Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.88
Peer-review started: July 4, 2015
First decision: August 25, 2015
Revised: October 20, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: February 26, 2016
Processing time: 240 Days and 23.4 Hours
Chemoprevention is one of the cancer prevention approaches wherein natural/synthetic agent(s) are prescribed with the aim to delay or disrupt multiple pathways and processes involved at multiple steps, i.e., initiation, promotion, and progression of cancer. Amongst environmental chemopreventive compounds, diet/beverage-derived components are under evaluation, because of their long history of exposure to humans, high tolerability, low toxicity, and reported biological activities. This compilation briefly covers and compares the available evidence on chemopreventive efficacy and probable mechanism of chemoprevention by selected dietary phytochemicals (capsaicin, curcumin, diallyl sulphide, genistein, green/black tea polyphenols, indoles, lycopene, phenethyl isocyanate, resveratrol, retinoids and tocopherols) in experimental systems and clinical trials. All the dietary phytochemicals covered in this review have demonstrated chemopreventive efficacy against spontaneous or carcinogen-induced experimental tumors and/or associated biomarkers and processes in rodents at several organ sites. The observed anti-initiating, anti-promoting and anti-progression activity of dietary phytochemicals in carcinogen-induced experimental models involve phytochemical-mediated redox changes, modulation of enzymes and signaling kinases resulting to effects on multiple genes and cell signaling pathways. Results from clinical trials using these compounds have not shown them to be chemopreventive. This may be due to our: (1) inability to reproduce the exposure conditions, i.e., levels, complexity, other host and lifestyle factors; and (2) lack of understanding about the mechanisms of action and agent-mediated toxicity in several organs and physiological processes in the host. Current research efforts in addressing the issues of exposure conditions, bioavailability, toxicity and the mode of action of dietary phytochemicals may help address the reason for observed mismatch that may ultimately lead to identification of new chemopreventive agents for protection against broad spectrum of exposures.
Core tip: Review compares the available evidence on the chemopreventive efficacy and probable mechanisms of chemoprevention by selected dietary phytochemicals in experimental systems and clinical trials. All the dietary phytochemicals covered have demonstrated chemopreventive efficacy against carcinogen-induced tumors in rodents at several organ sites. Mechanism of observed chemopreventive action(s) involve phytochemical-mediated redox changes, modulation of enzymes and signaling kinases leading to effects on multiple genes and cell signaling pathways. Clinical trials with these compounds have not demonstrated their chemopreventive efficacy. Probable reasons for observed mismatch between the two systems and areas of current research efforts and recommendations have been presented.
- Citation: Maru GB, Hudlikar RR, Kumar G, Gandhi K, Mahimkar MB. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: From experimental models to clinical trials. World J Biol Chem 2016; 7(1): 88-99
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/88.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.88
Cancer is a disease characterized by out-of-control cell growth leading to spread of abnormal cells to other body parts by local invasion and/or distant metastasis. It is one of the major and growing public health problem, currently accounting for over 12% deaths globally. With steady rise in life expectancy globally, it is estimated that the number of deaths due to cancer may double in next 50 years and new cases of cancer may rise to 15 million by 2020[1]. Majority of human cancers are caused by environmental and life-style factors. The etiology of all cancers is associated with inherited genetic aberrations (5%-10%) and acquired genetic abnormality (90%-95%) caused by exogenous and/or endogenous environmental agents[2].
Number of epidemiological studies have successfully shown that exposures to certain environmental chemicals/mixtures (exogenous-societal, occupational, lifestyle, industrial, agricultural, medicinal, etc., endogenous), physical agents (UV/solar, ionizing radiations, heat) and biological agents (certain viruses - EBV, HBV, HCV, HPV, HIV1, bacteria - H. pylori, parasites-liver fluke, schistosoma) increase the cancer risk at specific organs[3,4]. Experimental studies in animals, tissues/cells (in-vitro) have also provided supportive evidence for organ specific increased risk of cancer. Several of these associations have been established to be causative, e.g., use of tobacco and oral cancer, while for several other human cancers such as breast, prostate, esophagus, etc. though associated factors have been documented, their causal association is yet to be determined.
Dose and duration of exposure to environmental carcinogens and their complex interactions with genetic and/or acquired host factors are some of the critical determinants, for the development of cancer[2]. Response to carcinogen exposure may further be modulated by host (physical activity, obesity) and life style (diet, tobacco, alcohol) factors leading to disease or no-disease.
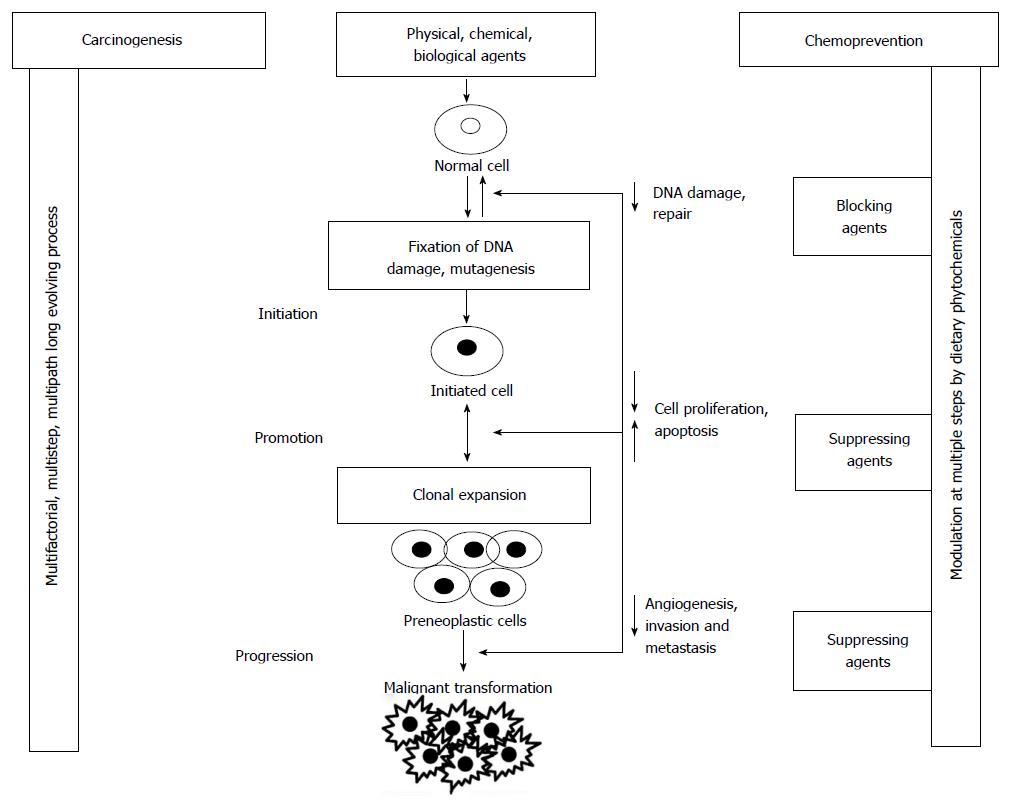
Carcinogenesis refers to environmental carcinogen [chemical/physical/biological agent(s)] -induced etiological pathways and processes that lead to cancer. It is a complex, multi-factorial, multi-step, multi-path and in humans a multi-years process comprised of at least three steps viz. initiation, promotion and progression[5] (Figure 1). Exposure of normal cells in tissues to carcinogenic agent results in genomic DNA damage (critical genes) and its fixation through a cycle of DNA replication leads to initiation and that is irreversible step. In the promotion process (reversible), clonal expansion of initiated cells occurs due to promotory stimuli resulting in formation of an actively proliferating, pre-malignant tumor cell population. While in progression (irreversible process) additional genetic changes lead to a new clone of tumor cells with increased proliferative, invasive, and metastatic potential.
Exposure to environmental carcinogens results in a series of genetic mutations and such alterations in at least two classes of genes, i.e., proto-oncogenes and/or tumor suppressor genes have been associated with tumor development. Activation of proto-oncogenes by qualitative or quantitative genetic changes results in enhancement of proliferative signals. Alternatively environmental carcinogen-mediated loss or inactivation of tumor suppressor genes (normal genes) also leads to tumor development[6,7]. Genetic mutations, genomic instability, and series of epigenetic events, such as chronic inflammation are known to play role in transformation of normal to malignant cells. All transformed cells exhibit certain common characteristics such as sustained proliferation signals, evasion of growth suppressors, resistance to cell death, replicative immortality, and ability to induce angiogenesis and activation of invasion and metastasis[8].
In spite of significant advancement in understanding the molecular mechanism of cancer development, very limited success has been achieved in early detection and treatment for most of the cancers. For patients with metastatic cancer, even the most advanced treatment strategies have not been successful in saving their lives while patients with less advanced cancer, treatment results in high morbidity and significant social and economic burden. Moreover, development of chemo/radio-resistance and recurrence of tumors are major challenges in the fight against cancer.
Considering limitations in early detection and successful treatment of cancer, preventive interventions have attracted increasing attention and significant research efforts. Several cancer prevention approaches and interventions have demonstrated potential for success in epidemiological and clinical trials across the globe[9,10] (Table 1).
| Cancer prevention approach | Measures undertaken | Demonstration in epidemiological studies/clinical trials | Ref. |
| Avoid/minimize exposure to known carcinogens/risk factors | Quit cigarettes smoking | Epidemiological study followed up for 40 yr on male British doctors demonstrated decrease in risk | [39-41] |
| Decreasing proportion of young smokers | Decrease in mortality due to lung cancer among younger male cohort | ||
| Reduction in number of cigarettes/day or duration of smoking and time since stopping smoking | Epidemiological study - decrease in the rate of lung cancer | [42] | |
| Ban the production and reduce the usage of carcinogenic aromatic amines | Reduction in bladder cancer among the dye workers | [43] | |
| Improvement in food preservation techniques | Significant reduction in incidence and mortality due to gastric cancers | [44] | |
| Vaccination | HPV vaccines | Have shown 95%-100% effectiveness in preventing the cervical cancer precursor lesions and in preventing cervical adenocarcinoma in situ. Protection conferred was highly variable in those with prior HPV infection | [45-47] |
| Gardasil (HPV 6,11,16,18) | |||
| Cervarix (HPV 16,18) | |||
| HBV vaccines | Rate of HCC decreased in children (6-14 yr) | [48] | |
| Engerix-B (HBV-DNA) | |||
| Recombivax (HBV surface antigen) | |||
| Surgical intervention | Prophylactic resection of high risk organs-bilateral mastectomy and oophorectomy in BRCA1/BRCA2 mutations carriers | Decrease in breast cancer risk and breast and ovarian cancer risk | [49-51] |
| Colostomy in FAP patients | Decrease in colorectal cancer risk in patients with APC mutations | [52,53] | |
| Chemoprevention | |||
| Non melanoma skin cancer | Topical application of 5-FU, Immiquimod, Diclofenac, PDT with delta-aminolevulinic acid, Ingenolmebutate | Partial or complete clearance of actinic keratosis leading to decrease in cancer | [54-64] |
| Skin melanoma | Daily or discretionary sunscreen (SPF15+) for 4 yr | Reduction in invasive melanoma in a community based trial | [22] |
| Breast cancer | Administration of selective estrogen receptor modulating agent(s) (Tamoxifen, Reloxifen)/aromatase inhibitors (Exemestane) | Trials have demonstrated efficacy of these agents in breast cancer prevention, reduction in recurrence and mortality | [25,65,66] |
| Prostate cancer | Administration of androgen receptor blockers (Finasteride, Dutasteride) | Reduction in prevalence and risk in clinical trials | [67-70] |
| Colorectal polyps, Adenomas, and cancer | Administration of NSAIDs- Aspirin, Celecoxib, Rofecoxib, Sulindac, DFMO | NSAIDs-mediated reduction in colorectal adenomas | [24,27,51,71-75] |
| Significant increase in time to first colorectal cancer occurrence |
Preventing cancers by avoiding or minimizing exposures, especially occupational and societal (habit-related) exposures to known environmental carcinogens is likely to be most practical and cost effective strategy[3,11]. However these approaches of elimination of carcinogen from the environment and/or minimizing the exposure to carcinogens have not achieved desired success due to lifestyle choices and modern developments. Additional cost effective and practical approaches in cancer prevention interventions include pursuing lifestyle or dietary changes, chemopreventive interventions or prophylactic resection of high-risk organs in certain germ-line mutation carriers[9,10,12]. One of these approaches with enormous potential is chemoprevention which can be defined as the use of natural/synthetic/biological agents to suppress or prevent either the initial phases of carcinogenesis (blocking agents) or delay the progression of premalignant cells to invasive disease(suppressing agents)[12-14]. Improved understanding of the molecular basis of carcinogenesis has further enhanced the interest and potential of preventive interventions, i.e.: (1) Process of carcinogenesis involving set of genetic and epigenetic changes has been shown to be modulated by several environmental agents and changes in lifestyle and host factors; and (2) Preventive strategies have achieved good measure of success in other environmental diseases such as infectious diseases (vaccines) and cardiovascular events (treating risk factors with statins, lifestyle changes). Chemoprevention of skin, breast, prostate, cervical, and colon cancer by various prevention agents have further stimulated the interest in the research area[10,12,15] (Table 1).
The aim of cancer chemoprevention is to delay or disrupt cancer causation associated pathways and multiple steps of carcinogenesis viz. initiation, promotion, and progression. Cancer preventive strategies can be classified into primary, secondary, and tertiary prevention. Primary cancer prevention is meant for general population and those at increased risk of cancer development. Secondary prevention is employed in patients with premalignant lesions that may progress to cancer. Tertiary cancer prevention is employed in preventing cancer recurrence (local invasion and/or distant metastasis) or second primary tumor among those who already have developed the disease.
This review article is targeted to briefly elaborate and compare the available evidence and understand the mechanism of chemoprevention by dietary phytochemicals in experimental and clinical investigations without presenting details on the experimental models, methods, and clinical trials.
Amongst environmental chemopreventive compounds, diet/beverage-derived agents due to their long history of exposure to humans, high tolerability, low toxicity, and reported biological activities are fast becoming lucrative targets for chemoprevention. Large number of pure compounds and extracts from dietary components has been evaluated in various experimental models for testing the chemopreventive efficacy of dietary phytochemicals[16,17]. Model systems have been employed based on their ability to rapidly answer, demonstrate expression of multi stage carcinogenesis, tissue/cell specificity, hormone responsiveness, invasiveness, modulation of tumor growth, histological types, and particular relevance to most common human cancers.
Chemopreventive activity of an agent is generally investigated employing carcinogens and/or spontaneously induced tumor and/or appropriate pathways or biomarkers in experimental animals/cell culture assays. In experimental models chemopreventive activity is ascertained based on observed increase in latency period and/or decrease in incidence and/or multiplicity of tumors or by modulation of disease/process associated biomarkers. Alternatively, development of pre-malignant lesions such as hyperplasia formation has been studied instead of carcinogenesis to study initial development[18]. In animal models regression of tumor or tumor xenografts and metastasis has also been reported[19-21].
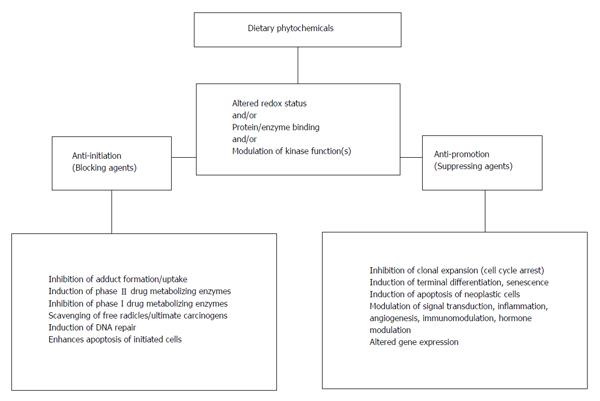
There are various dietary phytochemicals of plant origin (various plant parts-leaves, vegetables, fruits, seeds, etc.) such as capsaicin, curcumin, diallyl sulphide, genistein, green/black tea polyphenols, indoles, lycopene, phenethyl isothiocyanate, resveratrol, retinoids and tocopherols which are most frequently studied. These dietary phytochemicals have been demonstrated to modulate incidence and/or multiplicity and/or latency period of carcinogen/spontaneously-induced tumors at various organ sites in experimental rodent models (Table 2). Surrogate markers like cell proliferation and apoptosis and various detoxication pathways (xenobiotic enzyme inductions/inhibition, lipid peroxidation, etc.) have also been successfully used for demonstrating protective action in in vivo system. Overall, the mechanisms observed in the dietary phytochemical-mediated inhibition are diverse (Figure 2). The observed anti-initiating and anti-promoting activity of dietary phytochemicals in experimental models involve modulation of redox status, direct interaction with proteins and modulation of signaling kinases leading to effects on genes and cell signaling pathways at multiple levels (Figure 2).
| Compounds | Chemopreventive agent administered (before + during + after carcinogen treatment) route-range of dose | Prevention of carcinogen induced tumors | Pathways modulated | No. of studies reporting protection | Selected ref. | |||
| Target organs | Tumor multiplicity/tumor incidence (#) | Latency period | Cell proliferation/apoptosis | Detoxication pathways | ||||
| Tocopherols | Diet: 0.3%, 100-4000 mg/kg | Lung, Vagina, Prostate, Colon, Mammary gland | ↓(3)/NR | NR | ↓(3)/↑(2) | ↑(2) | 8 | [76,77] |
| Indoles | Diet: 0.25%, 500- 4000 ppm, 0.014 mmol/g, 112 μmol/g, Gavage 50-250 mg/kg BW | Endometrium, Liver, Mammary gland, Lung | ↓(5)/↓(2) | ↑ | ↓(4)/↑(3) | ↑(3) | 14 | [31] |
| Genistein | s.c.: 100-400 mg/mL (100 μL), Gavage: 1-500 mg/kg BW | Mammary gland, Prostate, Seminal vesicles, Buccal pouch, Uterous, Colon, Lung | ↓(5)/↓(2) | NR | ↓(5)/↑(3) | ↑(3) | 20 | [78] |
| Diet: 100-300 mg/kg | ||||||||
| Curcumin | Diet: 0.05%-2%, 500-2000 ppm, Topical: 1-100, 3000 nmol | Skin, Digestive system, Intestine1, Mammary gland, Liver, Kidney, Cheek pouch, Esophagus | ↓(14)/↓(8) | NR | ↓(12)/↑(8) | ↑(9) | 23 | [79-82] |
| Dially sulphide | i.g.: 150-200 mg/kg BW, Topical: 0.5%-1% (100 μL) | Colon, Kidney, Forestomach, Urinary bladder, Thymus, Lung, Esophagus, Buccal pouch, Skin, Liver, Colon, Kidney | ↓(6)/↓(2) | NR | ↓(5)/↑(3) | ↑(2) | 17 | [83,84] |
| Resveratrol | Diet: 60-200 mg/kg | Urinary bladder, Skin, Mammary gland1, Buccal pouch, Tounge, Prostate, Lung, Salivary gland | ↓(5)/↓(1) | ↑ | ↓(7)/↑(6) | ↑(4) | 24 | [85-88] |
| Retinoids | Topical (Oral): 0.5-1.0 mmol/kg BW | Urinary bladder, Skin, Mammary glands1, Buccal puch, Tounge, Prostate, Lung, Salivary gland | ↓(6)/↓(1) | ↑ | -/- | NR | 16 | [89-91] |
| Diet: 150-200 mg/kg | ||||||||
| Capsaicin | Diet: 1% capsaicinoids (64.5% capsaicin), Topical (Oral): 10-102 mg/kg BW | Lung, Skin, Tongue, Colon | ↓(4)/NR | - | ↓(4)/↑(3) | NR | 10 | [92,93] |
| Phenethy isothiocyanate | Diet: 5-25 μmol/g, 0.01%-0.1% | Lung, Esophagus, Urinary bladder, Colon | ↓(3)/↓(1) | NR | -/- | NR | 9 | [94-96] |
| Lycopene | Drinking water: 17 ppm | Prostate, Lung, Colon, Mammary gland1, Buccal pouch, Liver | ↓(5)/↓(2) | ↑ | ↓(4)/↑(5) | ↑(5) | 18 | [97] |
| Diet: 10-300 mg/kg Gavage: 15-20 mg/kg BW | ||||||||
| Green, Black tea polyphenols | Drinking water: 1%-5%, 200-1000 ppm | Liver, Skin, Mammary gland, Lung, Buccal pouch, Colon, Esophagus, Prostate gland | ↓(15)/↓(6) | ↑ | ↓(9)/↑(10) | ↑(8) | 24 | [98-104] |
| Diet: 0.05%, Topical: 1-200 mg/animal | ||||||||
Following findings are noted after comparing and analyzing the outcome of all available experimental studies (Table 2) evaluating the chemopreventive efficacy of dietary phytochemicals: (1) Effects are moderate and generally decrease in multiplicity, burden of tumor, and/or moderate increase in latency period have been observed without major change in incidence of tumor; (2) Relatively long and repeated exposures (prior, during, and post-carcinogen) to dietary phytochemicals have generally been needed for observing protective effects; (3) Most of the dietary phytochemicals have been demonstrated to be effective against several classes of environmental carcinogens at multiple organ sites; (4) Bioavailability of dietary phytochemicals and their metabolites have not been reported from most of the experimental studies that demonstrated their chemopreventive efficacy; and (5) In most of these studies doses of chemopreventive agent(s) administered or effective doses appear to be much higher than normal dietary exposures received in human, although chemopreventive doses have not been reported to be toxic.
Comprehensive evaluation of preclinical efficacy and safety of dietary phytochemicals, the promising compounds have been evaluated in appropriate clinical trials. A brief summary of the outcome from these trials has been presented (Table 3) and following points may be summarized: (1) In most of the trials chemopreventive agents were administered to patients with cancer or high risk individuals, i.e., after occurrence of damage or disease; (2) Though there are several clinical trials conducted using dietary phytochemicals, results from only approximately 20% trials have been reported. Other trials have either not been completed or their results are not reported due to issues of toxicity, bioavailability and other unknown reasons under the conditions employed for the trials; (3) Bioavailability issues have been reported for curcumin, genistein, resveratrol, lycopene and green tea; (4) Toxicity issues have been reported in clinical trials conducted with tocopherols and retinoids; and (5) Very few trials with curcumin and green tea have shown beneficial effects as judged by modulation of biomarkers and symptoms.
| Compounds | No. of clinical trials | Chemopreventive agent administered | Target organs | Efficacy | Bioavailability/toxicity issues | Ref. | ||
| Conducted | Results reported | High risk individuals/patients1 | Route-dose range | |||||
| Curcumin | 52 | 3 | -/+ | Oral: 0.036-8 g daily | Multiple organ sites | +(2)/-(1) | +(1)/-(3) | [105-107] |
| Genistein | 29 | 7 | -/+ | Oral: 160-600 mg/d | Breast, urothelial bladder, Mammary gland | NR | +(1)/-(1) | [108,109] |
| Indoles | 4 | 0 | -/+ | Oral: 2 serving (½ cup/serving) daily | Prostate, breast, blood | NR | NR/NR | NR |
| Tocopherols | 28 | 9 | +/+ | Oral: 50-500 mg/d, 400-1000 IU/d | Prostate, lung | NR | NR/+(2) | [110] |
| Dially Sulfide | 0 | 0 | -/- | NR | NR | NR | NR/NR | NR |
| Resveratrol | 5 | 2 | -/+ | Oral: 20-80 mg/d | Colorectal, colon | +(2) | +(1)/-(2) | [32,111] |
| Retinoids | 102 | 17 | +/+ | Oral: 1-80 mg/d | Lung, blood, prostate, kidney, skin, blood, head and neck, liver | +(3)/-(7) | NR/+(2) | [112,113] |
| Capsaicin | 2 | 0 | -/+ | Oral: 112 mg twice daily for 6 mo, one capsaicin lozenge 4 times daily | Prostate, head and neck | NR | NR/NR | NR |
| PEITC | 9 | 1 | -/+ | Oral: Broccoli (300 g) soup four times daily for 5 d, Broccoli seed extract (250 mg), 40 mg PEITC capsules 4 times a day | Lung, oral | -(1) | NR/-(1) | NR |
| Lycopene | 26 | 24 | -/+ | Oral: 15-90 mg/d | Prostate | +(1)/-(1) | +(2)/NR | [114,115] |
| Green tea | 85 | 6 | -/+ | Oral: 400-2000 mg twice a day, (daily dose is equivalent of 9 cup-of-green tea per day (0.9 g/d GTE, 0.6 g/d EGCG) | Blood, colorectal, prostate, pancreas | +(3)/-3 | +(3)/-(3) | [116-118] |
If direction of results from clinical trials evaluating the chemopreventive potential of dietary phytochemicals is compared with that of their chemopreventive effects in experimental models, it is clear that efficacy observed in experimental studies has not been observed in clinical trials. To understand the differences between experimental studies and clinical trials that may have contributed in the observed mismatch in the direction of outcome, the probable major differences have been listed below: (1) Experimental systems are generally simple and homogenous in terms of genetic, host and lifestyle factors and conditions are controlled, while in clinical trials additional variables of host and lifestyle factors, exposure complexity and metabolic competence makes the system more complex; (2) Exposure to potential chemopreventive agent in experimental systems is generally before, during and/or after carcinogen treatment when cells/tissues/processes are generally normal while in clinical trials considered in this review administration of chemopreventive agent is after damage/disease, i.e., high-risk individuals/cancer patients; and (3) Chemopreventive efficacy of dietary phytochemicals in experimental systems has been generally at doses higher than their normal dietary exposure to human while bioavailability and toxicity issues have probably affected the outcome in clinical trials.
To understand the role or contribution of listed factors (differences between two systems), a comparison of similarities and differences in the properties and biological effects of dietary phytochemicals with approved chemopreventive agents has been made (Table 4).
| Dietary phytochemicals | Approved chemopreventive agents |
| Occurrence | |
| Natural compounds of plant origin | Majority are synthetic compounds while some are biological and/or natural agents |
| Long History of exposure to humans through food | Human exposure as prescribed drug/vaccine |
| Easily available and relatively cheaper | Available on prescription and relatively expensive |
| Properties | |
| Anti-oxidant/polyphenolic in nature, sparingly soluble in water | Widely differing properties and characteristics |
| Chemopreventive efficacy | |
| Established in experimental models, yet to prove efficacy in clinical trials | Established both in experimental models and clinical trials |
| Effects-weak to moderate | Effects-moderate to strong |
| Successful against different classes of carcinogens and at multiple organ sites | May not be successful against different classes of carcinogens and at multiple organ sites |
| Mechanism(s) | |
| Modulators of redox status and kinase functions, inducer of phase II enzymes | Mechanisms are diverse and may not be related to redox status and/or kinase functions, may not be modulating phase II enzymes |
| Modulate multiple pathways/targets | Modulation of specific targets/pathways |
| Specificity | |
| Relatively less specific/non-specific (Pleotropic effects) | Relatively specific to defined agent/exposure and/or molecular pathways |
| Toxicity | |
| Depends on dose and duration of exposure. Non-toxic at the doses present in food | Depends on dose and duration of exposure |
Considering the chemopreventive efficacy of approved chemopreventive agents both in experimental studies as well as clinical trials[22-29], it appears that differences in host and lifestyle factors and the time of exposure to chemopreventive agent, i.e., before and after damage/disease, may not be the major determinants in the direction of outcome between the two systems. Based on these comparisons and reports in literature on the properties and bioavailability of dietary phytochemicals in clinical trials, bioavailability, metabolic processing and toxicity related differences appear to be the major and important factors.
Several clinical trials have found that administration of dietary phytochemicals resulted in low undetectable levels in blood[30-32], which probably curtailed its progress from laboratory to the clinic. Search for potential factors contributing to low bioavailability suggest: (1) low aqueous solubility (polarity, poor dissolution rate) of compounds; (2) poor absorption (lipophilic nature); and (3) extensive metabolic conversion to conjugates/metabolites. Number of approaches are currently being evaluated for enhancing the bioavailability such as use of nano-dietary phytochemicals (particles)/dietary phytochemicals in nano-carriers, incorporation of polymers, proteins/amino acids, phospholipids and/or solid lipids, use of liposomes for delivery of dietary phytochemicals or using phytochemicals coated with essential natural oil and use of synthetic analogues[33]. With continuing efforts to enhance the bioavailability of dietary phytochemicals using various approaches, we also need to understand if there is linear relationship between bioavailability and bioefficacy and identify the factors that are responsible for observed toxicity of these compounds in clinical trials but not in experimental studies.
Considering the limitations of currently available/employed animal models in identification of environmental chemopreventive agents, existing models need to be improved and new experimental models need to be developed. Efforts to achieve/incorporate exposure complexity similar to those in humans and replication of human host and/or lifestyle factors in animal models if successful may help in addressing the current limitations. Before embarking on clinical trials, experimentally successful chemopreventive agent(s) need to be thoroughly investigated for the mechanism(s) of observed chemopreventive actions and/or toxicity. Similarities and differences in circulating levels of chemopreventive agent(s) and metabolic products attained in experimental vs real life also need to be understood. Since process of carcinogenesis involves complex interplay between various factors it is suggested to study and understand the role of diet (animal vs plant sources), calorie content and physical activity on the dietary phytochemical(s) (single or mixture)-mediated effects in experimental systems and in humans. This suggestion is based on the available experimental and epidemiological observations on modulatory effects of calorie content[34,35], diets rich in fruits and vegetables[36] and moderate physical activity[37,38]. Experimental and clinical observations based on mechanistic studies, gene-environment interactions and complementary nutritional studies may help in identifying environmental chemopreventive agents. However, cancer being multi-factorial, multi-step complex evolving process, establishing or understanding the protective effect of one such factor or class of agent alone may not address the problem satisfactorily. Identifying novel prevention approaches and developing number of dietary chemopreventive agents differing in mechanism of their chemoprotectory action may help in slowing down and delaying /prevention of environmental cancers.
Dr. Maru GB thanks Director, ACTREC for the opportunity and support. Authors thank Dr. Priyanka Tilak for critical reading of the manuscript. Authors also thank Photography and IT department, ACTREC, Mr. Venkatesh Pai and Mr. Ashish Kulkarni for technical improvements in the manuscript.
P- Reviewer: Poirot M, Ruiz RB S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | International Agency for Researvh on Cancer. Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available from: www.globocan.iarc.fr. |
| 2. | Stewart B, Wild CP Ed. World Cancer Report, Chapter 2, Etiology of cancer. 82-169. |
| 3. | Tomatis L, Huff J, Hertz-Picciotto I, Sandler DP, Bucher J, Boffetta P, Axelson O, Blair A, Taylor J, Stayner L. Avoided and avoidable risks of cancer. Carcinogenesis. 1997;18:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 481] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 5. | Weinstein IB. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988;48:4135-4143. [PubMed] |
| 6. | Bowden GT, Schneider B, Domann R, Kulesz-Martin M. Oncogene activation and tumor suppressor gene inactivation during multistage mouse skin carcinogenesis. Cancer Res. 1994;54:1882s-1885s. [PubMed] |
| 7. | Solomon H, Brosh R, Buganim Y, Rotter V. Inactivation of the p53 tumor suppressor gene and activation of the Ras oncogene: cooperative events in tumorigenesis. Discov Med. 2010;9:448-454. [PubMed] |
| 8. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 9. | Lippman SM, Hong WK. Cancer prevention science and practice. Cancer Res. 2002;62:5119-5125. [PubMed] |
| 10. | Maru G. An Update on Cancer Prevention Approaches. Biomed Res J. 2014;2:146-72. |
| 11. | Tomatis L. Etiologic evidence and primary prevention of cancer. Drug Metab Rev. 2000;32:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Wu X, Patterson S, Hawk E. Chemoprevention--history and general principles. Best Pract Res Clin Gastroenterol. 2011;25:445-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Wattenberg LW. Chemoprevention of cancer. Cancer Res. 1985;45:1-8. [PubMed] |
| 14. | Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 296] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Patterson SL, Colbert Maresso K, Hawk E. Cancer chemoprevention: successes and failures. Clin Chem. 2013;59:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2044] [Cited by in RCA: 1899] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 17. | Patel R, Garg R, Erande S, B Maru G. Chemopreventive herbal anti-oxidants: current status and future perspectives. J Clin Biochem Nutr. 2007;40:82-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Crowell JA. The chemopreventive agent development research program in the Division of Cancer Prevention of the US National Cancer Institute: an overview. Eur J Cancer. 2005;41:1889-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Li Y, Che M, Bhagat S, Ellis KL, Kucuk O, Doerge DR, Abrams J, Cher ML, Sarkar FH. Regulation of gene expression and inhibition of experimental prostate cancer bone metastasis by dietary genistein. Neoplasia. 2004;6:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Saleem M, Kweon MH, Yun JM, Adhami VM, Khan N, Syed DN, Mukhtar H. A novel dietary triterpene Lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005;65:11203-11213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Maru GB, Kumar G, Ghantasala S, Tajpara P. Polyphenol-mediated in vivo cellular responses during carcinogenesis Polyphenols in Human Health and Disease. : Academic Press 2014; 1141-1173. |
| 22. | Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 463] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 23. | Klug HL, Tooze JA, Graff-Cherry C, Anver MR, Noonan FP, Fears TR, Tucker MA, De Fabo EC, Merlino G. Sunscreen prevention of melanoma in man and mouse. Pigment Cell Melanoma Res. 2010;23:835-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 725] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 25. | Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4008] [Cited by in RCA: 3604] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 26. | Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409-412. [PubMed] |
| 27. | Gann PH, Manson JE, Glynn RJ, Buring JE, Hennekens CH. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85:1220-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 259] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Reddy BS, Rao CV, Rivenson A, Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993;14:1493-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 214] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Jordan VC, Naylor KE, Dix CJ, Prestwich G. Anti-oestrogen action in experimental breast cancer. Recent Results Cancer Res. 1980;71:30-44. [PubMed] |
| 30. | Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894-1900. [PubMed] |
| 31. | Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 32. | Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392-7399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 33. | Aqil F, Munagala R, Jeyabalan J, Vadhanam MV. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013;334:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 34. | Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Hursting SD, Dunlap SM, Ford NA, Hursting MJ, Lashinger LM. Calorie restriction and cancer prevention: a mechanistic perspective. Cancer Metab. 2013;1:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Norat T, Aune D, Chan D, Romaguera D. Fruits and vegetables: updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Cancer Treat Res. 2014;159:35-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Standard J, Jiang Y, Yu M, Su X, Zhao Z, Xu J, Chen J, King B, Lu L, Tomich J. Reduced signaling of PI3K-Akt and RAS-MAPK pathways is the key target for weight-loss-induced cancer prevention by dietary calorie restriction and/or physical activity. J Nutr Biochem. 2014;25:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Food, nutrition, physical activity, and the prevention of cancer: a global perspective. World Cancer Research Fund and American Institute for Cancer Research. : Washington DC 2007; 289-298. |
| 39. | Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 372] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 40. | Coleman MP, Estève J, Damiecki P, Arslan A, Renard H. Trends in cancer incidence and mortality. IARC Sci Publ. 1993;1-806. [PubMed] |
| 41. | Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001;93:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Lubin JH. Modifying risk of developing lung cancer by changing habits of cigarette smoking. Br Med J (Clin Res Ed). 1984;289:921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Swerdlow AJ. Effectiveness of primary prevention of occupational exposures on cancer risk. IARC Sci Publ. 1990;23-56. [PubMed] |
| 44. | Hwang H, Dwyer J, Russell RM. Diet, Helicobacter pylori infection, food preservation and gastric cancer risk: are there new roles for preventative factors? Nutr Rev. 1994;52:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1294] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 46. | Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 520] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 47. | FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1457] [Cited by in RCA: 1379] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 48. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1196] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 49. | Haber D. Prophylactic oophorectomy to reduce the risk of ovarian and breast cancer in carriers of BRCA mutations. N Engl J Med. 2002;346:1660-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Meijers-Heijboer H, van Geel B, van Putten WL, Henzen-Logmans SC, Seynaeve C, Menke-Pluymers MB, Bartels CC, Verhoog LC, van den Ouweland AM, Niermeijer MF. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 653] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 51. | Metcalfe KA. Oophorectomy for breast cancer prevention in women with BRCA1 or BRCA2 mutations. Womens Health (Lond Engl). 2009;5:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1815] [Cited by in RCA: 1715] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 53. | Lynch PM, Ayers GD, Hawk E, Richmond E, Eagle C, Woloj M, Church J, Hasson H, Patterson S, Half E. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am J Gastroenterol. 2010;105:1437-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Kurwa HA, Yong-Gee SA, Seed PT, Markey AC, Barlow RJ. A randomized paired comparison of photodynamic therapy and topical 5-fluorouracil in the treatment of actinic keratoses. J Am Acad Dermatol. 1999;41:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Loven K, Stein L, Furst K, Levy S. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin Ther. 2002;24:990-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J Am Acad Dermatol. 2012;66:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Stockfleth E, Sterry W, Carey-Yard M, Bichel J. Multicentre, open-label study using imiquimod 5% cream in one or two 4-week courses of treatment for multiple actinic keratoses on the head. Br J Dermatol. 2007;157 Suppl 2:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Tutrone WD, Saini R, Caglar S, Weinberg JM, Crespo J. Topical therapy for actinic keratoses, I: 5-Fluorouracil and imiquimod. Cutis. 2003;71:365-370. [PubMed] |
| 59. | Tutrone WD, Saini R, Caglar S, Weinberg JM, Crespo J. Topical therapy for actinic keratoses, II: Diclofenac, colchicine, and retinoids. Cutis. 2003;71:373-379. [PubMed] |
| 60. | Gupta AK, Davey V, Mcphail H. Evaluation of the effectiveness of imiquimod and 5-fluorouracil for the treatment of actinic keratosis: Critical review and meta-analysis of efficacy studies. J Cutan Med Surg. 2005;9:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Hanke CW, Beer KR, Stockfleth E, Wu J, Rosen T, Levy S. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 3-week cycles. J Am Acad Dermatol. 2010;62:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Berlin JM, Rigel DS. Diclofenac sodium 3% gel in the treatment of actinic keratoses postcryosurgery. J Drugs Dermatol. 2008;7:669-673. [PubMed] |
| 63. | Kaufmann R, Spelman L, Weightman W, Reifenberger J, Szeimies RM, Verhaeghe E, Kerrouche N, Sorba V, Villemagne H, Rhodes LE. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol. 2008;158:994-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Ulrich C, Busch JO, Meyer T, Nindl I, Schmook T, Sterry W, Stockfleth E. Successful treatment of multiple actinic keratoses in organ transplant patients with topical 5% imiquimod: a report of six cases. Br J Dermatol. 2006;155:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1580] [Cited by in RCA: 1400] [Article Influence: 116.7] [Reference Citation Analysis (0)] |
| 66. | Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 672] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 67. | Lønning PE, Eikesdal HP. Aromatase inhibition 2013: clinical state of the art and questions that remain to be solved. Endocr Relat Cancer. 2013;20:R183-R201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Foley CL, Kirby RS. 5 alpha-reductase inhibitors: what’s new? Curr Opin Urol. 2003;13:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 794] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 70. | Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2044] [Cited by in RCA: 1883] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 71. | Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081-2087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 699] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 72. | Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, Ford LG, Jacobs EJ, Jankowski JA, La Vecchia C. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26:47-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 73. | Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, Bolognese JA, Oxenius B, Horgan K, Loftus S. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 313] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 74. | Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 783] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 75. | Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila). 2008;1:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 76. | Yang CS, Suh N. Cancer prevention by different forms of tocopherols. Top Curr Chem. 2013;329:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Lu G, Xiao H, Li GX, Picinich SC, Chen YK, Liu A, Lee MJ, Loy S, Yang CS. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 2010;31:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Kim SH, Kim CW, Jeon SY, Go RE, Hwang KA, Choi KC. Chemopreventive and chemotherapeutic effects of genistein, a soy isoflavone, upon cancer development and progression in preclinical animal models. Lab Anim Res. 2014;30:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 735] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 80. | Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 322] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 81. | Garg R, Gupta S, Maru GB. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 82. | Garg R, Ramchandani AG, Maru GB. Curcumin decreases 12-O-tetradecanoylphorbol-13-acetate-induced protein kinase C translocation to modulate downstream targets in mouse skin. Carcinogenesis. 2008;29:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 84. | Dorant E, van den Brandt PA, Goldbohm RA, Hermus RJ, Sturmans F. Garlic and its significance for the prevention of cancer in humans: a critical view. Br J Cancer. 1993;67:424-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 124] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 85. | Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953-957. [PubMed] |
| 86. | Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila). 2009;2:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 357] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 87. | Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review). Int J Oncol. 2003;23:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 489] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 89. | Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305-7315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 90. | Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 585] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 91. | Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 460] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 92. | Bode AM, Dong Z. The two faces of capsaicin. Cancer Res. 2011;71:2809-2814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 93. | Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 94. | Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 95. | Zhang Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis. 2012;33:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 96. | Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 302] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 97. | Cohen LA. A review of animal model studies of tomato carotenoids, lycopene, and cancer chemoprevention. Exp Biol Med (Maywood). 2002;227:864-868. [PubMed] |
| 98. | Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 830] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 99. | Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523-524:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Lin JK, Liang YC. Cancer chemoprevention by tea polyphenols. Proc Natl Sci Counc Repub China B. 2000;24:1-13. [PubMed] |
| 101. | Kumar G, Dange P, Kailaje V, Vaidya MM, Ramchandani AG, Maru GB. Polymeric black tea polyphenols modulate the localization and activity of 12-O-tetradecanoylphorbol-13-acetate-mediated kinases in mouse skin: mechanisms of their anti-tumor-promoting action. Free Radic Biol Med. 2012;53:1358-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Kumar G, Pillare SP, Maru GB. Black tea polyphenols-mediated in vivo cellular responses during carcinogenesis. Mini Rev Med Chem. 2010;10:492-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Patel R, Krishnan R, Ramchandani A, Maru G. Polymeric black tea polyphenols inhibit mouse skin chemical carcinogenesis by decreasing cell proliferation. Cell Prolif. 2008;41:532-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Patel R, Maru G. Polymeric black tea polyphenols induce phase II enzymes via Nrf2 in mouse liver and lungs. Free Radic Biol Med. 2008;44:1897-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847-6854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 900] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 106. | Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491-4499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 914] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 107. | Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1228] [Article Influence: 94.5] [Reference Citation Analysis (1)] |
| 108. | Messing E, Gee JR, Saltzstein DR, Kim K, diSant’Agnese A, Kolesar J, Harris L, Faerber A, Havighurst T, Young JM. A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer Prev Res (Phila). 2012;5:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 109. | Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 421] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 110. | Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1222] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 111. | Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. 2009;1:25-37. [PubMed] |
| 112. | Ryningen A, Stapnes C, Paulsen K, Lassalle P, Gjertsen BT, Bruserud O. In vivo biological effects of ATRA in the treatment of AML. Expert Opin Investig Drugs. 2008;17:1623-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1035] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 114. | Magbanua MJ, Richman EL, Sosa EV, Jones LW, Simko J, Shinohara K, Haqq CM, Carroll PR, Chan JM. Physical activity and prostate gene expression in men with low-risk prostate cancer. Cancer Causes Control. 2014;25:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Magbanua MJ, Roy R, Sosa EV, Weinberg V, Federman S, Mattie MD, Hughes-Fulford M, Simko J, Shinohara K, Haqq CM. Gene expression and biological pathways in tissue of men with prostate cancer in a randomized clinical trial of lycopene and fish oil supplementation. PLoS One. 2011;6:e24004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila). 2009;2:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 117. | Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, Suganuma M, Fujiki H, Moriwaki H. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17:3020-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 118. | Stingl JC, Ettrich T, Muche R, Wiedom M, Brockmöller J, Seeringer A, Seufferlein T. Protocol for minimizing the risk of metachronous adenomas of the colorectum with green tea extract (MIRACLE): a randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population. BMC Cancer. 2011;11:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |