Published online Sep 5, 2025. doi: 10.4331/wjbc.v16.i3.105875
Revised: April 20, 2025
Accepted: June 25, 2025
Published online: September 5, 2025
Processing time: 201 Days and 22 Hours
Fruits of Gardenia jasminoides (G. jasminoides) have extremely high medicinal value. However, the quality and traits of the plants vary significantly based on their provenances. In addition, the behaviour of the known bioactive components, such as geniposide and crocin, has been the primary focus of the research on G. jasminoides. However, the identification of unknown bioactive components and their metabolomics remains underexplored. Therefore, analysing the metabolic differences between gardenias from different sources is essential to provide a comprehensive theoretical basis for the evaluation of G. jasminoides and germ
To systematically evaluate the morphology, secondary metabolites, typical active ingredients, and antioxidant activity of wild G. jasminoides fruits.
Gardenia fruits were collected from different provenances. Metabolites were identified via ultra-high-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS). The metabolic differences were compared using hierarchical cluster analysis (HCA). The antioxidant capacity was evaluated using 2,2-diphenyl-1-picrylhydrazyl radical scavenging assays and ferric reducing antioxidant power assays, and its correlation with typical active ingredients was analysed.
A total of 444 and 240 metabolites were identified using UPLC-MS/MS in positive and negative ion modes, respectively. The HCA results of the flavonoids indicated that the higher content of flavonoids was in the fruits from Lukou. The differential analysis of metabolites in fruits from Shaoyang, Miluo and Lukou showed that the fruit from Miluo had the highest upregulated differential metabolites.
The metabolic characteristics of the Ningxiang and Xiangxi extracts were similar, while those of Lukou, Miluo and Shaoyang extracts differed significantly.
Core Tip: This study investigated the metabolomic profiles of wild Gardenia jasminoides (G. jasminoides) fruits from five regions in the Hunan Province, China, using ultra high-performance liquid chromatography–tandem mass spectrometry, and further analysed the relationship between the typical active ingredients and antioxidant activity. Contrary to conventional assumptions, chlorogenic acid exhibited a significant negative correlation to ferric reducing antioxidant power, indicating that the antioxidant capacity depends on synergistic interactions of multiple components. The fruits from Miluo demon
- Citation: Bi WY, He WL, Wang W, Wang XR, Zhou ZJ, Zeng YL. Chemical composition differences in wild Gardenia jasminoides fruits across provenances using metabolomics. World J Biol Chem 2025; 16(3): 105875
- URL: https://www.wjgnet.com/1949-8454/full/v16/i3/105875.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v16.i3.105875
Gardenia jasminoides (G. jasminoides) J. Ellis is an evergreen shrub of the genus Gardenia and family Rubiaceae. The Chinese gardenia has been grown for more than a millennium; it reached Europe and America by the mid-1700s[1-3]. Many iridoids, flavonoids and organic acids, which have complex chemical compositions, have been isolated from G. jasminoides.; however, terpenes are the primary bioactive components in it[4]. G. jasminoides contains three main types of active components, namely, iridoid glycosides, organic acids and pigments. Geniposide, a secondary metabolite of monoterpenes, is a cyclic ether terpenoid compound[5]; this can be used as an indicator component of G. jasminoides, as reported in the 2020 edition of the Chinese Pharmacopoeia. Crocin is a bimolecular gentian disaccharide of saffron acid that can be converted into saffron acid under enzymatic hydrolysis conditions. It is an important anti-inflammatory, anticancer, antibacterial and antiviral agent since it contains various flavonoids, alkaloids and terpenoids. In addition, gardenia fruits can be used in the treatment of various illnesses and diseases, such as headache, fever, inflammation, jaundice and hypertension[6-9].
The presence of metabolites in plants is strongly influenced by their growth environment. Altitude, temperature and light levels are the primary factors that affect the accumulation of secondary metabolites in plants[10]. The quality and traits of plants from different provenances may vary significantly, providing the basis for breeding superior varieties[6]. In addition, research on G. jasminoides has primarily focused on the behaviour of its known bioactive components, such as geniposide and crocin. However, the identification of unknown bioactive components and their metabolomics remains underexplored[11]. Ultra-high performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) facilitates the comprehensive detection of metabolites in specific plant organs and is therefore widely employed in metabolomics[12]. This research employed UPLC-MS/MS untargeted metabolomics to analyse metabolites of G. jasminoides procured from five regions in the Hunan Province, China. The variations in the types and quantities of G. jasminoides were analysed using multiple statistical methods, including principal component analysis (PCA), hierarchical cluster analysis (HCA) and pathway analysis. The differences in the distribution and composition of the metabolites present in the fruits of G. jasminoides from five different sources were analysed, and the key metabolic pathways were identified. This research primarily aimed to establish a comprehensive theoretical basis for the identification and evaluation of G. jasminoides, germplasm resource identification and by-product development and utilisation.
For metabolome analysis, fruits of wild G. jasminoides were collected from Shaoyang (SY) (27.237842N, 111.46923E), Lukou (LK) (28.24595N, 113.08093E), Miluo (ML) (28.80642N, 113.06711E), Xiangxi (XX) (28.31173N, 109.7389E) and Ningxiang (NX) (28.27741N, 112.55183E) (Figure 1).
Chromatographically pure methanol, acetonitrile, formic acid, phosphoric acid, acetic acid and ammonium nitrate were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Ultrapure water was used in all experiments. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging (BC4750) and fluorine-resistant acid phosphatase (FRAP) (BC5450) kits were obtained from Solarbio Science and Technology Co., Ltd., Beijing, China.
The following instruments were used in this study: Ultra-high-performance liquid chromatograph (HPLC) (Vanquish, Thermo Fisher Scientific), high-resolution mass spectrometer (Orbitrap Exploris 120, Thermo Fisher Scientific), centrifuge (Heraeus Fresco 17, Thermo Fisher Scientific), balance (BSA124S-CW, Sartorius), grinding instrument (JXFSTPRP-24, Shanghai Jingxin Industrial Development Co., Ltd.), pure water equipment (Mingche D24UV, Merck Millipore), visible spectrophotometer (722N, YOKE Instrument), centrifuge (Allegra X-22R, Beckman), digital display constant temperature water bath (XMTD203, Jiangsu Kexi Instrument Co., Ltd.), optical glass cuvette (Purshee Optical Elements Co., Ltd.) and ultrasonic instrument (YM-080S, Fang Ao Microelectronics Co., Ltd.). Chromatography was performed using an ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 100 mm, Waters).
The main morphological characteristics, including length, width, sepal length, and hundred-grain weight of 50 fresh wild G. jasminoides fruits, were determined per sample. The longitudinal length of the sample, measured using a vernier calliper, served as the length parameter. The width was measured at the broadest point of the sample, orthogonal to its length. The distance from the top to the bottom of the persistent calyx, measured using a vernier calliper, was defined as the sepal length. The hundred-grain weight was calculated by weighing 100 individual fruits per sample on a precision electronic scale, with three replicates taken for each species. The mean value was then derived to determine the standard weight per hundred fruits.
We used the DPPH kit to measure the DPPH free-radical scavenging ability. DPPH was dissolved in ethanol to prepare the DPPH stock solution. Briefly, 25 μL and 975 μL of the extract were added to the test tube, mixed in a vortex and incubated in the dark for 30 min. The absorbance at 515 nm was measured with a spectrophotometer. Anhydrous ethanol was used as the control, and Trolox was used as the standard. The DPPH free radical scavenging ability of the sample was calculated using the formula:
Here, D (%) = [A0 - (Ad - Ac)] ÷ A0 × 100%. A0 – the blank tube absorbance; Ad – the determination tube absorbance; Ac – the control tube absorbance.
We used the FRAP kit to measure the FRAP activity. Briefly, 1 mL of extract was added to approximately 0.1 g of the tissue and an ice bath homogenate was generated. Following centrifugation at a rotating speed of 8000 g for 10 minutes at 4 °C, the supernatant was taken and mixed with the reagents in the kit on ice in order to make the determination, control, standard and standard blank tubes; their absorbance at 400 nm was measured. The FRAP activity of the sample to DPPH was calculated using the formula:
FRAP activity (U/g) = (∆Ad × Cs ÷ ∆As) × Vd ÷ (W ÷ Ve × Vs) ÷ T × 103. Here, ∆Ad = Ad – Ac; ∆As = As – A0; As: The determination tube absorbance; Ac: The control tube absorbance; As: Standard tubes absorbance; A0: The blank tube absorbance; Cs: Phenol standard solution, 0.625 μmol/mL; Vs: The sample volume added to the reaction system, 0.05 mL; Ve: The extraction volume added to the reaction system, 1 mL; T: Response time, 30 minutes; W: Sample quality, 0.1 g.
The chlorogenic acid, geniposide and crocin contents of the samples were determined by HPLC. To determine the chlorogenic acid content, gardenia powder (0.2 g) was dissolved in 70% methanol (50 mL) and extracted at 25 °C for 1 hour. Further ultrasonic extraction was performed for 1 hour with 79% methanol at a constant volume, and the filtrate was obtained.
To determine the geniposide content, the fruit powder (5 g) was dissolved in methanol (30 g), and the solution was shaken on a shaking table for 3 hours. After filtering, the filtrate was concentrated to 1 mL by rotary evaporation, transferred to a 2 mL volumetric bottle and diluted with methanol to volume and then diluted with methanol 100 times after taking the constant volume.
To determine the crocin content, the fruit powder (1.0 g) was dissolved in methanol (70%, 10 mL), followed by ultrasonic treatment for 30 minute. The mixture was then shaken using a shaking table for 4 hour and filtered to obtain the extract. Subsequently, the filtrate was collected in a 50 mL volumetric bottle and diluted with methanol (70%) to volume.
Geniposide, crocin and chlorogenic acid were analysed under the following chromatographic conditions: A (acetonitrile): B (pure water) = 10:90, 238 nm, A (acetic acid ammonium acetate buffer): B (acetonitrile) = 15:85, 440 nm and A (acetonitrile): B (0.2% phosphoric acid aqueous solution) = 13:87, 327 nm, respectively. The flow rate, column temperature, and sample size were 1.0 mL/minute, 30 °C and 10 μL, respectively.
The samples were freeze-dried and ground (60 Hz, 150 seconds). Subsequently, 100 mg samples were mixed with 500 μL of the extraction solvent (methanol: Water, 4:1; internal standard: 10 μg/mL). Following that, the solution was swirled for 30 s, homogenised at 45 Hz for 4 minutes, ultrasonicated in an ice water bath for 1 hour and left undisturbed at -40 °C for 1 hour. After centrifugation at 12000 rpm (centrifugal force: 13800 g; radius: 8.6 cm) for 15 minutes, the supernatant was filtered through a 0.22 μm microporous membrane to obtain the extract for testing. Each extract (30 μL) was mixed into a quality control (QC) sample and stored at -80 °C prior to testing.
The flow velocity and composition of the Vanquish ultra-high-performance liquid phase were adjusted according to the parameters listed in Table 1. Formic acid (0.1%) was added to both A and B phases, and the samples (sample volume: 5 μL) were analysed using a UPLC BEH C18 column. The flow compatibility values are presented in Table 1. Primary and secondary mass spectrum data were acquired using an Orbitrap Exploris 120 mass spectrometer controlled by Xcalibur software. The parameters were as follows: Sheathing gas flow rate: 30 Arb, argon flow rate: 10 Arb, ion transport tube temperature: 350 °C, carburettor temperature: 350 °C, full mass spectrum resolution: 60000, mass spectrum/mass spectrum resolution: 15000, collision energy: 16/38/42 in the NCE mode and spray voltage: 5.5 kV (positive) or -4 kV (negative). The retention time and signal intensity of the total ion chromatogram peaks of the QC samples overlapped significantly (Figure 2), validating the high stability of the instrument.
| Time (minute) | Flow velocity (μL/minute) | A: Water (%) | B: Acetonitrile (%) |
| 0.0 | 400 | 95 | 5 |
| 3.5 | 400 | 85 | 15 |
| 6.0 | 400 | 70 | 30 |
| 6.5 | 400 | 70 | 30 |
| 12.0 | 400 | 30 | 70 |
| 12.5 | 400 | 30 | 70 |
| 18.0 | 400 | 0 | 100 |
| 22.0 | 400 | 0 | 100 |
| 25.0 | 400 | 0 | 100 |
| 26.0 | 400 | 95 | 5 |
| 30.0 | 400 | 95 | 5 |
The spectral data was processed using XCMS software, which handled retention time adjustments, peak detection, extraction, integration and alignment. Compound identification was achieved by cross-referencing against a custom MS/MS database and applying fragmentation pattern analysis. To analyse the data more accurately, we prepared and organised the original data (data management): The deviant values were filtered based on the relative standard deviation, which is the coefficient of variation. A single peak was filtered, the peak area of a single group with no more than 50% empty values or all groups with no more than 50% hollow values were retained and recoding was performed by filling the missing values in the original data with half of the minimum value; finally, data normalisation was performed.
SIMCA (V16.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden) software was used for logarithmic conversion and centralised formatting of the data. PCA and orthogonal partial least squares–discriminant analysis were performed on the processed data.
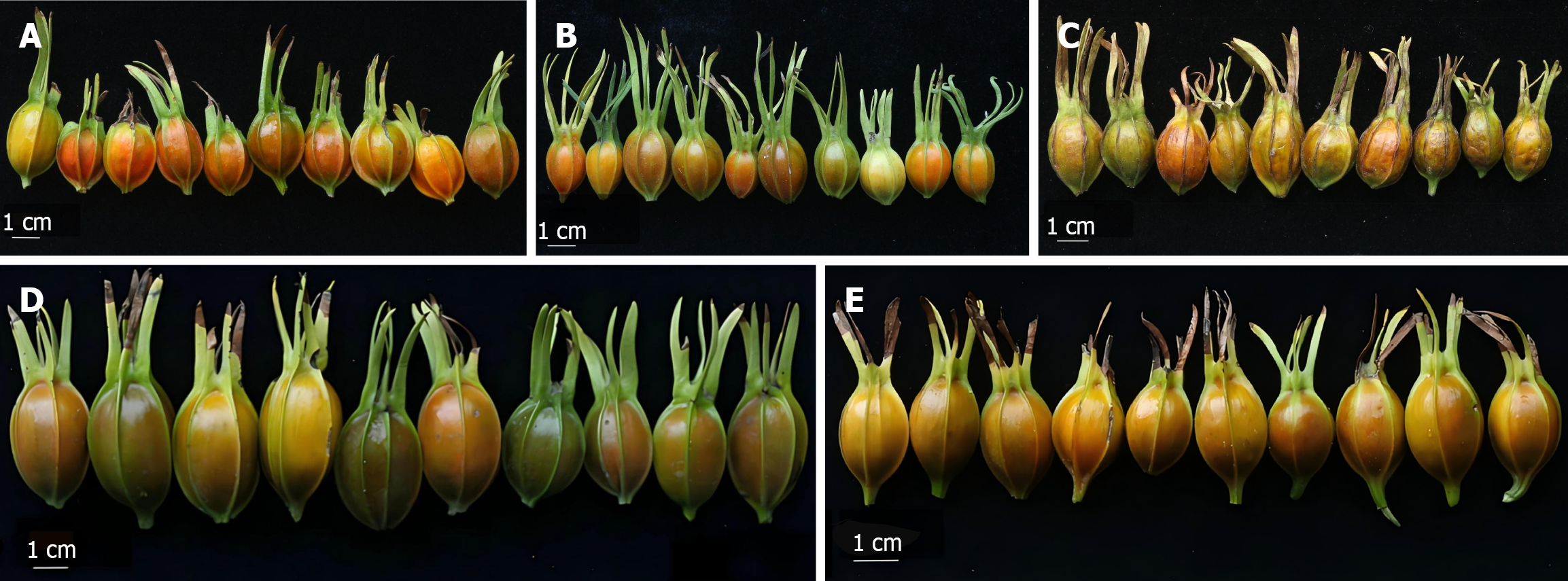
The morphological characteristics of the five kinds of wild G. jasminoides fruits are shown in Figure 1 and Table 2. The fruits varied significantly in shape—predominantly oblong, spherical, ellipsoid and obovate. NX fruits were the longest (29.11 mm), widest (16.75 mm) and heaviest (328.33 g), while XX fruits had the longest sepal (24.99 mm).
| Abbreviate name | Length (mm) | Width (mm) | Sepal length (mm) | Weight per hundred kernels (g) |
| LK | 19.23 ± 1.83c,e | 13.12 ± 1.38d | 15.66 ± 0.95c | 164.67 ± 27.75c |
| ML | 23.56 ± 3.29e | 13.30 ± 1.39d | 18.69 ± 0.24a | 186.67 ± 32.15c |
| XX | 16.94 ± 1.51d | 12.15 ± 0.82d | 24.99 ± 0.79e | 128.33 ± 7.64e |
| NX | 29.11 ± 2.57a | 16.75 ± 2.22b | 19.38 ± 0.41a | 328.33 ± 18.93b |
| SY | 18.49 ± 4.71c,d | 13.24 ± 0.86d | 21.90 ± 0.65d | 51.67 ± 12.58d |
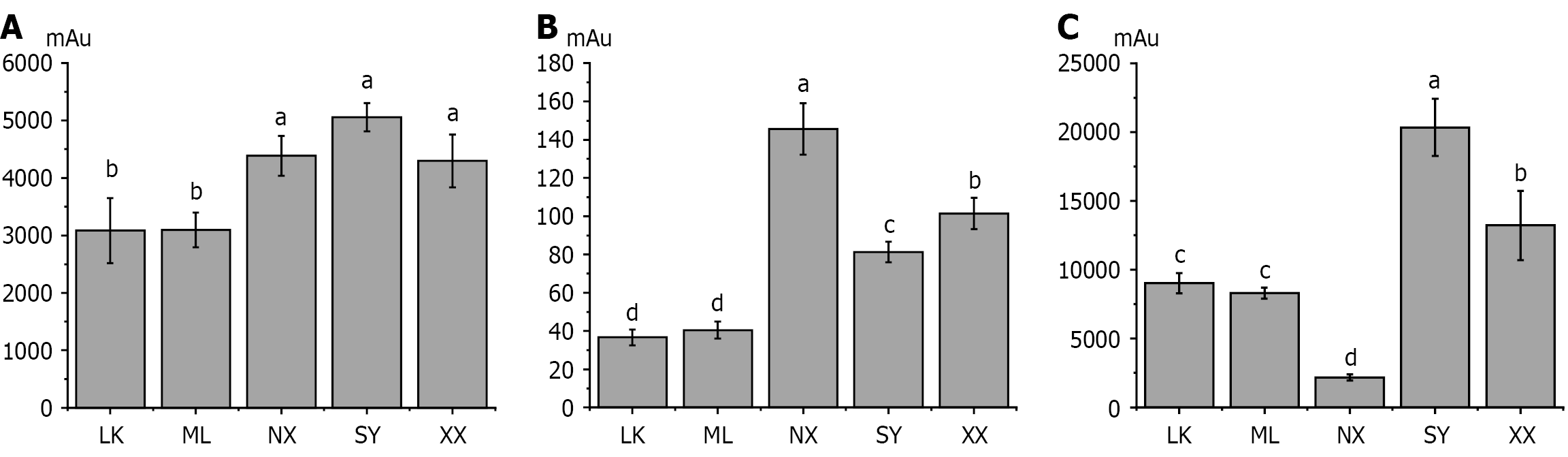
The relative contents of the typical organic acids and terpenoids (chlorogenic acid, crocetin and geniposide) in wild G. jasminoides fruits from the five sources were determined using HPLC. No significant difference was observed in the contents of three active ingredients in ML and LK extracts, while significant differences were observed in those of SY, NX and XX extracts. The concentrations of the components are indicated by the chromatographic peak areas illustrated in Figure 3. Geniposide and crocetin contents in the SY extract are significantly higher than those in the other four extracts; the NX extract shows the most intense chlorogenic acid peak, but the lowest intensity crocetin peak.
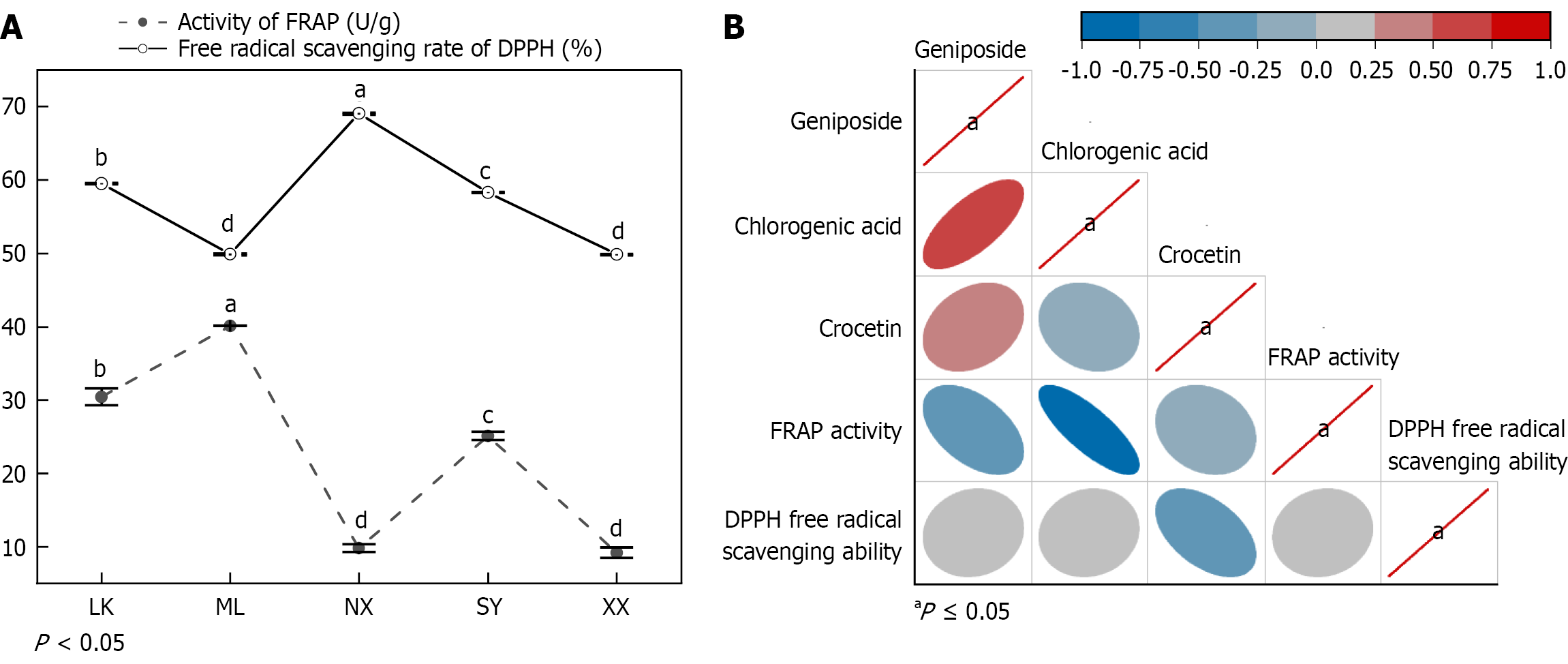
Figure 3 illustrates the DPPH free radical scavenging ability of the five wild G. jasminoides fruit extracts, ranging from 49.84% to 69.04%. The DPPH scavenging ability of the XX extract was the lowest, while the DPPH free radical scavenging rate of the NX extract was the highest. There were significant differences in the DPPH free radical scavenging rate between the ML and XX extracts (P < 0.05). The FRAP activity of 5 G. jasminoides fruit extracts ranged from 9.20 U/g to 40.13 U/g. The FRAP activity of the XX extract was the lowest, while that of the ML extract was the strongest (Figure 4A). No significant differences were observed in the FRAP activities of the NX and XX extracts, while significant differences were observed in those of the other three wild G. jasminoides fruit extracts (P < 0.05; Figure 4B).
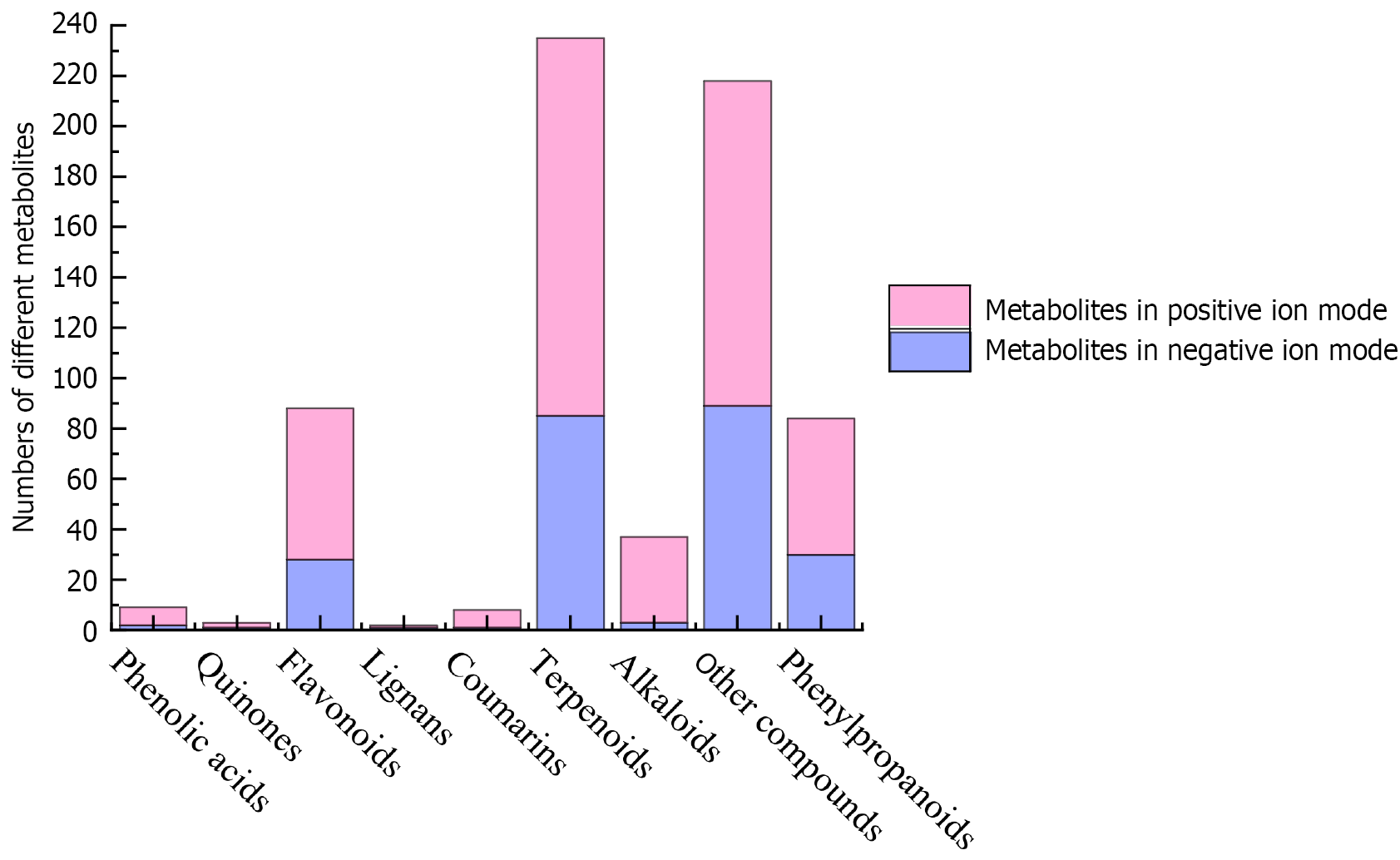
To determine the diversity of metabolites in the fruits of the wild G. jasminoides procured from different sources in the Hunan Province, a non-targeted metabolomics analysis was conducted, following standard procedures used in the study of traditional Chinese medicines. After impurity removal noise reduction, a total of 444 metabolites were detected with reference to the Kyoto Encyclopaedia of Genes and Genomes (KEGG) database in the positive ion mode (Figure 5), including 7 phenolic acids, 2 quinones, 60 flavonoids, 1 Lignan, 7 coumarins and derivatives, 150 terpenoids, 34 alkaloids, 54 phenylpropanoids and 129 other compounds. In the negative ion mode, 240 metabolites were detected, including 2 phenolic acids, 1 quinone, 28 flavonoids, 1 Lignan, 1 coumarin, 85 terpenoids, 3 alkaloids, 30 phenylpropanoids and 89 other compounds. In general, terpenoids were the most abundant in the wild G. jasminoides fruit, followed by flavonoids.
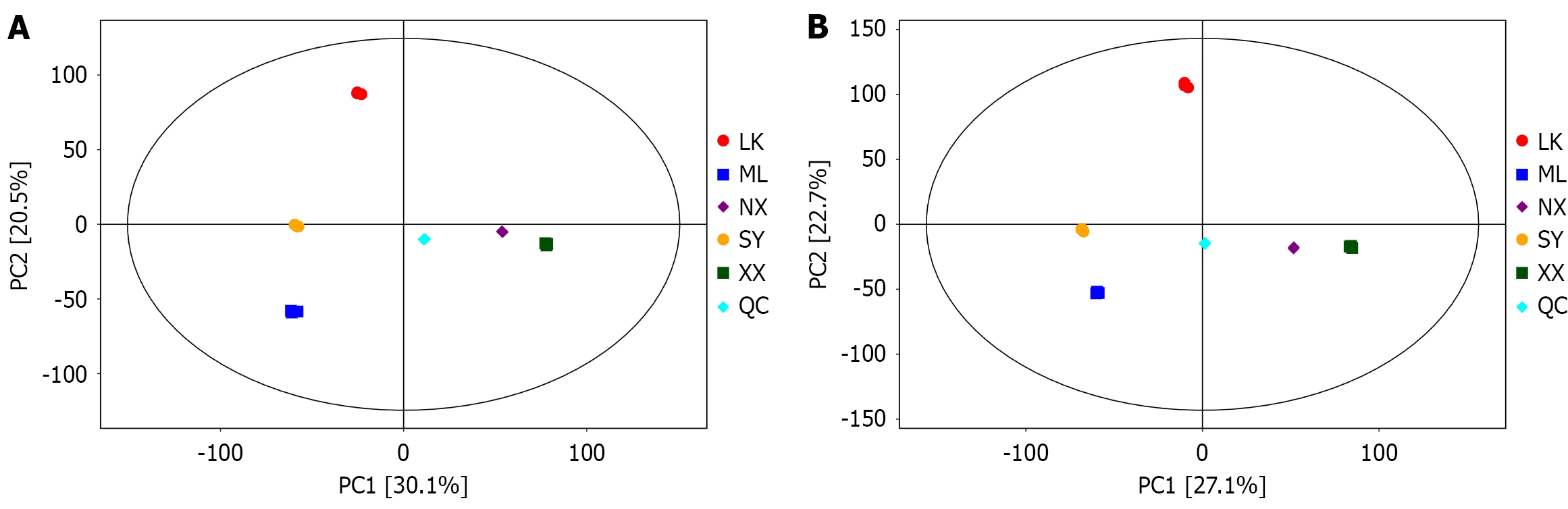
To further explore the diversity of the chemical components in the wild G. jasminoides fruits from the five different sources, the metabolites of the five extracts were analysed by PCA (Figure 6). PC1 and PC2 contributed 30.1% and 20.5%, respectively. The LK, ML and SY extracts of PC1 showed a significant separation trend from the NX and XX extracts in both positive and negative ion modes. This demonstrates that the accumulation patterns of metabolites in the LK, ML and SY extracts differ significantly from those of XX and NX extracts.
In this study, five kinds of terpenes were detected, namely moniterpenes, diterpenes, triterpenes, sesquiterpenes and iridoid terpenes (Figure 7A), among which moniterpenes were the most abundant. To further explore the composition difference of G. jasminoides fruits procured from different areas, the monoterpenoids of each extract were analysed by HCA. The analysis results show that the monoterpenoid metabolic profiles of the XX and NX extracts were similar, agreeing with the result of PCA. In the positive ion mode, oleanane-2H, +1O, 1COOH, O-HexA-HexA, fusaprolife
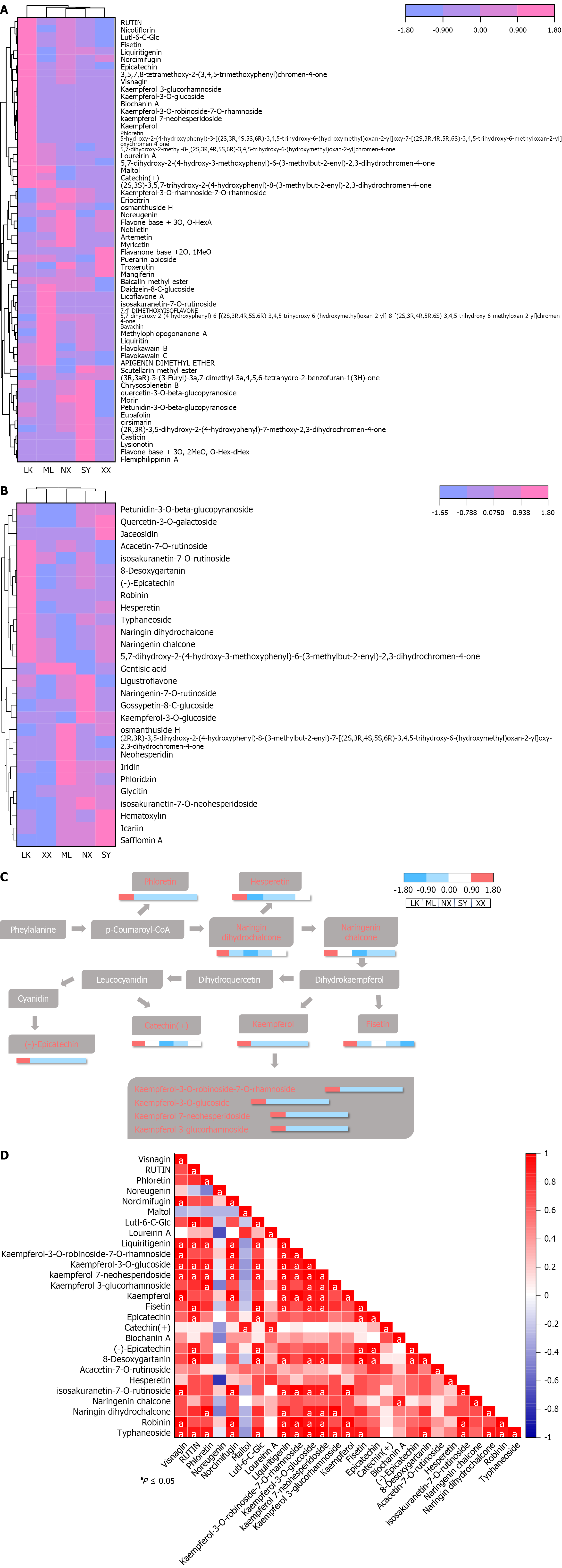
Significant differences were observed in the content of flavonoids in G. jasminoides fruits procured from the five provenances (Figure 8). In the positive ion mode, the levels of 23 metabolites, including visnagin, rutin, phloretin, noreugenin, norcimifugin, maltol, lutl-6-c-glc, loureirin a, liquiritigenin, kaempferol-3-o-robinoside-7-o-rhamnoside, kaempferol-3-o-glucoside, kaempferol 7-neohesperidoside, kaempferol 3-glucorhamnoside, kaempferol, fisetin, epi
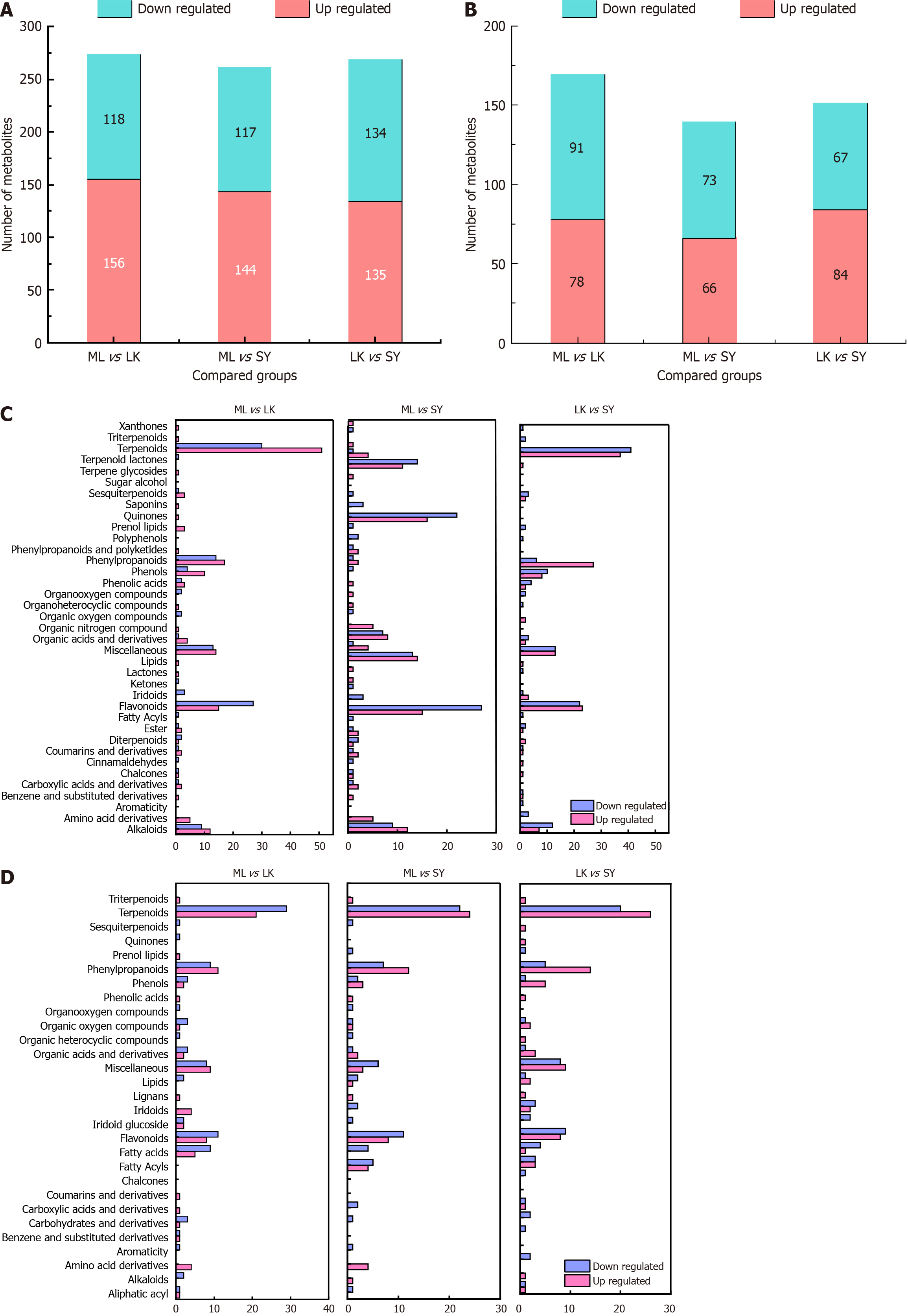
The results of PCA and HCA indicate a more significant separation trend among LK, ML and SY extracts compared with that between NX and XX extracts. To investigate the underlying causes for the significant differences between the three fruits, we used fold change (FC) and variable performance in the projection to screen for differential metabolites; metabolites that met FC > 2 or FC < 0. 5 with a P value less than 0.05 were considered differential metabolites (Figure 9). In the positive ion mode, a total of 274 differential metabolites were screened in the ML vs LK comparison, of which 156 were upregulated and 118 were downregulated. The comparison between ML and SY showed a total of 261 differential metabolites, of which 144 were upregulated and 117 were downregulated. The LK vs SY comparison had a total of 270 differential metabolites, of which 135 were upregulated and 135 were downregulated. In the negative ion mode, a total of 169 differential metabolites were screened in the ML and LK comparison, of which 78 were upregulated and 91 were downregulated. The ML vs SY comparison had a total of 139 differential metabolites, of which 66 were upregulated and 73 were downregulated. A total of 151 differential metabolites were screened in the LK vs SY comparison, of which 84 are upregulated and 67 are downregulated. The ML vs LK comparison revealed the highest number of differential metabolites. In general, the profiles of the differential metabolites in the comparisons of ML vs SY, LK vs SY and ML vs LK show an upward trend. Hence, ML is likely to have higher medicinal value. To gain deeper insights into the metabolic differences in each extract of SY, ML and LK, metabolites were grouped into subcategories (Figure 9C and D). We found that these differentially expressed metabolites were predominantly alkaloids, flavonoids, phenols, phenylpropanoids, terpenoids and a few amino acid derivatives. Among them, the LK extract displayed the highest upregulation of fla
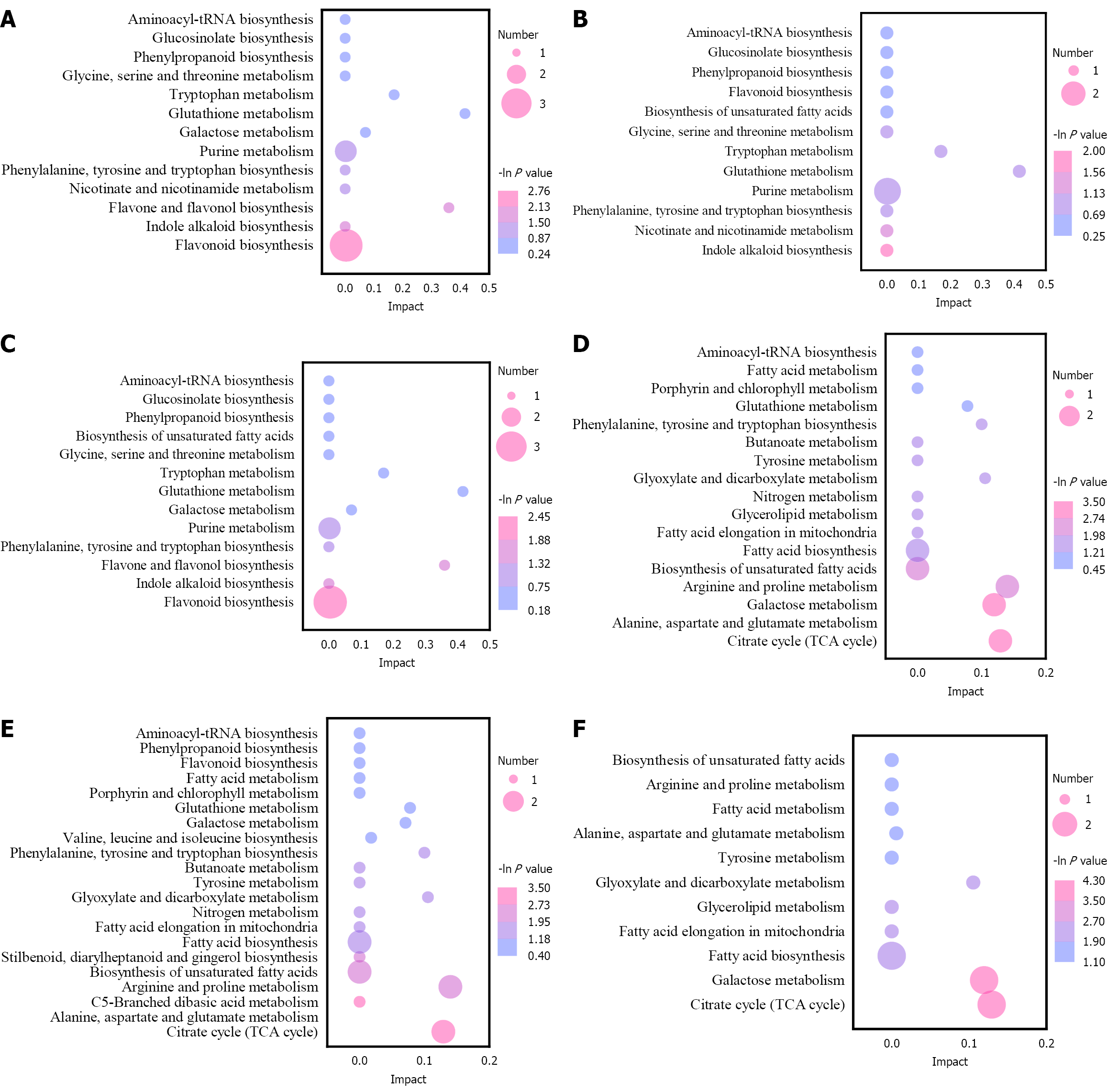
The differential metabolites in each group were analysed by KEGG annotation and enrichment analyses. In the positive ion mode, the differential metabolites of LK vs SY, ML vs LK and ML vs SY comparisons were enriched to 13, 13 and 12 metabolic pathways, respectively (Figure 10A-C). In the negative ion mode, LK vs SY, ML vs LK and ML vs SY compa
In the positive ion mode, flavonoid biosynthesis was the most enriched metabolic pathway in the LK vs SY and ML vs LK comparisons, whereas indole alkaloid biosynthesis was the most enriched metabolic pathway in the ML vs SY comparison. The former pathway involved the differential metabolites kaempferol, epicatechin and myricetin, while the latter involved L-tryptophan, indicating that kaempferol, epicatechin and myricetin were marker metabolites that could be used to distinguish LK and the other two samples. In the negative ion mode, the most enriched metabolic pathway in the LK vs SY, ML vs LK and ML vs SY comparisons was the citrate cycle (TCA cycle), which involved the differential metabolites citric acid and fumaric acid. The results indicated that the TCA cycle was an important metabolic pathway for distinguishing LK, SY and ML; hence, citric and fumaric acids could be used as marker metabolites to distinguish the three samples.
Fruits of G. jasminoides have high medicinal value, and their quality directly affects the safety and effectiveness of the medication[3]. Owing to the comprehensive impact of growth environments and cultivation techniques, secondary metabolites develop a strong correlation and correspondence with the growth environment during long-term evolution through interactions with their biological and abiotic environments[13,14]. Environmental conditions strongly affect the accumulation of secondary metabolites. Thus, high-quality seed sources, good planting environments and appropriate field management are essential to improve the quality of the fruit of G. jasminoides at the planting stage.
In this study, significant differences in the appearance of wild G. jasminoides fruits procured from five producing areas were observed. NX fruits were the longest, widest and heaviest, while XX fruits had the longest sepal, indicating that appearance can be used to distinguish G. jasminoides fruits from different regions.
The antioxidant capacity of G. jasminoides fruits was also analysed. The focus of DPPH radical-scavenging and FARP iron-reduction abilities was different. According to the experimental results, the DPPH radical scavenging activity of the NX extract was significantly higher than that of the others, while no significant difference was observed between ML and XX extract DPPH radical scavenging activities, and these were the lowest of the five. Contrastingly, the FARP iron reduction ability of ML was significantly higher than that of the others, while no significant difference was observed in that between the NX and XX extracts, and these were also the lowest of the five.
G. jasminoides fruits are well-known for their rich active ingredients. Studies have shown that components such as chlorogenic acid in G. jasminoides fruits have strong antioxidant activity[15,16]. However, in this study, no significant correlation between DPPH radical scavenging activity and the contents of geniposide, crocetin and chlorogenic acid was observed. Interestingly, the FARP iron reduction ability and the presence of chlorogenic acid demonstrated a significant negative correlation, which contradicts the reports from previous studies. This discrepancy could have resulted from methodological differences. This phenomenon also indicates that although certain substances possess strong antioxidant capabilities, the antioxidant capacity of plants is not determined by a single component, but rather by a combination of different components.
The biosynthesis of chlorogenic acid occurs downstream of the phenylpropanoid pathway, in which phenylalanine ammonia-lyase produces cinnamic acid, which is subsequently hydroxylated and methylated to produce ρ-coumaric acid, which in turn is converted to chlorogenic acid[17,18]. The higher content of chlorogenic acid in the NX extract therefore results from the abundant cinnamic acid, which provides a substrate for the synthesis of chlorogenic acid.
Phenolic acids play a vital role in plant defence against biotic (including microorganisms, bacteria, viruses, insects and fungi) and abiotic (including salt, temperature, dehydration and ultraviolet radiation) stresses and are thus widespread in the plant kingdom[19]. In the present study, the chlorogenic acid content of different producing areas varies greatly, likely owing to a combination of multiple factors such as light, temperature, water and nutrient elements in the growth environment[20-25]. In this study, the content of chlorogenic acid was evidently influenced by the locality of growth, corroborating previous findings.
In this study, eight types of differential metabolites were detected in G. jasminoides fruits procured from five different regions in the Hunan Province, China, by UPLC-MS/MS, including phenylpropanoids, terpenoids, flavonoids, benzene and substituted derivatives, alkaloids, esters, organic acids and derivatives, phenolic acids and prenol lipids; terpenoids were the most abundant. HCA was performed on the most abundant monoterpenes, and the result was similar to that of PCA, showing that the NX and XX extracts were clustered into one class. In addition, the HCA result of flavonoids showed that flavonoids of G. jasminoides fruits from different producing areas were significantly different. The content of 33 flavonoids in the LK extract was significantly higher than that of G. jasminoides fruits from the other four regions, including kaempferol-3-o-robinoside-7-o-rhamnoside, kaempferol-3-o-glucoside, kaempferol 7-neohesperidoside, kaempferol 3-glucorhamnoside, kaempferol, epicatechin, catechin(+), (-)-epicatechin, hesperetin, naringenin chalcone, naringin and dihydrochalcone; this may be related to the stress resistance function of flavonoids in the wild growth environment[26]. Research has shown that flavonoids play a vital role in the resistance of plants to salt, drought and UV-B stress[27,28]. Naringin, a primary node compound in the flavonoid pathway, plays a significant role in the process of resisting environmental stress by generating compounds such as hesperetin, kaempferol, fisetin catechin (+) and (-) - epicachin in wild G. jasminoides fruits from Lukou, Hunan, China. Meanwhile, only three types of catechins [catechin (+), (-) - epicatechin, and epigallocatechin] significantly increased in the fruits in this area, which can be caused by the accumulation of a large amount of flavonoids in the form of flavonol glycosides, leading to a decrease in the synthesis of flavanols. Therefore, we speculate that the stress on plants in this region is stronger than in the other four regions.
The differential metabolites identified in the fruits of SY, ML and LK were predominantly alkaloids, flavonoids, phenols, terpenoids, and amino acid derivatives. These differential metabolites could be used to distinguish the fruits of SY, ML and LK. In addition, the LK extract had the highest amount of flavonoids and the lowest amount of phenols and amino acid derivatives. Nitrogen deficiency can facilitate the synthesis of higher levels of flavonoids[29]. In this study, there were fewer amino acid derivatives in the LK extract, while flavonoids were the most abundant, especially naringenin, naringen dihydrochalcone, Lutl-6-C-Glc, kaempferol and its related derivative glycosides, which were significantly higher. Among them, kaempferol was significantly annotated in flavonoid biosynthesis, leading to the derivation of more flavonoid glycosides, such as robinin, typhaninoside, and rutin. This was also consistent with the conclusion drawn in previous studies that nitrogen limitation usually induces the production of flavonoid glycosides, such as rutin[30].
This study used UPLC-MS/MS to investigate the metabolites of wild G. jasminoides fruits procured from five regions in the Hunan Province, China, and explore their differences. The results showed that although previous studies have shown that geniposide, crocin and chlorogenic acid have strong antioxidant properties, the correlation between the content of geniposide and crocetin, DPPH free radical scavenging rate and FRAP activity was not significant in this study. Concurrently, the content of chlorogenic acid and FRAP activity exhibited a significant negative correlation. This discrepancy could have resulted from methodological differences. Furthermore, while individual phytochemicals may exhibit potent antioxidant properties, the overall antioxidant capacity of plants is not governed by a single constituent but is strongly influenced by the synergistic interactions among multiple components. The fruits of G. jasminoides contain abundant terpenes and flavonoids. The HCA of terpenes exhibited similar results to the overall HCA and PCA, indicating that the metabolic characteristics of the NX and XX extracts were similar, while those of LK, ML and SY extracts differed sig
| 1. | Chen J, Tang W, Li C, Kuang D, Xu X, Gong Y, Liu F, Gao S. Multi-omics analysis reveals the molecular basis of flavonoid accumulation in fructus of Gardenia (Gardenia jasminoides Ellis). BMC Genomics. 2023;24:588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Jiang W, Jiang L, Yin X, Zhang S, Duan X, Chen J, Liu Y, Zheng H, Tao Z. Untargeted Metabolomics Reveals the Metabolic Characteristics and Biomarkers of Antioxidant Properties of Gardeniae Fructus from Different Geographical Origins in China. Metabolites. 2025;15:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Li K, Gao L, Yang X, Wang M, Feng X, Deng S. Genome-Wide Resequencing Reveals Genetic Relationships between Main Gardenia jasminoides Ellis Cultivars. Horticulturae. 2023;9:754. [DOI] [Full Text] |
| 4. | Yin F, Liu J. Research and application progress of Gardenia jasminoides. Chin Herb Med. 2018;10:362-370. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Peng X, Tan L, Song J, Lai Y, Yu S, Xu F, Wei Q, He Z, Cheng W, Zhang W, Yang X. Geniposide alleviated hydrogen peroxide-induced apoptosis of human hepatocytes via altering DNA methylation. Food Chem Toxicol. 2023;182:114158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Ai Y, Chen Y, Li C, Li P, Chen J, Jiang L, Pan Y, Sun A, Yang Y, Liu Q. Elucidation of Geniposide and Crocin Accumulation and Their Biosysnthsis-Related Key Enzymes during Gardenia jasminoides Fruit Growth. Plants (Basel). 2023;12:2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Chen L, Li M, Yang Z, Tao W, Wang P, Tian X, Li X, Wang W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J Ethnopharmacol. 2020;257:112829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 8. | Qin S, Tian J, Zhao Y, Wang L, Wang J, Liu S, Meng J, Wang F, Liu C, Han J, Pan C, Zhang Y, Yi Y, Li C, Liu M, Liang A. Gardenia extract protects against intrahepatic cholestasis by regulating bile acid enterohepatic circulation. J Ethnopharmacol. 2024;319:117083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Wang L, Chen S, Liu S, Biu AM, Han Y, Jin X, Liang C, Liu Y, Li J, Fang S, Chang Y. A comprehensive review of ethnopharmacology, chemical constituents, pharmacological effects, pharmacokinetics, toxicology, and quality control of gardeniae fructus. J Ethnopharmacol. 2024;320:117397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 10. | He Q, Lu A, Qin L, Zhang Q, Lu Y, Yang Z, Tan D, He Y. An UPLC-Q-TOF/MS-Based Analysis of the Differential Composition of Dendrobium officinale in Different Regions. J Anal Methods Chem. 2022;2022:8026410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Fan X, Gao X, Deng Y, Ma B, Liu J, Zhang Z, Zhang D, Yang Y, Wang C, He B, Nie Q, Ye Z, Liu P, Wen J. Untargeted plasma metabolome identifies biomarkers in patients with extracranial arteriovenous malformations. Front Physiol. 2023;14:1207390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Li XY, Fu YJ, Fu YF, Wei W, Xu C, Yuan XH, Gu CB. Simultaneous quantification of fourteen characteristic active compounds in Eucommia ulmoides Oliver and its tea product by ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry (UPLC-QqQ-MS/MS). Food Chem. 2022;389:133106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Rabeh K, Hnini M, Oubohssaine M. A comprehensive review of transcription factor-mediated regulation of secondary metabolites in plants under environmental stress. Stress Biol. 2025;5:15. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Luo Z, Liu L, Nie Q, Huang M, Luo C, Sun Y, Ma Y, Yu J, Du F. HPLC-based metabolomics of Dendrobium officinale revealing its antioxidant ability. Front Plant Sci. 2023;14:1060242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Reddy YM, Kumar SPJ, Saritha KV, Gopal P, Reddy TM, Simal-Gandara J. Phytochemical Profiling of Methanolic Fruit Extract of Gardenia latifolia Ait. by LC-MS/MS Analysis and Evaluation of Its Antioxidant and Antimicrobial Activity. Plants (Basel). 2021;10:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Saravanakumar K, Park S, Sathiyaseelan A, Kim KN, Cho SH, Mariadoss AVA, Wang MH. Metabolite Profiling of Methanolic Extract of Gardenia jaminoides by LC-MS/MS and GC-MS and Its Anti-Diabetic, and Anti-Oxidant Activities. Pharmaceuticals (Basel). 2021;14:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | He L, Xu X, Li Y, Li C, Zhu Y, Yan H, Sun Z, Sun C, Song J, Bi Y, Shen J, Cheng R, Wang Z, Xiao W, Chen S. Transcriptome analysis of buds and leaves using 454 pyrosequencing to discover genes associated with the biosynthesis of active ingredients in Lonicera japonica Thunb. PLoS One. 2013;8:e62922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Peng X, Li W, Wang W, Bai G. Cloning and characterization of a cDNA coding a hydroxycinnamoyl-CoA quinate hydroxycinnamoyl transferase involved in chlorogenic acid biosynthesis in Lonicera japonica. Planta Med. 2010;76:1921-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Arbona V, Manzi M, Ollas Cd, Gómez-Cadenas A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int J Mol Sci. 2013;14:4885-4911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 20. | Tinyane PP, Sivakumar D, Soundy P. Influence of photo-selective netting on fruit quality parameters and bioactive compounds in selected tomato cultivars. Sci Hortic. 2013;161:340-349. [DOI] [Full Text] |
| 21. | Vithana MD, Singh Z, Johnson SK. Regulation of the levels of health promoting compounds: lupeol, mangiferin and phenolic acids in the pulp and peel of mango fruit: a review. J Sci Food Agric. 2019;99:3740-3751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Yan K, Zhao S, Bian L, Chen X. Saline stress enhanced accumulation of leaf phenolics in honeysuckle (Lonicera japonica Thunb.) without induction of oxidative stress. Plant Physiol Biochem. 2017;112:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Grace SC, Logan BA, Adams WW. Seasonal differences in foliar content of chlorogenic acid, a phenylpropanoid antioxidant, in Mahonia repens. Plant Cell Envir. 1998;21:513-521. [DOI] [Full Text] |
| 24. | Bartley GE, Avena-bustillos RJ, Du W, Hidalgo M, Cain B, Breksa AP. Transcriptional regulation of chlorogenic acid biosynthesis in carrot root slices exposed to UV-B light. Plant Gene. 2016;7:1-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Rodríguez-Calzada T, Qian M, Strid Å, Neugart S, Schreiner M, Torres-Pacheco I, Guevara-González RG. Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol Biochem. 2019;134:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Togami J, Okuhara H, Nakamura N, Ishiguro K, Hirose C, Ochiai M, Fukui Y, Yamaguchi M, Tanaka Y. Isolation of cDNAs encoding tetrahydroxychalcone 2′-glucosyltransferase activity from carnation, cyclamen, and catharanthus. Plant Biotechnol. 2011;28:231-238. [DOI] [Full Text] |
| 27. | Ma B, Song Y, Feng X, Guo P, Zhou L, Jia S, Guo Q, Zhang C. Integrated Metabolome and Transcriptome Analyses Reveal the Mechanisms Regulating Flavonoid Biosynthesis in Blueberry Leaves under Salt Stress. Horticulturae. 2024;10:1084. [DOI] [Full Text] |
| 28. | Chen G, Li D, Yao P, Chen F, Yuan J, Ma B, Yang Z, Ding B, He N. Metabolic and Transcriptional Analysis Reveals Flavonoid Involvement in the Drought Stress Response of Mulberry Leaves. Int J Mol Sci. 2024;25:7417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Liu J, Liu M, Fang H, Zhang Q, Ruan J. Accumulation of Amino Acids and Flavonoids in Young Tea Shoots Is Highly Correlated With Carbon and Nitrogen Metabolism in Roots and Mature Leaves. Front Plant Sci. 2021;12:756433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Tewari RK, Yadav N, Gupta R, Kumar P. Oxidative Stress Under Macronutrient Deficiency in Plants. J Soil Sci Plant Nutr. 2021;21:832-859. [DOI] [Full Text] |