1%) or hepatotoxicity emerge[80]. Statins also confer a 9%-12% relative increase in new-onset type 2 diabetes mellitus over 4 to 5 years, particu
Historically, CVD management has prioritized LDL-C as the primary modifiable target, a focus rooted in the lipid hypothesis and validated by decades of statin trials. However, this cholesterol-centric paradigm overlooks the arterial microenvironment’s broader contribution to plaque progression and rupture[82]. Endothelial dysfunction, oxidative stress, and plaque instability, driven by cellular and extracellular dynamics, persist despite low LDL-C. For instance, angiotensin-converting enzyme inhibitors, beyond their antihypertensive effects, improve endothelial function and reduce MACE by 20%-25% in CAD[83], suggesting a role for therapies targeting vascular biology. The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial further demonstrated that icosapent ethyl reduced MACE by 25% in statin-treated patients with triglycerides ≥ 150 mg/dL[84], pointing to triglyceride-rich lipoproteins as an additional therapeutic target.
Despite these advances, gaps remain in identifying patients at high residual risk and developing therapies that comprehensively address non-LDL-C pathways[85]. Future efforts should leverage advanced biomarkers [e.g., hsCRP, Lp(a)], imaging modalities like coronary artery calcium scoring, or polygenic risk scores to refine risk stratification[86]. Consequently, a more integrated approach, combining lipid-lowering agents with therapies modulating inflammation, endothelial health, and plaque stability, holds promise for reducing the global CVD burden and enhancing patient outcomes beyond the capabilities of statin-centered pharmacotherapy alone.
Enhanced external counter pulsation (EECP) is a noninvasive therapeutic modality that employs pneumatic cuffs wrapped around the patient’s lower extremities to enhance cardiac perfusion and systemic vascular function[87,88]. The cuffs are meticulously timed to inflate during diastole, the phase of cardiac relaxation, and deflate during systole, thereby augmenting diastolic coronary blood flow and venous return to the heart[89,90]. This Food and Drug Administration-approved therapy is primarily deployed for patients with refractory angina, a debilitating condition marked by persistent chest pain despite OMT and maximal revascularization efforts[91]. Affecting an estimated 600000 to 1.8 million individuals in the United States alone, refractory angina imposes a substantial burden on quality of life, often leaving patients with few viable options[92]. EECP emerges as a compelling alternative, leveraging mechanical means to sti
Clinical trials have consistently underscored EECP’s efficacy in ameliorating symptoms of refractory angina. Approximately 70% of patients experience at least a one-class improvement in angina severity, as assessed by the Canadian Cardiovascular Society grading system, which ranges from class I (mild) to class IV (severe). Moreover, studies have reported a reduction in the frequency of angina episodes by 50% or more, alongside a notable decrease in reliance on nitroglycerin, a cornerstone of acute angina management[94]. In a landmark randomized controlled trial (RCT), patients undergoing EECP exhibited a mean increase in exercise duration of approximately 60 seconds compared to controls, with sustained benefits observed over follow-up periods extending up to one year[95]. A standard EECP regimen entails 35 one-hour sessions, typically administered five days per week over seven weeks, offering a structured yet flexible out
The physiological underpinnings of EECP’s effectiveness are rooted in its capacity to modulate vascular dynamics. The rhythmic inflation and deflation of the cuffs generate heightened shear stress on endothelial cells lining the blood vessels, a mechanical force that triggers the release of nitric oxide (NO), a potent vasodilator that enhances coronary perfusion and reduces vascular resistance[96]. Concurrently, the pulsatile flow induced by EECP is hypothesized to stimulate angiogenesis, fostering the development of collateral vessels that provide alternative conduits for blood to reach ischemic myocardial regions[97]. Evidence of improved endothelial function, quantifiable through a 20%-30% enhancement in flow-mediated dilation, further bolsters EECP’s therapeutic profile, supporting vascular health by mitigating thrombosis and atherosclerosis progression[98].
EECP’s noninvasive nature distinguishes it as a scalable adjunct or alternative to invasive interventions such as CABG. Administered in an outpatient setting, the therapy circumvents risks inherent to surgery, such as infection, bleeding, or anesthesia-related complications, with adverse effects limited primarily to minor skin bruising or cuff-related discomfort in fewer than 5% of cases. For patients ineligible for further revascularization due to diffuse CAD, multiple comorbidities, or personal preference, EECP offers a pragmatic solution. When integrated with OMT, it has the potential to delay or obviate the need for more aggressive procedures, enhancing symptom control across a diverse patient cohort[99].
Long-term studies, such as those by Michaels et al[100], indicate that EECP-induced improvements in angina and exercise tolerance persist for up to two years, likely due to sustained coronary collateral vessel formation, with collateral flow indices rising by approximately 20%[101,102]. However, adoption remains limited by logistical challenges, requiring 35 one-hour outpatient sessions[103], and inconsistent insurance coverage, with only approximately 50% of United States Medicare plans reimbursing it[104], hindering broader clinical translation. Despite its demonstrated efficacy in symptom palliation, EECP’s impact on hard clinical endpoints, such as MI or all-cause mortality, remains incompletely delineated[105], necessitating larger, long-term studies. Similarly, while the therapy’s promotion of angiogenesis and endothelial repair is well-supported, the precise molecular pathways and predictors of patient response warrant deeper exploration to refine its application and optimize outcomes[106].
Historically, cardiovascular medicine has prioritized lesion-specific interventions like stenting and bypass surgery[107]; however, EECP signals a paradigm shift toward noninvasive, physiology-driven strategies that harness the body’s intrinsic adaptive mechanisms. Beyond refractory angina, preliminary investigations suggest potential applications in heart failure[108], peripheral artery disease, and vascular-related cognitive impairment[109], broadening its therapeutic horizon. With continued research to determine its long-term benefits and refine patient selection, EECP stands poised to integrate seamlessly into comprehensive cardiovascular care, offering a lifeline to those with limited conventional options and redefining the management of circulatory insufficiency.
Between 1909 and 1999, per-capita availability of soybean oil, the primary vector for dietary LA, rose by more than a thousand-fold[110]. This surge elevated LA from roughly 2.8% to 7.2% of total energy intake, a near three-fold increase mirroring mid-century public-health guidance, historically predicated on the lipid hypothesis, that promoted polyun
LA contains two bisallylic double bonds that render it highly susceptible to hydrogen abstraction[115]. Once esterified into membrane phospholipids, particularly the tetraacyl lipid cardiolipin (CL) that forms roughly 10%-15% of the inner mitochondrial membrane, the molecule becomes a ready substrate for lipid peroxidation[116]. Reactive oxygen species (ROS) generated during normal oxidative phosphorylation convert LA-containing CL into electrophilic aldehydes such as 4-hydroxynonenal (4-HNE), which covalently modifies complex I subunits and disrupts electron transfer. Experimental models demonstrate that 4-HNE adduction depresses adenosine triphosphate output and amplifies superoxide genera
Chronically highfat diets exacerbate this injury through reductive stress[119]. Excess β-oxidation funnels large quantities of nicotinamide adenine dinucleotide and flavin adenine dinucleotide into the respiratory chain; when their delivery outpaces complex I/II capacity, electrons leak and partially reduce molecular oxygen[120]. LA-derived 4-HNE simultaneously handicaps the very complexes needed to resolve the backlog, creating an environment in which both reducing equivalents and ROS accumulate[121]. Reductive-oxidative synergy thus links elevated LA consumption to the mitochondrial dysfunction characteristic of insulin resistance, nonalcoholic steatohepatitis, and myocardial contractile failure[122].
OxLDL provides the next mechanistic bridge to overt atherosclerosis[123]. LA is preferentially incorporated into low-density lipoprotein (LDL) particles; the higher the LA content, the more rapidly myeloperoxidase or transition metals convert LDL to its oxidized form[124]. OxLDL binds scavenger receptors on macrophages, drives foam-cell formation, and fuels the expansion of necrotic cores[125]. A re-analysis of the Sydney Diet Heart Study revealed that replacing saturated fat with LA-rich vegetable oil increased cardiovascular mortality by 62% over five years, despite lowering serum cholesterol by 8 mg/dL[126]. Meta-analytic synthesis of controlled trials has since associated high-LA substitution with a 13% rise in coronary death, challenging the notion that total-cholesterol reduction guarantees clinical benefit[127,128].
Even the most potent lipid-lowering pharmacotherapies leave substantial residual risk. In the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk trial, evolocumab plus statin therapy drove median LDL-C to 30 mg/dL yet MACE continued at 3.4% per year[129]. Persistent arterial inflammation, oxLDL burden, and Lp(a) activity all contribute to this residual risk[130]; each pathway is potentiated by excessive LA, which supplies the fattyacid substrate for oxLDL and pro-inflammatory oxylipins such as leukotriene B4 and thromboxane A2[131]. Consequently, attenuating LA intake targets a facet of atherogenesis that pharmacological LDL-C suppression cannot fully address.
Historical RCTs provide compelling evidence of the potential toxicity of LA. In a seminal 1965 RCT, Rose et al[132] administered approximately 95 g/day of corn oil, equivalent to 19 teaspoons, to patients diagnosed with ischemic heart disease, observing a statistically significant increase in mortality within the intervention group compared to controls (P < 0.05), a finding that necessitated the study’s premature termination. This observation, grounded in mid-20th-century dietary intervention research, suggests that excessive LA intake may directly precipitate adverse health outcomes, extending beyond its postulated contribution to carcinogenesis. In a similar vein, the Los Angeles Veterans Administration Trial revealed that men assigned to a diet deriving approximately 15% of total energy from LA exhibited a 25% higher incidence of cancer and cancer-related mortality compared to counterparts consuming a diet rich in saturated fatty acids (P < 0.01), with neoplastic events including lung and prostate carcinomas[133]. These data bolster the hypothesis that elevated LA consumption may amplify oncogenic processes, potentially mediated by mechanisms such as oxidative stress and chronic inflammation.
Similarly, a re-analysis of the Sydney Diet Heart Study demonstrated that replacing dietary saturated fats with safflower oil, a concentrated LA source supplying approximately 13% of energy, was associated with heightened all-cause mortality [hazard ratio (HR) = 1.62, 95% confidence interval: 1.08-2.43], CVD mortality (HR = 1.70), and coronary heart disease mortality (HR = 1.74), despite a measurable decrease in serum cholesterol concentrations[126]. This counterintuitive result challenges the long-standing assumption that reductions in cholesterol levels induced by LA universally enhance health outcomes. Furthermore, the Minnesota Coronary Experiment showed that a corn oil-based diet, which reduced LDL-C by 14%, correlated with a 22% increased mortality risk per 30 mg/dL LDL reduction (P < 0.05), contradicting the diet-heart hypothesis that historically linked lowered LDL with improved longevity[127]. These findings indicate that LA’s metabolic influence extends beyond lipid profiles, potentially aggravating systemic risk factors for chronic conditions, such as atherosclerosis and neoplasia. In this context, a meta-analysis involving over 76000 par
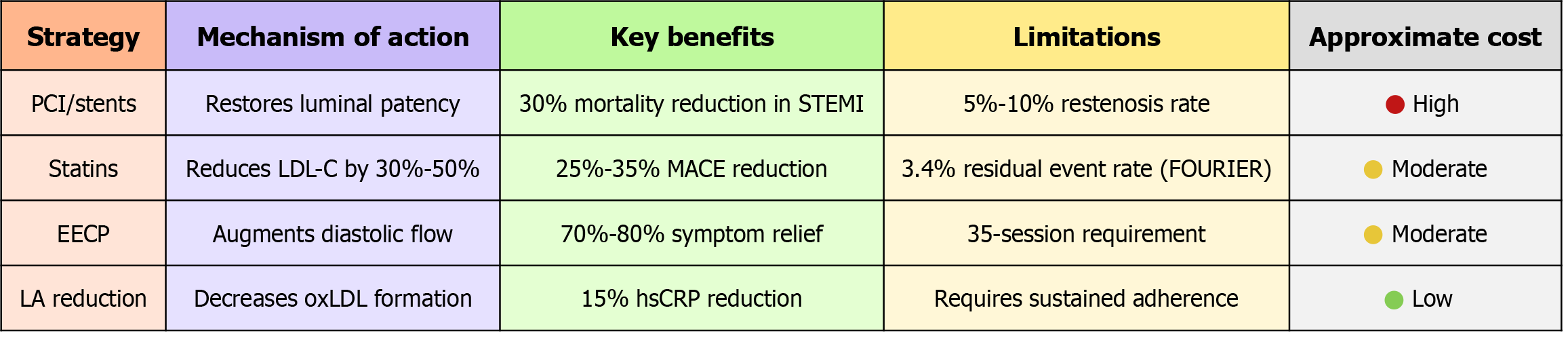
Randomized evidence supports this dietary strategy[134]. As depicted in Table 1, in a 12-week trial enrolling 100 adults with metabolic syndrome, reducing LA to < 5 g/day lowered hsCRP by 15%[135] and IL-6 by 10%[136] without altering totalfat energy[137]. These biomarker shifts mirror mechanistic expectations: Fewer LArich LDL particles undergo oxidation, macrophage uptake of oxLDL decreases[138], and cytokine signaling subsides. Importantly, the trial achieved these changes through isocaloric replacement of soybean, corn, and safflower oils with extra-virgin olive oil, avocado oil, grass-fed butter, and ruminant fats, foods whose monounsaturated or saturated profiles resist peroxidation[139].
| Study design | Intervention | Outcomes measured | Results |
| Randomized controlled trial, n = 100 | LA < 5 g/day vs control (usual diet) | hsCRP | hsCRP ↓15% (intervention vs control) |
| Adults with metabolic syndrome | Isocaloric substitution: Olive/avocado oil, grass-fed butter, ruminant fats instead of seed oils | IL-6 | IL-6 ↓10% (intervention vs control) |
The temporal dimension of tissue LA must also be considered. Adipose depots store LA with an estimated halflife of two years[140], meaning that a single year of low-LA eating only clears about 30% of prior accumulation. Sustained adherence is therefore required to remodel adipocyte triglycerides toward more stable fatty-acid species[141]. Nonetheless, measurable benefits emerge within months as circulating LA and oxLDL fall faster than adipose clearance would predict, indicating that plasma pools reflect recent intake[142]. Endothelial physiology furnishes a complementary vantage point. 4-HNE adducts deactivate endothelial nitricoxide synthase, diminishing bioavailable NO and promoting vasoconstriction, platelet aggregation, and leukocyte adhesion[143]. While EECP augments NO release mechanically, lowering LA addresses the biochemical bottleneck directly and may potentiate EECP benefits[144].
Practical implementation is straightforward: Discard industrial seed oils and seek to lower daily LA content to less than 5 g daily. One tablespoon of soybean oil supplies approximately 7 g LA, already exceeding the proposed daily cap[145]; the same volume of non-adulterated olive oil provides approximately 1 g[146]. Poultry and pork mirror their feed composition, so selecting pasture-raised or low-LA-fed animals further trims intake[147]. Nuts such as macadamia (< 2% LA) or pili (< 1%) substitute for high-LA peanuts (> 30%), enabling palatable adherence without energy restriction[148].
Although intermediate markers improve rapidly, definitive trials powered for MI or stroke endpoints remain scarce. Ongoing studies should stratify participants by fatty acid desaturase 1/2 polymorphisms, antioxidantenzyme capacity, and baseline adipose LA to refine personalized thresholds[149]. Investigations into the interaction between LA restriction and odd-chain fatty acids (e.g., pentadecanoic acid, C15:0) may also clarify whether replacement lipids confer additive plaque-stabilizing effects[150].
Until such data mature, clinicians can monitor erythrocyte-membrane LA, oxLDL, hsCRP, and lipoproteinassociated phospholipase A2 at six-month intervals[151]. In practice, erythrocyte LA tends to decline 15%-20% per quarter during consistent adherence, paralleling improvements in inflammatory biomarkers and endothelial-function assays like flow-mediated dilation[152]. Coronary computed-tomography angiography offers a structural read-out; case series report 5%-10% plaque-volume regression over two years when low-LA eating accompanies OMT.
In summary, the rise of LA-rich seed oils constitutes a century-long natural experiment, the outcomes of which, elevated oxidative burden, mitochondrial dysfunction, and residual cardiovascular risk, are now measurable. By curtailing daily LA intake to ancestral levels below 5 g, clinicians remove a central oxidizable substrate[153], restore mitochondrial redox balance[154], dampen endothelial inflammation[155], and shrink the biochemical foundation of atherogenesis[156]. This nutritional adjustment, simple in execution yet profound in scope, complements existing phar
Intravenous ethylenediaminetetra-acetic acid (EDTA) chelation entered cardiology lore after the Trial to Assess Chelation Therapy-1 (TACT-1) signaled a modest 18% relative reduction in MACE among post-MI patients. Hopes for replication, however, were tempered in 2024 when the larger, diabetes-enriched TACT-2 cohort showed no difference in the same composite endpoint despite a 61% fall in blood lead concentrations. These neutral data, presented as a late-breaker at the 2024 Annual Scientific Session and Expo of the American College of Cardiology, have largely relegated classic EDTA protocols to the periphery of evidence-based care. EDTA indiscriminately chelates divalent cations, calcium, iron, and copper, and must circulate system-wide for hours to reach appreciable plaque levels. The resulting low intralesional concentrations, coupled with repeated chair-time (40 infusions over one year in TACT), render the benefit-to-burden ratio unattractive. Furthermore, broad metal depletion risks perturbing essential metalloprotein functions[158].
Consequently, research focus has shifted toward precision delivery systems capable of depositing chelators, or other anti-calcific agents, directly within the atheroma while sparing systemic pools[159]. To operationalize this precision concept, investigators are engineering liposomal and polymeric nanoparticles that encapsulate EDTA or alternative chelators and display ligands for endothelial adhesion molecules or macrophage scavenger receptors over-expressed in inflamed plaques[160]. By resolving inflammation and clearing cellular debris, these advanced formulations could amplify the regressive potential of chelation, heralding a shift toward precision cardiovascular medicine[161].
Historically, chelation required protracted intravenous infusions; contemporary formulations now encapsulate EDTA within sub-150 nm liposomal vesicles coated with pH-responsive polymers[162]. These enteric coatings preserve vesicle integrity throughout the acidic gastric lumen and dissolve at intestinal pH ≥ 6.5[163], enabling M-cell-mediated transcytosis across Peyer’s patches[164]. By obviating parenteral access, the approach mitigates infusion-related complications and reduces per-course expenditure by an estimated 90%[165]. Consequently, oral nanoliposomal chelation emerges as a scalable, patient-centric strategy for long-term management of atherosclerotic disease[166].
Atherosclerotic plaques possess a fibrous cap enriched in fibrillar collagens, predominantly types I and IV, that can constitute nearly 60% of total plaque protein[167]. Once the overlying endothelium erodes, these collagens become exposed to the circulation, creating high-affinity docking sites for therapeutic nanocarriers[168]. Peptide-nanoparticle conjugates, typically assembled via maleimide-thiol or carbodiimide linkages, exploit this niche and can deliver low-microgram payloads without destabilizing colloidal properties[169]. Beyond the cap, plaque macrophages, especially CD206-positive, alternatively activated subsets, readily internalize such constructs by receptor-mediated endocytosis. In this canonical pathway, ligand-bound receptors cluster into clathrin-coated pits that invaginate and pinch off within 1-2 minutes, routing cargo to early endosomes and enabling cells to concentrate dilute extracellular ligands by several orders of magnitude[170].
Complementing pharmacological targeting, dietary modulation, particularly lowering LA intake to < 2% of total energy, may curb new plaque formation, thereby synergizing with chelation strategies that erode legacy calcifications[171]. This dual intervention addresses both initiation and persistence of atherosclerotic disease: Nutritional measures attenuate the inflammatory environment, whereas precision chelation dismantles established lesions[172]. Preclinical studies in apolipoprotein E-knockout mice Keuth et al[173] and Zohora et al[159] show that elastin-targeted, nanoparticle-encapsulated EDTA/diethylenetriaminepentaacetic acid regresses arterial plaque calcium by approximately 30% within 12 weeks while eliciting no detectable anti-polyethylene glycol immunoglobulin M after repeated dosing, indicating minimal immunogenicity[174,175]. However, safety concerns remain: Only approximately 9% of gold-nanoparticle mass is cleared from the liver over six months, leaving > 80% retained in the reticuloendothelial system[176], and PEGylated nanocarriers can trigger the accelerated-blood-clearance phenomenon upon subsequent injections[177]. Scalability is likewise constrained, current ligand-conjugation chemistries add roughly 500 dollars/g in raw-material costs and push per-dose prices above 200 dollars at pilot scale[178], limiting widespread adoption until continuous-flow or microfluidic Good Manufacturing Practice manufacturing drives costs down.
Notwithstanding these benefits, knowledge gaps persist regarding the long-term safety, scalability, and biodegradability of emerging nanoparticle platforms[179]. Targeted chelation therapy, augmented by nanotechnology and strategic dietary adjustment, thus represents an innovative frontier in non-invasive management of atherosclerosis. As formulation science matures and clinical validation accrues, this multifaceted approach could redefine therapeutic paradigms, deli
CVD remains the leading cause of mortality worldwide, likely claiming over 20 million lives in 2025. Despite significant advancements in acute interventions, such as PCI and CABG these treatments primarily address symptomatic manifestations, namely, occluded vessels and dysregulated lipid profiles, without fully extinguishing the underlying atherosclerotic process[181]. Historically, clinical strategies have prioritized lesion-centric metrics, such as reducing luminal stenosis, over addressing systemic drivers like oxidative stress and vascular inflammation.
Consequently, the global burden of CVD persists, underscoring the limitations of crisis-oriented management[182]. To transform CVD from a pervasive killer into a manageable rarity, a paradigm shift toward preventive and restorative strategies is imperative[183]. By integrating metabolic correction, physiologic conditioning, and plaque remodeling, a composite approach can target the root causes of atherosclerosis, offering a path to sustained vascular health[184]. By fostering a more resilient vascular system, EECP complements metabolic correction in reducing the functional im
Compared to CABG, costing approximately 100000 dollars per procedure[185] with a 50% compliance rate for postoperative lifestyle changes[186], EECP offers symptom relief at approximately 5000 dollars per 35-session course[104,187] with 80% patient adherence due to its noninvasive nature[188]. Dietary LA restriction (< 5 g/day) incurs negligible cost and achieves 70%-90% compliance with dietary counseling[189], reducing oxLDL by approximately 15% long-term[190] vs statins’ 3.4% annual residual event rate[67]. Public health policies should incentivize low-LA food labeling and expand EECP insurance coverage, currently at approximately 50% of United States plans[12,191], to maximize access and impact.
Furthermore, conventional intravenous EDTA chelation, celebrated after TACT-1 but neutralized by the 2024 TACT-2 data, now serves chiefly as a historical control. Current innovation centers on macrophage- and collagen-homing nanoliposomes that ferry chelators straight into plaques. Paired with dietary LA restriction (< 2% of energy) to throttle new oxidized-lipid influx[192]. This precision platform simultaneously could help dissolve legacy calcifications and starves nascent lesions[193]. Crucially, the latest formulations are orally administered, eliminating parenteral access, and costing an estimated 80% less than drip-based therapy[194]. Although long-term safety and scalable manufacturing still require validation, targeted nanochelation plus LA restriction presents a realistic, multi-mechanistic pathway toward non-invasive reversal of atherosclerotic CVD[195]. Taken together, these developments may move plaque regression from bench curiosity to everyday clinical cardiology within the coming decade.
Despite these promising developments, the integration of metabolic correction, physiologic conditioning, and plaque remodeling into mainstream CVD management remains nascent[196]. Current clinical trials often focus on single interventions, neglecting the synergistic potential of a composite strategy. Moreover, policy frameworks continue to emphasize lesion-centric outcomes, such as stent patency rates, over holistic measures of vascular health[197]. To bridge this gap, there is an urgent need for integrative clinical trials that evaluate the combined efficacy of these approaches in reducing oxidative stress and promoting vascular healing[198]. Additionally, policy shifts that prioritize preventive and restorative care, through incentives for dietary optimization and access to innovative therapies, are essential to curb the CVD epidemic. Nevertheless, challenges persist, including the need for standardized protocols and long-term safety data for novel interventions like advanced chelation. Furthermore, the scalability of personalized metabolic correction requires robust biomarkers to tailor dietary recommendations effectively[199]. Addressing these limitations will be crucial for the widespread adoption of a root-cause approach to CVD management.
By marrying conventional therapies with innovative solutions that address the core drivers of atherosclerosis, the medical community stands poised to redefine CVD management. Embracing a composite strategy that integrates meta
The persistent global burden of CVD underscores the limitations of lesion-centric interventions like PCI and statins, which reduce MACE by 25%-30% but leave a 20% five-year residual risk. Historically prioritized for acute symptom relief, these modalities fail to address upstream atherogenic processes such as mitochondrial oxidative stress and arterial inflammation. This paper advocates an integrative framework that synergizes metabolic correction, physiologic conditioning, and plaque remodeling to target these root causes. For example, restricting dietary LA to < 5 g/day reduces hsCRP by approximately 15%, while EECP alleviates angina in 70%-80% of refractory cases by enhancing NO-mediated vasodilation.
Historically, chelation therapy faced skepticism due to variable results. Yet, nanoparticle-facilitated chelation, targeting EDTA directly to atherosclerotic plaques, presents a theoretically promising, non-invasive method for plaque regression. Oral formulations may cut costs by approximately 80% vs intravenous approaches. Still, standardized protocols and long-term safety data are lacking. Though conceptually sound, its efficacy awaits clinical trial confirmation. Future research should prioritize multicenter RCTs evaluating the combined efficacy of LA restriction, EECP, and targeted chelation on MACE reduction, with 5-year follow-ups. Long-term safety studies must assess nanoparticle clearance rates in humans, targeting < 5% organ retention. Interdisciplinary collaboration among cardiologists, nutritionists, and nanomedicine specialists is essential to standardize protocols and translate findings into practice. Integrative trials with existing therapies and a preventive care focus are key. If proven, this could make CVD manageable, potentially slashing its incidence within a generation by tackling root causes, not just symptoms.
| 1. | Chong B, Jayabaskaran J, Jauhari SM, Chan SP, Goh R, Kueh MTW, Li H, Chin YH, Kong G, Anand VV, Wang JW, Muthiah M, Jain V, Mehta A, Lim SL, Foo R, Figtree GA, Nicholls SJ, Mamas MA, Januzzi JL, Chew NWS, Richards AM, Chan MY. Global burden of cardiovascular diseases: projections from 2025 to 2050. Eur J Prev Cardiol. 2024;zwae281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 2. | Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan GA, Dweck MR, Galbraith M, Gilard M, Hinterbuchner L, Jankowska EA, Jüni P, Kimura T, Kunadian V, Leosdottir M, Lorusso R, Pedretti RFE, Rigopoulos AG, Rubini Gimenez M, Thiele H, Vranckx P, Wassmann S, Wenger NK, Ibanez B; ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 1884] [Article Influence: 942.0] [Reference Citation Analysis (0)] |
| 3. | Thrane PG, Olesen KKW, Thim T, Gyldenkerne C, Mortensen MB, Kristensen SD, Maeng M. Mortality Trends After Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2023;82:999-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 4. | Soroush N, Nekouei Shahraki M, Mohammadi Jouabadi S, Amiri M, Aribas E, Stricker BH, Ahmadizar F. Statin therapy and cardiovascular protection in type 2 diabetes: The role of baseline LDL-Cholesterol levels. A systematic review and meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2024;34:2021-2033. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Cholesterol Treatment Trialists' Collaboration. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet. 2022;400:832-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 6. | Bae S, Ahn JB, Joseph C, Whisler R, Schnitzler MA, Lentine KL, Kadosh BS, Segev DL, McAdams-DeMarco MA. Incidence of Statin-Associated Adverse Events in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2023;18:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 7. | Giubilato S, Lucà F, Abrignani MG, Gatto L, Rao CM, Ingianni N, Amico F, Rossini R, Caretta G, Cornara S, Di Matteo I, Di Nora C, Favilli S, Pilleri A, Pozzi A, Temporelli PL, Zuin M, Amico AF, Riccio C, Grimaldi M, Colivicchi F, Oliva F, Gulizia MM. Management of Residual Risk in Chronic Coronary Syndromes. Clinical Pathways for a Quality-Based Secondary Prevention. J Clin Med. 2023;12:5989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Choi KH, Kwon W, Shin D, Lee SH, Hwang D, Zhang J, Nam CW, Shin ES, Doh JH, Chen SL, Kakuta T, Toth GG, Piroth Z, Hakeem A, Uretsky BF, Hokama Y, Tanaka N, Lim HS, Ito T, Matsuo A, Azzalini L, Leesar MA, Daemen J, Collison D, Collet C, De Bruyne B, Koo BK, Park TK, Yang JH, Song YB, Hahn JY, Choi SH, Gwon HC, Lee JM. Differential Impact of Fractional Flow Reserve Measured After Coronary Stent Implantation by Left Ventricular Dysfunction. JACC Asia. 2024;4:229-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Bi L, Geng Y, Wang Y, Li S, Sun K, Guo Y, Zhang O, Zhang P. An updated meta-analysis of optimal medical therapy with or without invasive therapy in patients with stable coronary artery disease. BMC Cardiovasc Disord. 2024;24:335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Mach F, Visseren FLJ, Cater NB, Salhi N, Soronen J, Ray KK, Delgado V, Jukema JW, Laufs U, Zamorano JL, Ros E, Plat J, Gesztes AG, Tokgozoglu L, Packard C, Libby P. Addressing residual risk beyond statin therapy: New targets in the management of dyslipidaemias-A report from the European Society of Cardiology Cardiovascular Round Table. J Clin Lipidol. 2024;18:e685-e700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Stone GW, Maehara A, Ali ZA, Held C, Matsumura M, Kjøller-Hansen L, Bøtker HE, Maeng M, Engstrøm T, Wiseth R, Persson J, Trovik T, Jensen U, James SK, Mintz GS, Dressler O, Crowley A, Ben-Yehuda O, Erlinge D; PROSPECT ABSORB Investigators. Percutaneous Coronary Intervention for Vulnerable Coronary Atherosclerotic Plaque. J Am Coll Cardiol. 2020;76:2289-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 12. | Vijayaraghavan K, Baum S, Desai NR, Voyce SJ. Intermediate and long-term residual cardiovascular risk in patients with established cardiovascular disease treated with statins. Front Cardiovasc Med. 2023;10:1308173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Lu ZF, Yin WH, Schoepf UJ, Abrol S, Ma JW, Yu XB, Zhao L, Su XM, Wang CS, An YQ, Xiao ZC, Lu B. Residual Risk in Non-ST-Segment Elevation Acute Coronary Syndrome: Quantitative Plaque Analysis at Coronary CT Angiography. Radiology. 2023;308:e230124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Eftekhari A, Holck EN, Westra J, Olsen NT, Bruun NH, Jensen LO, Engstrøm T, Christiansen EH. Instantaneous wave free ratio vs. fractional flow reserve and 5-year mortality: iFR SWEDEHEART and DEFINE FLAIR. Eur Heart J. 2023;44:4376-4384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 15. | Batty M, Bennett MR, Yu E. The Role of Oxidative Stress in Atherosclerosis. Cells. 2022;11:3843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 197] [Article Influence: 65.7] [Reference Citation Analysis (1)] |
| 16. | Higashi Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants (Basel). 2022;11:1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 17. | Noothi SK, Ahmed MR, Agrawal DK. Residual risks and evolving atherosclerotic plaques. Mol Cell Biochem. 2023;478:2629-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Weber C, Habenicht AJR, von Hundelshausen P. Novel mechanisms and therapeutic targets in atherosclerosis: inflammation and beyond. Eur Heart J. 2023;44:2672-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 19. | Rakocevic J, Dobric M, Borovic ML, Milutinovic K, Milenkovic S, Tomasevic M. Anti-Inflammatory Therapy in Coronary Artery Disease: Where Do We Stand? Rev Cardiovasc Med. 2023;24:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Buckler AJ, Doros G, Kinninger A, Lakshmanan S, Le VT, Libby P, May HT, Muhlestein JB, Nelson JR, Nicolaou A, Roy SK, Shaikh K, Shekar C, Tayek JA, Zheng L, Bhatt DL, Budoff MJ. Quantitative imaging biomarkers of coronary plaque morphology: insights from EVAPORATE. Front Cardiovasc Med. 2023;10:1204071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Wang J, Wu Q, Wang X, Liu H, Chen M, Xu L, Zhang Z, Li K, Li W, Zhong J. Targeting Macrophage Phenotypes and Metabolism as Novel Therapeutic Approaches in Atherosclerosis and Related Cardiovascular Diseases. Curr Atheroscler Rep. 2024;26:573-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Drexel H, Mader A, Larcher B, Festa A, Vonbank A, Fraunberger P, Leiherer A, Saely CH. Remnant cholesterol and long-term incidence of death in coronary artery disease patients. Atherosclerosis. 2025;401:119048. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Yoshimitsu Y, Awaya T, Kawagoe N, Kunimasa T, Iijima R, Hara H. Coronary Plaque Regression and Fractional Flow Reserve Improvement in a Chronic Coronary Syndrome Case: Early Optimal Medical Therapy and Fractional Flow Reserve-Computed Tomography Follow-Up Strategy. Diseases. 2024;12:297. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Ortiz M, Álvarez D, Muñoz Y, Crisosto N, Valenzuela R, Maliqueo M. Linoleic and Arachidonic Fatty Acids and their Potential Relationship with Inflammation, Pregnancy, and Fetal Development. Curr Med Chem. 2024;31:5046-5060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 25. | Shi F, Chowdhury R, Sofianopoulou E, Koulman A, Sun L, Steur M, Aleksandrova K, Dahm CC, Schulze MB, van der Schouw YT, Agnoli C, Amiano P, Boer JMA, Bork CS, Cabrera-Castro N, Eichelmann F, Elbaz A, Farràs M, Heath AK, Kaaks R, Katzke V, Keski-Rahkonen P, Masala G, Moreno-Iribas C, Panico S, Papier K, Petrova D, Quirós JR, Ricceri F, Severi G, Tjønneland A, Tong TYN, Tumino R, Wareham NJ, Weiderpass E, Di Angelantonio E, Forouhi NG, Danesh J, Butterworth AS, Kaptoge S. Association of circulating fatty acids with cardiovascular disease risk: analysis of individual-level data in three large prospective cohorts and updated meta-analysis. Eur J Prev Cardiol. 2025;32:233-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J, Lee KL; TACT Investigators. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 27. | Lamas GA, Anstrom KJ, Navas-Acien A, Boineau R, Nemeth H, Huang Z, Wen J, Rosenberg Y, Stylianou M, Jones TLZ, Joubert BR, Yu Q, Santella RM, Mon AC, Ujueta F, Escolar E, Nathan DM, Fonseca VA, Aude YW, Ehrman JK, Elliott T, Prashad R, Lewis EF, Lopes RD, Farkouh ME, Elliott AM, Newman JD, Mark DB; TACT2 Investigators. Edetate Disodium-Based Chelation for Patients With a Previous Myocardial Infarction and Diabetes: TACT2 Randomized Clinical Trial. JAMA. 2024;332:794-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Theofilis P, Oikonomou E, Sagris M, Papageorgiou N, Tsioufis K, Tousoulis D. Novel Concepts in the Management of Angina in Coronary Artery Disease. Curr Pharm Des. 2023;29:1825-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Rahman M, Alatiqi M, Al Jarallah M, Hussain MY, Monayem A, Panduranga P, Rajan R. Cardiovascular Effects of Smoking and Smoking Cessation: A 2024 Update. Glob Heart. 2025;20:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 30. | Lin CC, Kinnett-Hopkins D, Alawamleh A, Siemen M, Lane A, Abou L. Physical activity improves cardiovascular fitness and reduces cardiovascular risk factors in adults with multiple sclerosis: A systematic review and meta-analysis. Mult Scler Relat Disord. 2024;92:106170. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Singh R, Chandi SK, Sran S, Aulakh SK, Nijjar GS, Singh K, Singh S, Tanvir F, Kaur Y, Sandhu APS. Emerging Therapeutic Strategies in Cardiovascular Diseases. Cureus. 2024;16:e64388. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Martin SS, Aday AW, Allen NB, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Bansal N, Beaton AZ, Commodore-Mensah Y, Currie ME, Elkind MSV, Fan W, Generoso G, Gibbs BB, Heard DG, Hiremath S, Johansen MC, Kazi DS, Ko D, Leppert MH, Magnani JW, Michos ED, Mussolino ME, Parikh NI, Perman SM, Rezk-Hanna M, Roth GA, Shah NS, Springer MV, St-Onge MP, Thacker EL, Urbut SM, Van Spall HGC, Voeks JH, Whelton SP, Wong ND, Wong SS, Yaffe K, Palaniappan LP; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Committee. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. 2025;151:e41-e660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 75] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 33. | Bruoha S, Galli M, Sabouret P, Yosefy C, Taha L, Gragnano F, Savage MP, Shuvy M, Biondi-Zoccai G, Glikson M, Asher E. Atherosclerotic Plaque Erosion: Mechanisms, Clinical Implications, and Potential Therapeutic Strategies-A Review. J Cardiovasc Pharmacol. 2024;83:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 34. | Wu H, Diao H, Zhang F, Jiang W, Pan T, Bian Y. Organelle interplay in cardiovascular diseases: Mechanisms, pathogenesis, and therapeutic perspectives. Biomed Pharmacother. 2025;185:117978. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Theodorakis N, Nikolaou M, Krentz A. Cardiovascular-Endocrine-Metabolic Medicine: Proposing a New Clinical Sub-Specialty Amid the Cardiometabolic Pandemic. Biomolecules. 2025;15:373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 36. | Burgess S, Zaman S, Towns C, Coylewright M, Cader FA. The under-representation of women in cardiovascular clinical trials: State-of-the-art review and ethical considerations. Am Heart J. 2025;282:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 37. | Mansfield BS, Mohamed F, Larouche M, Raal FJ. The Hurdle of Access to Emerging Therapies and Potential Solutions in the Management of Dyslipidemias. J Clin Med. 2024;13:4160. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Huerne K, Eisenberg MJ. Advancing telemedicine in cardiology: A comprehensive review of evolving practices and outcomes in a postpandemic context. Cardiovasc Digit Health J. 2024;5:96-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 39. | Dimagli A, Spadaccio C, Myers A, Demetres M, Rademaker-Havinga T, Stone GW, Spertus JA, Redfors B, Fremes S, Gaudino M, Masterson Creber R. Quality of Life After Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting. J Am Heart Assoc. 2023;12:e030069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Llerena-Velastegui J, Zumbana-Podaneva K, Velastegui-Zurita S, Mejia-Mora M, Perez-Tomassetti J, Cabrera-Cruz A, Haro-Arteaga P, de Jesus ACFS, Coelho PM, Sanahuja-Montiel C. Comparative Efficacy of Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting in the Treatment of Ischemic Heart Disease: A Systematic Review and Meta-Analysis of Recent Randomized Controlled Trials. Cardiol Res. 2024;15:153-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 41. | Occhipinti G, Brugaletta S, Abbate A, Pedicino D, Del Buono MG, Vinci R, Biondi Zoccai G, Sabate M, Angiolillo D, Liuzzo G. Inflammation in coronary atherosclerosis: diagnosis and treatment. Heart. 2025;heartjnl-2024. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Cheng AM, Doll JA. When to Consider Coronary Revascularization for Stable Coronary Artery Disease. Med Clin North Am. 2024;108:517-538. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Vafaei P, Naderi S, Ambrosy AP, Slade JJ. Implications of the Landmark ISCHEMIA Trial on the Initial Management of High-Risk Patients with Stable Ischemic Heart Disease. Curr Atheroscler Rep. 2021;23:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Hochman JS, Anthopolos R, Reynolds HR, Bangalore S, Xu Y, O'Brien SM, Mavromichalis S, Chang M, Contreras A, Rosenberg Y, Kirby R, Bhargava B, Senior R, Banfield A, Goodman SG, Lopes RD, Pracoń R, López-Sendón J, Maggioni AP, Newman JD, Berger JS, Sidhu MS, White HD, Troxel AB, Harrington RA, Boden WE, Stone GW, Mark DB, Spertus JA, Maron DJ; ISCHEMIA-EXTEND Research Group. Survival After Invasive or Conservative Management of Stable Coronary Disease. Circulation. 2023;147:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 91] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 45. | Hirao Y, Seki T, Watanabe N, Matoba S. Health-Related Quality of Life After Percutaneous Coronary Intervention for Stable Ischemic Heart Disease: A Systematic Review and Meta-analysis. Can J Cardiol. 2023;39:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Jolly SS, Lee SF, Mian R, Kedev S, Lavi S, Moreno R, Montalescot G, Hillani A, Henry TD, Asani V, Storey RF, Silvain J, Spratt JCS, d'Entremont MA, Stankovic G, Zafirovska B, Natarajan MK, Sabate M, Shreenivas S, Pinilla-Echeverri N, Sheth T, Altisent OA, Ribas N, Skuriat E, Tyrwhitt J, Mehta SR. SYNERGY-Everolimus-Eluting Stent With a Bioabsorbable Polymer in ST-Elevation Myocardial Infarction: CLEAR SYNERGY OASIS-9 Registry. Am J Cardiol. 2024;220:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 47. | Souteyrand G, Mouyen T, Honton B, Mulliez A, Lattuca B, Dilinger JG, Levesque S, Range G, Combaret N, Marliere S, Lamallem O, Quillot M, Gerbaud E, Motreff P, Amabile N. Stent Underexpansion Is an Underestimated Cause of Intrastent Restenosis: Insights From RESTO Registry. J Am Heart Assoc. 2024;13:e036065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 48. | Dinc R. A review of the current state in neointimal hyperplasia development following endovascular intervention and minor emphasis on new horizons in immunotherapy. Transl Clin Pharmacol. 2023;31:191-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Rubino F, Pompei G, Brugaletta S, Collet C, Kunadian V. The role of physiology in the contemporary management of coronary artery disease. Heart. 2024;110:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Kjøller-Hansen L, Maehara A, Kelbæk H, Matsumura M, Maeng M, Engstrøm T, Fröbert O, Persson J, Wiseth R, Larsen AI, Jensen LO, Nordrehaug JE, Omerovic E, Held C, James S, Mintz GS, Ali ZA, Stone GW, Erlinge D. Impact of Lipidic Plaque on In-Stent and Stent Edge-Related Events After PCI in Myocardial Infarction: A PROSPECT II Substudy. Circ Cardiovasc Interv. 2024;17:e014215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 51. | Alzahrani AH, Itagaki S, Egorova NN, Chikwe J. Choice of revascularization strategy for ischemic cardiomyopathy due to multivessel coronary disease. J Thorac Cardiovasc Surg. 2025;169:639-647.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 52. | Chan J, Dimagli A, Dong T, Fudulu DP, Sinha S, Angelini GD. Trend and early clinical outcomes of off-pump coronary artery bypass grafting in the UK. Eur J Cardiothorac Surg. 2023;64:ezad272. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Ivert T, Berge A, Bratt S, Dalén M. Incidence and healing times of postoperative sternal wound infections: a retrospective observational single-centre study. Scand Cardiovasc J. 2024;58:2330349. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Brown CH, Lewis A, Probert J, Parish M, Tian J, Mandal K, Everett A, Colantuoni E, Kamath V, Hogue C, Moghekar A. Perioperative Neurofilament Light Plasma Concentrations and Cognition before and after Cardiac Surgery: A Prospective Nested Cohort Study. Anesthesiology. 2022;137:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Harik L, Perezgrovas-Olaria R, Soletti G Jr, Dimagli A, Alzghari T, An KR, Cancelli G, Gaudino M, Sandner S. Graft thrombosis after coronary artery bypass surgery and current practice for prevention. Front Cardiovasc Med. 2023;10:1125126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Kirov H, Caldonazo T, Mukharyamov M, Toshmatov S, Fleckenstein P, Kyashif T, Siemeni T, Doenst T. Cardiac Surgery 2024 Reviewed. Thorac Cardiovasc Surg. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 57. | Katkenov N, Mukhatayev Z, Kozhakhmetov S, Sailybayeva A, Bekbossynova M, Kushugulova A. Systematic Review on the Role of IL-6 and IL-1β in Cardiovascular Diseases. J Cardiovasc Dev Dis. 2024;11:206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 58. | Gyldenkerne C, Maeng M, Kjøller-Hansen L, Maehara A, Zhou Z, Ben-Yehuda O, Erik Bøtker H, Engstrøm T, Matsumura M, Mintz GS, Fröbert O, Persson J, Wiseth R, Larsen AI, Jensen LO, Nordrehaug JE, Bleie Ø, Omerovic E, Held C, James SK, Ali ZA, Rosen HC, Stone GW, Erlinge D. Coronary Artery Lesion Lipid Content and Plaque Burden in Diabetic and Nondiabetic Patients: PROSPECT II. Circulation. 2023;147:469-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 59. | Jamialahmadi T, Reiner Ž, Simental-Mendia LE, Almahmeed W, Karav S, Eid AH, Giammarile F, Sahebkar A. Effect of statins on arterial wall inflammation as assessed by 18F-FDG PET CT: an updated systematic review and meta-analysis. J Inflamm (Lond). 2024;21:52. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Potere N, Bonaventura A, Abbate A. Novel Therapeutics and Upcoming Clinical Trials Targeting Inflammation in Cardiovascular Diseases. Arterioscler Thromb Vasc Biol. 2024;44:2371-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 61. | Xing Y, Lin X. Challenges and advances in the management of inflammation in atherosclerosis. J Adv Res. 2025;71:317-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 62. | Pang B, Dong G, Pang T, Sun X, Liu X, Nie Y, Chang X. Emerging insights into the pathogenesis and therapeutic strategies for vascular endothelial injury-associated diseases: focus on mitochondrial dysfunction. Angiogenesis. 2024;27:623-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 63. | Hernandez-Sómerson MA, Montoya-Agudelo F, Huertas-Rodriguez G. Efficacy and safety of drugs in residual cardiovascular risk: A systematic review of the literature. Int J Cardiol Cardiovasc Risk Prev. 2024;22:200298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 64. | Osnes JB, Andressen KW, Levy FO. Statins: 50 years old and with new surprises in store. Tidsskr Nor Laegeforen. 2024;144. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 65. | Hong SJ, Lee YJ, Lee SJ, Hong BK, Kang WC, Lee JY, Lee JB, Yang TH, Yoon J, Ahn CM, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK; LODESTAR Investigators. Treat-to-Target or High-Intensity Statin in Patients With Coronary Artery Disease: A Randomized Clinical Trial. JAMA. 2023;329:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 66. | Dugré N, Lindblad AJ, Perry D, Allan GM, Braschi É, Falk J, Froentjes L, Garrison SR, Kirkwood JEM, Korownyk CS, McCormack JP, Moe SS, Paige A, Potter J, Thomas BS, Ton J, Young J, Weresch J, Kolber MR. Lipid-lowering therapies for cardiovascular disease prevention and management in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2023;69:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 67. | Bashir B, Schofield J, Downie P, France M, Ashcroft DM, Wright AK, Romeo S, Gouni-Berthold I, Maan A, Durrington PN, Soran H. Beyond LDL-C: unravelling the residual atherosclerotic cardiovascular disease risk landscape-focus on hypertriglyceridaemia. Front Cardiovasc Med. 2024;11:1389106. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 68. | Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4679] [Cited by in RCA: 4788] [Article Influence: 281.6] [Reference Citation Analysis (0)] |
| 69. | Gaba P, O'Donoghue ML, Park JG, Wiviott SD, Atar D, Kuder JF, Im K, Murphy SA, De Ferrari GM, Gaciong ZA, Toth K, Gouni-Berthold I, Lopez-Miranda J, Schiele F, Mach F, Flores-Arredondo JH, López JAG, Elliott-Davey M, Wang B, Monsalvo ML, Abbasi S, Giugliano RP, Sabatine MS. Association Between Achieved Low-Density Lipoprotein Cholesterol Levels and Long-Term Cardiovascular and Safety Outcomes: An Analysis of FOURIER-OLE. Circulation. 2023;147:1192-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 84] [Reference Citation Analysis (0)] |
| 70. | Giordano S, Ielapi J, Salerno N, Cersosimo A, Lucchino A, Laschera A, Canino G, Di Costanzo A, De Rosa S, Torella D, Sorrentino S. Rationale for Early Administration of PCSK9 Inhibitors in Acute Coronary Syndrome. Rev Cardiovasc Med. 2024;25:374. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 71. | Kumar R, Krishnaperumal G, Vellapandian C. Innovative mRNA Vaccine Approaches in Targeting Atherosclerosis: A New Era in Cardiovascular Therapy. Cureus. 2024;16:e74141. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Ridker PM. Targeting residual inflammatory risk: The next frontier for atherosclerosis treatment and prevention. Vascul Pharmacol. 2023;153:107238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Csengo E, Lorincz H, Csosz E, Guba A, Karai B, Toth J, Csiha S, Paragh G, Harangi M, Nagy GG. Newly Initiated Statin Treatment Is Associated with Decreased Plasma Coenzyme Q10 Level After Acute ST-Elevation Myocardial Infarction. Int J Mol Sci. 2024;26:106. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 74. | Razavi AC, Reyes MP, Wilkins JT, Szklo MS, Tsai MY, Whelton SP, Sperling LS, Tsimikas S, Bhatia HS. Traditional risk factors, optimal cardiovascular health, and elevated lipoprotein(a). Eur J Prev Cardiol. 2025;32:724-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Horton WB, Love KM, Gregory JM, Liu Z, Barrett EJ. Metabolic and vascular insulin resistance: partners in the pathogenesis of cardiovascular disease in diabetes. Am J Physiol Heart Circ Physiol. 2025;328:H1218-H1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 76. | Mohammadnia N, Opstal TSJ, El Messaoudi S, Bax WA, Cornel JH. An Update on Inflammation in Atherosclerosis: How to Effectively Treat Residual Risk. Clin Ther. 2023;45:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Dutka M, Zimmer K, Ćwiertnia M, Ilczak T, Bobiński R. The role of PCSK9 in heart failure and other cardiovascular diseases-mechanisms of action beyond its effect on LDL cholesterol. Heart Fail Rev. 2024;29:917-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | Iatan I, Mancini GBJ, Yeoh E, Hegele RA. Statin associated muscle symptoms (SAMS): strategies for prevention, assessment and management. Expert Rev Cardiovasc Ther. 2023;21:423-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Rakhshanda S, Liaw ST, Rhee J, Rye KA, Jonnagaddala J. Strategies to Address Statin Medication Intolerance Among Patients at Risk of Cardiovascular Disease Identified Through Electronic Health Records: A Literature Review and Pooled Analysis. Stud Health Technol Inform. 2024;316:132-136. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 80. | Wang B, Huang S, Li S, Deng Y, Li Z, Wang Y, Shi X, Zhang W, Shi L, Wang X, Tang X. Hepatotoxicity of statins: a real-world study based on the US Food and Drug Administration Adverse Event Reporting System database. Front Pharmacol. 2024;15:1502791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 81. | Tsui L, Wang D, Fan C, Huang Y, Zhang Z, Fang Z, Xie W. Evaluation of statin-induced muscle and liver adverse drug reactions in the Chinese population: a retrospective analysis of clinical trial data from 1992 to 2023. Eur J Hosp Pharm. 2025;ejhpharm-2024. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 82. | Wang H, Li Y, Zhang L, Lu M, Li C, Li Y. Anti-Inflammatory Lipid Mediators from Polyunsaturated Fatty Acids: Insights into their Role in Atherosclerosis Microenvironments. Curr Atheroscler Rep. 2025;27:48. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Abouzid MR, Eldahtoury S, Elshafei SM, Devi S, Saleh A, Esteghamati S, Kamel I. Efficacy of Angiotensin-Converting Enzyme Inhibitors in Coronary Microvascular Dysfunction: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Cureus. 2024;16:e52684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 84. | Averna M, Cefalù AB. LP(a): The new marker of high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2025;35:103845. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 85. | Zubirán R, Neufeld EB, Dasseux A, Remaley AT, Sorokin AV. Recent Advances in Targeted Management of Inflammation In Atherosclerosis: A Narrative Review. Cardiol Ther. 2024;13:465-491. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 86. | Hughes J, Shymka M, Ng T, Phulka JS, Safabakhsh S, Laksman Z. Polygenic Risk Score Implementation into Clinical Practice for Primary Prevention of Cardiometabolic Disease. Genes (Basel). 2024;15:1581. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Cao F, Liu Y, Wei W, Liang J. Effect of enhanced external counterpulsation on coronary microcirculation dysfunction (CMD) in patients with coronary artery disease (EECP-CMD II): study protocol of a single-centre, open-label, parallel group, randomised controlled trial. BMJ Open. 2024;14:e086901. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 88. | Ashokprabhu ND, Fox J, Henry TD, Schmidt CW, Tierney D, Gallatin J, Alvarez YR, Thompson L, Hamstra M, Shah SA, Quesada O. Enhanced External Counterpulsation for the Treatment of Angina With Nonobstructive Coronary Artery Disease. Am J Cardiol. 2024;211:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Zhang Q, Zhang YH, Hao LL, Xu XH, Wu GF, Lin L, Xu XL, Qi L, Tian S. A numerical study on the siphonic effect of enhanced external counterpulsation at lower extremities with a coupled 0D-1D closed-loop personalized hemodynamics model. J Biomech. 2024;166:112057. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 90. | Ren Z, Wu Z, Wang Y, Jakhongirkhon I, Zhou Q, Du J. Enhanced External Counterpulsation Intervention Induces the Variation of Physiological Parameters and Shear Stress Metrics in the Carotid Artery. Bioengineering (Basel). 2025;12:386. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 91. | Tartaglia JT, Eisenberg CA, DeMarco JC, Puccio G, Tartaglia CE, Hamby CV. Mobilization of Endogenous CD34+/CD133+ Endothelial Progenitor Cells by Enhanced External Counter Pulsation for Treatment of Refractory Angina. Int J Mol Sci. 2024;25:10030. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 92. | Vervaat FE, van der Gaag A, Teeuwen K, van Suijlekom H, Wijnbergen I. Neuromodulation in patients with refractory angina pectoris: a review. Eur Heart J Open. 2023;3:oeac083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 93. | Yin Q, Jiang H, Zhang Z, Zhang L, Wu Z, Huang L, Chen X. Influence of enhanced external counterpulsation on endothelial function: a meta-analysis of randomized controlled trials. Scand Cardiovasc J. 2023;57:2273223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Akula A, Grafft HR, Tak N, Haberman DA, Tak T. Enhanced External Counterpulsation Outcomes Study: Retrospective Analyses of Data Obtained from Patients at a Single Medical Center in United States. Int J Angiol. 2024;33:182-188. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 95. | Pecha A, White B, Yan H. Exploring Functional Improvements by Sex in Six-Minute Walk Test, Exertional Angina, and Dyspnea After Enhanced External Counterpulsation Therapy. J Cardiopulm Rehabil Prev. 2024;44:333-338. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 96. | Wang Q, Hao J, Jiang W, Tan Q. Enhanced external counterpulsation increases coronary flow reserve in coronary microvascular disease. Saudi Med J. 2023;44:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 97. | Sharma VK, Gopinathan A, Tan BYQ, Loh PH, Hung J, Tang D, Chua C, Chan ACY, Ong JJY, Chin A, Jing M, Goh Y, Sunny S, Keat CH, Ka Z, Pandya S, Wong LYH, Chen JT, Yeo LLL, Chan BPL, Teoh HL, Sinha AK. Enhanced external counter pulsation therapy in patients with symptomatic and severe intracranial steno-occlusive disease: a randomized clinical trial protocol. Front Neurol. 2023;14:1177500. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 98. | Zhang Y, Zhang Y, Zhong C, Wang Y, Wei W, Wu G. Effect of enhanced external counterpulsation versus individual shear rate therapy on the peripheral artery functions. Sci Rep. 2024;14:31197. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 99. | Hao X, Zhang Y, Huang D, Gu W, Lu Y. Effect of enhanced external counterpulsation on the rehabilitation of patients with acute myocardial infarction after drug-coated balloon-based percutaneous coronary intervention. J Cardiothorac Surg. 2025;20:210. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 100. | Michaels AD, Linnemeier G, Soran O, Kelsey SF, Kennard ED. Two-year outcomes after enhanced external counterpulsation for stable angina pectoris (from the International EECP Patient Registry [IEPR]). Am J Cardiol. 2004;93:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Gloekler S, Meier P, de Marchi SF, Rutz T, Traupe T, Rimoldi SF, Wustmann K, Steck H, Cook S, Vogel R, Togni M, Seiler C. Coronary collateral growth by external counterpulsation: a randomised controlled trial. Heart. 2010;96:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 102. | Buschmann EE, Utz W, Pagonas N, Schulz-Menger J, Busjahn A, Monti J, Maerz W, le Noble F, Thierfelder L, Dietz R, Klauss V, Gross M, Buschmann IR; Arteriogenesis Network (Art. Net.). Improvement of fractional flow reserve and collateral flow by treatment with external counterpulsation (Art.Net.-2 Trial). Eur J Clin Invest. 2009;39:866-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Sharma U, Ramsey HK, Tak T. The role of enhanced external counter pulsation therapy in clinical practice. Clin Med Res. 2013;11:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Lawson WE. Current use of enhanced external counterpulsation and patient selection. Clin Cardiol. 2002;25:II16-II21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 105. | Chen Y, Ge Y, Chao T, Huan N, Liu W, Chu G, Wang C. Refractory angina pectoris: a 20-year (2003-2022) bibliometric analysis. Front Cardiovasc Med. 2023;10:1228201. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 106. | Huang J, Fan Y, Wang Y, Liu J. The effects of enhanced external counter-pulsation on post-acute sequelae of COVID-19: A narrative review. Open Med (Wars). 2025;20:20241067. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Purmessur R, Wijesena T, Ali J. Minimal-Access Coronary Revascularization: Past, Present, and Future. J Cardiovasc Dev Dis. 2023;10:326. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 108. | Xu L, Cui M, Zhao W. The Effect of EECP on Ischemic Heart Failure: a Systematic Review. Curr Cardiol Rep. 2023;25:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Sathyamoorthy M, Sevak RJ, Cabrera J, Lopez M, Fox J, Shah SA, Verduzco-Gutierrez M. Enhanced External Counterpulsation Improves Cognitive Function of Persons With Long COVID. Am J Phys Med Rehabil. 2024;103:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 110. | Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 111. | Teicholz N. A short history of saturated fat: the making and unmaking of a scientific consensus. Curr Opin Endocrinol Diabetes Obes. 2023;30:65-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 112. | Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6994] [Cited by in RCA: 6391] [Article Influence: 1278.2] [Reference Citation Analysis (0)] |
| 113. | Zhang H, Zhou XD, Shapiro MD, Lip GYH, Tilg H, Valenti L, Somers VK, Byrne CD, Targher G, Yang W, Viveiros O, Opio CK, Mantzoros CS, Ryan JD, Kok KYY, Jumaev NA, Perera N, Robertson AG, Abu-Abeid A, Misra A, Wong YJ, Ruiz-Úcar E, Ospanov O, Kızılkaya MC, Luo F, Méndez-Sánchez N, Zuluaga M, Lonardo A, Al Momani H, Toro-Huamanchumo CJ, Adams L, Al-Busafi SA, Sharara AI, Chan WK, Abbas SI, Sookoian S, Treeprasertsuk S, Ocama P, Alswat K, Kong AP, Ataya K, Lim-Loo MC, Oviedo RJ, Szepietowski O, Fouad Y, Zhang H, Abdelbaki TN, Katsouras CS, Prasad A, Thaher O, Ali A, Molina GA, Sung KC, Chen QF, Lesmana CRA, Zheng MH. Global burden of metabolic diseases, 1990-2021. Metabolism. 2024;160:155999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 114. | Yamashima T. 4-Hydroxynonenal from Mitochondrial and Dietary Sources Causes Lysosomal Cell Death for Lifestyle-Related Diseases. Nutrients. 2024;16:4171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 115. | Wagner BA, Buettner GR, Burns CP. Free radical-mediated lipid peroxidation in cells: oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry. 1994;33:4449-4453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 239] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 116. | Fuentes JM, Morcillo P. The Role of Cardiolipin in Mitochondrial Function and Neurodegenerative Diseases. Cells. 2024;13:609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 117. | Tang Y, Wu J, Sun X, Tan S, Li W, Yin S, Liu L, Chen Y, Liu Y, Tan Q, Jiang Y, Yang W, Huang W, Weng C, Wu Q, Lu Y, Yuan H, Xiao Q, Chen AF, Xu Q, Billiar TR, Cai J. Cardiolipin oxidized by ROS from complex II acts as a target of gasdermin D to drive mitochondrial pore and heart dysfunction in endotoxemia. Cell Rep. 2024;43:114237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 118. | Mercola J. Reductive stress and mitochondrial dysfunction: The hidden link in chronic disease. Free Radic Biol Med. 2025;233:118-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 119. | Grayson C, Chalifoux O, Russo MST, Avizonis DZ, Sterman S, Faerman B, Koufos O, Agellon LB, Mailloux RJ. Ablating the glutaredoxin-2 (Glrx2) gene protects male mice against non-alcoholic fatty liver disease (NAFLD) by limiting oxidative distress. Free Radic Biol Med. 2024;224:660-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 120. | Weng SW, Wu JC, Shen FC, Chang YH, Su YJ, Lian WS, Tai MH, Su CH, Chuang JH, Lin TK, Liou CW, Chu TH, Kao YH, Wang FS, Wang PW. Chaperonin counteracts diet-induced non-alcoholic fatty liver disease by aiding sirtuin 3 in the control of fatty acid oxidation. Diabetologia. 2023;66:913-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 121. | Yang Y, Li N, Song J, Tian Y, Chen B, Li J, Lin L, Qin Z. Hemolysis-associated release of hemoglobin induces mitochondrial oxidative phosphorylation (OXPHOS) disturbance and aggravates cell oxidative damage in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2025;157:110043. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | Kim OY, Song J. Important roles of linoleic acid and α-linolenic acid in regulating cognitive impairment and neuropsychiatric issues in metabolic-related dementia. Life Sci. 2024;337:122356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 123. | Pyrpyris N, Dimitriadis K, Beneki E, Iliakis P, Soulaidopoulos S, Tsioufis P, Adamopoulou E, Kasiakogias A, Sakalidis A, Koutsopoulos G, Aggeli K, Tsioufis K. LOX-1 Receptor: A Diagnostic Tool and Therapeutic Target in Atherogenesis. Curr Probl Cardiol. 2024;49:102117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 124. | Jackson KH, Harris WS, Belury MA, Kris-Etherton PM, Calder PC. Beneficial effects of linoleic acid on cardiometabolic health: an update. Lipids Health Dis. 2024;23:296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 125. | Patterson MT, Xu Y, Hillman H, Osinski V, Schrank PR, Kennedy AE, Barrow F, Zhu A, Tollison S, Shekhar S, Stromnes IM, Tassi I, Wu D, Revelo XS, Binstadt BA, Williams JW. Trem2 Agonist Reprograms Foamy Macrophages to Promote Atherosclerotic Plaque Stability-Brief Report. Arterioscler Thromb Vasc Biol. 2024;44:1646-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 126. | Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013;346:e8707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 127. | Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM, Hibbeln JR. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ. 2016;353:i1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 128. | Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr. 2010;104:1586-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 129. | McClintick DJ, O'Donoghue ML, De Ferrari GM, Ferreira J, Ran X, Im K, López JAG, Elliott-Davey M, Wang B, Monsalvo ML, Atar D, Keech A, Giugliano RP, Sabatine MS. Long-Term Efficacy of Evolocumab in Patients With or Without Multivessel Coronary Disease. J Am Coll Cardiol. 2024;83:652-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Lian PA, Zhu WQ, Zhao WX, Huang PP, Ran JL, Tang YX, Huang XS, Li R. Lipoprotein(a) in atherosclerotic cardiovascular disease and proprotein convertase subtilisin/kexin-type 9 inhibitors. Clin Chim Acta. 2025;565:119982. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 131. | Naeem Z, Zukunft S, Huard A, Hu J, Hammock BD, Weigert A, Frömel T, Fleming I. Role of the soluble epoxide hydrolase in keratinocyte proliferation and sensitivity of skin to inflammatory stimuli. Biomed Pharmacother. 2024;171:116127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 132. | Rose GA, Thomson WB, Williams RT. Corn Oil in Treatment of Ischaemic Heart Disease. Br Med J. 1965;1:1531-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 133. | Pearce ML, Dayton S. Incidence of cancer in men on a diet high in polyunsaturated fat. Lancet. 1971;1:464-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 113] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 134. | Caldas APS, Alves RDM, Hermsdorff HHM, de Oliveira LL, Bressan J. Effects of high-oleic peanuts within a hypoenergetic diet on inflammatory and oxidative status of overweight men: a randomised controlled trial. Br J Nutr. 2020;123:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 135. | Urpi-Sarda M, Casas R, Sacanella E, Corella D, Andrés-Lacueva C, Llorach R, Garrabou G, Cardellach F, Sala-Vila A, Ros E, Ruiz-Canela M, Fitó M, Salas-Salvadó J, Estruch R. The 3-Year Effect of the Mediterranean Diet Intervention on Inflammatory Biomarkers Related to Cardiovascular Disease. Biomedicines. 2021;9:862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 136. | Sarapis K, George ES, Marx W, Mayr HL, Willcox J, Esmaili T, Powell KL, Folasire OS, Lohning AE, Garg M, Thomas CJ, Itsiopoulos C, Moschonis G. Extra virgin olive oil high in polyphenols improves antioxidant status in adults: a double-blind, randomized, controlled, cross-over study (OLIVAUS). Eur J Nutr. 2022;61:1073-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 137. | Baer DJ, Henderson T, Gebauer SK. Consumption of High-Oleic Soybean Oil Improves Lipid and Lipoprotein Profile in Humans Compared to a Palm Oil Blend: A Randomized Controlled Trial. Lipids. 2021;56:313-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 138. | Ramírez-Tortosa MC, Suárez A, Gómez MC, Mir A, Ros E, Mataix J, Gil A. Effect of extra-virgin olive oil and fish-oil supplementation on plasma lipids and susceptibility of low-density lipoprotein to oxidative alteration in free-living spanish male patients with peripheral vascular disease. Clin Nutr. 1999;18:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Violi F, Loffredo L, Pignatelli P, Angelico F, Bartimoccia S, Nocella C, Cangemi R, Petruccioli A, Monticolo R, Pastori D, Carnevale R. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr Diabetes. 2015;5:e172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 140. | Mercola J, D'Adamo CR. Linoleic Acid: A Narrative Review of the Effects of Increased Intake in the Standard American Diet and Associations with Chronic Disease. Nutrients. 2023;15:3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 141. | Liu Y, Gu X, Li Y, Rimm EB, Willett WC, Stampfer MJ, Hu FB, Wang DD. Changes in fatty acid intake and subsequent risk of all-cause and cause-specific mortality in males and females: a prospective cohort study. Am J Clin Nutr. 2025;121:141-150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 142. | Tillander V, Holmer M, Hagström H, Petersson S, Brismar TB, Stål P, Lindqvist C. Associations between dietary fatty acid and plasma fatty acid composition in non-alcoholic fatty liver disease: secondary analysis from a randomised trial with a hypoenergetic low-carbohydrate high-fat and intermittent fasting diet. Br J Nutr. 2024;132:1-13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 143. | Bontor K, Gabryel B. Sulodexide protects endothelial cells against 4-hydroxynonenal-induced oxidative stress and glutathione-dependent redox imbalance by modulation of sestrin2/nuclear factor erythroid 2-related factor 2 pathway. J Physiol Pharmacol. 2024;75. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 144. | Nesbeth PC, Ziegler TR, Tripathi AK, Dabeer S, Weiss D, Hao L, Smith MR, Jones DP, Maner-Smith KM, Tu CL, Chang W, Weitzmann MN, Alvarez JA. Linoleic acid blunts early osteoblast differentiation and impairs oxidative phosphorylation in vitro. Prostaglandins Leukot Essent Fatty Acids. 2024;201:102617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 145. | Nguyen ATL, Kaleda A, Onuh JO, Aryee ANA. Characterization of quality parameters and phytosterol content in oils and their formulated margarines. Food Sci Nutr. 2024;12:10110-10122. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 146. | Gunduz G, Konuskan DB. Fatty Acid and Sterol Compositions of Turkish Monovarietal Olive Oils with Regard to Olive Ripening. J Oleo Sci. 2023;72:79-85. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 147. | Cui XY, Gou ZY, Abouelezz KFM, Li L, Lin XJ, Fan QL, Wang YB, Cheng ZG, Ding FY, Jiang SQ. Alterations of the fatty acid composition and lipid metabolome of breast muscle in chickens exposed to dietary mixed edible oils. Animal. 2020;14:1322-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 148. | Pan F, Zhao L, Cai S, Tang X, Mehmood A, Alnadari F, Tuersuntuoheti T, Zhou N, Ai X. Prediction and evaluation of the 3D structure of Macadamia integrifolia antimicrobial protein 2 (MiAMP2) and its interaction with palmitoleic acid or oleic acid: An integrated computational approach. Food Chem. 2022;367:130677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 149. | Fallah Z, Vasmehjani AA, Aghaei S, Amiri M, Raeisi-Dekordi H, Moghtaderi F, Zimorovat A, Yazd EF, Madadizadeh F, Khayyatzadeh SS, Salehi-Abargouei A. Cardiometabolic risk factors are affected by interaction between FADS1 rs174556 variant and dietary vegetable oils in patients with diabetes: a randomized controlled trial. Sci Rep. 2024;14:27531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 150. | Venn-Watson S, Schork NJ. Pentadecanoic Acid (C15:0), an Essential Fatty Acid, Shares Clinically Relevant Cell-Based Activities with Leading Longevity-Enhancing Compounds. Nutrients. 2023;15:4607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 151. | Valenzuela R, Walbaum B, Farias C, Acevedo F, Vargas C, Bennett JT, Bravo ML, Pinto MP, Medina L, Merino T, Ibañez C, Parada A, Sanchez C. High linoleic acid levels in red blood cells predict a poor response to neoadjuvant chemotherapy in human epidermal growth factor receptor type 2-positive breast cancer patients. Nutrition. 2024;121:112357. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 152. | Lupoli R, Calcaterra I, Ambrosino P, Giacco R, Vitale M, Della Pepa G, Rivellese AA, Iannuzzo G, Bozzetto L, Di Minno M. Effects of Mediterranean Diet on Endothelial Reactivity in Individuals with High Cardiometabolic Risk: A Randomized Controlled Parallel-Group Preliminary Trial. Biomedicines. 2024;12:2595. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 153. | Qian X, Klatt S, Bennewitz K, Wohlfart DP, Lou B, Meng Y, Buettner M, Poschet G, Morgenstern J, Fleming T, Sticht C, Hausser I, Fleming I, Szendroedi J, Nawroth PP, Kroll J. Impaired Detoxification of Trans, Trans-2,4-Decadienal, an Oxidation Product from Omega-6 Fatty Acids, Alters Insulin Signaling, Gluconeogenesis and Promotes Microvascular Disease. Adv Sci (Weinh). 2024;11:e2302325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 154. | Sidorkiewicz M. The Cardioprotective Effects of Polyunsaturated Fatty Acids Depends on the Balance Between Their Anti- and Pro-Oxidative Properties. Nutrients. 2024;16:3937. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 155. | Li Q, Du Y, Xiang P, Chen G, Qian X, Li S, Mao Y, Ling W, Wang D. Re-Visiting Antioxidant Therapy in Murine Advanced Atherosclerosis with Brussels Chicory, a Typical Vegetable in Mediterranean Diets. Nutrients. 2023;15:832. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 156. | Kim J, Kim JY, Byeon HE, Kim JW, Kim HA, Suh CH, Choi S, Linton MF, Jung JY. Inhibition of Toll-like Receptors Alters Macrophage Cholesterol Efflux and Foam Cell Formation. Int J Mol Sci. 2024;25:6808. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 157. | Chen DK, Metherel AH, Rezaei K, Parzanini C, Chen CT, Ramsden CE, Horowitz M, Faurot KR, MacIntosh B, Zamora D, Bazinet RP. Analysis of omega-3 and omega-6 polyunsaturated fatty acid metabolism by compound-specific isotope analysis in humans. J Lipid Res. 2023;64:100424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 158. | Ceramella J, De Maio AC, Basile G, Facente A, Scali E, Andreu I, Sinicropi MS, Iacopetta D, Catalano A. Phytochemicals Involved in Mitigating Silent Toxicity Induced by Heavy Metals. Foods. 2024;13:978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 159. | Zohora FT, Arora S, Swiss A, Vyavahare N. Reversal of heavy arterial calcification in a rat model of chronic kidney disease using targeted ethylene diamine tetraacetic acid-loaded albumin nanoparticles. Cardiovasc Diagn Ther. 2024;14:489-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 160. | Voicu G, Mocanu CA, Safciuc F, Rebleanu D, Anghelache M, Cecoltan S, Droc I, Simionescu M, Manduteanu I, Calin M. VCAM-1 targeted nanocarriers of shRNA-Smad3 mitigate endothelial-to-mesenchymal transition triggered by high glucose concentrations and osteogenic factors in valvular endothelial cells. Int J Biol Macromol. 2024;281:136355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 161. | Su S, Chen Z, Ke Q, Kocher O, Krieger M, Kang PM. Nanoparticle-Directed Antioxidant Therapy Can Ameliorate Disease Progression in a Novel, Diet-Inducible Model of Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2024;44:2476-2488. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 162. | Nijhawan HP, Shyamsundar P, Prabhakar B, Yadav KS. PEGylated pH-Responsive Liposomes for Enhancing the Intracellular Uptake and Cytotoxicity of Paclitaxel in MCF-7 Breast Cancer Cells. AAPS PharmSciTech. 2024;25:216. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 163. | Nguyen HT, Van Duong T, Taylor LS. Enteric coating of tablets containing an amorphous solid dispersion of an enteric polymer and a weakly basic drug: A strategy to enhance in vitro release. Int J Pharm. 2023;642:123139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 164. | Tong T, Qi Y, Rollins D, Bussiere LD, Dhar D, Miller CL, Yu C, Wang Q. Rational design of oral drugs targeting mucosa delivery with gut organoid platforms. Bioact Mater. 2023;30:116-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 165. | Ling J, Schroder R, Wuelfing WP, Higgins J, Kesisoglou F, Templeton AC, Su Y. Molecular Investigation of SNAC as an Oral Peptide Permeation Enhancer in Lipid Membranes via Solid-State NMR. Mol Pharm. 2025;22:459-473. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 166. | Ding R, Li Y, Zheng W, Sun Y, Zhao Z, Zhang H, Yuan R, Wang A, Sun K, Wang H, Shi Y. Design of Auto-Adaptive Drug Delivery System for Effective Delivery of Peptide Drugs to Overcoming Mucus and Epithelial Barriers. AAPS J. 2024;26:102. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 167. | Singh P, Sun J, Cavalera M, Al-Sharify D, Matthes F, Barghouth M, Tengryd C, Dunér P, Persson A, Sundius L, Nitulescu M, Bengtsson E, Rattik S, Engelbertsen D, Orho-Melander M, Nilsson J, Monaco C, Goncalves I, Edsfeldt A. Dysregulation of MMP2-dependent TGF-ß2 activation impairs fibrous cap formation in type 2 diabetes-associated atherosclerosis. Nat Commun. 2024;15:10464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 168. | Wang A, Yue K, Zhong W, Zhang G, Zhang X, Wang L. Targeted delivery of rapamycin and inhibition of platelet adhesion with multifunctional peptide nanoparticles for atherosclerosis treatment. J Control Release. 2024;376:753-765. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 169. | Hoover EC, Roy Chowdhury C, Ruggiero OM, Day ES. Conjugation of Antibodies and siRNA Duplexes to Polymer Nanoparticles via Maleimide-Thiol Chemistry. ACS Omega. 2024;9:47637-47646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 170. | Naudi-Fabra S, Elena-Real CA, Vedel IM, Tengo M, Motzny K, Jiang PL, Schmieder P, Liu F, Milles S. An extended interaction site determines binding between AP180 and AP2 in clathrin mediated endocytosis. Nat Commun. 2024;15:5884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 171. | Valencia R, Kranrod JW, Fang L, Soliman AM, Azer B, Clemente-Casares X, Seubert JM. Linoleic acid-derived diol 12,13-DiHOME enhances NLRP3 inflammasome activation in macrophages. FASEB J. 2024;38:e23748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 172. | Lin Y, Liu J, Chong SY, Ting HJ, Tang X, Yang L, Zhang S, Qi X, Pei P, Yi Z, Huang C, Hou X, Gao L, Torta F, Liu X, Liu B, Kah JCY, Wang JW. Dual-Function Nanoscale Coordination Polymer Nanoparticles for Targeted Diagnosis and Therapeutic Delivery in Atherosclerosis. Small. 2024;20:e2401659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 173. | Keuth J, Nitschke Y, Mulac D, Riehemann K, Rutsch F, Langer K. Reversion of arterial calcification by elastin-targeted DTPA-HSA nanoparticles. Eur J Pharm Biopharm. 2020;150:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 174. | Shi D, Beasock D, Fessler A, Szebeni J, Ljubimova JY, Afonin KA, Dobrovolskaia MA. To PEGylate or not to PEGylate: Immunological properties of nanomedicine's most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev. 2022;180:114079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 255] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 175. | Shiraishi K, Yokoyama M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: a review. Sci Technol Adv Mater. 2019;20:324-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 176. | Sadauskas E, Danscher G, Stoltenberg M, Vogel U, Larsen A, Wallin H. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine. 2009;5:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 177. | Wang C, Cheng X, Su Y, Pei Y, Song Y, Jiao J, Huang Z, Ma Y, Dong Y, Yao Y, Fan J, Ta H, Liu X, Xu H, Deng Y. Accelerated blood clearance phenomenon upon cross-administration of PEGylated nanocarriers in beagle dogs. Int J Nanomedicine. 2015;10:3533-3545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 178. | Paliwal R, Babu RJ, Palakurthi S. Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech. 2014;15:1527-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 179. | Liu CH, Rethi L, Weng PW, Trung Nguyen H, Chuang AE. Cutting-edge advances in nano/biomedicine: A review on transforming thrombolytic therapy. Biochem Pharmacol. 2024;229:116523. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 180. | Yang X, Chi C, Li W, Zhang Y, Yang S, Xu R, Liu R. Metabolomics and lipidomics combined with serum pharmacochemistry uncover the potential mechanism of Huang-Lian-Jie-Du decoction alleviates atherosclerosis in ApoE(-/-) mice. J Ethnopharmacol. 2024;324:117748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 181. | Abbasciano RG, Layton GR, Torre S, Abbaker N, Copperwheat A, Lucarelli C, Bhandari S, Nijjer S, Mikhail G, Casula R, Zakkar M, Viviano A. Fractional flow reserve and instantaneous wave-free ratio in coronary artery bypass grafting: a meta-analysis and practice review. Front Cardiovasc Med. 2024;11:1348341. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 182. | Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Barone Gibbs B, Beaton AZ, Boehme AK, Commodore-Mensah Y, Currie ME, Elkind MSV, Evenson KR, Generoso G, Heard DG, Hiremath S, Johansen MC, Kalani R, Kazi DS, Ko D, Liu J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Perman SM, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Tsao CW, Urbut SM, Van Spall HGC, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Palaniappan LP; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. 2024;149:e347-e913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1087] [Cited by in RCA: 986] [Article Influence: 986.0] [Reference Citation Analysis (35)] |
| 183. | Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, Coresh J, Mathew RO, Baker-Smith CM, Carnethon MR, Despres JP, Ho JE, Joseph JJ, Kernan WN, Khera A, Kosiborod MN, Lekavich CL, Lewis EF, Lo KB, Ozkan B, Palaniappan LP, Patel SS, Pencina MJ, Powell-Wiley TM, Sperling LS, Virani SS, Wright JT, Rajgopal Singh R, Elkind MSV; American Heart Association. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation. 2023;148:1606-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 460] [Article Influence: 230.0] [Reference Citation Analysis (0)] |
| 184. | Ng YH, Koay YC, Marques FZ, Kaye DM, O'Sullivan JF. Leveraging metabolism for better outcomes in heart failure. Cardiovasc Res. 2024;120:1835-1850. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 185. | Wei C, Paranjpe I, Sharma P, Milligan M, Lam M, Heidenreich PA, Kalwani N, Schulman K, Sandhu A. Assessment of Price Variation in Coronary Artery Bypass Surgery at US Hospitals. J Am Heart Assoc. 2024;13:e031982. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 186. | Ali MA, Yasir J, Sherwani RN, Fareed M, Arshad F, Abid F, Arshad R, Ismail S, Khan SA, Siddiqui U, Muhammad MG, Fatima K. Frequency and predictors of non-adherence to lifestyle modifications and medications after coronary artery bypass grafting: A cross-sectional study. Indian Heart J. 2017;69:469-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 187. | Lawson WE, Hui JC, Kennard ED, Linnemeier G; IEPR-II Investigators. Enhanced External Counterpulsation Is Cost-Effective in Reducing Hospital Costs in Refractory Angina Patients. Clin Cardiol. 2015;38:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 188. | Lawson WE, Barsness G, Michaels AD, Soran O, Kennard ED, Kelsey SF, Hui JC; IEPR Investigators. Effectiveness of repeat enhanced external counterpulsation for refractory angina in patients failing to complete an initial course of therapy. Cardiology. 2007;108:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 189. | Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I; DIRECT Group. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT). J Am Coll Nutr. 2009;28:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 190. | Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung YM, Berk M, Mann JD. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 191. | Medicare now provides limited EECP (enhanced external counterpulsation) coverage. Patient Acc. 1999;22:1, 3. [PubMed] |
| 192. | Courville AB, Majchrzak-Hong S, Yang S, Turner S, Wilhite B, Ness Shipley K, Horneffer Y, Domenichiello AF, Schwandt M, Cutler RG, Chen KY, Hibbeln JR, Ramsden CE. Dietary linoleic acid lowering alone does not lower arachidonic acid or endocannabinoids among women with overweight and obesity: A randomized, controlled trial. Lipids. 2023;58:271-284. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 193. | Li Y, Wang Y, Xia Z, Xie Y, Ke D, Song B, Mu D, Yu R, Xie J. Noninvasive platelet membrane-coated Fe(3)O(4) nanoparticles identify vulnerable atherosclerotic plaques. Smart Med. 2024;3:e20240006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 194. | Ray S, Moonshi SS, Ta HT. "Therapies Through Gut:" Targeted Drug Delivery for Non-Gastrointestinal Diseases by Oral Administration. Adv Healthc Mater. 2025;14:e2403162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 195. | Zhen J, Li X, Yu H, Du B. High-density lipoprotein mimetic nano-therapeutics targeting monocytes and macrophages for improved cardiovascular care: a comprehensive review. J Nanobiotechnology. 2024;22:263. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 196. | Barkas F, Sener YZ, Golforoush PA, Kheirkhah A, Rodriguez-Sanchez E, Novak J, Apellaniz-Ruiz M, Akyea RK, Bianconi V, Ceasovschih A, Chee YJ, Cherska M, Chora JR, D'Oria M, Demikhova N, Kocyigit Burunkaya D, Rimbert A, Macchi C, Rathod K, Roth L, Sukhorukov V, Stoica S, Scicali R, Storozhenko T, Uzokov J, Lupo MG, van der Vorst EPC, Porsch F. Advancements in risk stratification and management strategies in primary cardiovascular prevention. Atherosclerosis. 2024;395:117579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 197. | Goldfarb MJ, Saylor MA, Bozkurt B, Code J, Di Palo KE, Durante A, Flanary K, Masterson Creber R, Ogunniyi MO, Rodriguez F, Gulati M; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Hypertension; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Council on Quality of Care and Outcomes Research. Patient-Centered Adult Cardiovascular Care: A Scientific Statement From the American Heart Association. Circulation. 2024;149:e1176-e1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 198. | Zhang Z, Guo J. Deciphering Oxidative Stress in Cardiovascular Disease Progression: A Blueprint for Mechanistic Understanding and Therapeutic Innovation. Antioxidants (Basel). 2024;14:38. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 199. | Coral DE, Smit F, Farzaneh A, Gieswinkel A, Tajes JF, Sparsø T, Delfin C, Bauvin P, Wang K, Temprosa M, De Cock D, Blanch J, Fernández-Real JM, Ramos R, Ikram MK, Gomez MF, Kavousi M, Panova-Noeva M, Wild PS, van der Kallen C, Adriaens M, van Greevenbroek M, Arts I, Le Roux C, Ahmadizar F, Frayling TM, Giordano GN, Pearson ER, Franks PW. Subclassification of obesity for precision prediction of cardiometabolic diseases. Nat Med. 2025;31:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 200. | Tesoro L, Hernandez I, Saura M, Badimón L, Zaragoza C. Novel cutting edge nano-strategies to address old long-standing complications in cardiovascular diseases. A comprehensive review. Eur J Clin Invest. 2024;54:e14208. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |