Published online Sep 26, 2021. doi: 10.4330/wjc.v13.i9.483
Peer-review started: March 10, 2021
First decision: May 6, 2021
Revised: May 25, 2021
Accepted: July 26, 2021
Article in press: July 26, 2021
Published online: September 26, 2021
Processing time: 192 Days and 2.3 Hours
The quality of warfarin therapy can be determined by the time in the therapeutic range (TTR) of international normalized ratio (INR). The estimated minimum TTR needed to achieve a benefit from warfarin therapy is ≥ 60%.
To determine TTR and the predictors of poor TTR among atrial fibrillation patie
A retrospective observational study was conducted at a cardiology referral center in Selangor, Malaysia. A total of 420 patients with atrial fibrillation and under follow-up at the pharmacist led Warfarin Medication Therapeutic Adherence Clinic between January 2014 and December 2018 were included. Patients’ clinical data, information related to warfarin therapy, and INR readings were traced through electronic Hospital Information system. A data collection form was used for data collection. The percentage of days when INR was within range was calculated using the Rosendaal method. The poor INR control category was defined as a TTR < 60%. Predictors for poor TTR were further determined by using logistic regression.
A total of 420 patients [54.0% male; mean age 65.7 (10.9) years] were included. The calculated mean and median TTR were 60.6% ± 20.6% and 64% (interquartile range 48%-75%), respectively. Of the included patients, 57.6% (n = 242) were in the good control category and 42.4% (n = 178) were in the poor control category. The annual calculated mean TTR between the year 2014 and 2018 ranged from 59.7% and 67.3%. A high HAS-BLED score of ≥ 3 was associated with poor TTR (adjusted odds ratio, 2.525; 95% confidence interval: 1.6-3.9, P < 0.001).
In our population, a high HAS-BLED score was associated with poor TTR. This could provide an important insight when initiating an oral anticoagulant for these patients. Patients with a high HAS-BLED score may obtain less benefit from warfarin therapy and should be considered for other available oral anticoagulants for maximum benefit.
Core Tip: This is a retrospective observational study to determine time in therapeutic range (TTR) and the predictors of poor TTR among patients with atrial fibrillation under follow-up at the Warfarin Medication Therapeutic Adherence Clinic of a tertiary cardiology referral center in Selangor, Malaysia. In this study cohort, we found that high HAS-BLED score (≥ 3) was a significant predictor of poor TTR.
- Citation: Lee SL, Ong TJ, Mazlan-Kepli W, Mageswaran A, Tan KH, Abd-Malek AM, Cronshaw R. Patients’ time in therapeutic range on warfarin among atrial fibrillation patients in Warfarin Medication Therapy Adherence Clinic. World J Cardiol 2021; 13(9): 483-492
- URL: https://www.wjgnet.com/1949-8462/full/v13/i9/483.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i9.483
Atrial fibrillation (AF) is usually asymptomatic and is associated with an increased risk of major adverse cardiovascular events (MACE) and mortality in patients with coronary artery disease (CAD)[1]. The prevalence of AF in the Malaysian population is low at 0.54%, compared to the global average of 1%[2].
Warfarin is the most widely used anticoagulant in the world[3-5]. Although novel oral anticoagulants are available, warfarin remains a viable oral anticoagulant for many patients because of its availability and cost[4,6,7]. The time in therapeutic range (TTR) estimates the percentage of time a patient’s international normalized ratio (INR) is within the desired treatment range or goal and is widely used as an indicator of anticoagulation control[4,9-12]. TTR is commonly used to evaluate the quality of warfarin therapy and is an essential tool for assessing the risks vs benefits of warfarin therapy[4,8,13,14]. A 10% increase in time spent out of TTR is associated with a 29% increase in the risk of mortality and a 10%-12% increase in the risk of an ischemic stroke and other thromboembolic events[13,15-17].
The aim of this study is to determine the percentage of time a patient’s INR is within the desired treatment range and the predictors of poor TTR among patients with AF who receive warfarin therapy.
This is a retrospective observational study that was conducted in Hospital Serdang located in Kajang, Selangor. Hospital Serdang is a cardiology reference center that covers patients from Serdang, Bangi, Putrajaya, Kajang, Dengkil, and Puchong area in the state of Selangor, Malaysia. Ethical approval for this study was obtained from the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR-19-839-46462). The study was carried out in accordance with the Declaration of Helsinki.
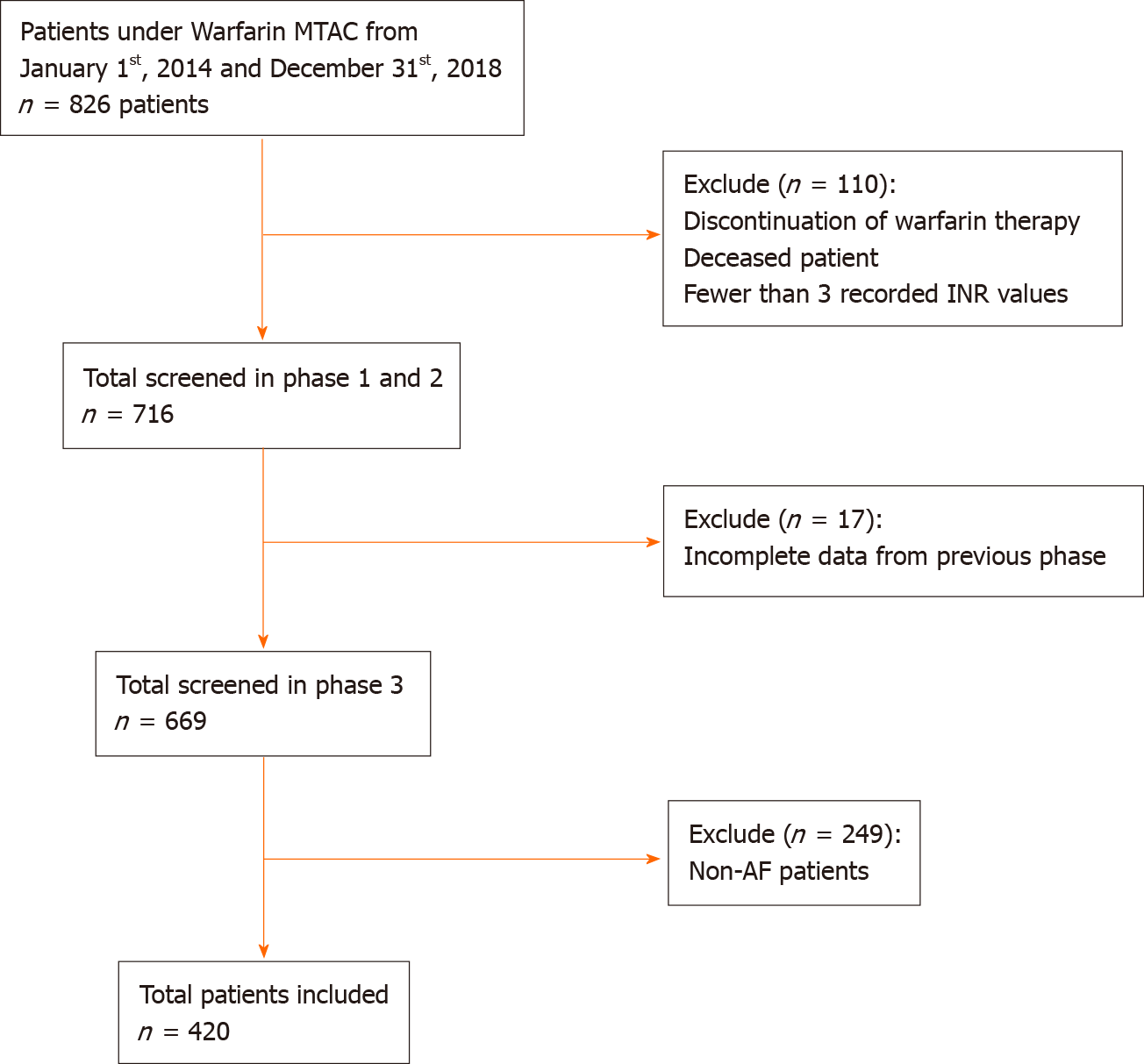
All AF patients under Warfarin Medication Therapeutic Adherence Clinic follow-up between 2014 to 2018 were included. The electronic Hospital Information System (eHIS) was used to extract patients’ demographics, comorbidities, information related to warfarin therapy, and INR readings. Baseline comorbidities were retrospectively retrieved according to available medical records in eHIS. The consecutive sampling method was used. A case report form was used for data collection. Each patient was allocated a patient identifier number that matched their patient registration number, in order to protect confidentiality. Patients were excluded if they discontinued warfarin therapy, died, or had less than three INR readings.
For patients with AF, the target INR range was between 2.0 to 3.0. TTR is defined as an estimate of the average time that a medication is dosed with optimal efficacy and safety[4]. From previous literature, the estimate of the minimum TTR needed to achieve a benefit from warfarin therapy is between 58% and 65%[18]. The percentage of days that INR was within range was calculated using the Rosendaal method[8]. Thus, based on these references, we defined the “poor” TTR category as TTR < 60% and the “good” TTR category as TTR > 60%.
This study aimed to determine to what extent the following factors are associated with poor TTR: Age, gender, ethnicity, diabetes mellitus, hypertension, previous ischemic heart disease, chronic kidney disease, peripheral vascular disease, stroke/transient ischemic attack, deep vein thrombosis/pulmonary embolism, heart failure, chronic obstructive pulmonary disease/asthma, chronic kidney disease, dyslipidaemia, chronic rheumatic heart disease, CHA2DS2-VASc score, and HAS-BLED score. A study by Peduzzi et al[19] on sample size for logistic regression suggests using a minimum event per variable value of ten. Since our study was interested in studying 17 risk factors, a minimum sample of 170 patients in the poor TTR category group was required. The study planned to recruit at least 400 samples in order to exceed the minimum sample size, as the prevalence of poor TTR was estimated at 38%[19].
Categorical variables were summarized using frequencies and proportions while continuous variables were summarized using mean ± standard deviation or median (inter-quartile range) values. Logistic regression analysis was used to calculate odds ratios (OR) and 95% confidence intervals (CI) to model the predictors of poor TTR. This analysis was repeated and adjusted to variables that were significant from the previous studies: Heart failure, chronic kidney disease, CHA2DS2-VASc score, and HAS-BLED score[20]. A P < 0.05 was considered significant. Point estimates and 95%CI are presented for all results. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 20 (Armonk, NY, United States).
A total of 420 patients with AF were included in this study (Figure 1). Baseline characteristics are shown in Table 1. The mean age of the study population was 65.7 (10.9) years. There were slightly more male patients (54%, n = 227) than female patients (46%, n = 193). The largest ethnic population was Malay, followed by Chinese and Indian. Hypertension accounted for the highest percentage of comorbidities among patients with AF, followed by diabetes mellitus, dyslipidaemia, and ischemic heart diseases. Median CHA2DS2-VASc score was 3 (moderate risk of stroke), and median HAS-BLED score was 2 (low bleeding risk).
| Baseline characteristic | All patients, n = 420 | TTR < 60%, n = 178 | TTR ≥ 60%, n = 242 |
| Age, yr | |||
| mean ± SD | 65.7 ± 10.9 | 65.7 ± 10.8 | 65.7± 10.9 |
| Median (IQR) | 67 (15) | 66 (14) | 67 (15) |
| Male | 227(54.0) | 99 (55.6) | 128 (52.9) |
| Female | 193 (46.0) | 79 (44.4) | 114 (47.1) |
| Ethnicity | |||
| Malay | 210 (50.0) | 96 (53.9) | 114 (47.1) |
| Chinese | 179 (42.6) | 64 (36.0) | 115 (47.5) |
| Indian | 26 (6.2) | 16 (9.0) | 10 (4.1) |
| Others | 5 (1.2) | 2 (1.1) | 3 (1.2) |
| Comorbidities | |||
| Diabetes mellitus | 170 (40.5) | 85 (47.8) | 85 (35.1) |
| Hypertension | 291 (69.3) | 128 (71.9) | 163 (67.4) |
| Ischemic heart disease | 90 (21.4) | 48 (27.0) | 42 (17.4) |
| Peripheral vascular disease | 8 (1.9) | 2 (1.1) | 6 (2.5) |
| Stroke/transient ischemic attack | 49 (11.7) | 20 (11.2) | 29 (12.0) |
| Deep vein thrombosis/pulmonary embolism | 2 (0.5) | 1 (0.6) | 1 (0.4) |
| Heart failure | 27 (6.4) | 12 (6.7) | 15 (6.2) |
| Chronic obstructive pulmonary disease/asthma | 22 (5.2) | 8 (4.5) | 14 (5.8) |
| Chronic kidney disease | 49 (11.7) | 28 (15.7) | 21 (8.7) |
| Dyslipidaemia | 125 (29.8) | 47 (26.4) | 78 (32.2) |
| Chronic rheumatic heart disease | 17 (4.0) | 10 (5.6) | 7 (2.9) |
| Liver disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CHA2DS2-VASc score | |||
| Mean ± SD | 2.6 ± 1.4 | - | - |
| Median (IQR) | 3 (1.0) | 3 (2) | 2(1) |
| Score 0-1 | 91 (21.7) | 34 (19.1) | 57 (23.6) |
| Score 2-4 | 297 (70.7) | 125 (70.2) | 172 (71.1) |
| Score ≥ 5 | 32 (7.6) | 19 (10.7) | 13 (5.4) |
| HAS-BLED score | |||
| Median (IQR) | 2 (2.0) | 3 (1.0) | 2 (2.0) |
| Low bleeding risk (score ≤ 2) | 257 (61.2) | 83 (46.6) | 174 (71.9) |
| High bleeding risk (score ≥ 3) | 163 (38.8) | 95 (53.4) | 68 (28.1) |
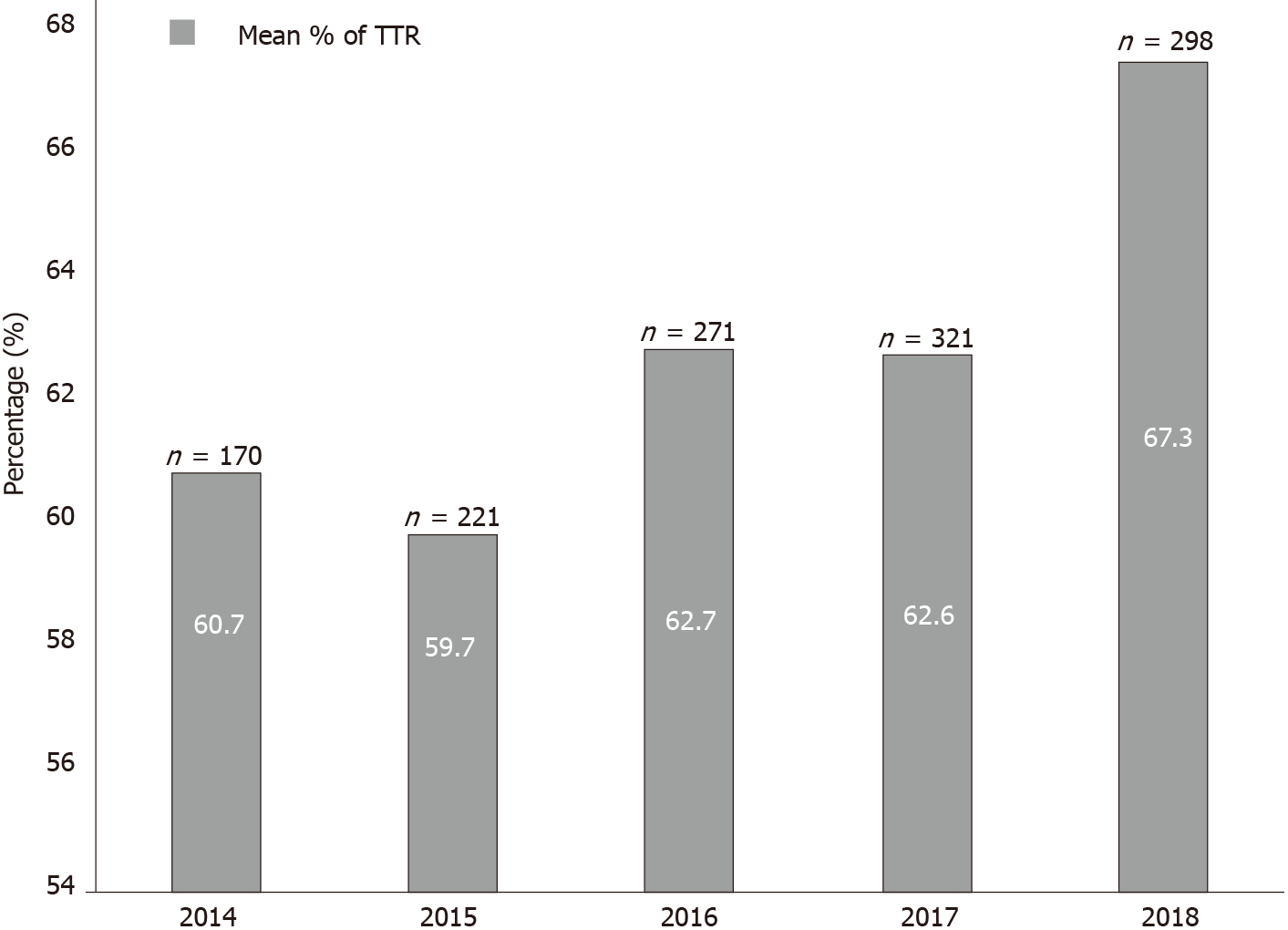
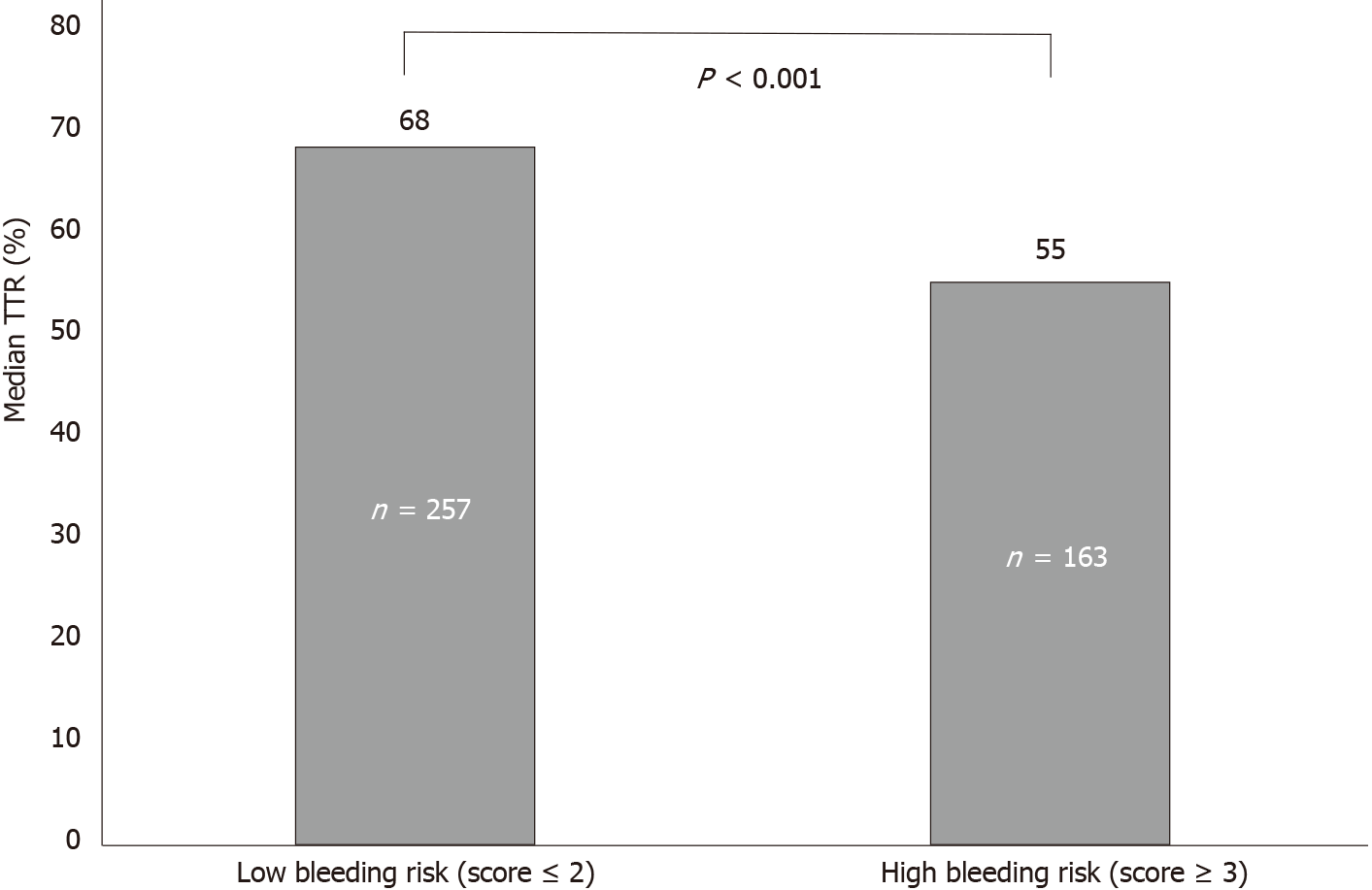
Over 5 years, the mean (standard deviation) TTR was 60.6% (20.6%) with a median of 64% (interquartile range 27%). Of the included patients, 57.6% (n = 242) were in the good control category, and 42.4% (n = 178) were in the poor control category (Table 2). The annual calculated mean TTR between the year 2014 and 2018 ranged between 59.7% and 67.3%. The highest percentage was in 2018, with 67.3% of days within therapeutic range in AF patients. Figure 2 shows the mean TTR for AF patients from 2014 to 2018. Patient with a high HAS-BLED (score ≥ 3) had a median TTR of 55% compared to patients with a low HAS-BLED score, who had a median TTR of 68% (Figure 3).
| Variables | All patients, n = 420 | TTR < 60%, n = 178 | TTR ≥ 60%, n = 242 |
| TTR | |||
| mean ± SD | 60.6 ± 20.6 | 41.1 ± 14.7 | 75.0 ± 9.6 |
| Median (IQR) | 64 (27) | 44 (22) | 74 (14) |
| Good (≥ 60%) | 242 (57.6) | - | - |
| Poor (< 60%) | 178 (42.4) | - | - |
From the multivariate analysis, a high HAS-BLED score (score ≥ 3) was significantly associated with a poor TTR (< 60%) (adjusted OR, 2.5;1.6-3.9, P < 0.001) compared to a low HAS-BLED score (score ≤ 2). Similarly, in the model adjusted for predictors previously described in previous reports[20] (model 2), only HAS-BLED score was found to be a significant predictor of poor TTR (adjusted OR, 2.9;1.8-4.7, P < 0.001) (Table 3).
| Baseline characteristic | Univariate | Multivariate | |||||||||
| Model 11 | Model 22 | ||||||||||
| OR | 95%CI | P | aOR | 95%CI | P | aOR | 95%CI | P | |||
| Age | 1.0 | 0.9-1.0 | 0.976 | ||||||||
| Ethnicity | |||||||||||
| Malay | 1.0 | Reference | - | ||||||||
| Chinese | 1.3 | 0.2-7.7 | 0.800 | ||||||||
| Indian | 0.8 | 0.1-5.1 | 0.845 | ||||||||
| Others | 2.4 | 0.3-16.9 | 0.380 | ||||||||
| Gender | |||||||||||
| Male | 1.0 | Reference | - | ||||||||
| Female | 0.9 | 0.6-1.3 | 0.580 | ||||||||
| Comorbidities | |||||||||||
| Diabetes mellitus | 1.7 | 1.1-2.5 | 0.009 | 1.3 | 0.8-1.9 | 0.240 | |||||
| Hypertension | 1.2 | 0.8-1.9 | 0.318 | ||||||||
| Ischemic heart disease | 1.8 | 1.1-2.8 | 0.018 | 1.3 | 0.8-2.1 | 0.317 | |||||
| Peripheral vascular disease | 0.4 | 0.1-2.2 | 0.328 | ||||||||
| Stroke/transient ischemic attack | 0.9 | 0.5-1.7 | 0.814 | ||||||||
| Deep vein Thrombosis/pulmonary embolism | 1.4 | 0.1-21.9 | 0.828 | ||||||||
| Heart failure | 1.1 | 0.5-2.4 | 0.823 | 1.0 | 0.5-2.4 | 0.913 | |||||
| Chronic obstructive pulmonary disease/asthma | 0.8 | 0.3-1.9 | 0.558 | ||||||||
| Chronic kidney disease | 1.9 | 1.1-3.6 | 0.028 | 1.2 | 0.6-2.3 | 0.585 | 1.2 | 0.7-2.4 | 0.514 | ||
| Dyslipidaemia | 0.7 | 0.5-1.2 | 0.197 | ||||||||
| Chronic rheumatic heart disease | 1.9 | 0.8-5.4 | 0.169 | ||||||||
| CHA2DS2-VASc score | 1.4 | 0.9-2.1 | 0.060 | 0.9 | 0.6-1.4 | 0.629 | |||||
| HAS-BLED Score | |||||||||||
| Low bleeding risk (score ≤ 2) | 1.0 | Reference | - | ||||||||
| High bleeding risk (score ≥ 3) | 2.9 | 1.9-4.4 | < 0.001 | 2.5 | 1.6-3.9 | < 0.001 | 2.9 | 1.8-4.7 | < 0.001 | ||
This study assessed the percentage of time a patient’s INR is within the desired treat
Hypertension had the highest prevalence, followed by diabetes mellitus, hyperlipidemia, and ischemic heart disease. The higher prevalence of cardiac-related comorbidities could be explained by Hospital Serdang’s status as a cardiology center, therefore catering towards patients with cardiovascular disease in the region. Addi
Interestingly, when compared to other Asian studies, the mean TTR of patients obtained in the study was 60.6%. In comparison, a study from Korea recorded a mean TTR of 49.1%[23], and a study from Singapore recorded a mean TTR of 58%[25]. Possible reasons for the higher mean TTR percentage may be due to the involvement of pharmacists in the pharmacist-led warfarin therapy. This translates to improved patient counselling, dosage adjustment, and identification of possible food-drug and drug-drug interactions.
This study found that patients with a high HAS-BLED score (≥ 3) had a lower median TTR of 55%, compared to patients with a low HAS-BLED score (score < 2) who had a higher median TTR of 68%. Previous reports have suggested that heart failure, chronic kidney disease, and CHA2DS2-VASc score in addition to high bleeding risk may also contribute to poor TTR[20]. After adjusting for these variables, we found only high HAS-BLED (score ≥ 3) was associated with poor TTR. This is supported by the study from Urbonas et al[15], which found median TTR was significantly lower in patients with high bleeding risk (36.4%) as compared to low-risk patients (55.6%). They suggested that the poor TTR in these patients may be due to biological variation in clotting factors[15].
Our patients’ data were derived from the eHIS database, an electronic storage system of patient records, enabling straightforward access to patient records. The INR results collected in the study were obtained either through point-of-care testing (POC) or clinical laboratory INR. There may be bias in INR measurements calculated using POC compared with laboratory INR, as POC measurements tend to be higher[10], which may affect subsequent INR readings. We were unable to measure objectively patient compliance to warfarin as a risk factor for poor TTR. Since it was a retrospective study, possible missed documentation may have occurred.
This study found the mean TTR was 60.6% in patients with AF and that a high HAS-BLED score (≥ 3) was associated with poor TTR. This could provide an important insight during the initiation of oral anticoagulant for patients with a high HAS-BLED score, who may obtain less benefit from warfarin therapy. They should therefore be considered for other available oral anticoagulants for maximum benefit.
The time in therapeutic range (TTR) is a quality measure for anticoagulation therapy with warfarin.
TTR of international normalized ratio (INR) needs to be achieved with a percentage of ≥ 60% for patient to receive a maximal benefit from warfarin such as preventing stroke, major bleeding, and even death.
TTR and the predictors of poor TTR need to be evaluated among atrial fibrillation (AF) patients that received warfarin therapy.
Eligible patients with AF from January 2014 to December 2018 for INR monitoring were included in this study. Demographic data, indication of warfarin therapy, INR target, and percentage of INR within range were collected using a data collection form. TTR was assessed using Rosendaal method.
In patients with AF, the mean TTR was 60.6% with the highest TTR score achieved in 2018, with a percentage of 67.3%.
This study showed that high HAS-BLED score was associated to poor TTR.
Patients with AF and high HAS-BLED score may have less benefit from warfarin therapy. Thus, other alternative oral anticoagulants should be considered for maxi
The authors would like to thank the Director-General of Health Malaysia for the permission to publish this paper and to those who contributed directly or indirectly in this study. Further on, the authors would also like to thank the colleagues Siaw SH, Tam AS, Mohamad RIA, Wong KY, Noor Hisham NA, So SH, Lim SY, Tee CY, and Tang MH for their contribution in data collection.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Luo W S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wu RR
| 1. | Vrsalović M, Presečki AV. Atrial fibrillation and risk of cardiovascular events and mortality in patients with symptomatic peripheral artery disease: A meta-analysis of prospective studies. Clin Cardiol. 2017;40:1231-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Lim CW, Kasim S, Ismail JR, Chua NY, Najme Khir R, Zainal Abidin HA, Abdul Rahman E, Mohd Arshad MK, Ibrahim Othman Z, Yusoff K. Prevalence of atrial fibrillation in the Malaysian communities. Heart Asia. 2016;8:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Pirmohamed M. Warfarin: almost 60 years old and still causing problems. Br J Clin Pharmacol. 2006;62:509-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Farsad BF, Abbasinazari M, Dabagh A, Bakshandeh H. Evaluation of Time in Therapeutic Range (TTR) in Patients with Non-Valvular Atrial Fibrillation Receiving Treatment with Warfarin in Tehran, Iran: A Cross-Sectional Study. J Clin Diagn Res. 2016;10:FC04-FC06. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Mwita JC, Francis JM, Oyekunle AA, Gaenamong M, Goepamang M, Magafu MGMD. Quality of Anticoagulation With Warfarin at a Tertiary Hospital in Botswana. Clin Appl Thromb Hemost. 2018;24:596-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | You JH. Novel oral anticoagulants vs warfarin therapy at various levels of anticoagulation control in atrial fibrillation--a cost-effectiveness analysis. J Gen Intern Med. 2014;29:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Shields LBE, Fowler P, Siemens DM, Lorenz DJ, Wilson KC, Hester ST, Honaker JT. Standardized warfarin monitoring decreases adverse drug reactions. BMC Fam Pract. 2019;20:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Gateman D, Trojnar ME, Agarwal G. Time in therapeutic range: Warfarin anticoagulation for atrial fibrillation in a community-based practice. Can Fam Physician. 2017;63:e425-e431. [PubMed] |
| 9. | Bahbahani H, AlTurki A, Dawas A, Lipman ML. Warfarin anticoagulation in hemodialysis patients with atrial fibrillation: comparison of nephrologist-led and anticoagulation clinic-led management. BMC Nephrol. 2018;19:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | McAlister FA, Wiebe N, Hemmelgarn BR. Time in therapeutic range and stability over time for warfarin users in clinical practice: a retrospective cohort study using linked routinely collected health data in Alberta, Canada. BMJ Open. 2018;8:e016980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Reiffel JA. Time in the Therapeutic Range for Patients Taking Warfarin in Clinical Trials: Useful, but Also Misleading, Misused, and Overinterpreted. Circulation. 2017;135:1475-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Macedo AF, Bell J, McCarron C, Conroy R, Richardson J, Scowcroft A, Sunderland T, Rotheram N. Determinants of oral anticoagulation control in new warfarin patients: analysis using data from Clinical Practice Research Datalink. Thromb Res. 2015;136:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Vestergaard AS, Skjøth F, Larsen TB, Ehlers LH. The importance of mean time in therapeutic range for complication rates in warfarin therapy of patients with atrial fibrillation: A systematic review and meta-regression analysis. PLoS One. 2017;12:e0188482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J Thromb Thrombolysis. 2003;15:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Urbonas G, Valius L, Šakalytė G, Petniūnas K, Petniūnienė I. The Quality of Anticoagulation Therapy among Warfarin-Treated Patients with Atrial Fibrillation in a Primary Health Care Setting. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Haas S, Ten Cate H, Accetta G, Angchaisuksiri P, Bassand JP, Camm AJ, Corbalan R, Darius H, Fitzmaurice DA, Goldhaber SZ, Goto S, Jacobson B, Kayani G, Mantovani LG, Misselwitz F, Pieper K, Schellong SM, Stepinska J, Turpie AG, van Eickels M, Kakkar AK; GARFIELD-AF Investigators. Quality of Vitamin K Antagonist Control and 1-Year Outcomes in Patients with Atrial Fibrillation: A Global Perspective from the GARFIELD-AF Registry. PLoS One. 2016;11:e0164076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Jones M, McEwan P, Morgan CL, Peters JR, Goodfellow J, Currie CJ. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 239] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S; ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 646] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 19. | Peduzzi P, Concato J, Kemper E, Holford TR, Feinstem AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373-1379. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4758] [Cited by in RCA: 5399] [Article Influence: 186.2] [Reference Citation Analysis (0)] |
| 20. | Pokorney SD, Simon DN, Thomas L, Fonarow GC, Kowey PR, Chang P, Singer DE, Ansell J, Blanco RG, Gersh B, Mahaffey KW, Hylek EM, Go AS, Piccini JP, Peterson ED; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators. Patients' time in therapeutic range on warfarin among US patients with atrial fibrillation: Results from ORBIT-AF registry. Am Heart J. 2015;170:141-148, 148.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 182] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 21. | Department of Statistics Malaysia Official Portal [Internet]. [cited 2020 Nov 4]. Available from: https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=430&bul_id=aFYzVjJ3anNyQytHZGxzcUZxTG9Ydz09&menu_id=L0pheU43NWJwRWVSZklWdzQ4TlhUUT09. |
| 22. | Björck F, Kadhim H, Själander A. Predictors for INR-control in a well-managed warfarin treatment setting. J Thromb Thrombolysis. 2019;47:227-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Hong KS, Kim YK, Bae HJ, Nam HS, Kwon SU, Bang OY, Cha JK, Yoon BW, Rha JH, Lee BC, Park JM, Park MS, Lee J, Choi JC, Kim DE, Lee KB, Park TH, Lee JS, Kim SE. Quality of Anticoagulation with Warfarin in Korean Patients with Atrial Fibrillation and Prior Stroke: A Multicenter Retrospective Observational Study. J Clin Neurol. 2017;13:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 25. | Bernaitis N, Ching CK, Chen L, Hon JS, Teo SC, Davey AK, Anoopkumar-Dukie S. The Sex, Age, Medical History, Treatment, Tobacco Use, Race Risk (SAMe TT2R2) Score Predicts Warfarin Control in a Singaporean Population. J Stroke Cerebrovasc Dis. 2017;26:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |